Abstract
Female astrocytes sustain less cell death from oxygen-glucose deprivation (OGD) than male astrocytes. Arimidex, an aromatase inhibitor, abolishes these sex differences. To verify sex-dependent differences in P450 aromatase function in astrocyte cell death following OGD, we developed a novel method that uses sex-specific and genotype-specific single pup primary astrocyte cultures from wild-type (WT) and aromatase-knockout (ArKO) mice. After determining sex by external and internal examination as well as PCR and genotype by PCR amplification of tail cDNA, we established cultures from 1−3 day-old male and female, WT and ArKO mice pups and grew them to confluence in estrogen-free media. Cell death was measured by lactate dehydrogenase (LDH) assay. Our study shows that, while WT female astrocytes are more resistant to OGD than WT male cells, sex differences disappear in ArKO cells. Cell death is significantly increased in ArKO compared to WT in female astrocytes but not male cells. Therefore, P450 aromatase appears to be essential in endogenous neuroprotection in females, and this finding may have clinical implications. This innovative technique may also be applied to other in vitro studies of sex-related functional differences.
Keywords: aromatase, sex, astrocyte, cell death, neuroprotection
1. Introduction
Stroke is a sexually dimorphic disease. In humans, for example, male and female differences in stroke risk and outcome have been reported (Holroyd-Leduc, et al., 2000; Di, et al., 2003; Kapral, et al., 2005). Female sex is associated with favorable outcome from ischemic stroke compared to male animals, and these sex differences are attributed in part to the protective effect of estrogen (Alkayed, et al., 2000; Murphy, et al., 2004).
In brain, estrogen can be synthesized locally from testosterone via P450 aromatase. Female mice with targeted deletion of P450 aromatase (encoded by cyp19) sustained increased brain damage after middle cerebral artery occlusion compared to wild-type (WT) female littermates or even ovariectomized females (McCullough, et al., 2003). Accordingly, brain aromatase may be important to ischemic pathophysiology. Consistent with this hypothesis, aromatase activity is induced in astrocytes after brain injury (Garcia-Segura, et al., 1999; Azcoitia, et al., 2003).
We recently reported that astrocytes isolated from neonatal cortex exhibit marked sex differences in sensitivity to oxygen-glucose deprivation (OGD) in part due to higher aromatase expression in female astrocytes compared to male cells. The aromatase inhibitor Arimidex abolished sex differences in OGD-induced cell death (Liu, et al., 2007). In this study, we developed a method to establish an in vitro ischemic model using P450 aromatase-knockout (ArKO) mice generated from heterozygous breeding harems. We grew sex-specific and genotype-specific WT and ArKO single pup primary astrocyte cultures to verify the neuroprotective effect of P450 aromatase in astrocytes following OGD. By applying this new method, we tested the hypothesis that P450 aromatase plays a key role in mediating the sex difference in astrocyte survival, and we demonstrated that P450 aromatase gene deletion abolishes the sex difference in astrocyte cell death.
In contrast to previous in vitro methods, our novel technique uses cultures that are both sex-specific and genotype-specific to study molecular mechanisms of cell death and cell survival that will lead to new therapeutic targets and improved outcomes following stroke and other neurologic diseases.
2. Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health guidelines for care and use of animals in research, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
2.1. Sex determination
Astrocytes were cultured from 1−3 day-old single male and female Sprague-Dawley pups separately (dams from Charles River, Wilmington, MA). Male and female rat pups were distinguished by a larger genital papilla and longer ano-genital distance in male vs. female pups. Sex was confirmed by inspecting internal organs and gonads after laporatomy, e.g., uterine horn in females and testis in males, and by multiplex polymerase chain reaction (PCR) using male-specific marker SRY (sex determination region on the Y chromosome responsible for testes formation) and the universal marker myogenin (Myog) (expressed in both males and females) as previously described (McClive and Sinclair, 2001).
2.2. P450 aromatase-deficient single pup genotyping by PCR
P450 aromatase-knockout (Ar−/−, ArKO) mice were generated as previously described (Fisher, et al., 1998). This strain originated on a C57BL/6J;129SvEv background and was backcrossed to C57BL/6J for at least 10 generations. In ArKO mice, exon IX of the Cyp 19 gene is disrupted by inserting a neo cassette using homologous recombination. Homozygous (Ar−/−) mice produce an abnormal transcript that generates a nonfunctional protein. Consequently, aromatase activity is lacking in all cell types, and estrogen synthesis is blocked. Ar+/− (heterozygote) breeder pairs were obtained from Dr. Orhan K. Oz, Department of Radiology, University of Texas Southwestern Medical Center. Female ArKO (Ar−/−) mice are infertile due to arrested folliculogenesis (Findlay, et al., 2001), and ArKO (Ar−/−) males are hypofertile due to impaired sexual behavior and an age-dependent disruption of spermatogenesis (Murata, et al., 2002). Therefore, the breeding colony was maintained with heterozygous (Ar+/−) breeding harems (2 females, 1 male).
Genotype was determined using PCR amplification of tail DNA, as previously described (Robertson, et al., 1999; Britt, et al., 2000), to identify exon IX and the neo insert in the Cyp 19 gene. Offspring were screened with two sets of primers. The first primer pair (5’ GTG ACA GAG ACA TAA AGA TCG 3’ and 5’ GTA AAT TCA TTG GGC TTA GGG 3’) produced a 220 base pair (bp) product for the WT allele and no product for the ArKO allele. The second primer pair (5’ ATC AGG ATG ATC TGG ACG AAG A 3’ and 5’ CCA CAG TCG ATG AAT CCA GAA 3’) produced a 170 bp product for the ArKO allele and no product for the WT allele. The PCR products were resolved by electrophoresis in 2.5% agarose gel and were visualized by ethidium bromide staining. The expected sizes of the PCR products are 220 bp in Ar+/+ (WT) and 170 bp in Ar−/− (ArKO); and both PCR products are present in Ar+/− (heterozygote). Every set of PCR reactions included a negative control (no DNA) and a positive control (heterozygous DNA).
2.3. Sex-specific and genotype-specific single pup primary astrocyte cultures
After segregation of male and female WT and ArKO mice using the methods described above, primary cultures of single pup male and female cortical astrocytes were prepared seperately, as previously published (Liu and Alkayed, 2005). The cell suspension was diluted with a feeding media consisting of 10% charcoal-stripped, estrogen-free fetal bovine serum (HyClone, Logan, Utah) in DMEM (Invitrogen) with 1% penicillin-streptomycin. The cells were seeded in 24-well plates for cell death assay and incubated at 37°C in 95% / 5% mixture of atmospheric air and CO2. . Feeding media were changed after 2 days and subsequently twice a week. Confluent monolayers [10 to 14 days in vitro (div)] of primary cortical cultures consisting of 98.4 ± 0.5% glial fibrillary acidic protein (GFAP)-positive cells were used, as previously described (Liu and Alkayed, 2005).
2.4. Oxygen-glucose deprivation (OGD) and cell death assay
At 10−14 div, the feeding media were replaced with oxygen-depleted, glucose-, serum-and phenol red-free DMEM, and the cultures were incubated for 12 hours in a Coy™ anoxia chamber filled with an anoxic gas mixture containing 5%H2/5%CO2/90%N2 at 37°C. Anoxic conditions were monitored using an oxygen sensor that maintained oxygen levels at 0 p.p.m (Coy Laboratories Products, Grass Lake, MI, USA). Immediately after OGD, the media were replaced with fresh feeding media containing glucose, and the cells were returned to normoxia for 24 hours. Cell death was measured by lactate dehydrogenase (LDH) release into the culture medium (LDH cytotoxicity detection kit, Roche). Experiments were repeated on 12 independent cultures from different litters, and data was averaged from 3 wells per condition per experiment to n = 1.
2.5. Statistical analysis
Cells prepared from single male vs. female pup constitute one experiment (n = 1). The number of experiments (n) for any sex refers to number of cultures prepared from different litters. Differences between male and female astrocytes were determined by ANOVA with post hoc Newman Keuls. Values are presented as mean ± SEM with statistical significance at p < 0.05.
3. Results
3.1. Sex determination
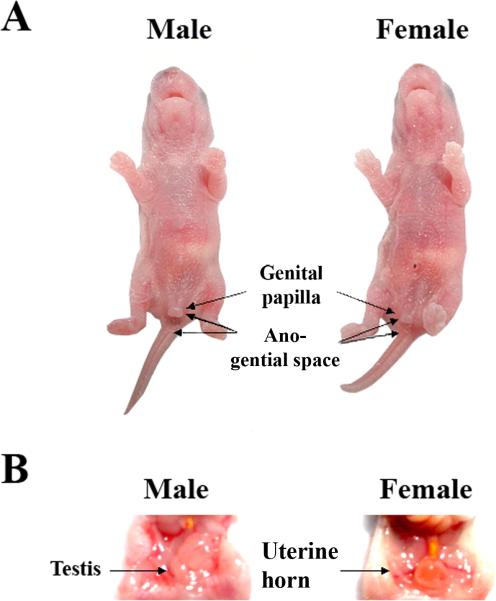
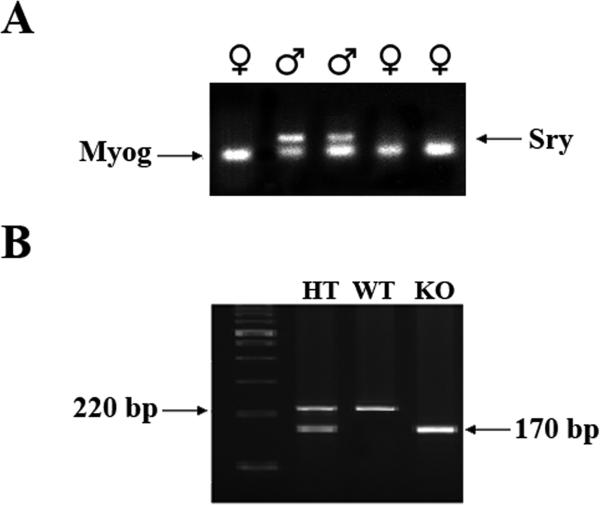
Visual sex identification (Fig. 1A and Fig. 1B) was highly reliable (100% accuracy, n = 10), as verified by subsequent PCR confirmation of sexual genotype (Fig. 2A). Male and female rat pups were distinguished by a larger genital papilla and longer ano-genital distance in male vs. female pups (Fig.1A). Sex was confirmed by inspecting internal organs and gonads after laporatomy, e.g., uterine horn in females and testis in males (Fig. 1B). Sex was determined by PCR using the male-specific marker SRY (sex determination region on the Y chromosome, responsible for testes formation) and the universal marker myogenin (Myog), which is expressed in both males and females. Female pups express Myog gene only, and male pups express both SRY gene and Myog gene (Fig. 2A).
Fig. 1A.
Male and female rat pups were distinguished by a larger genital papilla and longer ano-genital distance in male vs. female pups.
Fig. 1B. Sex was confirmed by inspecting internal organs and gonads after laporatomy, e.g., uterine horn in females and testis in males.
Fig. 2A.
Sex was determined by PCR using the male-specific marker SRY (appears in the sex determination region on the Y chromosome and is responsible for testes formation) and the universal marker myogenin (Myog) (expressed in both males and females). ♀ pups express Myog gene only, ♂ pups express both SRY gene and Myog gene.
Fig. 2B. PCR genotyping for tail DNA, the PCR products are 220 base pairs (bp) for Ar+/+ (WT), 170 bp for Ar−/− (ArKO), and both of these PCR products for Ar+/−(heterozygote, HT).
3.2. Genotyping by PCR
Fig. 2B illustrates the single pup PCR genotyping for tail DNA analysis. As expected, the PCR products were 220 bp for Ar+/+ (WT) and 170 bp for Ar−/− (ArKO). Both PCR products were present for Ar+/− (heterozygote, HT).
3.3. Response of sex-specific and genotype–specific ArKO and WT single pup primary astrocyte cultures to OGD
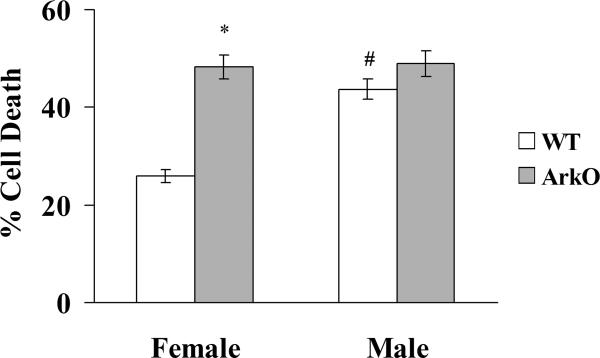
When subjected to 12-hour OGD, WT female astrocytes were more resistant to OGD than WT male cells. Cell death at 24 hours was 25.9±1.3% (n = 12) in female and 43.7±2.1% (n = 12) in male (Fig. 3) (p < 0.05).
Fig. 3.
WT female astrocytes are less susceptible to cell death compared to WT male astrocytes after OGD (n = 12, p < 0.05, # - Significant difference in WT female astrocytes compared to WT male cells). Cell death markedly increased in ArKO female astrocytes compared to WT female cells, but there was no significant difference in cell death between ArKO male astrocytes compared to WT male cells. Sex-specific responses to OGD were abolished in ArKO astrocyte cultures, (n = 12, p < 0.05, * - Significant difference in ArKO female astrocytes compared to WT female cells).
To determine if differences in aromatase activity mediate the sex-specific response to OGD, we used aromatase-knockout mice (ArKO). Cell death markedly increased following OGD in ArKO female astrocytes compared to WT female cells, but not in ArKO male astrocytes compared to WT male cells. Therefore, aromatase-deficiency abolished sex-specific responses to OGD (48.2±2.5%, n = 12 in F vs. 49.1±2.6%, n = 12 in M, p > 0.05) (Fig. 3).
Discussion
Using our new sex-specific, genotype-specific method for culturing single pup primary astrocytes to achieve an in vitro ischemic model, we found that sex-specific responses to OGD can be altered by genotype. We also observed for the first time that female but not male astrocytes are dependent on the integrity of the aromatase gene.
There are gender differences in many diseases. For example, stroke is a sexually dimorphic disease, starting from male and female differences in stroke incidence and outcome in humans, to experimental studies that clearly show differences in ischemic sensitivity between male and female animals. This sex difference in ischemic brain injury persisted in genetic in vivo models of hypertension (Alkayed, et al., 1998) and diabetes (Hurn and Macrae, 2000; Toung, et al., 2000; Vannucci, et al., 2001; Sieber, et al., 2001), suggesting that fundamental molecular mechanisms of ischemic cell death may be gender dependent. These differences are in part due to the protective effect of the female sex hormone estrogen (Hurn and Macrae, 2000; McCullough, et al., 2003; Liu, et al., 2004), since ovariectomy increases ischemic brain damage in female rats (Alkayed, et al., 1998) and mice (McCullough, et al., 2005), and estrogen replacement is protective against cerebral ischemia in ovariectomized rats (Rusa, et al., 1999) and mice (McCullough, et al., 2005) and in reproductively senescent male and female rats (Alkayed, et al., 2000).
In recent in vitro studies, neuronal survival was different in male vs. female brain cells despite similar culture media composition (Zhang, et al., 2003). XX vs. XY cells respond differently to simulated ischemia and toxicity. For example, XY neurons are more susceptible to glutamate or peroxynitrite exposure than are XX cells, whereas XX cells are more sensitive to proapoptotic stimuli (Du, et al., 2004). We reported that astrocytes isolated from neonatal cortex exhibited marked sex differences in sensitivity to oxygen glucose deprivation (OGD) in part due to higher aromatase expression in female astrocytes compared to male cells. The aromatase inhibitor Arimidex abolished sex differences in OGD-induced cell death (Liu, et al., 2007), suggesting that sex differences in ischemic cell death are related to aromatase expression (Liu, et al., 2007). Such experiments conducted in steroid-free media indicate that some mechanisms of injury (or survival) may be linked to the genetic sex of the cell.
In this study, we further delineated our sex-specific in vitro ischemic model by using aromatase-knockout (ArKO) mice to develop single pup primary astrocyte cultures that are also genotype-specific. We bred these ArKO mice from heterozygous colonies, since female ArKO−/− mice are infertile due to arrested folliculogenesis (Findlay, et al., 2001) and ArKO−/− males are hypofertile due to impaired sexual behavior and an age-dependent disruption of spermatogenesis (Murata, et al., 2002). Three types of pups were born: wild-type (WT), knockout (ArKO), and heterozygote (HT). In agreement with data generated in sex-specific rat astrocytes (Liu, et al., 2007), we found that female WT mouse astrocytes are more resistant to oxygen-glucose deprivation (OGD) than male WT mouse astrocytes. It is important to note that male sensitivity and female resistance to OGD are observed across species. Using the new technique developed for this study, we demonstrated that P450 aromatase gene deletion significantly increased cell death after OGD in female astrocytes and abolished the sex differences in sensitivity to OGD seen in WT female and male astrocytes. These findings, then, confirm that P450 aromatase is instrumental in mediating astrocyte survival following OGD and suggest that P450 aromatase is neuroprotective against ischemic brain injury. This is consistent with an earlier in vivo study of ischemia in which brain damage was greater in female ArKO mice compared to wild-type (WT) female mice (McCullough, et al., 2003).
Cell response to injury in vitro can be sex-specific. When gathering preclinical data, therefore, it is crucial to use sex-specific cell cultures to characterize these mechanisms and to avoid erroneous conclusions, as clinical trials of new therapies and drugs require representation of both sexes. Pharmacological inhibition or gene deletion may be important to further delineate sex-specific molecular mechanisms. This study is the first to combine sex-specific and genotype-specific techniques to investigate cell death and cell survival pathways following ischemia in single pup primary astrocyte cell cultures.
In conclusion, results of this study indicate that the biosynthetic enzyme P450 aromatase has neuroprotective effects in females and may have therapeutic implications for clinical stroke. These findings underscore the importance of studying male and female cells separately and in genotype-specific cultures to better understand sex-specific pathways of cell death and survival following ischemia.
This sex-specific, genotype-specific in vitro method can also be a very useful tool to improve accuracy and reduce interpretation errors in other studies on sex-related differences in molecular mechanisms.
ACKNOWLEDGMENTS AND FUNDING
This research was funded by American Heart Association grant 0535284N and National Institutes of Health, National Institute of Neurological Disorders and Stroke, grant NS33668, NS49210. The authors would like to thank Dr. Orhan K. Oz, Department of Radiology, University of Texas Southwestern Medical Center, for providing Ar+/−(heterozygous) breeder pairs. The authors would also like to acknowledge the excellent service and care provided by the Department of Anesthesiology and Peri-Operative Medicine Mouse Colony Core, which oversaw management of the P450 estrogen aromatase knockout (ArKO) mouse breeding colony. Finally, we thank Ms. Kathy Gage, Grant and Publications Writer in the Department of Anesthesiology and Peri-Operative Medicine, for her consultation in writing the final version of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–66. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–8. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann. N. Y. Acad. Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–23. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- Di CA, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–9. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 2004;279:38563–70. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Britt K, Kerr JB, O'Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reprod. Fertil. Dev. 2001;13:543–7. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6965–70. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–78. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–7. doi: 10.1161/01.str.31.8.1833. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J. Cereb. Blood Flow Metab. 2000;20:631–52. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–14. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J. Cereb. Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Alkayed NJ. Cytochrome p450 in neurological disease. Curr. Drug Metab. 2004;5:225–34. doi: 10.2174/1389200043335540. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J. Cereb. Blood Flow Metab. 2007;27:135–41. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- McClive PJ, Sinclair AH. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol. Reprod. Dev. 2001;60:225–6. doi: 10.1002/mrd.1081. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005;25:502–12. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Mol. Cell Endocrinol. 2002;193:7–12. doi: 10.1016/s0303-7207(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR. J. 2004;45:147–59. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O'Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7986–91. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–70. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sieber FE, Hurn P, Alkayed NJ, Traystman RJ. Gender-based differences in Na+ -K+ adenosine triphosphatase activity occur in the microcirculation of the diabetic rat brain. Anesthesiology. 2001;94:372–5. doi: 10.1097/00000542-200102000-00037. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31:2701–6. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J. Cereb. Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR. Sex-related differences in neuronal cell survival and signaling in rats. Neurosci. Lett. 2003;337:65–8. doi: 10.1016/s0304-3940(02)01179-5. [DOI] [PubMed] [Google Scholar]