Abstract
Individuals with autism spectrum disorder (ASD) have impaired ability to use context, which may manifest as alterations of relatedness within the semantic network. However, impairment in context use may be more difficult to detect in high-functioning adults with ASD. To test context use in this population, we examined the influence of context on memory by using the “false memory” test. In the false memory task, lists of words were presented to high-functioning subjects with ASD and matched controls. Each list consists of words highly related to an index word not on the list. Subjects are then given a recognition test. Positive responses to the index words represent false memories. We found that individuals with ASD are able to discriminate false memory items from true items significantly better than are control subjects. Memory in patients with ASD may be more accurate than in normal individuals under certain conditions. These results also suggest that semantic representations comprise a less distributed network in high-functioning adults with ASD. Furthermore, these results may be related to the unusually high memory capacities found in some individuals with ASD. Research directed at defining the range of tasks performed superiorly by high-functioning individuals with ASD will be important for optimal vocational rehabilitation.
Autism is associated with disordered social interaction and communication (1). These behaviors have been attributed to “weak central coherence” or impaired ability to use context (2, 3), which may manifest as dysfunction of the neural networks that interrelate the meanings of words (semantics). When words are placed into syntactic or semantic context, normal individuals will remember more words than when words are not placed into context (4). Autistic children demonstrate less of an increase in recall than nonautistic controls when words are placed into syntactic or semantic context (5–7). However, this difference is less detectable among high-functioning adults with autism spectrum disorder (ASD; ref. 3). We wished to assess this high-functioning population with a more sensitive test for alterations in context use.
The “false memory” test depends on semantic and associative context to induce illusory recognition of certain index words. Specifically, a list of words that are closely related in meaning to (semantically related) or frequently co-occur with (associatively related) an index word can induce illusory recognition of that word. Our hypothesis was that comparison of illusory and true recognition from this test might prove sensitive to variations in use of semantic and associative context. Therefore, subjects with ASD would be expected to discriminate true items from “false” items better than matched control subjects on this test.
Methods
Subjects.
Eight high-functioning adults with ASD and 16 nonautistic adults, matched for age, gender, performance scale Wechsler Adult Intelligence Scale-Revised Intelligence Quotient (WAIS-R IQ), verbal scale WAIS-R IQ, full-scale WAIS-R IQ, and educational level, were studied (Table 1). Seven of the subjects with ASD were diagnosed with the Autism Diagnostic Interview-Revised (ADI-R; ref. 8; interviews performed by D.Q.B., validated administrator for this test), and one was diagnosed by medical records and personal history (including personal recall of ADI-R items by the subject). [ASD includes autism, Asperger syndrome, and pervasive developmental disorder. Although all subjects met the diagnostic criteria for autism through their reported behavior during childhood (ADI-R), most subjects had demonstrated significant improvement in function over time, such that the distinction between the various forms of ASD was not as clear. Therefore, the more general term ASD is used to describe these patients.] Before participation, informed consent was obtained from each subject after the nature of the study (described as a test of memory for words) was explained in accordance with the regulations of the University of Florida Human Subjects Committee.
Table 1.
Demographic and psychometric data for ASD and control groups
| Subject information | ASD | Nonautistic subjects |
|---|---|---|
| No. | 8 | 16 |
| Gender, M/F | 6/2 | 12/4 |
| Age, years | 31.8 ± 8.6 | 31.4 ± 12.1 [t (22) = 0.078, n.s.] |
| Education level | 14.6 ± 1.8 | 15.6 ± 2.6 [t (22) = −0.990, n.s.] |
| WAIS-R IQ scales | ||
| Full scale | 110.9 ± 18.1 | 112.9 ± 13.5 [t (22) = −0.315, n.s.] |
| Performance | 106.1 ± 16.0 | 107.4 ± 10.3 [t (22) = −0.244, n.s.] |
| Verbal | 114.0 ± 19.7 | 114.9 ± 16.4 [t (22) = −0.115, n.s.] |
Values shown are means ± SD. n.s., not significant (P > 0.05).
Tasks.
The false memory test was administered by using the stimuli and procedure modified from Roediger and McDermott (9). Subjects were presented with an audiotape consisting of the first 12 of the 15 words from each of the 24 word lists of Roediger and McDermott (9). After each word list, the tape was stopped and subjects were given a seven item recognition test for that list. Two of the test words from this recognition test were items from the list (the first item on the list was selected along with another chosen from among the first six items). Two words were distantly related to the index items but were not on the list (selected from the 13th, 14th, and 15th words from the word lists of Roediger and McDermott, ref. 9). Two words were unrelated to the index items and were not on the list (selected from the 13th, 14th, and 15th words from other word lists of Roediger and McDermott, ref. 9, and other words not from any list were selected for being unrelated to any index word). One word was the index item, a closely related item that was not on the list. The placement of the index item among the seven recognition test words was varied from fifth to seventh in order of presentation, such that subjects would be less likely to detect a pattern.
For example, subjects heard the words (at the rate of one per second) “thread, pin, eye, sewing, sharp, point, prick, thimble, haystack, thorn, hurt, injection” (list 15 from Roediger and McDermott, ref. 9) and were prompted subsequently with “Did you hear ‘thread’? Did you hear ‘pie’? Did you hear ‘sewing’? Did you hear ‘syringe’? Did you hear ‘needle’? Did you hear ‘ugly’? Did you hear ‘knitting’?” In this case, “needle” was not on the list but was the index word for which subjects had illusory recognition.
For each recognition trial, subjects were asked to respond verbally “four” if they were certain the prompted word was on the list, “three” if they thought the word was probably on the list, “two” if they thought the word was probably not on the list, and “one” if they were certain the word was not on the list.
Half of the subjects heard an audiotape starting with list 1 and proceeding through list 24 of Roediger and McDermott (9). The other half heard a tape starting with list 24 and proceeding in reverse order.
To compare results of this test with previously studied tests of the role of semantic relatedness in memory, subjects were also given the California Verbal Learning Test (CVLT; ref. 10). This test entails learning of word lists in which several items come from the same semantic category. In normal individuals, semantic relatedness improves recall performance.
Results
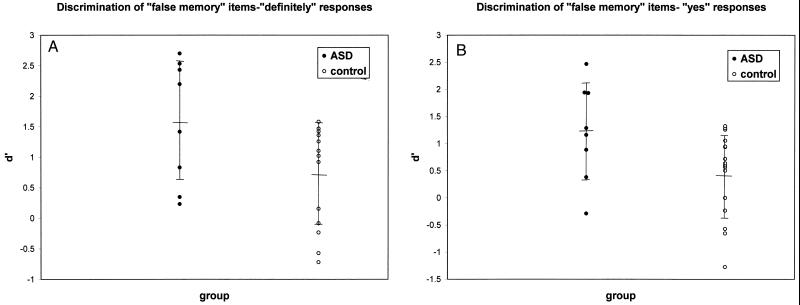
To generate an index for comparison of the ability of subjects with ASD and control subjects to discriminate true items from false items, d′ was calculated for each subject comparing recognition of true items and index (false memory) items. Separate calculations of d′ were performed for “definitely” responses (replies of “four”) and “yes” responses (sum of replies of “three” and “four”; Fig. 1). Discrimination of true items from false index items (d′) for each subject was compared between groups in a manner derived from Wagner et al.m ANOVA revealed that subjects with ASD discriminated true items from false index items (d′) significantly better than control subjects for “definitely” responses [subjects are certain that the word was on the list; Fig. 1A; F(1,22) = 4.600; P = 0.043] and “yes” responses [subjects either are certain or feel that the word was probably on the list; F(1,22) = 4.809; P = 0.039]). When the groups were compared with P(A) as a discrimination index (11, 12), ANOVA revealed that subjects with ASD discriminated true items from false index items significantly better for “yes” responses [Fig. 1B; F(1,22) = 4.410; P = 0.047] but not “definitely” responses [F(1,22) = 2.147; not significant].
Figure 1.
Comparison of d′ between ASD and control groups for “definitely” responses (replies of “four”; A) and of d′ for “yes” responses (sum of replies of “three” and “four”; B). Values shown are means ± SD.
Despite the fact that the subjects with ASD performed better than controls at discriminating true items from index items, control subjects did not recognize significantly more false index items than subjects with ASD for “definitely” responses (ASD, 7.5 ± 8.9; controls, 11.5 ± 6.5; t(22) = −0.923; not significant), but control subjects did recognize significantly more false index items than subjects with ASD for “yes” responses (ASD, 13.8 ± 7.0; controls, 16.8 ± 4.2; t(22) = −7.368; P = 0.013). Subjects with ASD did not recognize significantly more true items than control subjects for “definitely” responses (ASD, 36.6 ± 7.9; controls, 36.3 ± 5.6; t(22) = 1.593; not significant) or “yes” responses (ASD, 44.5 ± 2.8; controls, 40.7 ± 4.4; t(22) = 0.127; not significant). (Data are summarized in Table 2.) Thus, the critical difference between subjects with ASD and those without ASD is the finding that those without ASD were more inclined to endorse the index items as previously viewed, when other differences in their pattern of responding were controlled statistically (13). Overall, the two groups did not differ significantly in terms of the criterion they used to distinguish between true and false recognition of items [F(1,22) = 2.549; not significant for “definitely” responses; F(1,22) = 0.016, not significant for “yes” responses], and none of the subjects showed any serious response biases.
Table 2.
Summary of responses to the false memory test for each group
| Response categories | ASD, % | Nonautistic subjects, % |
|---|---|---|
| Index item (false) responses | ||
| Definitely (“four”) | 31.2 ± 37.1 | 47.9 ± 27.1 |
| Yes (“three” and “four”) | 57.5 ± 29.2 | 70.0 ± 17.5 |
| No (“one” and “two”) | 42.5 ± 29.2 | 30.0 ± 17.5 |
| True item responses | ||
| Definitely (“four”) | 76.3 ± 16.5 | 75.6 ± 11.7 |
| Yes (“three” and “four”) | 92.7 ± 5.8 | 84.8 ± 9.2 |
| No (“one” and “two”) | 7.3 ± 5.8 | 15.2 ± 9.2 |
| Related item responses | ||
| Definitely (“four”) | 10.4 ± 19.4 | 5.4 ± 7.3 |
| Yes (“three” and “four”) | 19.6 ± 23.8 | 10.0 ± 7.5 |
| No (“one” and “two”) | 80.4 ± 23.8 | 90.0 ± 7.5 |
| Unrelated item responses | ||
| Definitely (“four”) | 1.0 ± 2.3 | 0.6 ± 0.8 |
| Yes (“three” and “four”) | 2.3 ± 5.0 | 0.6 ± 0.8 |
| No (“one” and “two”) | 97.7 ± 5.0 | 99.4 ± 0.8 |
Values shown are means ± SD.
Verbal working memory was also compared between groups (derived from the CVLT), because subjects with greater verbal working memory would be expected to perform better at discriminating true from false index items. Control subjects and subjects with ASD showed no significant difference in verbal working memory (ASD, 6.6 ± 2.5 words; controls, 7.8 ± 1.4 words; t(22) = −1.162; not significant). When d′ scores were compared while controlling for individual differences in verbal working memory by using analysis of covariance (ANCOVA), subjects with ASD again demonstrated better discrimination of false memories for both “definitely” responses with d′ [F(1,22) = 4.233; P = 0.029] and “yes” responses with d′ [F(1,22) = 4.434; P = 0.025]. A similar ANCOVA also demonstrated better discrimination of false memories for both “definitely” responses with P(A) [F(1,22) = 3.927; P = 0.036] and “yes” responses with P(A) [F(1,22) = 4.809; P = 0.019].
To test whether subjects with ASD performed better because of a change in strategy during the test, we used repeated measures ANOVA to look for an interaction between group (ASD vs. control) and order within the test (first half vs. second half). No group–order interaction effect was found for “yes” responses or “definitely” responses for either true or false index items [F(1,21) = 0.242, not significant for false index “definitely” responses; F(1,21) = 0.352, not significant for false index “yes” responses; F(1,21) = 0.316, not significant for true “definitely” responses; F(1,21) = 0.255, not significant for true “yes” responses].
No differences existed between subjects with ASD and control subjects in age- and gender-adjusted scores for semantic and serial clustering, or list B semantic clustering ratios on the CVLT [semantic clustering: F(1,22) = 0.122, P = 0.730; serial clustering: F(1,22) = 1.536, P = 0.228; list B semantic clustering ratios: F(1 20) = 0.205, P = 0.662]. Therefore, subjects with ASD did not seem to use related meanings of words to help with recall on the CVLT to a different extent than did control subjects.
Discussion
Memory in patients with ASD may be more accurate than in normal individuals under certain conditions. The finding that subjects with ASD had better discrimination of true items from false items in our study is in accord with the theory of weak central coherence or decreased use of context in efforts to understand the environment in autism (2, 3). With decreased use of context, subjects with ASD are less susceptible to the influences of associatively related (frequently co-occurring) and semantically related (similar in meaning) words in inducing illusory recognition of index items not presented on the word list. Therefore, individuals with ASD have an advantage on this test. This advantage may be related to the unusually high category-specific memory capacities (hypermnesia) observed in some patients with ASD (14). However, the same mechanism that allows superior performance on this test may impair their performance in daily life. For example, context is crucial for some forms of learning, problem solving, and determining appropriate responses in a particular social setting. Research directed at defining the range of tasks performed superiorly by high-functioning subjects with ASD will be critical for optimal vocational rehabilitation.
Greater than normal discrimination on the false memory test is a finding that, to our knowledge, has not been reported previously in any other patient population. Further studies in nonverbal domains, such as context use in remembering a visual scenen, will be required to learn whether this advantage generalizes to other domains. Whereas amnestic patients show a decrease in false recognition, there is also a decrease in true recognition resulting in no improvement in discrimination of false memories (15, 16). Increased age also results in increases in false memories (17–19). Increased discrimination of false memories in normals can occur after warning of the false-memory phenomenon (20), after encoding associated with other distinctive information (21), or after repeated testing (16). In our study, groups did not differ in warning, other associated information given, or number of tests given.
The absence of a difference in semantic clustering on the CVLT between groups agrees with previous studies demonstrating that context-related language impairments can be more difficult to detect in high-functioning adults with ASD (3). A previous study had demonstrated a decreased semantic clustering ratio from list B of the CVLT in high-functioning subjects with ASD (22). However, the mean IQ of the ASD subjects included in our study was higher than that in the previous CVLT study. Therefore, we suspect that the use of higher-functioning subjects with ASD in our study may have been the reason that the CVLT list B clustering finding was not seen. The ability of the false memory test to detect differences readily between groups in our study suggests that it may be more sensitive to alterations in semantic networks than many tests currently used for this purpose. However, the reason why it is more sensitive has yet to be determined.
Decreased use of context among individuals with ASD suggests that word representations in the semantic network may be associated in an aberrant manner, leading to restricted semantic-associative networks. Neural network models have been proposed to account for this finding, which may have a neuroanatomical explanation (23). For example, Hebb (24) suggested that memories are stored by forming associative connections between neurons that are simultaneously active. An increase in synaptic strength or long-term potentiation has been described in the hippocampus. Furthermore, the hippocampus may be an essential component in the development of semantic networks (25). Decreased dendritic arborization and increased neuronal cell-packing density have been shown in the CA4 and CA1 subfields of the hippocampus in ASD (26, 27). These findings in the hippocampus have been proposed to be related to the memory findings in autism (28). The findings in autism would differ from the destructive processes seen in acquired amnesia, which can result in decreases in both false and true recognition (15, 16). It is possible that, in ASD, the diminished degree of hippocampal neuronal arborization results in a reduction in the amount of associative information stored in neocortical areas being used in CA1-subfield (29) N-methyl-d-aspartate-mediated associative long-term potentiation. However, we cannot exclude the possibility that decreased use of context in subjects with ASD results from other anatomical abnormalities observed elsewhere in the limbic system or in the cerebellum (26, 27) or as a result of an as-yet unknown pathology in other corticocortical connections. Further studies are required to test this hypothesis more directly.
Note Added in Proof.
Since these studies were carried out, Bowler et al. (30) reported that, unlike recognition, discrimination performance on free recall in the false memory test is not improved in ASD, suggesting a contribution from frontal-executive impairment in free recall in ASD.
Acknowledgments
We thank Sheri Anderson for comments on this manuscript. This research was supported by a grant from the Stallone Fund. These data were presented initially at the Society for Neuroscience's 28th annual meeting (1998).
Abbreviations
- ASD
autism spectrum disorder
- WAIS-R IQ
Wechsler Adult Intelligence Scale-Revised Intelligence Quotient
- ADI-R
Autism Diagnostic Interview-Revised
- CVLT
California Verbal Learning Test
Footnotes
Wagner, A. D., Stebbins, G. T., Carillo, M. C., Dirksen, C., Gabrieli, J. D. E. & Schacter, D. L., Fifth Meeting of the Cognitive Neuroscience Society, April 5–7, 1998, San Francisco, p. 57.
Miller, M. B., Wolford, G. L., Heinrich, J. & Gazzaniga, M. S., Fifth Meeting of the Cognitive Neuroscience Society, April 5–7, 1998, San Francisco, p. 56.
References
- 1.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: Am. Psychol. Assoc.; 1995. [Google Scholar]
- 2.Frith U, Happé F. Cognition. 1994;50:115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 3.Beversdorf D Q, Anderson J M, Manning S E, Anderson S L, Nordgren R E, Felopulos G J, Nadeau S E, Heilman K M, Bauman M L. J Neurol Neurosurg Psychiatry. 1998;65:685–692. doi: 10.1136/jnnp.65.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller G A, Selfridge J A. Am J Psychol. 1950;63:176–185. [PubMed] [Google Scholar]
- 5.Hermelin B, Frith U. Focus Autistic Behav. 1991;6:6–13. [Google Scholar]
- 6.Hermelin B, O'Connor N. Psychological Experiments with Autistic Children. Oxford: Oxford Univ. Press; 1970. [Google Scholar]
- 7.O'Connor N, Hermelin B. J Ment Defic Res. 1967;11:126–131. doi: 10.1111/j.1365-2788.1967.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Lord C, Rutter N, LeCouteur A. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 9.Roediger H L, III, McDermott K B. J Exp Psychol Learn Mem Cogn. 1995;21:803–814. doi: 10.1037//0278-7393.20.6.1379. [DOI] [PubMed] [Google Scholar]
- 10.Delis D C, Kramer J H, Kaplan E, Ober B A. California Verbal Learning Test. San Antonio, TX: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 11.Craig A. Hum Factors. 1979;21:69–78. doi: 10.1177/001872087902100109. [DOI] [PubMed] [Google Scholar]
- 12.Calderia J D. Hum Factors. 1980;22:119–130. doi: 10.1177/001872088002200113. [DOI] [PubMed] [Google Scholar]
- 13.Green D M, Swets J A. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 14.Mottron L, Belleville S, Stip E. Brain Lang. 1996;53:326–350. doi: 10.1006/brln.1996.0052. [DOI] [PubMed] [Google Scholar]
- 15.Melo B, Winocur G, Moscovitch M. Cogn Neuropsychol. 1999;15:343–359. [Google Scholar]
- 16.Schacter D L, Verfaellie M, Anes M D, Racine C. J Cogn Neurosci. 1998;10:668–679. doi: 10.1162/089892998563086. [DOI] [PubMed] [Google Scholar]
- 17.Balota D A, Cortese M J, Duchek J M, Adams D, Roediger H L, III, McDermott K B, Yerys B E. Cogn Neuropsychol. 1999;16:361–384. [Google Scholar]
- 18.Norman K A, Schacter D L. Mem Cogn. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- 19.Tun P A, Wingfield A, Rosen M J, Blanchard L. Psychol Aging. 1998;13:230–241. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- 20.McDermott K B, Roediger H L., III J Mem Lang. 1998;39:508–520. [Google Scholar]
- 21.Schacter D L, Israel L, Racine C. J Mem Lang. 1999;40:1–24. [Google Scholar]
- 22.Minshew N J, Goldstein G. Neuropsychology. 1993;7:209–216. [Google Scholar]
- 23.Cohen I L. Biol Psychiatry. 1994;36:5–20. doi: 10.1016/0006-3223(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 24.Hebb D O. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 25.Gabrieli J D E, Cohen N J, Corkin S. Brain Cogn. 1998;7:157–177. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 26.Bauman M L, Kemper T L. In: The Neurobiology of Autism. Bauman M L, Kemper T L, editors. Baltimore: Johns Hopkins Univ. Press; 1994. pp. 119–145. [Google Scholar]
- 27.Kemper T L, Bauman M L. In: Neurobiology of Infantile Autism. Naruse H, Ornitz E M, editors. Amsterdam: Elsevier Science; 1992. pp. 43–57. [Google Scholar]
- 28.DeLong G R. Neurosci Biobehav Rev. 1992;16:63–70. doi: 10.1016/s0149-7634(05)80052-1. [DOI] [PubMed] [Google Scholar]
- 29.Kandel E R. In: Principles of Neural Science. 3rd Ed. Kandel E R, Schwartz J H, Jessell T M, editors. Amsterdam: Elsevier Science; 1991. pp. 1009–1031. [Google Scholar]
- 30.Bowler, D. M., Gardiner, J. M. & Grice, S. (2000) J. Abnorm. Psychol., in press. [DOI] [PubMed]