Abstract
A variety of 1-allenyl-2-propargyl-substituted cyclopentanol derivatives were found to undergo facile intramolecular microwave-assisted 2+2 alleneyne cycloaddition reactions to generate tricyclic 5-6-4 ring systems present in the sterpurenes.
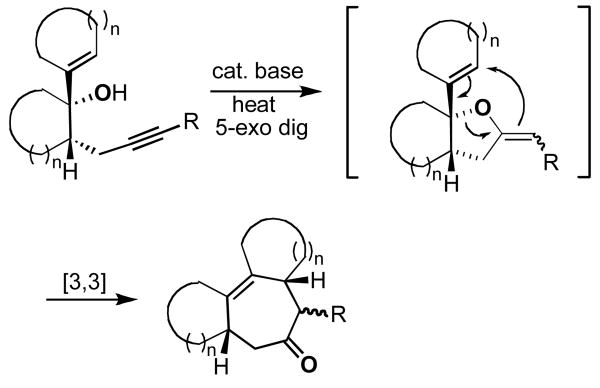
We have recently investigated a known but largely ignored tandem reaction that involves a base-catalyzed intramolecular 5-exo cyclization of appropriately substituted 4-pentyn-1-ols, followed by in situ thermal Claisen rearrangement of the intermediate 2-methylenetetrahydrofurans (Figure 1).1–8 These reactions, which provide convenient access to a number of interesting cyclohept-4-enone systems, are most conveniently performed under microwave irradiation that typically allows for greatly shortened reaction times and increased product yields.5–8
Figure 1.
General method for the generation of polycyclic ring systems via tandem 5-exo dig cyclization/Claisen rearrangement sequence.
We were intrigued about the possibility of extending this methodology to systems that would involve the allene variant of the Claisen rearrangement.9–12 It was envisioned that such reactions might provide a facile route to 3-alkylidene substituted cyclohept-4-enone derivatives.
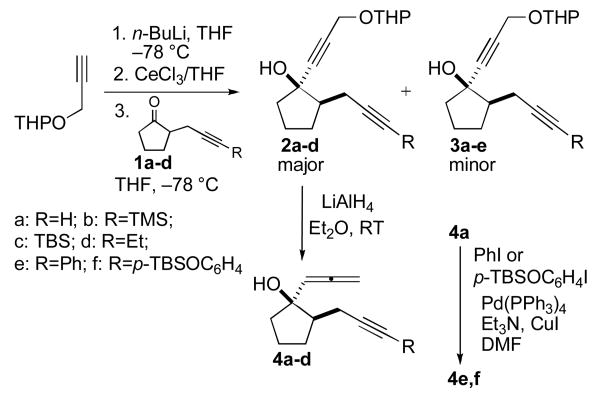
The requisite allene derivatives used for this study were prepared in a straightforward fashion by first reacting the acetylide anion derived from a THP protected propargyl alcohol with different 2-propargyl-substituted cyclopentanone derivatives 1a-d (Scheme 1). These reactions resulted in the formation of diastereomeric mixtures from which products having the propargyl and hydroxyl substitutents either cis (major) or trans (minor) were easily separated by column chromatography. Subsequent reaction of 2a-d with LiAlH4 at room temperature for 30 min afforded the expected allene products 4a-d as single diastereomers typically in 70-90 % isolated yields. Allene products bearing aromatic substituents on the triple bond terminus were prepared in a straightforward fashion from 4a via the Sonogashira reaction.13
Scheme 1.
Synthesis of 1-allenyl-2-propargyl-substituted cyclopentanol derivatives.
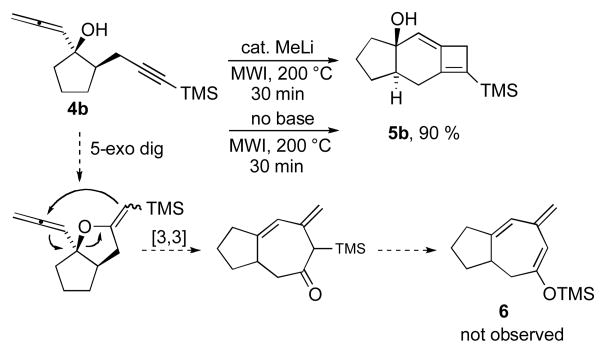
To assess whether these alleneynes would undergo the anticipated tandem 5-exo cyclization/Claisen rearrangement reaction, compound 4b was subjected to microwave irradiation in the presence of catalytic MeLi. After 30 min of heating at 210 °C in the microwave oven, the starting material was consumed, and a single new product had been formed in nearly quantitative yield (Scheme 2). Interestingly, spectroscopic analysis of the isolated product was consistent with compound 5b having the tricyclic 5-6-4 structure; none of the initially expected 6 was produced under these conditions. The reaction outcome, including yield, was further found to be independent of added base, suggesting that formation of the observed product was mechanistically consistent with a thermal intramolecular [2+2] allenyne cycloaddition process.
Scheme 2.
Preparation of compound 6 via intramolecular [2+2] alleneyne cycloaddition reaction.
At the time these studies were conducted, only a handful of reports involving [2+2] cycloaddition reactions of allenes and alkynes were known in the literature14–17 and of these, only two involved an intramolecular variant of this process. In both cases, the [2+2] cycloaddition reactions were observed as by-products in molybdenum mediated Pauson-Khand reactions.16,17 Brummond et al.18 later showed that bicyclo[4.2.0]octa-1,6-dienes and bicyclo[5.2.0]nona-1,7-dienes could be accessed through [2+2] allenic cycloaddition reaction under microwave irradiation. In addition, Oh et al.19 discovered approximately at the same time that appropriately substituted allenynes underwent similar thermal cycloaddition reactions with or without transition metal catalysts, providing a facile route to a number of bicyclic compounds. Very recently, Mukai et al.20 demonstrated that bicyclo[6.2.0]deca-1,8-dienes, bicyclo[5.2.0]nona-1,7-dienes, and bicyclo[4.2.0]octa-1,6-dienes could be prepared using thermal [2+2] cycloaddition of allenynes.
In addition to the TMS derivative described above, we found that other allenynes bearing different substitutents at the triple bond terminus could also be used to generate analogous tricyclic products. The reaction was shown to be rather general and independent of the type of substituent on the triple bond with the exception of terminal alkynes, which decomposed under the reaction conditions employed. The results from these experiments are summarized in Table 1.21
Table 1.
Intramolecular [2+2] alleneyne cycloaddition reactions of alleneynes 4a-f.a
| Entry | Substrate | Product | Yield (%) |
|---|---|---|---|
| 1 | 4b | 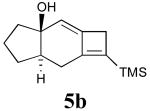 |
90 |
| 2 | 4c | 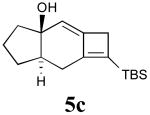 |
85 |
| 3 | 4e | 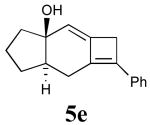 |
92 |
| 4 | 4d | 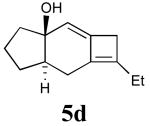 |
70 |
| 5 | 4f | 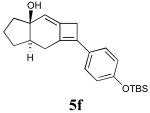 |
75 |
| 6 | 4a | 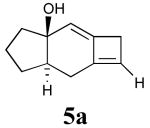 |
0 |
All reactions were conducted in 10 mL base-washed microwave vials under MWI using phenetole as the solvent at 200 °C for 30 min except reactions affording 5e and 5f (entries 3 and 5), which were conducted at 150 °C for 45 min.
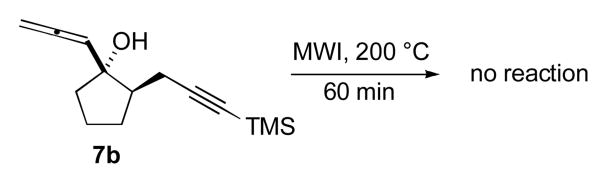
Interestingly, only those allenic systems having the OH and the propargylic moieties in a cis orientation were found to be reactive under the conditions investigated. In fact, only unreacted starting material was recovered when 7b, the trans analogue of 4b, was subjected to microwave irradiation at 200 °C. Preliminary examination of molecular models suggests that the ring geometry of 4b allows for better overlap of the terminal p-orbitals of both the allene and acetylene moieties compared to those in 7b, which may explain the observed resistance of 7b toward cycloaddition in this relatively rigid system.
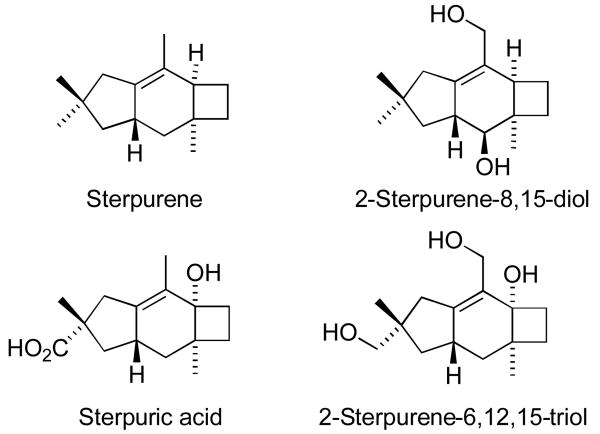
Notably, the methodology described in this communication allows a straightforward entry to the carbon skeleton of sterpurene, which is one of several closely related structures that have been isolated as metabolites of the fungus Chondrostereum purpureum (Figure 2).22 This fungus is responsible for the “silver leaf” disease, which is widespread in North America. Although the 5-6-4 ring system and sterpurene have been synthesized before,23 the present methodology represents a novel approach to this unique ring system.
Figure 2.
Structures of sterpurene and three related fungal metabolites.
Supplementary Material
Scheme 3.
Attempted [2+2] cycloaddition of 7b.
Acknowledgments
This research was supported by grants from the donors of the Petroleum Research Fund, administered by the American Chemical Society, the National Institutes of Health (NIGMS), and the Camille and Henry Dreyfus Foundation (Scholar-Fellow Program). T.V.O also gratefully acknowledges support from the Hans and Ella McCollum-Vahlteich '21 endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ovaska TV, Roark JL, Shoemaker CM. Tetrahedron Lett. 1999;39:5705–5708. [Google Scholar]
- 2.Ovaska TV, Roses JB. Org Lett. 2000;2:2361–2364. doi: 10.1021/ol006139w. [DOI] [PubMed] [Google Scholar]
- 3.Ovaska TV, Reisman SE, Flynn MA. Org Lett. 2001;3:115–117. doi: 10.1021/ol006823a. [DOI] [PubMed] [Google Scholar]
- 4.Ovaska TV, Ravi Kumar JS, Hulford CA, O'Sullivan MF, Reisman SE. Tetrahedron Lett. 2002;43:1939–1941. [Google Scholar]
- 5.McIntosh CE, Martinez I, Ovaska TV. Synlett. 2004:2579–2581. [Google Scholar]
- 6.Martinez I, Alford PE, Ovaska TV. Org Lett. 2005;7:1133–1135. doi: 10.1021/ol050144o. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Kyne RE, Ovaska TV. Org Lett. 2006;8:5153–5156. doi: 10.1021/ol0620848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Kyne RE, Ovaska TV. Tetrahedron. 2007;63:1899–1906. [Google Scholar]
- 9.Huche M. Tetrahedron Lett. 1976;17:2607–2610. [Google Scholar]
- 10.Sleeman MJ, Meehan GV. Tetrahedron Lett. 1989;30:3345–3348. [Google Scholar]
- 11.Behrens U, Wolff C, Hoppe D. Synthesis. 1991:644–646. [Google Scholar]
- 12.Parsons PJ, Thomson P, Taylor A, Sparks T. Org Lett. 2000;2:571–572. doi: 10.1021/ol990305m. [DOI] [PubMed] [Google Scholar]
- 13.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467–4470. [Google Scholar]
- 14.Kimura M, Horino Y, Wakamiya Y, Okajima T, Tamaru Y. J Am Chem Soc. 1997;119:10869–10870. [Google Scholar]
- 15.Horino Y, Kimura M, Tanaka S, Okajima T, Tamaru Y. Chem Eur J. 2003;9:2419–2438. doi: 10.1002/chem.200304586. [DOI] [PubMed] [Google Scholar]
- 16.Cao H, Flippen-Anderson J, Cook JM. J Am Chem Soc. 2003;125:3230–3231. doi: 10.1021/ja021159+. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Hammond GB. J Am Chem Soc. 2002;124:6534–6535. doi: 10.1021/ja0263893. [DOI] [PubMed] [Google Scholar]
- 18.Brummond KM, Chen D. Org Lett. 2005;7:3473–3475. doi: 10.1021/ol051115g. [DOI] [PubMed] [Google Scholar]
- 19.Oh CH, Gupta AK, Park DI, Kim N. Chem Commun. 2005:5670–5672. doi: 10.1039/b508306k. [DOI] [PubMed] [Google Scholar]
- 20.Mukai C, Hara Y, Miyashita Y, Inagaki F. J Org Chem. 2007;72:4454–4461. doi: 10.1021/jo070513p. [DOI] [PubMed] [Google Scholar]
- 21.General procedure for the allenyne [2+2] cycloaddition reaction: Compound 4b (63 mg, 0.27 mmol) was dissolved in 1.0 mL of phenetole and the mixture was heated under MWI at 200 °C for 30 min in a base-washed (NaOH) 10 mL microwave vial. Phenetole was then removed in vacuo and the residue was passed through a short plug of deactivated silica gel (MeOH), eluting with 10% ethyl acetate in hexanes. Solvent evaporation afforded compound 5b (57 mg, 90%) as a clear oil. 1H NMR (CDCl3, 500 MHz) δ 5.04 (s, 1H), 2.92–3.00 (m, 2H), 2.40–2.51 (m, 1H), 2.11–2.21 (m, 2H), 1.82–1.92 (m, 2H), 1.75–1.82 (m, 1H), 1.65–1.75 (m, 1H), 1.61 (s, 1H), 1.48–1.60 (m, 1H), 1.23–1.35 (m, 1H); 13C NMR (CDCl3, 125 MHz) δ 156.17, 146.77, 143.38, 112.23, 78.69, 45.51, 39.41, 37.06, 28.67, 24.52, 20.10, -1.56. HRMS calc'd for C14H22OSi 234.1440, found 234.1334.
- 22.Ayer WA, Saeedi-Ghomi MH. Can J Chem. 1981;59:2536–2538. [Google Scholar]
- 23.For existing synthetic approaches to sterpurene, see e.g. Harmata M, Bohnert GJ. Org Lett. 2003;5:59–61. doi: 10.1021/ol027176l.Mehta G, Sreenivas K. Tetrahedron Lett. 2002;43:703–706.Singh V, Alam SQ. J Chem Soc, Chem Commun. 1999:2519–2520.Zhao SK, Helquist PJ. Org Chem. 1990;55:5820–5821.Murata Y, Ohtsuka T, Shirahama H, Matsumoto T. Tetrahedron Lett. 1981;22:4313–4314.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.