Abstract
B lymphocytes can function independently as efficient APCs. However, our previous studies demonstrate that both dendritic cells and macrophages are necessary to propagate immune responses initiated by B cell APCs. This finding led us to identify a process in mice whereby Ag-specific B cells transfer Ag to other APCs. In this study, we report the ability and mechanism by which human B lymphocytes can transfer BCR-captured Ag to macrophages. The transfer of Ag involves direct contact between the two cells followed by the capture of B cell-derived membrane and/or intracellular components by the macrophage. These events are abrogated by blocking scavenger receptor A, a receptor involved in the exchange of membrane between APCs. Macrophages acquire greater amounts of Ag in the presence of specific B cells than in their absence. This mechanism allows B cells to amplify or edit the immune response to specific Ag by transferring BCR-captured Ag to other professional APCs, thereby increasing the frequency of its presentation. Ag transfer may perpetuate chronic autoimmune responses to specific self-proteins and help explain the efficacy of B cell-directed therapies in human disease.
Immune responses are mediated by the communication between various cell types involving cell contact, cytokine production, and recognition of Ag. Ag presentation plays a critical role in this communication process thought to be mediated independently by dendritic cells (DCs),3 macrophages, and B lymphocytes. Each of these APCs serves a specific role in the immune system and, as such, acquires Ag by distinct means. Macrophages and DCs primarily capture a diverse range of Ags without bias by phagocytosis (1) and pinocytosis (2), whereas B cells acquire specific Ags by virtue of their BCR. This feature enables B lymphocytes to trigger T cell immunity by selectively binding Ags that are only available at limiting concentrations (3–7). Ag-specific B cells have been shown to be critical in the induction of autoimmunity and pathology in various disease models, including lupus, rheumatoid arthritis, diabetes, and multiple sclerosis (8–14). B lymphocytes can initiate an early immune response to soluble Ag earlier than other APCs (15, 16), although their effective APC functions may be short lived in vivo (15). These results indicate that B cells can acquire Ag early due to BCR specificity before nonspecific uptake by other APCs.
The exchange of proteins and/or Ag has been characterized for a variety of cells types, both immune and nonimmune. These include the transfer of peptides from endothelial cells to macrophages through gap junctions (17), viral Ags from live and apoptotic CD4+ T cells to DCs mediated by scavenger receptors (18), immune complexes from DCs to B lymphocytes (19), and membrane-associated proteins from one DC to another in a process termed “nibbling” (20). Finally, DCs can acquire Ag from B lymphocytes or other dying cells by endocytosis of cell debris (21–23). Furthermore, B cells can serve as recipients of Ag transferred from macrophages within the subcapsular sinus of lymph nodes in the form of immune complexes or large particulate Ag, such as fluorescent avidin microspheres (24, 25). The interaction of these two APCs had been demonstrated earlier in vitro as well as in vivo and was shown to be mediated through the scavenger receptors SR-AI and MARCO, respectively, on the macrophages (26–29).
Based on our prior studies of murine systems and those of others (21, 23, 30), we sought to define a mechanism for Ag transfer occurring between human B lymphocytes and macrophages. Our results indicate that Ag transfer is dependent on cell contact with live B cells and that membrane and/or intracellular proteins of the Ag donor cells are exchanged through a mechanism requiring class A scavenger receptors (SR-A). The transfer of Ag from B lymphocytes resulted in a greater accumulation of fluorescent protein in the recipient macrophages than in those that acquired Ag by phagocytosis alone. We propose that the transfer of Ag from specific B cells to other APCs leads to a more focused immune response. Receptors of B cells can acquire selected Ags (or autoantigens) from a vast milieu of cellular proteins and thereby edit the ongoing immune response. This pathway in humans may explain the efficacy of B cell-directed immunotherapies for the treatment of autoimmune disease (8–14).
Materials and Methods
Isolation and tissue culturing of cells
Human PBMC were isolated from leukopacks (New York Blood Center, Long Island City, NY) by a Ficoll-Hypaque method previously described (31). Macrophages were typically generated by an adherence-based method (32) in chamber slides (BD Bioscience-Falcon) with 1.75 × 107 PBMC per milliliter plated in 10% heat-inactivated human male AB serum (Sigma-Aldrich)/RPMI 1640 medium for 6−8 days before use. For quiescent macrophages, 10% FBS was substituted for human serum as described previously (33). Primary human B cells were isolated from PBMC by negative selection using magnetic beads (StemCell Technologies) and cultured in same medium as typical macrophages unless noted otherwise. The EBV-transformed human B lymphoblastoid cell line (B-LCL) and the EBV-negative human B cell lymphoma cell line BJAB were both maintained in 10% FBS/RPMI 1640 medium. The human monocytic leukemia cell line THP-1 (American Type Culture Collection (ATCC)) were grown in 10% FBS/RPMI 1640 supplemented with 10 mM HEPES and 1 mM sodium pyruvate (ATCC medium).
Preparation of fluorescent proteins
Anti-human IgG/IgM F(ab′)2 Ab fragments (anti-Ig; Jackson Immuno-Research Laboratories) were conjugated with Alexa Fluor 488 (AF488; Molecular Probes) according to manufacturer's protocol at a 1:6 molar ratio, respectively, using the succinimidyl ester form of the fluorophore. The Abs were separated from the unreacted fluorophore by centrifugation through a concentrator (5,000 m.w. cutoff; Millipore) and resuspended in PBS at 1 mg/ml for storage at −20°C. Pigeon cytochrome c (PCC; Sigma-Aldrich) was conjugated with pHrodo (Molecular Probes) in a similar manner as that described for anti-Ig.
Uptake of Ag by B lymphocytes
B-LCL or BJAB cells were cultured for 15 min in the presence of 10% human serum/RPMI 1640 medium and 1 mg/ml human Ig (Sigma-Aldrich) to block Fc receptors. Cells were washed twice in prewarmed HBSS and once in 10% FBS/RPMI 1640 medium to remove excess Ig. For various time points, B cells (2 × 107 cells/ml) were pulsed with 10 μg/ml either anti-Ig or anti-FITC Ig conjugated with AF488 (nonspecific Ab; Molecular Probes) at 37°C in 5% CO2 followed by four washes with ice-cold HBSS and a wash with 10% human serum/RPMI 1640 medium. Level of Ag uptake was determined for fixed cells by fluorescence microscopy of wet mounts and by flow cytometry. Optimal incubation time of B cells with Ag was found to be 60 min.
Ag transfer assays with human macrophages
Macrophages (1 × 106 cells per chamber) were used following differentiation from adherent monocytes as described above. Undifferentiated THP-1 cells (1 × 106 cells per well) were used as nonadherent cells, whereas differentiated THP-1 (adherent) were generated by the addition of 100 nM PMA to medium 24 h before use. The macrophages were cocultured for 18 h with B cells (2 × 106 cells per chamber or well) that had been pulsed with one of the following: no Ag, nonspecific Ab, or anti-Ig. All cells were harvested in ice-cold PBS by scraping and then stained for flow cytometry with anti-CD19-PE (BD Pharmingen) and anti-CD14-PECy5 (Invitrogen-Caltag Laboratories) Abs. The level of Ag (AF488+) was evaluated for the CD19−CD14+-gated population that was derived from nonlymphocyte forward scatter (FSC)/side scatter (SSC)-gated cells. For cocultures with soluble Ag, macrophages were cultured for 4 h under the following conditions: no Ag, 10 μg/ml anti-Ig (no B cells), and 5 μg/ml Ag in the absence or presence of B cells. For inhibitor studies, macrophages were pretreated for 1 h with the following: 50 μl of anti-SR-A antiserum (Chemicon), 25 μg/ml anti-CD36 Ab (20) (Immunotech-Coulter), or 100 μM 2-aminoethyl diphenylborinate (2-APB; Sigma-Aldrich) (17). All inhibitors were maintained in cocultures with B cells.
Live cell imaging of Ag transfer by confocal microscopy
Macrophages were detached from chamber slides using trypsin and plated into glass-bottom (1.5-mm thickness) dishes (Mattek) (1 × 106 cells per dish) 24 h before use. BJAB cells were labeled with 5 μM CellTrace Far Red (CTFR) (DDAO-SE; Molecular Probes) according to the manufacturer's protocol and pulsed with Ag as described above. The B cells (2 × 106) were cocultured with macrophages in the presence of 5 μg/ml PCC-pHrodo for 18 h, after which the live cell cultures were examined by confocal microscopy (LSM 510 META; Zeiss) at ×600 magnification and ×2 zoom. Signals for each fluorophore were collected simultaneously.
Assay for Ag transfer from apoptotic B cells
BJAB cells (2 × 106 cells) were pulsed with Ag and induced to undergo apoptosis by hyperthermia (30 min at 44°C) as previously described (34). Induction of apoptosis was confirmed by flow cytometry using the vital dye TO-PRO-3 (Molecular Probes). Given that 15% of BJAB cells (3 × 105 cells of 2 million cells) undergo apoptosis/necrosis when cultured alone (B. P. Harvey and M. J. Mamula, unpublished data), macrophages were cocultured with 3 × 105 apoptotic B cells for 18 h before being harvested for flow cytometry.
Preventing cell contact between Ag donor and recipient by Transwell system
Macrophages were detached from chamber slides using trypsin and seeded into a 24-well plate 1 day before use. Ag-pulsed B cells were added to the Transwell system (1, 3, or 8 μm; BD Falcon) above the wells containing macrophages and cultured for 18 h. Cells in the Transwell system were collected and analyzed by flow cytometry separately from those within the plate wells. Each set was stained with anti-CD19-PE and anti-CD14-PECy5 Abs, and the level of Ag was evaluated for the CD19−CD14+-gated population.
Membrane/cytosolic protein exchange assay
BJAB cells were labeled with 5 μM CellTracker Orange (CTO) (CMRA; Molecular Probes) according to manufacturer's protocol and pulsed with Ag. Macrophages were labeled with 2.5 μM CTFR (DDAO-SE; Molecular Probes) on chamber slides as follows: cells were washed with prewarmed (37°C) HBSS (no phenol red), stained for 7.5 min with CellTrace in HBSS, and the reaction was stopped with the addition of FBS. The B cells were cocultured with macrophages for 18 h before being harvested for flow cytometry. Acquisition of Ag (AF488+) as well as B cell membrane/cytosolic protein (CTO+) was determined for the CTFR+ cells derived from the nonlymphocyte FSC/SSC gated population.
Results
Human B cells capture and transfer fluorescent Ag to primary human macrophages
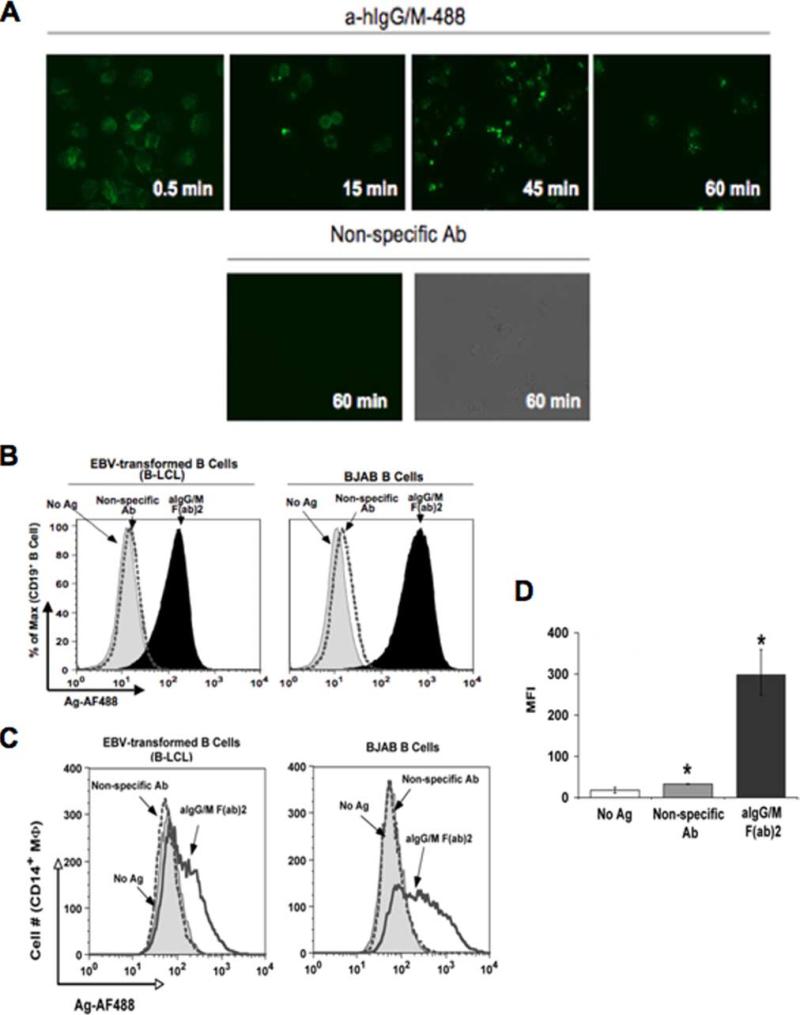
We as well as others have previously demonstrated in murine systems that transgenic B cells can transfer Ag to macrophages by an undefined mechanism (21, 23, 30). In our current investigation, we sought to determine whether this process could be identified with human APCs. For these studies, we monitored the movement of fluorescently labeled Ag from human B lymphocytes (Ag donor) to primary macrophages (Ag recipients). The Ag of choice was F(ab′)2 of anti-human Ig (anti-Ig) to target human BCR and prevent FcR-mediated Ag uptake. Ag acquisition by two different human B cell lines, EBV-transformed B-LCL and EBV-negative B cell lymphoma BJAB, was assessed at various time points. As seen in the upper panels of Fig. 1A, Ag was captured by B-LCLs within 30 s followed by BCR capping and Ag accumulation within 45 min. In contrast, binding of nonspecific Ab was absent in cells even after1hof incubation (Fig. 1A, lower panels), demonstrating that specific Ag uptake was mediated through the BCR. Both B-LCL and BJAB cells acquired and maintained similar levels of Ag (Fig. 1B, left and right panels, respectively) 18 h after being pulsed with Ag. Moreover, the level of Ag fluorescence remained stable following an overnight incubation of the human B cells.
FIGURE 1.
Human B cells acquire and transfer Ag to macrophages. A, Kinetics of Ag uptake by B cells. B-LCLs pulsed with either AF488-congugated anti-human Ig (a-hIgG/M-488; upper panels) or nonspecific Ab (lower panels) for the indicated time points were washed and observed by fluorescence microscopy (×450 original magnification). B, Ag uptake by various B cell lines. B-LCL (left panel) and BJAB (right panel) were pulsed with no Ag (gray-shaded curve), nonspecific Ab (dotted curve), or anti-IgG/M (aIgG/M; filled curve) and stained with anti-CD19 Ab for flow cytometry. C, Various B cell lines transfer Ag to macrophages (MΦ). Human PBMC-derived macrophages were cocultured for 18 h at a 1:2 ratio with B-LCL (left panel) or BJAB (right panel) pulsed with no Ag (gray-shaded curve), nonspecific Ab (dotted curve), or anti-Ig (aIgG/M F(ab)2; open curve). Cells were stained with anti-CD19 and CD14 Abs for flow cytometry. Levels of Ag (AF488) was assessed for the CD19−CD14+-gated population. D, Levels of transferred Ag are significant. MFI values for transferred Ag (AF488) are given for three independent experiments (*, p = 0.0012).
To determine whether B cells could transfer the Ag to other APCs, Ag-pulsed B-LCL and BJAB B cells were extensively washed to remove unbound Ag and then cocultured separately with PBMC-derived human macrophages at a 2:1 ratio. At various time points, cells were harvested, stained, and analyzed by flow cytometry. As seen in Fig. 1C, only macrophages cultured in the presence of anti-Ig-pulsed B cells were found to contain Ag compared with those with B cells pulsed with nonspecific Ab (negative control) (mean fluorescence intensity (MFI) 299 and 33, respectively, p = 0.0012; Fig. 1D). Although the highest percentage of Ag-bearing macrophages (54% of total number of CD14+CD19− cells) occurred following an 18-h coin-cubation period, we were able to identify this subset population as early as 2 h (26%) with an incremental increase after 4 h (35%) (data not shown). These results indicate that human macrophages are capable of acquiring Ag from the B lymphocytes with rapid kinetics. Because Ag transfer was independent of the donor B cell type, we will not distinguish between B-LCL and BJAB cells and will refer to them collectively as B cells throughout the text unless otherwise noted.
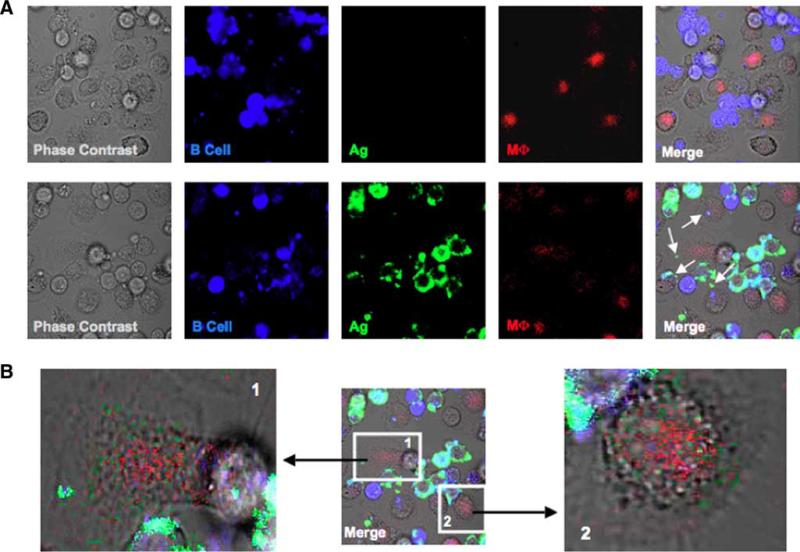
To confirm that the Ag-bearing macrophage population identified by flow cytometry was attributable to macrophages solely acquiring Ag from the B cells and not a consequence of B cell-macrophage clusters, we performed live cell imaging of cocultures as performed earlier. Macrophages were identified based on their ability to phagocytize the soluble pHRodo-conjugated PCC (red) that was present throughout the duration of the coculture; pHRodo only fluoresces in low pH conditions typically found in phagolysosomes, thereby limiting the cells detected to those that are functionally active. Ag-bearing macrophages could be identified when cocultured with B cells pulsed with anti-Ig (Fig. 2A, lower panels) as compared with those with B cells pulsed with nonspecific Ab (Fig. 2A, upper panels). Interestingly, a few of the macrophages contained both Ag (green) as well as small B cell fragments (blue), suggesting that the macrophages were capturing B cell membrane and/or cytosol along with the Ag. Enhancement of the AF488 signal reveals that a majority of macrophages in culture receive detectable amounts of Ag from B cells (Fig. 2B). Intriguingly, the anti-Ig (green) spots do not colocalize with those of the PCC (red), suggesting that the Ag is being acquired from B cells by a mechanism independently of direct phagocytosis. Noticeably absent from our images are macrophages that contained whole B cells or large fragments of these cells, which would be indicative of macrophages that had acquired Ag by phagocytizing the entire B lymphocyte. Overall, these confocal images of live cell cultures verify our flow cytometry findings that macrophages are acquiring Ag directly from the B cells.
FIGURE 2.
Live cell images depict Ag transfer from B cells to macrophages. A, BJAB cells were labeled with CTFR and pulsed with Ag. Macrophages (MΦ) were plated into glass-bottom dishes 24 h before the assay and then cocultured with either nonspecific Ab (upper panels)- or anti-Ig (lower panels) B cells in the presence of 5 μg/ml PCC-pHrodo (macrophage “stain”). After 18 h, live cell images were taken by confocal microscopy (×600 original magnification, ×2 zoom; arrows point to macrophages that bear Ag). B, Transferred Ag and phagocytized PCC localize to different cellular compartments. The AF488 signal was enhanced for cells that appeared to have Ag (panel 1) and those that did not (panel 2).
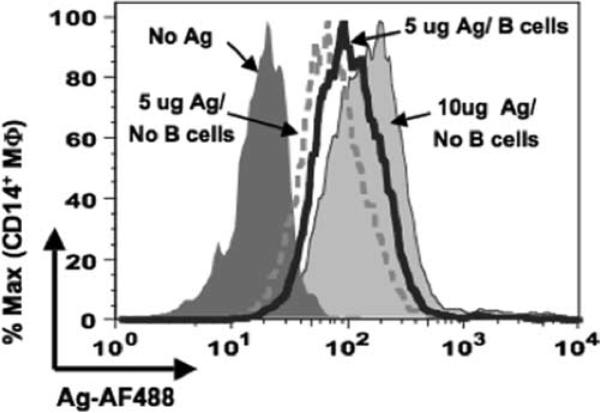
Our culture conditions use macrophages and Ag-pulsed human B cells washed free of unbound Ag. However, under physiological conditions, both of these APCs would likely be exposed to the soluble Ag simultaneously. We next cultured unpulsed B cells, macrophages, and soluble Ag to determine whether the kinetics of the macrophage uptake of Ag is altered. At lower concentrations of Ag, macrophages acquired a greater amount of Ag with B cells present than that of Ag without B cells (Fig. 3). Under conditions of Ag excess, the macrophages were capable of capturing more Ag than that transferred from B cells. No difference was observed between the two groups when nonspecific Ab was used as the soluble Ag (data not shown).
FIGURE 3.
Macrophages acquire greater Ag in presence of B cells. Macrophages were cultured for 4 h with the following: no Ag (dark gray curve), 10 μg/ml soluble Ag alone (light gray curve), 5 μg/ml soluble Ag in absence (dotted curve) or presence of BJAB cells (open curve with heavy line). Cells were stained for flow cytometry and the levels of transferred Ag were assessed for the CD19−CD14+-gated population.
Mechanisms of Ag transfer from B cells to macrophages
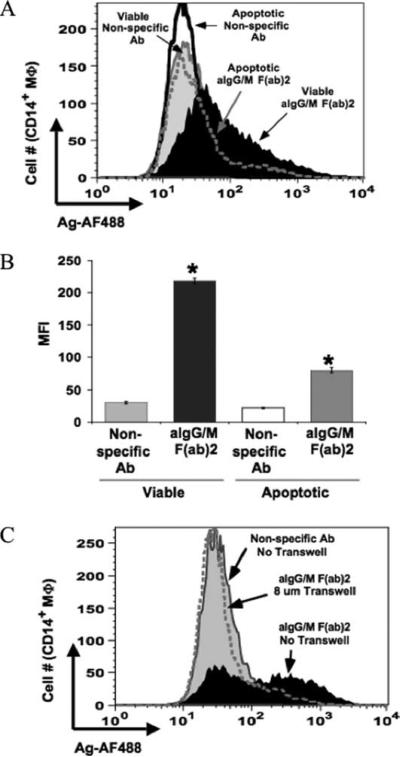
The exchange of Ag from B cells via BCR to other APCs may play a significant role in focusing or editing immune response to particular Ags. Live cell culture images indicate that macrophages acquire minute cellular fragments of the B lymphocytes along with Ag. One explanation could be that the macrophages are capturing apoptotic bodies produced by dying B cells. To assess this possibility, macrophages were cocultured with Ag-pulsed apoptotic B cells that were equivalent in cell number to those found within B cell culture. We found that on average 15% of the total B cell population (∼3 × 105 cells) undergoes apoptosis when cultured alone for 18 h (data not shown). With this number of Ag-pulsed apoptotic B cells as the sole source of Ag, acquisition by the macrophages was diminished in comparison with those cocultured with viable B cell populations (Fig. 4A) as reflected by a significant reduction in MFI of Ag (Fig. 4B). This finding demonstrates that phagocytosis of apoptotic B cells is not the primary means by which macrophages are acquiring fluorescent Ag.
FIGURE 4.
Direct contact between viable B cells and macrophages (MΦ) is required for Ag transfer. A, Apoptotic B cells do not contribute to Ag transfer. Apoptosis of Ag pulsed B-LCLs was induced by hyper-thermia. Macrophages were cocultured for 18 h with viable (1 × 106 cells) B cells pulsed with nonspecific Ab (gray curve) or anti-Ig (aIgG/M (F(ab)2; filled curve) or with apoptotic B cells (3 × 105 cells; number of apoptotic cells within viable B cell population) pulsed with the same Ags (open curve with heavy line or gray-dotted curve, respectively). Cells were stained for flow cytometry and levels of Ag were assessed for the CD19−CD14+-gated population. B, Ag transfer from apoptotic B cells was significantly less compared with that from viable cells. MFI values for transferred Ag (AF488) are given for three independent experiments (*, p < 0.0001). C, Ag transfer is abrogated upon the separation of B cells from macrophages by use of a Transwell system. Macrophages were cocultured in the absence of the Transwell system with B cells pulsed with nonspecific Ab (gray shaded curve) or in the absence (filled curve) or presence (dotted gray line curve) of Transwell with B cells pulsed with anti-Ig.
To determine whether cell contact is required fort he transfer of Ag from B cell to macrophage, Ag-pulsed B cells were kept separated from macrophages by Transwell membranes of various pore sizes. Even with a pore size as large as 8 μm, Ag transfer was completely blocked (Fig. 4C). These findings indicate that neither cellular debris nor soluble Ag is contributing to Ag transfer but that cell contact between the Ag donor and recipient is required.
Ag transfer involves acquisition of B cell membrane/cytosol
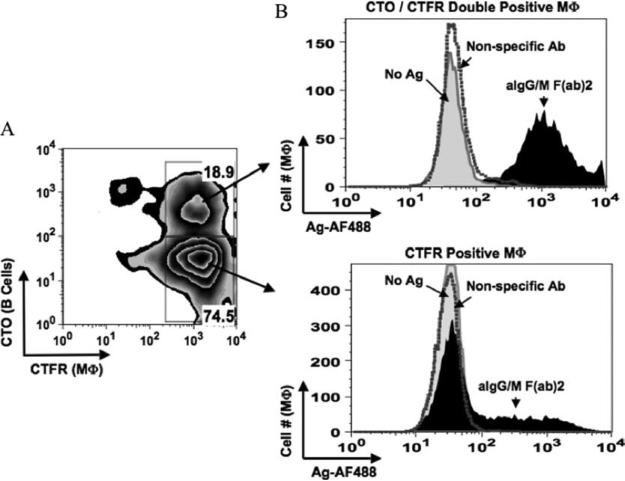
Our confocal microscopy data suggest that macrophages acquire Ag and fragments of B cell membrane or cytosol in a contact-dependent manner. To further assess this mechanism, we stained B cells and macrophages with fluorescent dyes (CTO and CTFR, respectively) that label both cytoplasmic and membrane-associated proteins and tracked the transfer of B cell-specific components to the macrophage population by flow cytometry. In cocultures such as those described above, we observed a double-labeled (CTO+CTFR+) macrophage population (Fig. 5A) that was nearly 100% positive for Ag (Fig. 5B, upper panel), indicating that macrophages are acquiring B cell membrane and/or cytosol along with Ag. Separating B cells from macrophages by an 8-μm Transwell membrane prevented the formation of the double-labeled macrophage population (data not shown), demonstrating that this population is not the result of CTO dye leaking from the B cells and staining the macrophages. Interestingly, 31% of the single CTFR-labeled population (Fig. 5B, lower panel) acquired Ag without the B cell-specific label, suggesting that another mechanism other than membrane/cytosol fusion and/or exchange may also be involved in Ag transfer.
FIGURE 5.
Macrophages (MΦ) acquire B cell-derived membrane and/or intracellular proteins along with Ag. BJAB cells were labeled with CTO and pulsed with Ag. Macrophages were labeled with CTFR and then cocultured for 18 h with the following Ag-pulsed B cell populations: no Ag (gray curve), nonspecific Ab (dotted curve), and anti-Ig (aIgG/M (F(ab)2; filled curve). Cells were harvested and analyzed by flow cytometry. A, B cell specific fluorescence is transferred to macrophages. Level of the B cell-derived label (CTO) was determined for the FSC/SSC-gated population corresponding to macrophages (CTFR). B, Macrophages positive for B cell-derived fluorescence acquire the highest amount of transferred Ag. Levels of transferred Ag (AF488) were assessed for the double stain (CTO+CTFR+; upper panel) gated macrophage population and for those that were single stain (CTFR+ alone; lower panel).
Class A scavenger receptor mediates Ag transfer
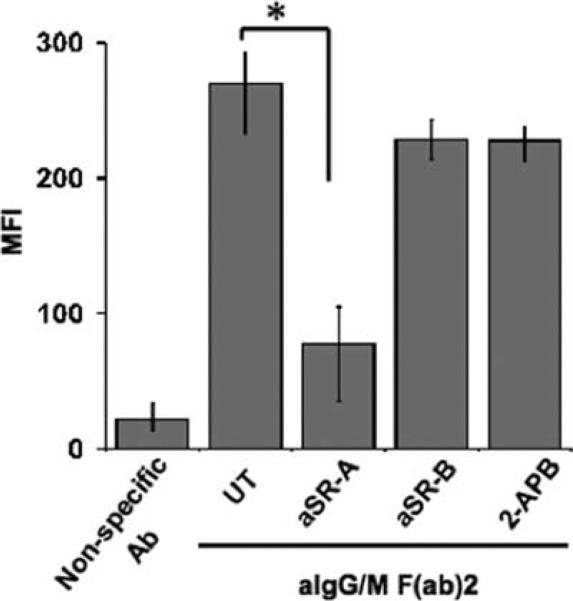
Previous studies using DCs have shown that lymphoid cell contact and the exchange of cellular components are mediated by a class A scavenger receptor (20). To determine whether the macrophages are acquiring Ag from B lymphocytes via this surface protein, we performed in vitro Ag transfer assays in the presence of an anti-human SR-A-blocking Ab as well as other inhibitors of the scavenger receptor family. Macrophages were pretreated with each of the inhibitors before being cocultured with Ag-pulsed B cells. Blocking SR-A caused a significant reduction (nearly 75%) in the level of Ag acquired by macrophages as compared with those left untreated (Fig. 6). Furthermore, SR-A ligands such as fucoidan (a polysaccharide component of Fucus vesiculosus) and acetylated low density lipoprotein (AcLDL) also reduced the amount of transferred Ag (data not shown). Failure of anti-human CD36-blocking Ab to inhibit Ag transfer (Fig. 6) suggests that only class A scavenger receptors are involved. Finally, Ag transfer still occurred in the presence of 2-APB, an inhibitor of gap junction proteins (35), (Fig. 6). Exposure to anti-SR-A Ab did not compromise the phagocytic function of the macrophages (uptake of FITC-conjugated dextran as Ag), nor did it induce apoptosis/necrosis in these APCs (data not shown).
FIGURE 6.
Class A scavenger receptor mediates Ag transfer from B cells to macrophages. Macrophages were treated with the following inhibitors for 1 h before being cocultured with BJAB cells pulsed with either nonspecific Ab or anti-Ig (aIgG/M F(ab)2): no inhibitor (untreated (UT)), 2-APB (gap junction protein inhibitor), and anti-SR-A (aSR-A)- or anti-SR-B (aSR-B; CD36)-blocking Abs. Cells were harvested and stained for flow cytometry with Abs for CD19 and CD14. MFI values for transferred Ag (AF488) were determined for the CD19−CD14+ gated populations from three independent experiments (*, p = 0.002).
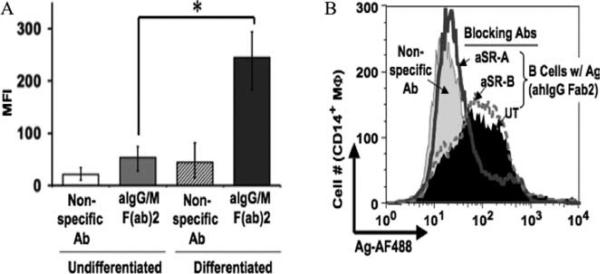
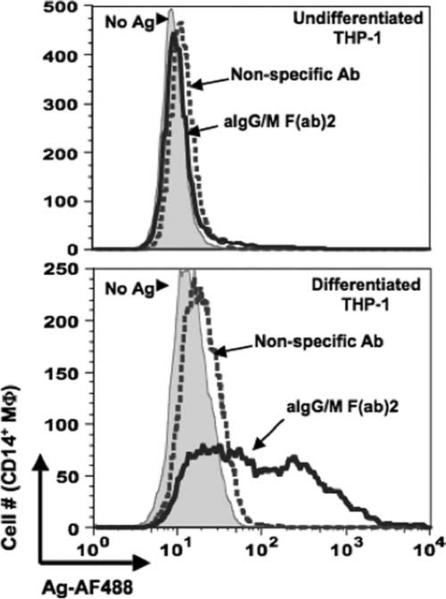
The critical role of SR-A in the transfer of Ag from B cells was further verified using the monocytic leukemia cell line THP-1 as the recipient APC. SR-A is not expressed on undifferentiated THP-1, whereas PMA-differentiated cells express high levels of this receptor (36). SR-A expression was evaluated for each THP-1 cell population (undifferentiated and PMA-differentiated) and correlated with the acquisition of Ag from B cells. The level of transferred Ag was significantly greater in the differentiated THP-1 cells than in the undifferentiated population (MFI 245 and 53, respectively; Fig. 7A). Of the macrophages that were positive for Ag, 95% expressed SR-A (data not shown). Furthermore, pretreatment of differentiated THP-1 cells with anti-SR-A-blocking Ab prevented the transfer of Ag (Fig. 7B), demonstrating the need for this specific scavenger receptor to mediate Ag transfer from B cells to THP-1 cells.
FIGURE 7.
Expression of SR-A is required for Ag transfer to differentiated THP-1 cells. A, Differentiated THP-1 cells acquire more transferred Ag than those left undifferentiated. THP-1 cells were cultured in the absence or presence of PMA for 24 h before being cocultured with B-LCL cells pulsed with either nonspecific Ab or anti-Ig (aIgG/m F(ab)2). Cells were harvested and stained for flow cytometry with Abs to CD19 and CD14. MFI values for transferred Ag (AF488) are given for the CD19−CD14+-gated populations from five independent experiments (*, p = 0.00003). B, SR-A mediates Ag transfer to differentiated THP-1 cells. PMA-differentiated THP-1 cells were left untreated (UT) (filled curve) or pretreated for 1 h with anti-SR-A (aSR-A; open curve with heavy line)- or anti-SR-B (aSR-B; dotted curve)-blocking Abs before being cocultured for 18 h with B-LCL cells pulsed with anti-Ig. As a negative control, PMA-differentiated THP-1 cells were left untreated and cocultured with B cells pulsed with nonspecific Ab (light gray-shaded curve). Cells were stained for flow cytometry and the levels of transferred Ag were assessed for the CD19−CD14+-gated population.
Activated macrophages serve as effective Ag recipients
Given the significant differences in Ag acquisition between un-differentiated and differentiated THP-1, we sought to determine whether the activation status of the human macrophages affected their ability to serve as recipients for transferred Ag. As such, the ability of a macrophage to receive Ag may be dependent on the presence of activation-specific surface receptor(s) in addition to SR-A. For this study, resting macrophages were generated as previously described (33) and then cocultured with Ag-pulsed B cells. Cells were analyzed by flow cytometry to evaluate the levels of transferred Ag as well as those of the activation markers. As expected, the resting cells expressed lower levels of both CD14 and CD80 as compared with the typical macrophages (data not shown). The decreased level of these markers directly correlated with a 55% reduction of transferred Ag in the resting cells relative to the activated set (data not shown). However, SR-A expressions were nearly identical between the two macrophage populations (data not shown). These findings would suggest that SR-A mediates the transfer of Ag in a process that is amplified by the activation of recipient macrophages.
Primary human B cells efficiently transfer Ag macrophages
The Ag donor cells used throughout our study have been either EBV-transformed B cells (B-LCL) or B cell lymphoma cells (BJAB). We next assessed whether the primary human B cells isolated from PBMCs could function as Ag donors in a manner similar to that of transformed B cells. As was observed with the transformed B cells (see Fig. 7), primary human B cells were capable of transferring Ag to differentiated THP-1 cells (Fig. 8, lower panel), whereas they were unable to do so with the undifferentiated APCs (Fig. 8, upper panel). Taken together (data from Figs. 7 and 8), these results indicate that primary B cells can function as Ag donors and suggest that class A scavenger receptors are critical in this process.
FIGURE 8.
Primary human B cells transfer Ag to macrophages. Primary human B cells were isolated from PBMC and pulsed for 60 min with the following: no Ag (gray-shaded curve), nonspecific Ab (dotted curve), or anti-Ig (open curve with heavy line). These cells were then cocultured with either undifferentiated (upper panel) or PMA-differentiated (lower panel) THP-1 cells for 18 h before being harvested for flow cytometry. Levels of transferred Ag were assessed for the CD19−CD14+-gated population.
Discussion
The APC function of Ag-specific B lymphocytes has been implicated in the initiation of immune responses, in particular those that are autoimmune (8, 9, 15). By virtue of its BCR, B cells capture and sequester specific foreign or self-Ag from a complex milieu of available Ags. Consequently, these APCs are more efficient at acquiring soluble Ag at low concentrations as compared with nonspecific APCs, DCs, and/or macrophages (16). By using membrane-restricted Ig transgenic mice, we have previously shown that the Ag-specific B lymphocytes capture soluble Ag and initiate a T cell response earlier than DCs. Although more immediate, Ag presentation by the B cells is shorter in duration in vivo, as other APCs begin to acquire and present Ag or autoantigens (15). This finding led us to investigate whether APCs such as DCs and/or macrophages were capable of acquiring Ag from Ag-specific B cells.
Recently, we demonstrated that live B cells expressing a membrane-restricted Ig are able to transfer fluorescently labeled Ag to murine macrophages (30). Furthermore, the transfer of Ag to APCs in vivo was found to induce an Ag-specific T cell response in the absence of cognate presentation by the B lymphocytes. Interestingly, the recipient macrophage population was found to be positive for the B cell marker B220. suggesting that membrane from the B lymphocytes had been acquired by the macrophages along with Ag. In this study, we demonstrate that human B cells transfer BCR-targeted Ag to human macrophages and that the macrophages do capture B cell-derived membrane and/or intracellular proteins along with Ag. Moreover, we identified a specific cell surface receptor, namely SR-A, on the macrophages that mediates Ag transfer from the B cells to these APCs. Human myeloid DCs can also acquire Ag from B cells through a similar mechanism as described herein for human macrophages (B. Harvey, T. E. Quan, B. J. Rudenga, R. M. Roman, J. Craft, and M. J. Mamula, submitted for publication).
The nonspecific shuttling of Ag between APCs has been characterized by other investigators (21–23, 37–40). These studies demonstrate that DCs and/or macrophages can carry out this process by several mechanisms: phagocytosis of B lymphocyte cellular fragments containing Ag (21, 22), uptake of soluble Ag by recipient APC as either whole protein (37) or immune complexes (38, 39), or peptide capture by MHC class II molecules (40). Few studies have examined the specific role of BCR-enriched Ag in this process (23, 40, 41). In the study by Moss et al. (40), the BCR-captured Ag was processed on the membrane surface and then transferred to a neighboring MHC class II molecule. Valdez et al. (23) demonstrated that Ag-specific murine B cells can transfer Ag along with membrane and/or cytosolic components to syngenic DCs in vivo. However, the specific mechanism as well as whether direct interaction between the B cell and a recipient APC was required remained unresolved.
Results from our current study indicate that human B cells can deliver BCR-captured Ag to human macrophages. MHC identity is not required for Ag transfer, and apoptotic B cells were excluded as a significant source of Ag transferred to macrophages. In addition, direct B cell contact with the macrophage is required for Ag transfer to occur. The direct interaction of the two APCs could mediate the exchange of Ag as well as cellular components. This would provide an explanation for our observation made during live cell imaging of B cell-macrophage cocultures that several macrophages contained Ag as well as fluorescent components derived from the B lymphocyte membrane and/or cytoplasm. Absent from our imaging studies were macrophages that had engulfed entire B cells, demonstrating that the macrophages are not acquiring Ag by this mechanism. In addition, the transferred Ag does not colocalize with Ag taken up by phagocytosis in the cell. Taken together with the Transwell experiments, these results suggest that the macrophages are actively capturing membrane and/or cytosolic proteins along with Ag from live B cells by a mechanism other than phagocytosis.
Our studies examining the role of SR-A in the transfer of Ag from B cells to macrophages were based on several prior reports demonstrating the importance of this surface protein in cell-to-cell adhesion and the exchange of macromolecules between various immune cell subsets (20, 28, 42). In particular, Harshyne et al. illustrated that SR-A mediates the ability of immature DCs to form contacts and acquire cell membrane (termed “nibbling”, or trogocytosis) from a variety of other immune cells, including other DCs, macrophages, T cells, and B cells (20). In some contrast to the present studies, human macrophages were found to be very inefficient at taking up membrane after contact with other immune cells (20). SR-A is found on a number of cell types, including both DCs and macrophages (43). Moreover, the presence of SR-A on macrophages has been shown to influence their adhesion to activated B lymphocytes (28). SR-A may also play an important role in Ag processing based on its role in representation of gp96 (endoplasmic reticulum chaperone)-associated peptides in macrophages (42). Each of these independent findings may offer insight into the functional significance of SR-A in Ag transfer. First, this receptor may contribute to the initiation of Ag transfer by mediating the attachment of the macrophage or DC to the Ag-bearing B cell through interaction with its surface ligand on the B lymphocyte. Second, SR-A may contribute to the processing and presentation of the transferred Ag by directing the newly acquired Ag to a particular pathway in a manner similar to that of gp96-associated peptides. The integration of these two processes through SR-A would allow for proficient acquisition and processing of transferred Ag.
The activation phenotype of a cell also plays a role in its ability to capture membrane from neighboring target cells whether mediated by SR-A or not (44, 45). We've observed that activated macrophages (i.e., increased levels of CD80 and CD14) acquire a greater amount of Ag from B cells. Interestingly, the levels of SR-A expression were the same regardless of the activation status, indicating that SR-A expression is necessary but may be amplified by other surface proteins of the activated macrophage. CD14 has been shown to mediate signaling through the receptor-linked PI3K pathway in response to SR-A receptor ligand binding (46). Therefore, CD14 may function as a coreceptor with SR-A in acquiring Ag from B cells.
Alternative mechanisms, besides SR-A-mediated membrane capture, that would require cell contact or at least very close proximity between the Ag donor and recipient cells would include the transfer of Ag through gap junctions or B cell-derived exosomes. Neijssen et al. (17) have shown that peptide Ags of 10-aa in length can be transferred to APCs through gap junctions following contact with nonimmune donor cells. In our study, the failure of 2-APB, an inhibitor of the gap junction subunit protein connexin 43, to block the transfer of Ag from the B lymphocytes to the macrophages indicates that this form of Ag transfer is not being mediated via gap junctions.
The remaining mechanism of B cell-derived exosomes has been proposed by us as well as others as a means by which B lymphocytes can transfer Ag to other APCs (30, 47). Exosomes are externalized vesicles generated by a variety of cell types including B and T lymphocytes, DCs, and macrophages as well as nonhematopoietic cells (reviewed by Denzer et al. in Ref. 48). B lymphocytes secrete exosomes that are formed by the direct fusion of the limiting membrane of the MIIC (MHC class II-enriched compartment) with that of the plasma membrane (49). These vesicles have been shown to induce Ag-specific MHC class II-restricted responses, suggesting that the MHC class II molecules carry antigenic peptides (49). More recently, Denzer et al. (47) demonstrated that follicular DCs can preferentially acquire B cell-derived exosomes when the two APCs are in close proximity to one another.
We propose that the acquisition of BCR-enriched Ag from B lymphocytes by other APCs, such as DCs and macrophages, could promote a focused immunological response to a specific foreign Ag (protective response) or self-Ag (tolerance or autoimmune response). B cells are able to capture specific Ag more efficiently than other APCs as a consequence of their BCR (3, 16, 30). In this study, we identified the mechanism of Ag transfer as the capture of human B cell-derived membrane and intracellular proteins by the recipient APC. Furthermore, we found that the macrophages acquire a greater amount of BCR-targeted Ag in the presence of B cells than in their absence. The immunologic relevance of Ag transfer may be inferred from studies demonstrating the importance of B cells in the development of autoimmune responses. In murine models of lupus and diabetes, T cell activation as well as pathology is significantly reduced in the absence B cells despite the presence of normal DC and macrophage populations (50, 51). Additionally, the presence of membrane-restricted Ig B cells has been shown to be sufficient to induce kidney pathology in lupus-prone mice, demonstrating that B cells play a significant role in autoimmunity independently of Ab secretion (8). One mechanism by which B cells could induce such responses may be through their ability to transfer BCR-enriched Ag to other professional APCs. Moreover, such a mechanism may help to explain the efficacy of B cell-directed therapies, such as rituximab, in various human auto-immune syndromes (8–14, 52–54). For example, these therapeutics eliminate not only autoantibody production but also cytokines, direct APC functions, and the transfer of autoantigens from B cells. Although Ag transfer is easily discernible within the context of autoimmunity, our findings have a broader implication for the role that B cells play in editing Ag presentation to foreign pathogens as well.
Acknowledgments
We thank Drs. Mark J. Shlomchik and Ruth R. Montgomery for contributing significantly to the intellectual aspects of our investigation as well as for technical assistance. We also thank Eva Sutton for technical expertise in imaging analysis.
Footnotes
These studies were supported by National Institutes of Health Grants AI-48120 and AR-41032. B.P.H. was supported by an Arthritis Foundation postdoctoral fellowship.
Abbreviations used in this paper: DC, dendritic cell; AF488, Alexa Fluor 488; anti-Ig, anti-human IgG/M F(ab′)2; 2-APB, 2-aminoethyl diphenylborinate; B-LCL, B lymphoblastoid cell line; CTFR, CellTrace Far Red; CTO, CellTracker Orange; FSC, forward scatter; MFI, mean fluorescence intensity; PCC, pigeon cytochrome c; SR-A, class A scavenger receptor; SSC, side scatter.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J. Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 4.Mamula MJ. Lupus autoimmunity: from peptides to particles. Immunol. Rev. 1995;144:301–314. doi: 10.1111/j.1600-065x.1995.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 5.Mamula MJ, Janeway CA. Do B cells drive the diversification of immune responses? Immunol. Today. 1993;14:151–152. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 6.Pierce SK, Morris JF, Grusby MJ, Kaumaya P, Buskirk AV, Srinivasan M, Crump M, Smolenski LA. Antigen-presenting function of B lymphocytes. Immunol. Rev. 1988;106:149–180. doi: 10.1111/j.1600-065x.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 7.Byersdorfer CA, Dipaolo RJ, Petzold SJ, Unanue ER. Following immunization antigen becomes concentrated in a limited number of APCs including B cells. J. Immunol. 2004;173:6627–6634. doi: 10.4049/jimmunol.173.11.6627. [DOI] [PubMed] [Google Scholar]
- 8.Chan O, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1647. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J. Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 10.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J. Immunol. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 11.Bour-Jordan H, Bluestone JA. B cell depletion: a novel therapy for autoimmune diabetes? J. Clin. Invest. 2007;117:3642–3645. doi: 10.1172/JCI34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 13.Pers JO, Daridon C, Bendaoud B, Devauchelle V, Berthou C, Saraux A, Youinou P. B-cell depletion and repopulation in autoimmune diseases. Clin. Rev. Allergy Immunol. 2008;34:50–55. doi: 10.1007/s12016-007-8015-4. [DOI] [PubMed] [Google Scholar]
- 14.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J. Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 16.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 18.Marañón C, Desoutter JF, Hoeffel G, Cohen W, Hanau D, Hosmalin A. Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 2004;101:6092–6097. doi: 10.1073/pnas.0304860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J. Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 21.Bickham K, Goodman K, Paludan C, Nikiforow S, Tsang ML, Steinman RM, Münz C. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J. Exp. Med. 2003;198:1653–1663. doi: 10.1084/jem.20030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdez Y, Mah W, Winslow MM, Xu L, Ling P, Townsend SE. Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8α+ dendritic cells in vivo. J. Exp. Med. 2002;195:683–694. doi: 10.1084/jem.20010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 25.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Boswell HS, Sharrow SO, Singer A. Role of accessory cells in B cell activation: I. Macrophage presentation of TNP-Ficoll: evidence for macrophage-B cell interaction. J. Immunol. 1980;124:989–996. [PubMed] [Google Scholar]
- 27.Weaver DJ, Voss EW. A novel cellular interaction involving antigen-pulsed macrophage and antigen-specific B-lymphocytes. Mol. Immunol. 2000;37:311–320. doi: 10.1016/s0161-5890(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 28.Yokota T, Ehlin-Henriksson B, Hansson GK. Scavenger receptors mediate adhesion of activated B lymphocytes. Exp. Cell Res. 1998;239:16–22. doi: 10.1006/excr.1997.3876. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson MCI, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey BP, Gee RJ, Haberman AM, Shlomchik MJ, Mamula MJ. Antigen presentation and transfer between B cells and macrophages. Eur. J. Immunol. 2007;37:1739–1751. doi: 10.1002/eji.200636452. [DOI] [PubMed] [Google Scholar]
- 31.Bennett WE, Cohn ZA. The isolation and selected properties of blood monocytes. J. Exp. Med. 1966;123:145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson WD, Mei B, Cohn ZA. The separation, long-term cultivation, and maturation of the human monocyte. J. Exp. Med. 1977;146:1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mufson RA. Preparation of quiescent human monocytes for studies of colony-stimulating factor responsiveness. J. Tissue Cult. Methods. 1992;14:37–38. [Google Scholar]
- 34.Zhao Q-L, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic. Biol. Med. 2006;40:1131–1143. doi: 10.1016/j.freeradbiomed.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 35.Bai D, Corsso C. d., Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2 APB). J. Pharmacol. Exp. Ther. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- 36.Miki S, Tsukada S, Nakamura Y, Aimoto S, Hojo H, Sato B, Yamamoto M, Miki Y. Functional and possible physical association of scavenger receptor with cytoplasmic tyrosine kinase Lyn in monocytic THP-1-derived macrophages. FEBS Lett. 1996;399:241–244. doi: 10.1016/s0014-5793(96)01332-4. [DOI] [PubMed] [Google Scholar]
- 37.Taylor GS, Long HM, Haigh TA, Larsen M, Brooks J, Rickinson AB. A role for intercellular antigen transfer in the recognition of EBV-transformed B cell lines by EBV nuclear antigen-specific CD4+ T cells. J. Immunol. 2006;177:3746–3756. doi: 10.4049/jimmunol.177.6.3746. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 39.Lindorfer MA, Jinivizian HB, Foley PL, Kennedy AD, Solga MD, Taylor RP. B cell complement receptor 2 transfer reaction. J. Immunol. 2003;170:3671–3678. doi: 10.4049/jimmunol.170.7.3671. [DOI] [PubMed] [Google Scholar]
- 40.Moss CX, Tree TI, Watts C. Reconstruction of a pathway of antigen processing and class II MHC peptide capture. EMBO J. 2007;26:2137–2147. doi: 10.1038/sj.emboj.7601660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend SE, Goodnow CC. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J. Exp. Med. 1998;187:1611–1621. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geng YJ, Hansson GK. High endothelial cells of postcapillary venules express the scavenger receptor in human peripheral lymph nodes. Scand. J. Immunol. 1995;42:289–296. doi: 10.1111/j.1365-3083.1995.tb03658.x. [DOI] [PubMed] [Google Scholar]
- 44.Tabiasco J, Espinosa E, Hudrisier D, Joly E, Fournié JJ, Vercellone A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol. 2002;32:1502–1508. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J. Immunol. 2002;169:6162–6169. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 46.Kim WS, Ordija CM, Freeman MW. Activation of signaling pathways by putative scavenger receptor class A (SR-A) ligands requires CD14 but not SR-A. Biochem. Biophys. Res. Commun. 2003;310:542–549. doi: 10.1016/j.bbrc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 47.Denzer K, Eijk M. v., Kleijmeer MJ, Jakobson E, Groot C. d., Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 2000;165:1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 48.Denzer K, Kleijmeer MJ, Heijnen HFG, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 49.Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding CV, Melief CJM, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J. Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 51.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J. Exp. Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 53.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 54.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]