Abstract
Aims
We investigated whether rapid cooling instituted by total liquid ventilation (TLV) improves cardiac and mitochondrial function in rabbits submitted to ischaemia-reperfusion.
Methods and results
Rabbits were chronically instrumented with a coronary artery occluder and myocardial ultrasonic crystals for assessment of segment length-shortening. Two weeks later they were re-anaesthetized and underwent either a normothermic 30-min coronary artery occlusion (CAO) (Control group, n = 7) or a comparable CAO with cooling initiated by a 10-min hypothermic TLV and maintained by a cold blanket placed on the skin. Cooling was initiated after 5 or 15 min of CAO (Hypo-TLV and Hypo-TLV15′ groups, n = 6 and 5, respectively). A last group underwent normothermic TLV during CAO (Normo-TLV group, n = 6). Wall motion was measured in the conscious state over three days of reperfusion before infarct size evaluation and histology. Additional experiments were done for myocardial sampling in anaesthetized rabbits for mitochondrial studies. The Hypo-TLV procedure induced a rapid decrease in myocardial temperature to 32–34°C. Throughout reperfusion, segment length-shortening was significantly increased in Hypo-TLV and Hypo-TLV15′ vs. Control and Normo-TLV (15.1 ± 3.3%, 16.4 ± 2.3%, 1.8 ± 0.6%, and 1.1 ± 0.8% at 72 h, respectively). Infarct sizes were also considerably attenuated in Hypo-TLV and Hypo-TLV15′ vs. Control and Normo-TLV (4 ± 1%, 11 ± 5%, 39 ± 2%, and 42 ± 5% infarction of risk zones, respectively). Mitochondrial function in myocardial samples obtained at the end of ischaemia or after 10 min of reperfusion was improved by Hypo-TLV with respect to ADP-stimulated respiration and calcium-induced opening of mitochondrial permeability transition pores (mPTP). Calcium concentration opening mPTP was, e.g., increased at the end of ischaemia in the risk zone in Hypo-TLV vs. Control (157 ± 12 vs. 86 ± 12 µM). Histology and electron microscopy also revealed better preservation of lungs and of cardiomyocyte ultrastructure in Hypo-TLV when compared with Control.
Conclusion
Institution of rapid cooling by TLV during ischaemia reduces infarct size as well as other sequelae of ischaemia, such as post-ischaemic contractile and mitochondrial dysfunction.
KEYWORDS: Cooling, Contractile function, Mitochondria, Infarction, Total liquid ventilation
1. Introduction
Among the numerous experimental strategies that have been proposed to reduce the size of myocardial infarct, one of the most potent is cooling the heart to 32–34°C during ischaemia.1–3 At these temperatures, the heart beats normally and no external support of the circulation is required. The cooling rates that can be achieved by cooling the skin4 or using intravascular thermodes1 are however too slow to be optimally clinically effective. To exert optimal protection, a cooling strategy should aim to lower temperature to the desired target very fast after the onset of coronary artery occlusion (CAO) to effectively shorten the normothermic ischaemic time. Total liquid ventilation (TLV) with temperature-controlled perfluorocarbons has been proposed for rapid cooling,5–7 as these liquids have a high thermal conductivity and can use the lungs as heat exchangers while still maintaining gas exchange (high solubility for O2 and CO2).5,8,9 In a previous report, we demonstrated that a left atrial temperature of 32°C could be achieved within 5 min using TLV in anaesthetized rabbits.7 This was associated with a dramatic decrease in infarct size when hypothermic TLV (Hypo-TLV) was performed during a 30-min CAO. It remains, however, unknown whether rabbits can recover after Hypo-TLV and whether it would protect the myocardium against regional contractile dysfunction. This latter endpoint must be determined as it is well known that infarct size reduction does not predict full left ventricular contractile recovery, i.e. myocardium could be salvaged but still remain dysfunctional.10,11
Moreover, the exact mechanism by which mild hypothermia protects the heart against ischaemia remains unknown and justifies further investigation. Reduction in ATP consumption,12 intracellular acidosis, and Na+ and Ca2+ overload13 would be likely components of a mechanism resulting in the reduction of myocardial energy utilization14 and inhibition of intracellular processes associated with cell necrosis. Preserved mitochondrial function has also been proposed to protect isolated hypothermic hearts against ischaemia,15 but this was investigated following only ex vivo 17°C deep hypothermia. The effect of moderate 32–34°C hypothermia on ischaemia-induced mitochondrial dysfunction has not been studied despite the observation that this organelle, and in particular the mitochondrial permeability transition pore (mPTP), is possibly the end-effector of cardioprotection.16–19 Such investigations would indeed provide new mechanistic insights to determine whether TLV-induced hypothermia, a cardioprotective manoeuvre not related to preconditioning or post-conditioning, would also protect mitochondria against mPTP opening.
The main goal of the present study was, therefore, to investigate whether rapid cooling induced by TLV improves regional left ventricular function and protects mitochondria in rabbits submitted to lethal ischaemia. Accordingly, we first investigated left ventricular recovery in a well-established model of myocardial infarction in chronically instrumented conscious rabbits. Secondly, we performed additional investigations on mitochondria from ischaemic myocardium of rabbits submitted to CAO. Mitochondrial structure and function were studied by evaluating oxygen consumption, calcium-induced opening of mPTP, and morphology.
2. Methods
Animal instrumentation and ensuing experiments were conducted in accordance with French official regulations after approval by the local ethical committee. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All experiments were performed in male New Zealand rabbits (2.5–3.0 kg).
2.1. Instrumentation
Rabbits were anaesthetized with a mixture of tiletamine (25 mg/kg iv) and zolazepam (25 mg/kg iv), intubated, and mechanically ventilated with oxygen. Maintenance of anaesthesia was done with pentobarbital and inhaled 2% isoflurane. A left thoracotomy was performed under sterile conditions and a pneumatic occluder was implanted around a major branch of the left coronary artery (marginal artery).10,11 A pair of 1-mm ultrasonic piezoelectric crystals was inserted within the left ventricular wall in the perfusion territory of the instrumented coronary artery. The chest was closed in layers. The occluder tubing and the crystal wires were exteriorized between the scapulae. During the post-operative period, rabbits received buprenorphine (0.02 mg/kg/12 h sc, 3 days) and spiramycine (60 000 IU/kg/day im, 5 days). Rabbits recovered for a minimum of 10 days after surgery before inclusion in the study.
2.2. Experimental protocol
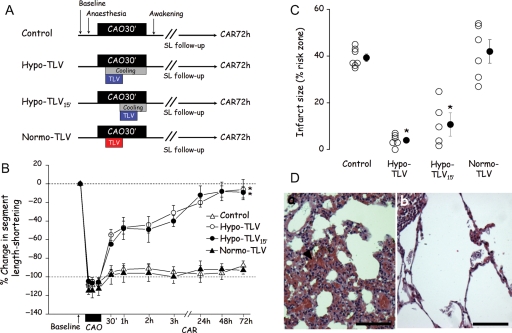
Baseline haemodynamic parameters were recorded in the conscious state in all rabbits after surgery. They were then re-anaesthetized using thiopentone (20 mg/kg iv) and intubated for conventional mechanical ventilation. As shown in Figure 1A rabbits were divided into four groups. All underwent 30 min of CAO to induce infarction. Reperfusion was then initiated and continued for 3 days in the conscious state. The first group (Control) was not subjected to any other treatment. In the second group (Hypo-TLV), cooling was started at the fifth minute of CAO using the combination of 10 min of hypothermic TLV and 25 min of surface cooling with ice-filled cold blankets initiated at the same time as TLV. In the third group (Hypo-TLV15′), a similar cooling protocol was induced at the 15th min of CAO using hypothermic TLV for 10 min combined with 15 min of surface cooling. The last group (Normo-TLV) was treated with 10 min of normothermic TLV initiated in the fifth min of CAO. In the Hypo-TLV, Hypo-TLV15′, and Normo-TLV groups, TLV episodes were performed using a mixture of perfluorobutyltetrahydrofurane and perfluoropropyltetrahydropyrane (RM101, Miteni, Milano, Italy).7 Rabbits were switched to liquid ventilation by filling the lung with 20 mL of perfluorocarbon and then connecting the endotracheal tube to the liquid ventilator. The ventilator was set to a tidal volume of 15 mL/kg body weight and 5 breaths/min. For each breath, the ventilator pumped into and out of the lungs the tidal volume of liquids. This protocol has previously been demonstrated to maintain normal blood gases.7 The perfluorocarbon mixture was bubbled with 100% O2. The temperature of the heat exchanger was set to either 15°C in Hypo-TLV and Hypo-TLV15′ or 39°C in the Normo-TLV group. At the end of the TLV procedure, the liquid was aspirated from the lung (approximately 20 mL of liquid) and resumption of conventional gas ventilation was permitted. In the Hypo-TLV and Hypo-TLV15′ groups, warm-up was initiated at the onset of reperfusion using infra-red lamps and a heating pad until the return of normothermia as monitored by the rectal temperature probe. Rabbits were allowed to wake-up and breath spontaneously as soon as possible after initiation of reperfusion. They were kept in a cage with supplemental oxygen for 24–48 h.
Figure 1.
Cardioprotective effect of cooling induced by hypothermic liquid ventilation in chronically instrumented rabbits. (A) Experimental protocol. (B) Change in segment length-shortening throughout the experimental protocol (expressed as % change from baseline). (C) Infarct size (expressed as % of the risk zone). (D) Lung histology showing severe congestion (arrowhead) in the Control group (a, Bar = 60 µm) and normal appearance in the Hypo-TLV group (b, Bar = 60 µm). CAO, coronary artery occlusion; CAR, coronary artery reperfusion; TLV, total liquid ventilation; Hypo-TLV, hypothermic TLV; Normo-TLV, normothermic TLV; SL, segment length-shortening; *P < 0.05 vs. Control and Normo-TLV (throughout reperfusion for the % change in SL shortening).
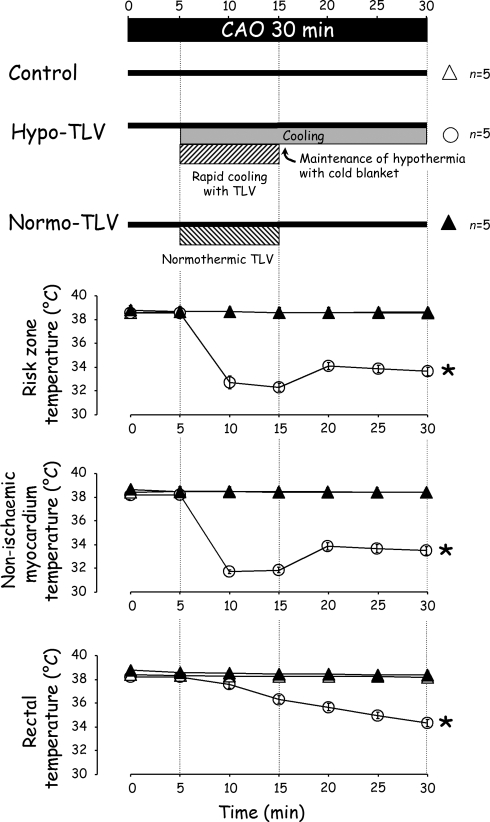
In order to measure myocardial temperature during the TLV procedure, additional rabbits were anaesthetized and mechanically ventilated. A left lateral thoracotomy was performed and two thermal probes were implanted within the left ventricular wall for measurement of myocardial temperatures in ischaemic and non-ischaemic territories during a subsequent CAO. After the institution of CAO, the chest was rapidly closed in layers, and rabbits were randomly divided into Control, Hypo-TLV, or Normo-TLV groups, as described above. In the Hypo-TLV group, the cooling procedure was started at the fifth minute of ischaemia. Rectal and myocardial temperatures were continuously monitored throughout the 30 min of CAO (Figure 2, upper panel).
Figure 2.
Myocardial and rectal temperatures in anaesthetized rabbits subjected to hypothermic or normothermic liquid ventilation. See legend of Figure 1; *P < 0.05 vs. Control and Normo-TLV.
Finally, in order to avoid blood-sampling in rabbits in which haemodynamics were investigated, we performed blood-gas analyses (ABL77, Radiometer Medical ApS, Brønshøj, Denmark) in additional rabbits submitted to Control or Hypo-TLV procedures (n = 4 in each condition). Blood-gas values were corrected for the actual body temperature.
2.3. Haemodynamic measurements in chronically instrumented rabbits
Data were digitized and analysed using the data acquisition software HEM 3.5 (Notocord Systems, Croissy sur Seine, France). A catheter was positioned in the artery of the rabbit's ear for arterial blood pressure measurement (Statham P23ID strain gauge; Statham Instruments, Oxnard, CA, USA) during CAO and during the first hour of reperfusion. An external electrocardiogram was also recorded. As previously described10,11 regional segment length was measured by connecting the crystal wires to an ultrasound module (Module 201, System 6; Triton Technology, San Diego, CA, USA). Segment systolic-shortening was calculated as the difference between end-diastolic and end-systolic lengths normalized for the end-diastolic length. A phonocardiogram was recorded for detection of the beginning and end of systole.10,11
2.4. Post-mortem analyses and histology
After completion of three days of reperfusion, the chronically instrumented rabbits were euthanized using pentobarbital (60 mg/kg iv) followed by potassium chloride. The hearts were excised and the coronary artery was ligated at the occluder site. The ascending aorta was cannulated and perfused retrogradely with Alcian blue (0.5%). The left ventricle was cut into slices, weighed, and incubated in 1% triphenyltetrazolium chloride. Slices were fixed in formaldehyde and photographed. Risk and infarcted zones were quantified by planimetry and expressed as percentages of the left ventricle and risk zone, respectively. To determine the extent of myocardial infarction between implanted crystals, we analyzed the formaldehyde-fixed myocardium between the crystals. As previously described, a computerized reconstruction of the complete haematoxylin–eosin-stained histological section was made by juxtaposition of digital photographs.10 Planimetry was performed and the infarcted area between crystals was quantitated. Lungs were also analysed by histology after H&E staining.
2.5. Investigation of mitochondrial oxygen consumption and calcium-induced mitochondrial permeability transition pores opening
A cardiac mitochondrial fraction was prepared from additional Control or Hypo-TLV rabbits acutely subjected to a 30-min CAO (Figure 3A). In a first set of experiments, hearts were submitted only to ischaemia and rapidly excised immediately after completion of CAO. In a second set of experiments, hearts were submitted to ischaemia-reperfusion and hearts were excised after 10 min of reperfusion. In both conditions, two samples of approximately 300 mg from ischaemic and non-ischaemic myocardium were rapidly minced and homogenized in a cold buffer (220 mM mannitol, 70 mM sucrose, 10 mM HEPES, 1 mM EGTA, 0.04 mM free fatty acid bovine serum albumin, pH = 7.4 at 4°C), as previously described.20 Homogenates were centrifuged at 1000 g for 5 min and supernatants were centrifuged at 10 000 g for 10 min. The final mitochondrial pellets were resuspended and oxygen consumption was measured at 37°C with a Clark type electrode in a respiration buffer (50 mM sucrose, 100 mM KCl, 10 mM HEPES, 5 mM KH2PO4, pH = 7.4) containing mitochondria (0.4 mg protein/ml). Substrate-respiration rate (state 4 oxygen consumption) and ATP synthesis (state 3) were investigated by the addition of 5 mM pyruvate/malate and 300 µM ADP, respectively. The corresponding respiration control ratio (state 3/state 4) was calculated. In the mitochondrial samples submitted to both ischaemia and reperfusion, ATP concentration was measured using an ATP determination kit (FluoProbes®, Interchim, Montluçon, France).
Figure 3.
Mitochondrial oxygen consumption and calcium-induced opening of permeability transition pore. (A) Experimental protocol before myocardial sampling of ischaemic and non-ischaemic territories in rabbits submitted to a 30-min CAO only (ControlIsch and Hypo-TLVIsch) or to a similar CAO followed by 10 min of reperfusion (ControlI/R and Hypo-TLVI/R). (B) Typical recording of an experiment of Ca2+-induced mitochondrial permeability transition pore (mPTP) opening in mitochondria extracted from a non-ischaemic zone of a Control heart. Ca2+ levels outside the mitochondria were monitored with Ca2+ green-5 N fluorescence expressed in arbitrary units (AU) as successive 10 µM Ca2+ pulses were added to the medium. (C, E) Respiratory control ratio (state 3/state 4 oxygen consumption) in mitochondria from ischaemic and non-ischaemic zones of hearts subjected to ischaemia only (panel C: ControlIsch and Hypo-TLVIsch) or to ischaemia-reperfusion (panel E: ControlI/R and Hypo-TLVI/R). (D, F) Mitochondrial ability to retain Ca2+ expressed as mean calcium concentration inducing opening of mPTP in mitochondria from ischaemic and non-ischaemic zones of hearts subjected to ischaemia only (panel D: ControlIsch and Hypo-TLVIsch) or to ischaemia-reperfusion (panel F: ControlI/R and Hypo-TLVI/R). See legend of Figure 1; *P < 0.05 vs. corresponding non-ischaemic zone; †P < 0.05 vs. corresponding Control value.
In other experiments, the ability of mitochondria to retain Ca2+ before exogenously induced mPTP opening was monitored, as previously described.20 Briefly, cardiac mitochondria (1 mg protein/mL) energized with 5 mM pyruvate/malate were incubated in the respiration buffer including 1 µM of the Ca2+ green-N fluorescent probe. The reaction was started by the addition of successive 10 µM Ca2+ pulses. After each addition, a rapid uptake was observed followed by a dynamic steady state corresponding to the equilibrium between influx and efflux of Ca2+. When sufficient Ca2+ loading was obtained to trigger mPTP opening, this equilibrium was disrupted and Ca2+ was released (Figure 3B). We calculated the total Ca2+ concentration triggering mPTP opening. The concentration of Ca2+ in the extramitochondrial medium was monitored by means of a Perkin-Elmer® LS 50B spectrofluorimeter at excitation and emission wavelengths of 506 nm and 532 nm, respectively.
2.6. Electron microscopy
In order to further investigate myocardial integrity following hypothermia, experiments were performed in additional Hypo-TLV or Control anaesthetized rabbits. After opening the chest, a major branch of the left coronary artery (marginal artery) was occluded for 30 min. The chest was immediately closed and rabbits were subjected to either no procedure (Control) or to cooling with Hypo-TLV initiated in the fifth min of CAO. Just before completion of the 30-min CAO, the chest was reopened in order to reperfuse the ligated artery. At 15 s after the onset of reperfusion, hearts were removed and perfused-fixed through the aorta with 2.5% glutaraldehyde. A 5 × 5 mm sample of myocardium was excised from both the ischaemic and non-ischaemic areas. Each sample was cut into 1 mm3 tissue blocks which were embedded in Epon for standard electron microscopy. Five blocks from each area were randomly selected for analysis.
2.7. Statistical analysis
Values are expressed as means ± SEM. Comparisons were made using either a one- or two-way analysis of variance followed by a Student's t-test with Bonferroni correction. Significant differences were determined at P < 0.05.
3. Results
As shown in Figure 2 (lower panel) myocardial and rectal temperatures were rapidly decreased in Hypo-TLV when compared with Control and Normo-TLV (n = 5 in each group, P < 0.05). Myocardial temperature in the ischaemic territory decreased by an average of −6.3 ± 0.2°C at the 15th min of CAO in the Hypo-TLV group.
During conventional mechanical ventilation, blood-gases were within normal ranges in Control hearts (e.g. pH 7.4 ± 0.1, pCO2 41 ± 4 mmHg, and pO2 531 ± 30 mmHg after 30 min of follow-up). Blood-gas values were also not altered at the end of the Hypo-TLV cooling procedure (pH 7.4 ± 0.1, pCO2 32 ± 3 mmHg, and pO2 597 ± 14 mmHg) and 1 h later (pH 7.4 ± 0.1, pCO2 33 ± 3 mmHg, and pO2 538 ± 59 mmHg).
Twenty-four rabbits underwent the complete protocol in the chronic study (seven, six, five, and six rabbits in Control, Hypo-TLV, Hypo-TLV15′, and Normo-TLV groups, respectively). As shown in Table 1, haemodynamic parameters and rectal temperature were not significantly different among groups at baseline. During CAO, those parameters were similar between groups except heart rate and rectal temperature that were significantly reduced after the onset of cooling in Hypo-TLV and Hypo-TLV15′. Conversely, segment length-shortening was significantly increased throughout reperfusion in Hypo-TLV and Hypo-TLV15′ when compared with Control. As shown in Figure 1B, this parameter recovered to 95% of its corresponding baseline value after 72 h of reperfusion in these groups. Sizes of risk regions were not significantly different among groups averaging 32 ± 3%, 35 ± 4%, 34 ± 2%, and 36 ± 4% of the left ventricle in Control, Hypo-TLV, Hypo-TLV15′, and Normo-TLV groups, respectively. As shown in Figure 1C, infarct size was significantly reduced in Hypo-TLV and Hypo-TLV15′ when compared with Control and Normo-TLV (4 ± 1%, 11 ± 5%, 39 ± 2%, and 42 ± 5% of region at risk, respectively). Infarction between crystals assessed by histology was also significantly reduced in the Hypo-TLV (1 ± 1%) and Hypo-TLV15′ (3 ± 2%) groups when compared with Control (45 ± 9%) and Normo-TLV (36 ± 6%). In all lungs from the Control group, histology demonstrated foci of minor to severe congestion (Figure 1D, panel a). In the Hypo-TLV group, three rabbits had normal lungs (Figure 1D, panel b) and three others had only minor congestion.
Table 1.
Haemodynamic parameters and rectal temperature in chronically instrumented rabbits
| Baseline | CAO |
Reperfusion |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 min | 25 min | 1 h | 2 h | 3 h | 24 h | 48 h | 72 h | ||
| Heart rate (beats/ min) | |||||||||
| Control | 234 ± 11 | 248 ± 16 | 242 ± 15 | 234 ± 13 | 239 ± 10 | 246 ± 9 | 242 ± 14 | 242 ± 12 | 243 ± 13 |
| Hypo-TLV | 243 ± 11 | 177 ± 13* | 200 ± 7* | 243 ± 9 | 248 ± 11 | 244 ± 15 | 251 ± 8 | 240 ± 13 | 244 ± 15 |
| Hypo-TLV15′ | 240 ± 10 | 228 ± 17 | 187 ± 13* | 211 ± 16 | 252 ± 17 | 242 ± 13 | 220 ± 15 | 227 ± 19 | 245 ± 30 |
| Normo-TLV | 234 ± 9 | 241 ± 15 | 238 ± 16 | 230 ± 17 | 250 ± 12 | 257 ± 10 | 243 ± 13 | 241 ± 18 | 243 ± 13 |
| Mean arterial blood pressure (mmHg) | |||||||||
| Control | 84 ± 2 | 68 ± 8 | 68 ± 8 | 73 ± 8 | |||||
| Hypo-TLV | 84 ± 4 | 73 ± 6 | 66 ± 4 | 69 ± 5 | |||||
| Hypo-TLV15′ | 76 ± 2 | 73 ± 3 | 70 ± 4 | 67 ± 5 | |||||
| Normo-TLV | 84 ± 3 | 74 ± 2 | 67 ± 5 | 68 ± 6 | |||||
| Segment length at the end of diastole (mm) | |||||||||
| Control | 7.5 ± 1.0 | 7.7 ± 1.1 | 7.7 ± 1.2 | 7.8 ± 1.1 | 7.9 ± 1.1 | 7.8 ± 1.1 | 7.9 ± 1.1 | 7.8 ± 1.1 | 8.0 ± 1.1 |
| Hypo-TLV | 6.8 ± 1.1 | 7.5 ± 1.2 | 7.4 ± 1.1 | 6.9 ± 1.0 | 6.8 ± 1.0 | 6.8 ± 1.1 | 6.7 ± 1.1 | 6.8 ± 1.1 | 6.7 ± 1.1 |
| Hypo-TLV15′ | 7.7 ± 0.9 | 8.2 ± 1.0 | 8.2 ± 1.0 | 7.8 ± 1.0 | 7.9 ± 0.9 | 7.9 ± 0.9 | 8.3 ± 1.1 | 7.9 ± 1.0 | 7.5 ± 0.9 |
| Normo-TLV | 7.1 ± 1.1 | 7.2 ± 0.9 | 7.3 ± 1.0 | 7.1 ± 1.0 | 7.2 ± 1.0 | 7.2 ± 1.0 | 7.4 ± 1.0 | 7.2 ± 1.0 | 7.0 ± 1.0 |
| Segment length systolic-shortening (%) | |||||||||
| Control | 15.2 ± 2.3 | -2.1 ± 0.3 | -1.4 ± 0.4 | 0.9 ± 0.6 | 0.9 ± 0.7 | 0.4 ± 0.8 | 0.4 ± 1.0 | 0.6 ± 0.8 | 1.8 ± 0.6 |
| Hypo-TLV | 15.8 ± 2.7 | -0.9 ± 0.4 | -1.3 ± 0.3 | 8.3 ± 1.8* | 8.8 ± 1.7* | 11.1 ± 2.2* | 12.5 ± 2.0* | 15.3 ± 4.0* | 15.1 ± 3.3* |
| Hypo-TLV15′ | 18.3 ± 2.7 | -1.0 ± 0.7 | -0.9 ± 0.7 | 10.2 ± 3.4* | 10.5 ± 3.8* | 11.4 ± 2.5* | 15.5 ± 1.9* | 16.6 ± 2.1* | 16.4 ± 2.3* |
| Normo-TLV | 14.9 ± 3.1 | -2.1 ± 0.5 | -1.7 ± 0.5 | 0.9 ± 1.1 | 0.7 ± 1.1 | 0.3 ± 0.8 | 1.0 ± 1.4 | 1.1 ± 1.0 | 1.1 ± 0.8 |
| Rectal temperature (°C) | |||||||||
| Control | 39.1 ± 0.3 | 38.8 ± 0.3 | 38.6 ± 0.3 | 39.0 ± 0.4 | 38.9 ± 0.8 | 39.0 ± 0.9 | |||
| Hypo-TLV | 39.2 ± 0.1 | 36.3 ± 0.5* | 34.8 ± 0.6* | 36.2 ± 0.6* | 38.1 ± 0.3 | 38.7 ± 0.3 | |||
| Hypo-TLV15′ | 38.7 ± 0.2 | 38.3 ± 0.2 | 35.5 ± 0.7* | 36.1 ± 0.3* | 38.0 ± 0.6 | 38.3 ± 0.3 | |||
| Normo-TLV | 39.3 ± 0.2 | 38.9 ± 0.2 | 38.7 ± 0.2 | 38.3 ± 0.3 | 38.5 ± 0.2 | 38.8 ± 0.2 | |||
CAO, coronary artery occlusion; Hypo-TLV, hypothermic total liquid ventilation; Hypo-TLV15′, Hypo-TLV instituted at the fifteenth min of CAO; Normo-TLV, normothermic total liquid ventilation.
*P < 0.05 vs. Control.
In 10 other rabbits mitochondria were isolated from myocardium obtained at the end of the 30-min CAO (n = 5 in both Control and Hypo-TLV groups). In Control normothermic hearts, mitochondrial dysfunction in the ischaemic territory was demonstrated by a −41% decrease in ATP synthesis (state 3 oxygen consumption) compared with the non-ischaemic zone (447 ± 30 vs. 763 ± 63 nmoles O2/min/mg of protein, respectively, P < 0.05). In Hypo-TLV hearts (n = 5), state 3 oxygen consumption was decreased by only −25% in ischaemic vs. non-ischaemic zones (631 ± 53 vs. 841 ± 50 nmoles O2/min/mg of protein, respectively, P < 0.05). However, substrate-respiration rate (state 4 oxygen consumption) was not altered in the ischaemic zone in either group (data not shown). As illustrated in Figure 3C, the respiratory control ratio (state 3/state 4) was therefore significantly decreased by 42% in the ischaemic zone in the Control group. In contrast, the respiratory control ratio was significantly less depressed in Hypo-TLV (−21%). As shown in Figure 3D, Ca2+ concentration required to open mPTP was significantly reduced by −49% in the ischaemic vs. non-ischaemic zones in the Control group (86 ± 12 vs. 170 ± 10 µM, respectively). In contrast, hypothermia protected mitochondria against mPTP opening as Ca2+ concentration required to open mPTP was not significantly different in the ischaemic and non-ischaemic zones in the Hypo-TLV group (157 ± 12 vs. 188 ± 14 µM, respectively).
Ten rabbits were also included for mitochondrial investigations after 30-min CAO followed by 10 min of reperfusion (n = 5 in both Control and Hypo-TLV groups). The respiratory control ratio (state 3/state 4) was significantly decreased by −58% in the reperfused when compared with non-ischaemic zone in the Control group, as shown in Figure 3E. In contrast, the respiratory control ratio was significantly less decreased in Hypo-TLV (−30%). The decrease in respiratory control ratio was again related to an alteration in state 3 oxygen consumption as state 4 was unchanged (data not shown). Interestingly, ATP concentrations in myocardium subjected to ischaemia-reperfusion was also significantly improved in Hypo-TLV hearts compared with Control (5.90 ± 1.63 and 0.65 ± 0.17 µM/g of tissue, respectively). As shown in Figure 3F, Ca2+ concentration required to open mPTP was significantly reduced by −68% in the territory undergoing ischaemia-reperfusion compared with the non-ischaemic zone in the Control group (57 ± 8 vs. 174 ± 17 µM, respectively). In contrast, it was only reduced by −37% in the Hypo-TLV group (117 ± 16 vs. 183 ± 12 µM, respectively).
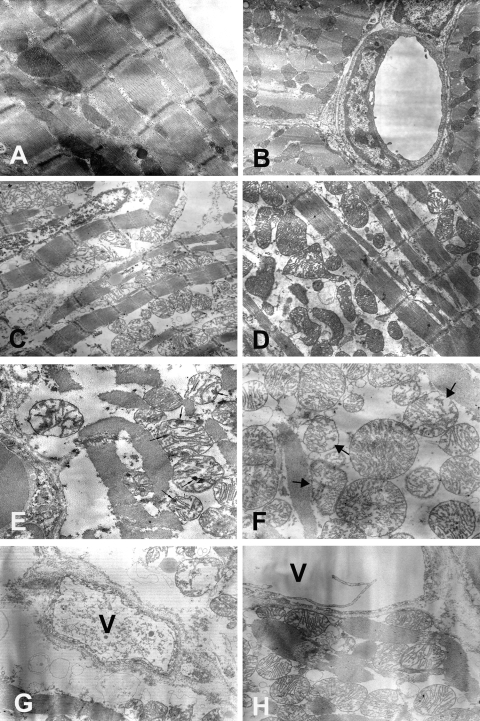
Finally, six other rabbits underwent myocardial sampling for electron microscopy (n = 3 in both Control and Hypo-TLV groups). In each heart, we analysed five samples issued from the ischaemic territory and five samples from the normally perfused myocardium (total number of blocks = 60). In all samples from Control and Hypo-TLV rabbits, cellular and extracellular architecture of normally perfused myocardium was intact with normal mitochondria, lack of intracellular oedema (Figure 4A), and normal microvessels (Figure 4B). In all samples of ischaemic myocardium from Control hearts we observed marked intracellular oedema (Figure 4C) with wide-spread irreversible ischaemic damage to the mitochondria characterized by membrane rupture and amorphous densities and (Figure 4E). In the Hypo-TLV group, intracellular oedema was more modest (Figure 4D) and mitochondrial damage was characterized by minor loss of mitochondrial cristae without amorphous densities (Figure 4F). Finally, we observed endothelial cell necrosis in Control samples (Figure 4G) but not in Hypo-TLV hearts (Figure 4H).
Figure 4.
Electron microscopic analysis of myocardium samples fixed immediately after the onset of reperfusion. (A, B) Normal ultrastructure of myocardium in the non-ischaemic area in both Control (A, ×12 500) and Hypo-TLV (B, ×8000) groups. (C, D) More marked intracellular oedema in the ischaemic area in the Control (C, ×6300) than in the Hypo-TLV group (D, ×6300). (E) In the ischaemic area in the Control group (E), there is widespread irreversible ischaemic damage to the mitochondria characterized by amorphous densities (small arrows) (×12 500). (F) In the ischaemic area in the Hypo-TLV group, the mitochondrial damage is characterized by minor loss of mitochondrial cristae in the ischaemic area (large arrows, ×12 500). (G) Damage of the microvessels (V) showing endothelial cell necrosis in the ischaemic area in the Control group (×12 500). (H) The microvessels (V) are normal in the ischaemic area in the Hypo-TLV group (×12 500).
4. Discussion
The present study demonstrates that institution of rapid cooling by a brief episode of Hypo-TLV during ischaemia not only reduces infarct size as previously reported2,3,21–23 but importantly also abolishes post-ischaemic regional contractile dysfunction. Interestingly, our results also demonstrate that hypothermia protects the myocardium against cellular damage and calcium-induced opening of mPTP at the end of the ischaemic period. To our knowledge this is the first study to investigate the functional recovery following myocardial infarction and cooling in a 3-day recovery model and to compare mitochondrial function following in vivo normothermic and hypothermic ischaemia (32–34°C).
Our results document that a short period of hypothermic TLV can be used to induce rapid cooling that can be maintained by less-invasive surface cooling. Importantly, the infarct size reduction elicited by Hypo-TLV was not related to a chemical effect of the perfluorocarbon mixture since infarct size was not altered with normothermic TLV. This result was expected as the cardioprotective effect of cooling during ischaemia has already been demonstrated in rabbits,2,3 dogs,21 pigs,22 and rats.23 The protection is dependent on the depth of cooling and maximal protection is reached at 32°C.3 In the present study, myocardial salvage was observed when cooling was initiated either at the fifth or fifteenth min of ischaemia. In our previous report in open-chest anaesthetized rabbits, Hypo-TLV did not reduce infarct size if instituted at the 25th min of a 30 min ischaemia.7 Importantly, we further observed in the present report a rapid and potent recovery of post-ischaemic regional contractility in Hypo-TLV and Hypo-TLV15′ groups when compared with normothermic intervention. The recovery was evident as early as 30 min after the onset of reperfusion and virtually complete after 3 days. Conversely, ischaemic preconditioning that can induce an ultimate infarct size reduction as potent as that observed with Hypo-TLV failed to improve regional contractility during the first hours of reperfusion in similarly instrumented rabbits.10,11 These data suggest that in addition to its infarct-sparing effect, cooling during ischaemia exerts a more potent protective effect than preconditioning against early post-ischaemic dysfunction. It has also been reported that a modest decrease in myocardial temperature exerts a positive inotropic and oxygen-saving effect in in situ dog hearts at 34°C.24 The proposed mechanisms include an increased Ca2+ sensitivity of myofilament proteins25 and improved Ca2+-activated force generation.26
In addition to infarct size reduction and improvement in post-ischaemic left ventricular function, we further demonstrated excellent preservation of cardiomyocyte and blood vessel ultrastructure in rabbits subjected to Hypo-TLV compared with Control. This effect was observed by electron microscopy in hearts fixed immediately after the onset of reperfusion, again suggesting that the beneficial effect of cooling occurs during ischaemia.22 This however does not definitely exclude any reperfusion injury in these conditions. We also demonstrate that hypothermia protects mitochondria by preserving respiratory control ratio and ATP synthesis and reducing mitochondrial Ca2+ sensitivity to mPTP opening. Mitochondrial preservation by hypothermia is an important finding since opening of mPTP is a well-known trigger of cell death following myocardial ischaemia.16–19 Preconditioning and post-conditioning exert, at least in part, their cardioprotective effect at reperfusion through inhibition of mPTP opening.17,27,28 The beneficial effect of cooling was unexpectedly observed in mitochondria from ‘non-reperfused’ as well as ‘reperfused’ myocardium. In ‘non-reperfused’ conditions, Ca2+ concentration required to open mPTP was importantly not significantly decreased in hypothermic hearts (−16%), whereas we observed a −49% drop in normothermic hearts. In hearts subjected to ischaemia-reperfusion, a significant decrease was observed in both hypothermic (−37%) and Control (−68%) hearts, although the decrease was greater in the latter. Hence reperfusion enhanced mitochondrial damage in both normothermic and hypothermic hearts again suggesting that hypothermia protects during ischaemia rather than against reperfusion injury. The exact mechanism by which hypothermia inhibits mPTP opening was not investigated. Inhibition of ischaemia-induced calcium overload could be involved since it was previously reported in isolated guinea pig hearts that hypothermia to 17°C abolished mitochondrial rise of calcium concentration during ischaemia.15 Interestingly, hypothermia also altered reactive oxygen species production in that study.15 However, we did not properly demonstrate that mitochondrial protection was a direct effect of hypothermia as one would expect any treatment against ischaemia to protect mitochondria directly or indirectly.
One of our important conclusions is also that brief TLV is safe. We and others have already demonstrated that haemodynamics and gas exchange were not altered during TLV in rabbits,7 lambs,6 and cats.5 However, our preliminary experiments demonstrated an increased mortality in rabbits subjected to prolonged periods of Hypo-TLV, i.e. >30 min. The cause of these deaths was not pinpointed but that problem was not seen in rabbits exposed to shorter periods of Hypo-TLV as in the present report. To our knowledge this is the first study demonstrating that short TLV is compatible with rapid resumption of spontaneous breathing without major hypoxia and with minimal lung trauma. Indeed, our rabbits resumed spontaneous breathing only a few hours after the end of TLV. They breathed normally and were maintained in a closed cage with air supplemented with oxygen during the initial 24–48 h of their recovery. This was to prevent any possibility of hypoxia since atelectasis may occur following TLV.7,29 Later, rabbits were kept in conventional cages and still breathed normally without hypoxia. Lung histology also demonstrated that Hypo-TLV did not exert a deleterious effect. We even observed protection against pulmonary oedema and congestion in the Hypo-TLV group, further demonstrating the potency of the protection against cardiac mechanical dysfunction.
A recent editorial emphasized the clinical relevance of inducing cooling by breathing chilled liquids.30 The present report further proposes a strategy that would be easy to manage by adding a short period of Hypo-TLV to conventional surface cooling. The translation would be easier in patients who are already intubated and mechanically ventilated, e.g. patients with atherosclerotic vascular disease and post-operative myocardial infarction following high-risk surgery for whom immediate coronary revascularization is not possible. Similarly, Hypo-TLV might be useful in myocardial ischaemia secondary to profound blood loss or hypovolaemia. Whether conscious patients awaiting revascularization for acute ST-segment elevation myocardial infarction would benefit from Hypo-TLV and the attendant delay would depend on the projected time before the proposed intervention and reperfusion could be accomplished. However, as rapid cooling virtually stops infarct extension, the time needed for anaesthesia and intubation might not represent a serious limitation. Liquid ventilation could also be a strategy to induce cooling as an aid to resuscitation. In the hours following cardiac arrest and resumption of spontaneous circulation, therapeutic hypothermia is indeed already recommended by international guidelines.31 The method of rapid induction described here would likely increase its effectiveness.
In conclusion, rapid cooling instituted by a brief episode of hypothermic TLV in rabbits abolished most consequences of ischaemia and resulted in potent infarct size reduction, early and complete contractile recovery, protection against myocardial cellular lesions, and prevention of mitochondrial dysfunction.
Conflict of interest: none declared.
Funding
Grants HL20648 from the Heart, Lung and Blood Institute of the National Institutes of Health, TLVenCool 06-JCJC-0078 from the French ‘Agence Nationale pour la Recherche’, and ET7-460 from the Fondation de l'Avenir.
References
- 1.Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–1934. doi: 10.1016/s0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 2.Hale SL, Dave RH, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol. 1997;92:351–357. doi: 10.1007/BF00788947. [DOI] [PubMed] [Google Scholar]
- 3.Miki T, Liu GS, Cohen MV, Downey JM. Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol. 1998;93:372–383. doi: 10.1007/s003950050105. [DOI] [PubMed] [Google Scholar]
- 4.Ly HQ, Denault A, Dupuis J, Vadeboncoeur A, Harel F, Arsenault A, et al. A pilot study: the Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (the NICAMI Study) Am Heart J. 2005;150:933.e9–933.e13. doi: 10.1016/j.ahj.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer TH, Forman DL, Wolfson MR. Physiological effects of ventilation with liquid fluorocarbon at controlled temperatures. Undersea Biomed Res. 1984;11:287–298. [PubMed] [Google Scholar]
- 6.Forman DL, Bhutani VK, Tran N, Shaffer TH. A new approach to induced hypothermia. J Surg Res. 1986;40:36–42. doi: 10.1016/0022-4804(86)90142-3. [DOI] [PubMed] [Google Scholar]
- 7.Tissier R, Hamanaka K, Kuno A, Parker JC, Cohen MV, Downey JM. Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol. 2007;49:601–605. doi: 10.1016/j.jacc.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Yang SS, Jeng MJ, McShane R, Chen CY, Wolfson MR, Shaffer TH. Cold perfluorochemical-induced hypothermia protects lung integrity in normal rabbits. Biol Neonate. 2005;87:60–65. doi: 10.1159/000081245. [DOI] [PubMed] [Google Scholar]
- 9.Hong S-B, Koh Y, Shim T-S, Lee SD, Kim WS, Kim DS, et al. Physiologic characteristics of cold perfluorocarbon-induced hypothermia during partial liquid ventilation in normal rabbits. Anesth Analg. 2002;94:157–162. doi: 10.1097/00000539-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Aouam K, Tissier R, Bruneval P, Mandet C, Berdeaux A, Ghaleh B. Preconditioning of salvaged myocardium in conscious rabbits with postinfarction dysfunction. Am J Physiol. 2005;288:H2763–H2769. doi: 10.1152/ajpheart.00657.2004. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MV, Yang X-M, Downey JM. Smaller infarct after preconditioning does not predict extent of early functional improvement of reperfused heart. Am J Physiol. 1999;277:H1754–H1761. doi: 10.1152/ajpheart.1999.277.5.H1754. [DOI] [PubMed] [Google Scholar]
- 12.Simkhovich BZ, Hale SL, Kloner RA. Metabolic mechanism by which mild regional hypothermia preserves ischemic tissue. J Cardiovasc Pharmacol Ther. 2004;9:83–90. doi: 10.1177/107424840400900203. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SE, Liu H, Beyschau A, Cala PM. Effects of cold cardioplegia on pH, Na, and Ca in newborn rabbit hearts. Am J Physiol. 2006;290:H1090–H1097. doi: 10.1152/ajpheart.00776.2004. [DOI] [PubMed] [Google Scholar]
- 14.Ning X-H, Chi EY, Buroker NE, Chen S-H, Xu C-S, Tien Y-T, et al. Moderate hypothermia (30 degrees C) maintains myocardial integrity and modifies response of cell survival proteins after reperfusion. Am J Physiol. 2007;293:H2119–H2128. doi: 10.1152/ajpheart.00123.2007. [DOI] [PubMed] [Google Scholar]
- 15.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol. 2003;35:339–341. doi: 10.1016/s0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 17.Boengler K, Gres P, Dodoni G, Konietzka I, Di Lisa F, Heusch G, et al. Mitochondrial respiration and membrane potential after low-flow ischemia are not affected by ischemic preconditioning. J Mol Cell Cardiol. 2007;43:610–615. doi: 10.1016/j.yjmcc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, Abellan A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Boengler K, Gres P, Cabestrero A, Ruiz-Meana M, Garcia-Dorado D, Heusch G, et al. Prevention of the ischemia-induced decrease in mitochondrial Tom20 content by ischemic preconditioning. J Mol Cell Cardiol. 2006;41:426–430. doi: 10.1016/j.yjmcc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Rande JL, Berdeaux A, et al. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther. 2008;326:252–258. doi: 10.1124/jpet.108.138008. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J Mol Cell Cardiol. 1997;29:1577–1583. doi: 10.1006/jmcc.1997.0391. [DOI] [PubMed] [Google Scholar]
- 22.Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol. 2006;101:61–68. doi: 10.1007/s00395-005-0550-7. [DOI] [PubMed] [Google Scholar]
- 23.van den Doel MA, Gho BCG, Duval SY, Schoemaker RG, Duncker DJ, Verdouw PD. Hypothermia extends the cardioprotection by ischaemic preconditioning to coronary artery occlusions of longer duration. Cardiovasc Res. 1998;37:76–81. doi: 10.1016/s0008-6363(97)00222-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura Y, Naito Y, Nishioka T, Okamura Y. The effects of cardiac cooling under surface-induced hypothermia on the cardiac function in the in situ heart. Interact Cardiovasc Thorac Surg. 2005;4:101–105. doi: 10.1510/icvts.2004.097188. [DOI] [PubMed] [Google Scholar]
- 25.Stowe DF, Fujita S, An J, Paulsen RA, Varadarajan SG, Smart SC. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol. 1999;277:H2321–H2332. doi: 10.1152/ajpheart.1999.277.6.H2321. [DOI] [PubMed] [Google Scholar]
- 26.Kusuoka H, Ikoma Y, Futaki S, Suga H, Kitabatake A, Kamada T, et al. Positive inotropism in hypothermia partially depends on an increase in maximal Ca2+-activated force. Am J Physiol. 1991;261:H1005–H1010. doi: 10.1152/ajpheart.1991.261.4.H1005. [DOI] [PubMed] [Google Scholar]
- 27.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KHH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 29.Salman NH, Fuhrman BP, Steinhorn DM, Papo MC, Hernan LJ, Leach CL, et al. Prolonged studies of perfluorocarbon associated gas exchange and of the resumption of conventional mechanical ventilation. Crit Care Med. 1995;23:919–924. doi: 10.1097/00003246-199505000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Lew WYW. A cool heart protected from infarction: clinical translation of breathing chilled liquids. J Am Coll Cardiol. 2007;49:606–607. doi: 10.1016/j.jacc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WGJ, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]