Abstract
We report the synthesis of racemic Alloc-Ncy(Tmob)-OH, the resolution of its methyl ester, and demonstrate its application to form a norcystine bridge in octreotide-amide using the Fmoc-strategy on solid phase. N-Alloc and S-Tmob protections of norcysteine (Ncy) were found to be a preferred choice for Fmoc-strategy over three other protected norcysteines synthesized i.e. Fmoc-Ncy(tBu)-OH, Alloc-Ncy(tBu)-OH and Alloc-Ncy(Trt)-OH.
Keywords: norcysteine, norcystine, SPPS, somatostatin, octreotide
Norcysteine (Ncy) or α-thiolglycine (H2N-CH(SH)-COOH) is an unnatural amino acid possessing an electronegative sulfur atom attached directly to the α-carbon atom. Recently, we described the synthesis of Boc-d, l-Ncy(Mob)-OH, the resolution of its methyl ester, and the introduction of both d- and l-Ncy in cyclic gonadotropin-releasing hormone (GnRH) analogues.1 Boc-Ncy(Mob)-OH is compatible with SPPS using Boc-strategy and can be introduced in peptides as a bridge head to constrain peptide conformation via norcysteine-containing disulfide bridges that are shorter in ring size than the cystine bridges by one or two methylene groups. Herein we describe the synthesis of N- and S-protected norcysteines for Fmoc strategy.
In studies directed towards the protected norcysteines for Fmoc-strategy, the α-amino group of norcysteine was protected with Fmoc2 and Alloc,3 as both can be easily removed during SPPS under basic and neutral conditions, respectively. The sulfhydryl function was protected either with tBu4 /Trt5 /Tmob6 for their stability during deprotection of Fmoc/Alloc groups and for their lability7 after the cleavage of the desired peptide from the resin or during the cleavage step. The synthesis of racemic N- and S-protected norcysteines (4a-d) is illustrated in Scheme 1. In short, refluxing Fmoc- or Alloc-carbamate (1) and glyoxylic acid monohydrate (2) in acetone for 5 h or stirring in diethylether at room temperature (RT) overnight yielded the α-hydroxy intermediate (3). The reaction of tert-butylthiol/tritylthiol/2,4,6-trimethoxybenzylthiol8 in toluene with 3 in the presence of PTSA afforded the racemic N- and S-protected norcysteines (4a-d). We attempted to separate the enantiomers of 4a-d by stereoselective enzymatic resolution of their methyl esters 5a-d using papain.1 The methyl esters (5a-d) which are the preferred substrate for the papain-catalyzed hydrolysis,9 were obtained by reacting the racemic amino acids (4a-d) with trimethylsilyldiazomethane.10 Our attempts to enzymatically resolve the racemic methyl esters 5a and 5c using papain were unsuccessfully. The enzymatic resolution of 5a and 5c with α-chymotrypsin and subtilisin also failed, and may be attributed to the bulkier Fmoc/Trt groups, which may affect the substrate recognition. However, we were successful in resolving racemic methyl esters 5b and 5d using papain1 to optically enriched protected l-norcysteines 6b and 6d11 with enantiomeric excess of 80% and >98%, respectively. The papain catalyzed hydrolysis was carried out in phosphate buffer (pH 6.2) containing 60% CH3CN at 25 °C and monitored by chiral RP-HPLC using chiralcel® OD-RH™ column, Figure 1. After 24 h, the reaction mixture contained 50% acid according to chiral RP-HPLC and was quenched by adding acetic acid. The crude products containing unreacted d-methyl esters and the resolved l-acids (6b and 6d) were separated by column chromatography.12
Scheme 1.
Synthesis of N- and S- protected norcysteines.
(i) acetone, reflux, 5 h or diethylether, RT, overnight; (ii) tert-butylthiol/tritylthiol/ 2,4,6 - trimethoxybenzylthiol, benzene, PTSA, Dean-Stark, reflux, 6 h; (iii) Me3SiCHN2, benzene:methanol (4:1); (iv) Papain, CH3CN:buffer(3:2), pH 6.2.
Figure 1.

Chiral HPLC profile of the enzymatic hydrolysis of racemic Boc-Alloc-Ncy(Tmob)-OCH3 (5d). Reaction progress (a) initial, retention time (tR) for racemic ester is 30.34 min and 30.96 min (b) after 24 h, tR for resolved Alloc-Ncy(Tmob)-OH is 25.32 min and tR for unreacted Alloc-DNcy(Tmob)-OCH3 is 30.31 min. The HPLC assay conditions for the chiralcel® OD-RH™ reversed-phase column (0.46 cm × 15 cm): buffer A, 0.1% TFA in H2O, buffer B, CH3CN in A; gradient elution from 10% B – 70% B in 30 min and then 95% B in 2 min at a flow rate of 0.5 mL/min. UV detection, 0.1 AUFS at 210 nm.
The compatibility of 6b and 6d in SPPS to form a norcystine bridge was investigated in somatostatin analogue octreotide-amide.13 All of the peptides 7-9 (Table 1) were synthesized manually on a 2, 4-dimehoxybenzhydrylamine resin (DMBHA-resin,14 substitution 0.25 mmol/g resin) or Rink Amide AM resin (Novabiochem, substitution, 0.70 mmol/g resin) using the Fmoc-strategy.15
Table 1.
Characterization of peptides.
| no. | peptides | ring size | % purity | tRc min | MSd (M + H)+ | ||
|---|---|---|---|---|---|---|---|
| HPLCa | CZEb | calcd. | obsd. | ||||
| 7 | cyclo(2-7)[DPhe1-Cys2-Phe3-DTrp4-Lys5-Thr6-Cys7-Thr8-NH2] (Octreotide-amide) | 20 | 99 | 99 | 17.4 | 1032.436 | 1032.404 |
| 8 | DPhe1-Ncy(tBu2-Phe3-DTrp4-Lys5-Thr6-Ncy(tBu)7-Thr8-NH2 | - | 98 | 99 | 35.8 | 1118.546 | 1118.502 |
| 9 | cyclo(2-7)[DPhe1-Ncy2-Phe3-DTrp4-Lys5-Thr6-Ncy7-Thr8-NH2] | 18 | 99 | 99 | 14.9 | 1004.405 | 1004.341 |
Percentage purity determined by HPLC using buffer A: TEAP, pH 2.30, buffer B: 60% CH3CN/40% A under gradient conditions (20% to 50% B over 30 min), at flow rate of 0.2 mL/min on a Vydac C18 column (0.21 cm × 15 cm, 5 μm particle size, 300 Å pore size). Detection at 214 nm.
Percentage purity determined by capillary zone electrophoresis (CZE) using a Beckman P/ACE System 2050 controlled by an IBM Personal system/2 model 50Z; field strength of 15 kV at 30 °C. Buffer, 100 mM sodium phosphate (85:15, H2O:CH3CN), pH 2.50, on a Agilent μSil bare fused-silica capillary (75 μm i.d. × 40 cm length). Detection at 214 nm.
Retention times under HPLC conditions described above.
Mass spectra (MALDI-MS) were measured on an ABI-Voyager DE-STR instrument using saturated solution of α-cyano-4-hydroxycinnamic acid in 0.3% trifluoroacetic acid and 50% acetonitrile as matrix. The calculated [M + H] of the monoisotope was compared with the observed [M + H]+ monoisotopic mass.
Each coupling reaction was achieved using a 3-fold excess of amino acid, and utilizing N, N′-diisopropylcarbodiimide (DIC)/1-hydroxybenzotriazole(HOBt)-mediated activation of the carboxyl group in DMF. Piperidine treatment (25% piperidine in DMF) was used for the Fmoc removal and the Alloc-deprotection was performed under neutral conditions i.e. Pd(PPh3)4/PhSiH3/DCM16 in the presence of argon. The fully protected peptido-resins [Boc-dPhe1-Ncy(tBu)2-Phe3-dTrp(Boc)4-Lys(Boc)5-Thr(tBu)6-Ncy(tBu)7-Thr(tBu)8-resin and Boc-dPhe1-Ncy(Tmob)2-Phe3-dTrp(Boc)4-Lys(Boc)5-Thr(tBu)6-Ncy(Tmob)7-Thr(tBu)8-resin] were cleaved with the cocktail mixture of TFA:H2O:EDT:TIS (94:2.5:2.5:1) for 3 h to give the crude peptide 8 and disulfide reduced form of the peptide 9, respectively. The excess of TFA was then removed in vacuo and the crude peptides were precipitated by the addition of excess of tert-butyl methyl ether. Analysis of the crude peptides by RP-HPLC and mass spectroscopy revealed deprotection of Tmob but not tBu protection from the sulfhydryl function of Ncy. The linear precursor peptide dPhe1-Ncy2-Phe3-dTrp4-Lys5-Thr6-Ncy7-Thr8-NH2 obtained from the Boc-dPhe1-Ncy(Tmob)2-Phe3-DTrp(Boc)4-Lys(Boc)5-Thr(tBu)6-Ncy(Tmob)7-Thr(tBu)8-resin was oxidatively cyclized in the presence of I217 to give the crude cyclic peptide 9, Figure 2.
Figure 2.
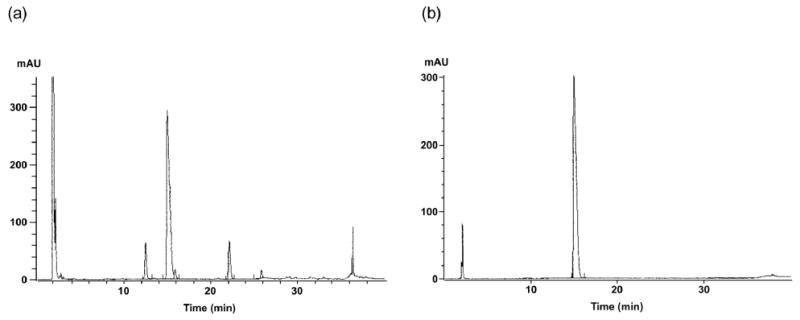
RP-HPLC profile of (a) crude and (b) purified peptide (9). RP-HPLC conditions: buffer A, TEAP pH 2.30; buffer B, 60% CH3CN/40% A; gradient elution from 20 to 50% buffer B in 30 min at a flow rate of 0.2 mL/min on a Vydac C18 column (0.21 cm × 15 cm, 5 μm particle size, 300 Å pore size). UV detection, 0.1 AUFS at 210 nm.
Our attempts to remove tBu protection from peptide 8 using DMSO/TFA mixtures18 or using Hg(II) acetate19 failed with noticeable side-products on RP-HPLC. Further experiments were directed towards on-resin oxidative deblocking of tBu protection using Tl(tfa)3.20 The fully protected Boc-dPhe1-Ncy(tBu)2-Phe3-dTrp(Boc)4-Lys(Boc)5-Thr(tBu)6-Ncy(tBu)7-Thr(tBu)8-DMBHA resin was treated with Tl(tfa)3 (1.2 euiv), 3 h in DMF-anisole (19:1) at 0 °C. The cleavage of the peptido resin with a cocktail mixture of TFA:H2O:TIS (95:2.5:2.5) followed by post cleavage workup did not show the desired [Ncy2, Ncy7]octreotide-amide (9) on RP-HPLC.
All of the crude peptides (7-9) were purified21 by RP-HPLC in at least two different solvent systems (TEAP pH 2.25 and 0.1% TFA on C18 silica). The analytical techniques used for the characterization of the analogs in Table 1 included RP-HPLC with two different solvent systems (0.1% TFA and TEAP pH 2.30) and capillary zone electrophoresis (CZE). Mass spectrometric analysis supported the identity of the intended structures, Table 1. Additionally, the structure of 9 was confirmed by coinjection experiment on RP-HPLC with [Ncy2, Ncy7]octreotide-amide synthesized in parallel using resolved Boc-Ncy(Mob)-OH1 and Boc-strategy.
In conclusion, we have shown that Alloc-Ncy(Tmob)-OH is compatible with the Fmoc strategy as an alternative to Boc-Ncy(Mob)-OH (used in the Boc strategy) for introducing a norcystine bridge in peptides by SPPS. The optical purity, easy removal of the Nα-Alloc protection (during chain elongation step) and the sulfhydryl Tmob protection (during the cleavage of peptide from resin) clearly demonstrate that Alloc-Ncy(TMob)-OH is a preferred choice for Fmoc-based SPPS.
Supplementary Material
Acknowledgments
We thank Ron Kaiser and Charleen Miller for technical assistance, Thomas Baiga and Dr. Joseph Noel for 1H, 13C NMR and chiral HPLC analysis. We thank Dr. David Chatenet for critical reading of the manuscript. We thank Debbie Doan for manuscript preparation. This work was supported by NIH Grants DK059953 and DK026741. JR is the Dr. Frederik Paulsen Chair in Neurosciences Professor.
Footnotes
Supplementary Data: Experimental procedures for the synthesis of amino acids in Scheme 1 and for the synthesis of peptide analog 9 in Table 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Samant MP, Rivier JE. Org Lett. 2006;8:2361–2364. doi: 10.1021/ol0606740. [DOI] [PubMed] [Google Scholar]
- 2.Carpino LA, Han GYJ. Am Chem Soc. 1970;92:5748–5749. [Google Scholar]
- 3.Stevens CM, Watanabe R. J Am Chem Soc. 1950;72:725–727. [Google Scholar]
- 4.Callahan FM, Anderson GW, Paul R, Zimmerman JE. J Am Chem Soc. 1963;85:201–207. [Google Scholar]
- 5.Zervas L, Theodoropoulos DM. J Am Chem Soc. 1956;78:1359–1363. [Google Scholar]
- 6.Munson MC, Garcia-Echeverria C, Albericio F, Barany G. J Org Chem. 1992;57:3013–3018. [Google Scholar]
- 7.Moroder L, Musiol HJ, Schaschke N, Chen L, Hargittai B, Barany G, 2.6.6 Thiol Group . In: Methods of Organic Chemistry: Synthesis of Peptides and Peptidomimetics. Goodman M, Felix A, Moroder L, Toniolo C, editors. E 22a. Houben-Weyl; New York: 2002. pp. 384–423. [Google Scholar]
- 8.Vetter S. Synthetic Commun. 1998;28:3219–3223. [Google Scholar]
- 9.Miyazawa T, Iwanaga H, Yamada T, Kuwata S. Biotechnol Lett. 1994;16:373–378. [Google Scholar]
- 10.3 and 5 were obtained in quantitative yields from 1 and 4, respectively. 4a-d and 5a-d were purified by column chromatography. The racemic amino acids 4a-d were obtained in 35-65% yield after purification (4a = 65%, 4b = 54%, 4c = 47% and 4d = 35%). The crude 4d contained approximately 15% of insoluble polymeric material.
- 11.6d. oil; [α]25D = + 42.40 (c = 1.0, MeOH); 1H NMR (300 MHz, DMSO-d6): 7.96 (d, 1H, J = 8.7 Hz), 6.21 (s, 2H), 5.98 - 5.85 (m, 1H), 5.32 (dd, 1H, J = 17.1, 1.2 Hz), 5.19 (dd, 1H, J = 10.5, 1.2 Hz), 5.12 (d, 1H, J = 8.7 Hz), 4.51 (d, 2H, J = 5.1 Hz), 3.76 (s, 2H), 3.75 (s, 9H); 13C NMR (75 MHz, DMSO-d6): 170.03, 160.23, 158.34, 158.30, 155.03, 133.34, 133.29, 117.07, 105.77, 90.67, 64.64, 56.17, 55.68, 55.16, 22.46; HRMS Calcd. for C16H21NO7S: 394.0931 (M + Na+), Found: 394.0920 (M + Na+), 2.8 ppm error.
- 12.The unreacted D-methyl esters were eluted with a mixture of EtOAc:hexane (25:75) and the resolved Alloc-Ncy(tBu/Tmob)-OH were eluted with EtOAc:MeOH (85:15).
- 13.Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless J. Life Sci. 1982;31:1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 14.Penke B, Rivier J. J Org Chem. 1987;52:1197–1200. [Google Scholar]
- 15.Chan WC, White PD. Fmoc solid phase peptide synthesis. Oxford University Press; New York: 2000. [Google Scholar]
- 16.Thieriet N, Alsina J, Giralt E, Guibe F, Albericio F. Tetrahed Lett. 1997;38:7275–7278. [Google Scholar]
- 17.Rivier J, Erchegyi J, Hoeger C, Miller C, Low W, Wenger S, Waser B, Schaer JC, Reubi JCJ. Med Chem. 2003;46:5579–5586. doi: 10.1021/jm030243c. [DOI] [PubMed] [Google Scholar]
- 18.Cuthbertson A, Indrevoll B. Tetrahed Lett. 2000;41:3661–3663. [Google Scholar]
- 19.Nishimura O, Kitada C, Fujino N. Chem Pharm Bull. 1978;26:1576. [Google Scholar]
- 20.Fujii N, Otaka A, Funakoshi S, Bessho K, Watanabe T, Akaji K, Yajima H. Chem Pharm Bull (Tokyo) 1987;35:2339–2347. doi: 10.1248/cpb.35.2339. [DOI] [PubMed] [Google Scholar]
- 21.The RP-HPLC purified peptide analogues 8 and 9 were obtained in 43% and 32% yields, respectively.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



