Abstract
Aims
We have previously shown that activation of leptin signalling in the heart reduces cardiac morbidity and mortality after myocardial infarction (MI). In the present study, we tested the hypothesis that leptin signalling limits cardiac apoptosis after MI through activation of signal transducer and activator of transcription (STAT)-3 responsive anti-apoptotic genes, including B-cell lymphoma (bcl)-2 and survivin, that serve to downregulate the activity of caspase-3.
Methods and results
Hearts from C57BL/6J and three groups of leptin-deficient Ob/Ob mice (food-restricted, ad libitum, and leptin-repleted) were examined 4 weeks after permanent left coronary artery ligation or sham operation. Inflammatory and apoptotic cell number was determined in cardiac sections by immunostaining. Expression of cardiac bcl-2, survivin, and pro and active caspase-3 was determined and correlated with in vitro caspase-3 activity. In the absence of MI, both lean and obese leptin-deficient mice exhibited increased cardiac apoptosis compared with wild-type mice. After MI, the highest rates of apoptosis were seen in the infarcted tissue of lean and obese Ob/Ob mice. Further, leptin-deficient hearts, as well as hearts from wild-type mice treated with the STAT-3 inhibitor WP1066, exhibited blunted anti-apoptotic bcl-2 and survivin gene expression, and increased caspase-3 protein expression and activity. The increased caspase-3 activity and apoptosis in hearts of leptin-deficient mice after MI was significantly attenuated in Ob/Ob mice replete with leptin, reducing apoptosis to levels comparable to that observed in wild-type mice after MI.
Conclusion
These results demonstrate that intact leptin signalling post-MI acts through STAT-3 to increase anti-apoptotic bcl-2 and survivin gene expression and reduces caspase-3 activity, consistent with a cardioprotective role of leptin in the setting of chronic ischaemic injury.
Keywords: Apoptosis, Leptin, Survivin, bcl-2, Heart failure
1. Introduction
A key feature in the pathogenesis of heart failure (HF) is cardiomyocyte loss.1 Both cellular necrotic and apoptotic processes account for the cardiomyocyte loss seen in HF, with apoptosis initiated by ischaemia, infarction, inflammation, and other pathologic events that occur early as well as later in the setting of left ventricular (LV) dysfunction.2 Apoptosis is a modality of cell death that differs from necrosis by being an active, highly regulated, and energy-requiring process.3 Apoptosis occurs continuously at a rate of up to 0.7% in chronic stable human HF4 and has been defined in cardiomyocytes by specific morphologic criteria, including DNA fragmentation, condensation of chromatin, maintenance of intact cytoplasmic structures, and intact cell membranes.5 In contrast, myocardial necrosis, which typically occurs early in the setting of cardiac injury, represents an acute process involving a large number of cardiomyocytes that undergo cellular destruction through rupture of the plasma membrane and release of intracellular contents into the surrounding tissue.6 Nevertheless, cardiomyocyte apoptosis, even amounts that occur at low levels, is recognized as an important component in the long-term development of HF.7
Cardiomyocyte viability is dependent upon the action of opposing apoptotic and survival pathways. Apoptosis is mediated at the biochemical level by a family of cysteinyl-aspartate-directed proteases known as caspases.8 Two major apoptotic pathways have been described in mammalian cells, an intrinsic and an extrinsic pathway, that converge to activate one final effector molecule, caspase-3.9 The intrinsic pathway in the heart is initiated by the release of cytochrome c from the mitochondria in response to hypoxia, ischaemia, and oxidative stress,10 whereas the extrinsic pathway is activated by members of the death receptor superfamily, including the Fas receptor and the tumour necrosis factor receptor.11 Activation of both the intrinsic and extrinsic apoptotic cascades is complex and regulated at many levels by various death antagonist and agonist proteins. For example, B-cell lymphoma (bcl)-2 is a potent anti-apoptotic protein that is anchored to the outer mitochondrial membrane and serves to limit activation of the intrinsic pathway by binding to proteins that mediate mitochondrial membrane disruption,12 including bcl-2 associated x (bax) and bcl-2 antagonist killer (bak) proteins. Regulating this availability is bcl-2 associated death (bad) promoter, a cytoplasmic protein that can bind to bcl-2. When phosphorylated, however, bad loses its ability to bind bcl-2 and contributes to cell survival. One other important regulatory protein is survivin, a member of the inhibitor of apoptosis protein gene family. Survivin blocks activation of effector caspases in both extrinsic and intrinsic pathways of apoptosis.13 More importantly, both survivin and bcl-2 have been shown to limit cardiomyocyte apoptosis in the setting of HF.14,15 Thus, apoptosis in the failing heart involves activation of caspase-3 through intrinsic and extrinsic pathways, and is regulated by the action of anti-apoptotic proteins, including bcl-2 and survivin.
bcl-216 and survivin17 are both signal transduction and activator of transcription (STAT)-3 responsive genes. In our prior study,18 we observed an increase in STAT-3 activation in wild-type mice at 4 weeks post-experimental myocardial infarction (MI) leading to HF. In leptin-deficient Ob/Ob mice, experimentally induced MI caused a blunted STAT-3 response that was associated with increased morbidity and mortality compared with wild-type mice. Leptin treatment in Ob/Ob mice restored the STAT-3 response, as well as morbidity and mortality, to that seen in infarcted wild-type animals. Here, we extend these previous findings by assessing apoptosis and inflammation in hearts from these same animals, and in a second group of wild-type mice treated with the STAT-3 inhibitor, WP1066. Whereas others have shown that leptin limits cardiomyocyte apoptosis induced by oxidative stress in vitro19 and that it reverses age-associated cardiomyocyte apoptosis in vivo,20 it is unknown what effect leptin has on cardiac apoptosis that occurs after MI and in HF. Hence, the present study was undertaken to test the hypothesis that leptin limits cardiac apoptosis post-MI through activation of STAT-3 responsive anti-apoptotic genes that downregulate the activity of caspase-3.
2. Methods
2.1. Animals
We have previously published18 the experimental protocol for the coronary artery ligation (CAL) and sham procedures performed on the wild-type and Ob/Ob mice whose hearts were subsequently used in this study. All animal use conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Care and Use Committee at the University of Pittsburgh. In our first set of previously reported experiments,18 seven groups of 5- to 6-week-old male leptin-deficient Ob/Ob and wild-type (littermate control C57BL/6) mice were subjected to sham or CAL surgery, and studied 4 weeks later. These included three groups of sham animals (lean wild-type food-provided ad libitum, lean Ob/Ob food-restricted, and obese Ob/Ob food-provided ad libitum) and four groups of CAL animals [lean wild-type food-provided ad libitum, obese Ob/Ob food-provided ad libitum, lean Ob/Ob food-restricted, and lean Ob/Ob leptin-replete (0.3 mg/kg/day sc by miniosmotic pump starting the day prior to CAL surgery and continuing to 4 weeks)]. Echocardiography on surviving animals was performed 4 weeks post-operatively, followed by animal euthanasia and tissue recovery. In a second, independent set of experiments, sixteen 5- to 6-week old male C57BL/6 mice were subjected to CAL surgery. At 3.5 weeks post-surgery, surviving mice underwent echocardiography and randomization in equal numbers to either treatment with the STAT-3 inhibitor WP1066 (20 mg/kg; Calbiochem) or equal volume vehicle (20% DMSO, 80% PEG-3000) given ip daily for 3 days. WP1066 dose was determined based on published toxicity and efficacy data in mice.21 Mice were then studied again by echocardiography, followed by euthanasia and tissue recovery.
2.2. STAT-3 DNA-binding assay
Protein extracts were assayed for STAT-3 DNA-binding activity using a STAT-3 DNA-binding ELISA kit (Active Motif) that is specific for the detection of activated STAT-3 as per manufacturer’s instructions. Specificity of binding was confirmed using competitor and mutant oligonucleotides provided by the manufacturer.
2.3. Immunofluorescence/TUNEL staining
Mouse cardiac tissue was harvested and fixed in 2% paraformaldehyde and cryoprotected in 30% sucrose, as described previously.18 Quantification of CD45- and TUNEL-positive cell numbers was performed on at least 10 × 20 fields per mouse (∼3000–4000 cells/field with a minimum of four mice in each experimental group) in both infarcted and non-infarcted tissue using the image analysis software Metamorph v7.5 (Molecular Devices). Results are reported as an apoptotic index or inflammation index, representing the percentage of nuclei that are associated with either TUNEL or CD45 staining, respectively. For further details, see Supplementary material online.
2.4. Quantitative real-time reverse transcriptase–polymerase chain reaction analysis
Total RNA was isolated from hearts and utilized for quantitative RT–PCR as described previously.18 For further details, see Supplementary material online.
2.5. Western blotting
Protein was extracted from hearts and utilized for immunoblotting as described previously.18 For further details, see Supplementary material online.
2.6. In vitro caspase-3 activity assay
Basal, caspase-8, and cytochrome c stimulated caspase-3 activity was determined using the Caspase-3/CPP32 Colorimetric Assay kit (Biovision), recombinant active human caspase-8, and horse cytochrome c as detailed in the Supplementary material online.
2.7. Statistical analysis
Data presented are mean ± standard error of the mean (SEM). Statistical significance of mean changes in apoptotic and CD45 cell number, mRNA and protein expression, and caspase-3 activity were determined by independent t-test when comparisons were limited to two, or by one-way ANOVA with post hoc comparisons between means using Bonferroni simultaneous tests, when comparisons exceeded two groups.
3. Results
3.1. Leptin deficiency results in greater morbidity and mortality after coronary artery ligation
The tissue utilized in this part of this study arose from mice that we had characterized previously.18 Table 1 summarizes our previously reported survival and echocardiographic measurements obtained 4 weeks after CAL and sham surgeries in 5- to 6-week-old male wild-type C57BL/6J and Ob/Ob mice.18 All three groups of sham mice (wild-type, Ob/Ob food-restricted, and Ob/Ob food-provided ad libitum) demonstrated 100% survival, an end-diastolic dimension (EDD) of ∼3.0 mm, and a fractional shortening (FS) of ∼50% at 4 weeks post-surgery. After CAL, wild-type mice demonstrated a survival rate of 75%, a 50% reduction in FS to ∼25%, and a 150% increase in the EDD of the LV to ∼4.4 mm. In contrast, both lean and obese leptin-deficient Ob/Ob mice subjected to CAL had worse survival (∼45%), decreased cardiac function (∼15% FS), and greater LV dilation (EDD of ∼5.4 mm). Demonstrating a beneficial effect of leptin in the setting of chronic ischaemic myocardial injury, Ob/Ob CAL mice replete with leptin demonstrated survival (69%), FS (∼27%), and EDD (∼4.3 mm) measurements that were comparable to those seen in wild-type CAL mice. These changes in cardiac structure, function, and mortality post-CAL were associated with an approximately two-fold increase in cardiac STAT-3 activation in wild-type and leptin-replete Ob/Ob mice post-CAL relative to lean and obese leptin-deficient Ob/Ob mice post-CAL.
Table 1.
Survival, echocardiographic, and relative cardiac activated STAT-3 levels in wild-type and Ob/Ob mice at 4 weeks post-sham and coronary artery ligation surgeriesa
| Group | Body weight (g) | Survival (%) | End-diastolic dimension (mm) | Fractional shortening (%) | STAT-3 activity (fold change vs. wild-type sham) |
|---|---|---|---|---|---|
| Wild-type sham (n = 10) | 27.5 ± 0.5 | 100 | 3.02 ± 0.01 | 50.3 ± 0.2 | 1.0 ± 0.1 |
| Ob/Ob food ad libitum sham (obese; n = 9) | 51.6 ± 0.5 | 100 | 3.03 ± 0.02 | 50.3 ± 0.5 | 1.1 ± 0.1 |
| Ob/Ob food-restricted sham (lean; n = 9) | 28.2 ± 0.3 | 100 | 2.99 ± 0.02 | 49.3 ± 0.3 | 1.1 ± 0.1 |
| Wild-type CAL (n = 11) | 28.1 ± 0.5 | 75 | 4.40 ± 0.05* | 24.5 ± 0.6* | 1.8 ± 0.1# |
| Ob/Ob leptin-replete CAL (n = 9) | 27.5 ± 0.5 | 69 | 4.26 ± 0.03* | 27.1 ± 0.4* | 1.9 ± 0.1# |
| Ob/Ob food ad libitum CAL (obese; n = 8) | 51.8 ± 0.5 | 44‡ | 5.37 ± 0.22‡ | 15.3 ± 1.0‡ | 1.1 ± 0.1 |
| Ob/Ob food-restricted CAL (lean; n = 7) | 27.5 ± 0.5 | 46‡ | 5.46 ± 0.08‡ | 16.4 ± 0.2‡ | 1.0 ± 0.1 |
aFrom reference 18.
*P < 0.05 vs. all shams.
#P < 0.05 vs. all shams and Ob food ad libitum CAL and Ob food-restricted CAL groups.
‡P < 0.05 vs. all shams and wild-type CAL and Ob leptin-replete CAL groups.
3.2. Leptin deficiency causes increased apoptosis and inflammatory cell number in sham mice
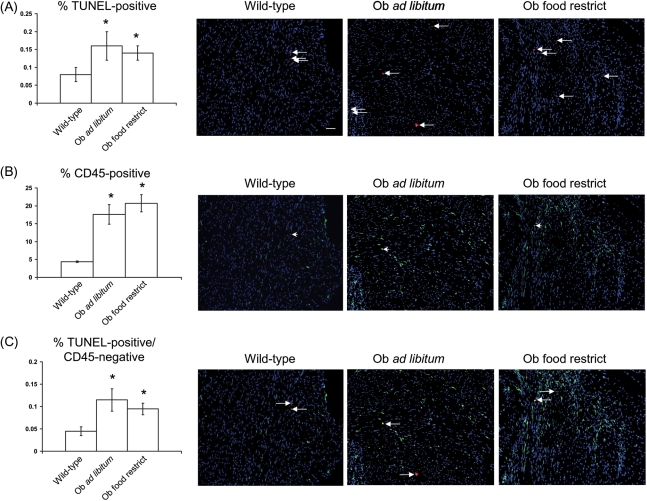
Hearts from wild-type sham mice had an apoptotic index of 0.08% (Figure 1A). In contrast, leptin-deficient sham mice, whether food-provided ad libitum (and therefore obese) or food-restricted (and therefore lean), had apoptotic indices of ∼0.14% and ∼0.17%, respectively (Figure 1A). This increase in apoptotic index in leptin-deficient hearts was accompanied by an increased inflammatory index (i.e. percentage of CD45-positive, pan-inflammatory cells) (Figure 1B and C). Despite increased inflammatory index in leptin-deficient sham hearts, the fraction of apoptotic cells that were CD45-negative was significantly increased relative to wild-type shams, demonstrating that the increased apoptosis observed in Ob/Ob mice is occurring in non-inflammatory resident cardiac cells (Figure 1C).
Figure 1.
Increased apoptosis and inflammation in leptin-deficient obese and lean sham mice. (A) Mean ± SEM per cent apoptosis (left panel) and ×20 power representative immunofluorescent staining of cardiac sections from sham mice (right three panels). Nuclei (stained with DAPI) are shown in blue, and all TUNEL-positive cells in the field are shown in red (left facing arrow), and accompanying scale bar (10 µM) in white. (B) As in (A), except that per cent CD45 cells (left panel) with representative images (right three panels, single example at left facing arrowhead) are shown in green. (C) As in (A), except that per cent TUNEL-positive/CD45-negative cells with representative images [right three panels, examples of TUNEL-positive/CD45-positive cell (in yellow; left facing arrow) and TUNEL-positive/CD45-negative cell (in red; right facing arrow)] are shown. *P < 0.05 vs. wild-type sham.
3.3. Coronary artery ligation causes increased apoptosis and inflammatory cell number in remote and infarcted cardiac tissue of leptin-deficient Ob/Ob mice that is attenuated with leptin repletion
3.3.1. Remote tissue
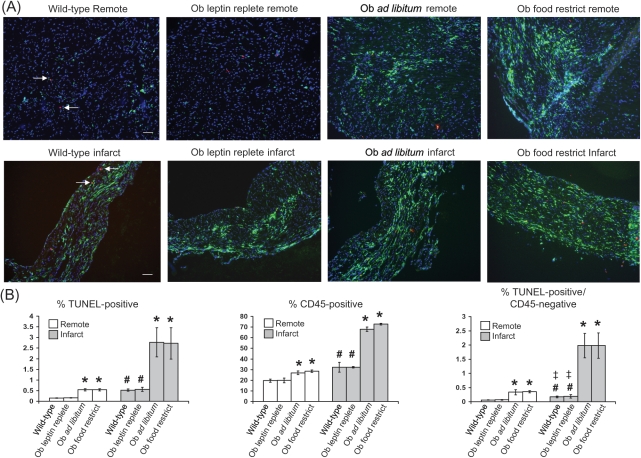
At 4 weeks post-CAL, tissue was examined from an area of the heart at the level of the mid-left ventricle outside of the zone of infarction (recognized by its wall thickness and nuclear density). In this non-infarcted tissue, wild-type mice had an apoptotic index of ∼0.15%, an inflammatory index of ∼20%, and a non-inflammatory cell apoptotic (TUNEL-positive/CD45-negative cells) index of ∼0.06% (Figure 2A and B). In contrast, non-infarcted tissue from leptin-deficient obese and lean Ob/Ob mice subjected to CAL demonstrated an increased inflammatory index (∼28% CD45-positive cells) and greater amounts of apoptosis in non-inflammatory cell types (∼0.54% apoptotic index, ∼0.34% TUNEL-positive/CD45-negative). These exacerbated indices of inflammation and apoptosis were attenuated with exogenous leptin. Specifically, non-infarcted tissue from the Ob/Ob mouse replete with leptin and subjected to CAL showed ∼20% inflammatory, ∼0.17% apoptotic, and ∼0.06% non-inflammatory cell apoptotic indices.
Figure 2.
Increased apoptosis and inflammation in remote and infarcted cardiac tissue from obese and lean leptin-deficient Ob/Ob mice post-coronary artery ligation (CAL) is attenuated with leptin replacement. (A) Representative ×20 power immunofluorescent staining of cardiac sections from remote and infarcted tissue in wild-type, Ob/Ob leptin-repleted, and Ob/Ob leptin-deficient mice. Nuclei (stained with DAPI) are shown in blue, an example of a TUNEL-positive cell (left facing arrow) is shown in red, CD45-positive cells are shown in green (unmarked), TUNEL/CD45 co-stained cells (single example at right facing arrow) are shown in yellow, and accompanying scale bar (10 µM) is shown in white. (B) Mean ± SEM per cent apoptotic (left panel), CD45-positive (middle panel) and TUNEL-positive/CD45-negative cells (right panel) are shown graphically for remote and infarct tissue from each of the four CAL groups. *P < 0.05 vs. wild-type CAL and Ob leptin-replete groups; #P < 0.05 vs. remote wild-type and Ob-leptin-replete groups; ‡P < 0.05 vs. Ob ad libitum and Ob food restrict groups.
3.3.2. Infarcted tissue
Wild-type mice demonstrated an inflammatory index of ∼32%, an apoptotic index of ∼0.54%, and a non-inflammatory cell apoptotic index of ∼0.17% (Figure 2A and B). Worse indices of inflammation (∼70% CD45-positive cells), total apoptosis (∼2.75%), and non-inflammatory cell apoptosis (∼1.98%) were seen in infarcted tissue from both obese and lean leptin-deficient Ob/Ob mice. These findings are in contrast to infarcted tissue from the Ob/Ob mouse replete with leptin and subject to CAL. Specifically, with leptin repletion in the Ob/Ob mouse, infarcted tissue demonstrated indices of inflammation (∼32% CD45-positive cells), total apoptosis (∼0.56% apoptotic cells), and non-inflammatory cell apoptosis (∼0.19%) comparable to those seen in the wild-type mouse.
3.4. Leptin-deficient hearts post-coronary artery ligation exhibit decreased anti-apoptotic bcl-2 and survivin expression and decreased bad phosphorylation
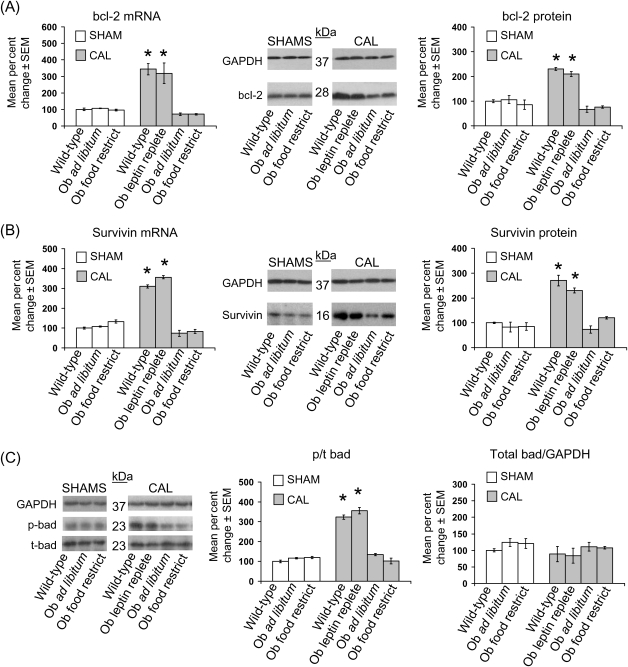
Cardiac bcl-2 mRNA expression in obese and lean leptin-deficient Ob/Ob sham mice was unchanged relative to wild-type sham mice (Figure 3A). CAL in wild-type mice resulted in a ∼300% increase in cardiac bcl-2 mRNA expression, whereas bcl-2 transcript levels were reduced by ∼30% in obese and lean leptin-deficient Ob/Ob mice subjected to CAL (Figure 3A). This reduction in anti-apoptotic bcl-2 expression was attenuated in leptin-replete Ob/Ob mice subjected to CAL, which showed ∼350% increase in cardiac bcl-2 mRNA relative to wild-type shams, a value comparable to wild-type mice subjected to CAL. These changes in cardiac bcl-2 mRNA across groups were paralleled by similar changes in bcl-2 protein. A pattern of mRNA and protein expression similar to bcl-2 was seen for survivin across all seven experimental groups (Figure 3B). Additionally, the ratio of phosphorylated (p) to total (t) cardiac bad protein was increased ∼350% in wild-type and leptin-replete Ob/Ob mice subjected to CAL relative to obese and lean leptin-deficient Ob/Ob subjected to CAL, with no change in total bad protein (Figure 3C). In contrast, whereas CAL resulted in a ∼200% increase in apoptotic bax and bak protein expression relative to sham groups, there were no leptin-dependent differences in bax and bak expression among the CAL groups (see Supplementary material online, Figure S1).
Figure 3.
Decreased anti-apoptotic bcl-2 and survivin expression and bad phosphorylation in obese and lean leptin-deficient Ob/Ob mice post-coronary artery ligation (CAL) are restored to wild-type levels with leptin replacement. Data for bar graphs are expressed as per cent changes in mean values ± SEM relative to wild-type sham mice, which were arbitrarily assigned a value of 100% after statistical calculations were performed. *P < 0.05 relative to all shams and Ob/Ob leptin-deficient CAL groups. (A) Per cent change in cardiac bcl-2 mRNA (left graph) and protein (right graph) with representative western blots are shown. (B) As in (A), except that survivin mRNA and protein were measured. (C) As in (B), except that phosphorylated and total bad were measured.
3.5. Leptin-deficient hearts demonstrate increased active caspase-3 expression and activity
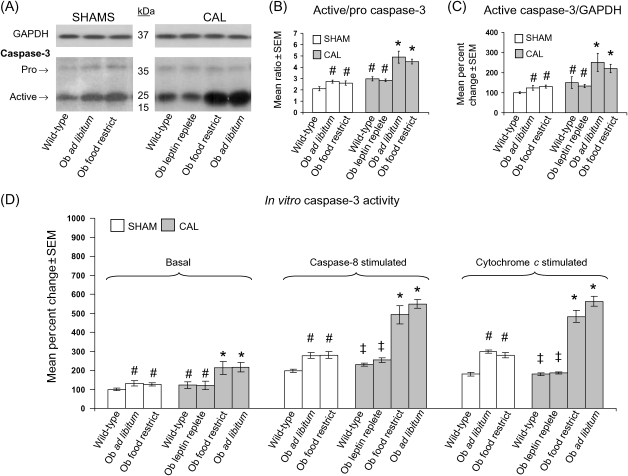
At baseline, wild-type sham mice hearts demonstrated a ratio of active- to pro-caspase-3 of ∼2 (Figure 4A and B). In agreement with the increased rates of myocardial apoptosis observed in obese and lean leptin-deficient Ob/Ob sham mice (Figure 1), cardiac tissue from these same mice demonstrated an increase in the ratio of active- to pro-caspase-3 to ∼2.5. With CAL, the ratio of active- to pro-caspase-3 in wild-type mice increased from ∼2 to ∼3 (Figure 4B). In comparison, the ratio of active- to pro-caspase-3 in lean and obese leptin-deficient Ob/Ob mice subjected to CAL was further increased to ∼4.6. This increased active- to pro-caspase-3 ratio in leptin-deficient Ob/Ob mice was attenuated with leptin replacement, as the leptin-replete Ob/Ob mouse subjected to CAL demonstrated a ratio of ∼2.8, a value similar to that seen in wild-type CAL mice. Paralleling the direction and magnitude of changes in the ratio of active- to pro-caspase-3, relative per cent changes in the amount of active caspase-3, when normalized to the housekeeping protein GAPDH, showed a similar pattern of response across groups (Figure 4C). Also consistent with these changes in the ratio of active- to pro-caspase-3 and the GAPDH normalized amount of active-caspase-3 protein present, the in vitro activity of caspase-3 was significantly increased in obese and lean leptin-deficient Ob/Ob sham and CAL mice relative to wild-type sham mice (Figure 4D). Finally, both the caspase-8 and cytochrome c stimulated in vitro caspase-3 activity was significantly increased in obese and lean leptin-deficient Ob/Ob sham and CAL mice relative to wild-type sham mice, but blunted in wild-type and leptin-replete Ob/Ob CAL mice.
Figure 4.
Leptin-deficient obese and lean Ob/Ob mice demonstrate increased caspase-3 expression and activity that is restored post-coronary artery ligation (CAL) to wild-type levels with leptin replacement. Data for bar graphs in (C) and (D) are expressed as per cent changes in mean values ± SEM relative to wild-type sham mice, which were arbitrarily assigned a value of 100% after statistical calculations were performed. #P < 0.05 relative to wild-type sham; *P < 0.05 relative to all shams and wild-type and Ob/Ob leptin-replete CAL groups; ‡P < 0.05 relative to leptin-deficient sham and CAL groups. (A) Representative western blots showing protein levels of pro- and active-caspase-3, along with GAPDH in cardiac tissue from sham and CAL groups of mice. (B) Ratio of active- to pro-caspase-3 in cardiac tissue from sham and CAL groups of mice. (C) Per cent change in active caspase-3 protein expression from sham and CAL groups of mice after normalizing to GAPDH. (D) Per cent change in basal and caspase-8 stimulated in vitro caspase-3 activity from sham and CAL groups after normalizing to total protein.
3.6. Short-term systemic administration of compound WP1066 in the setting of chronic ischaemic injury inhibits cardiac STAT-3 activation
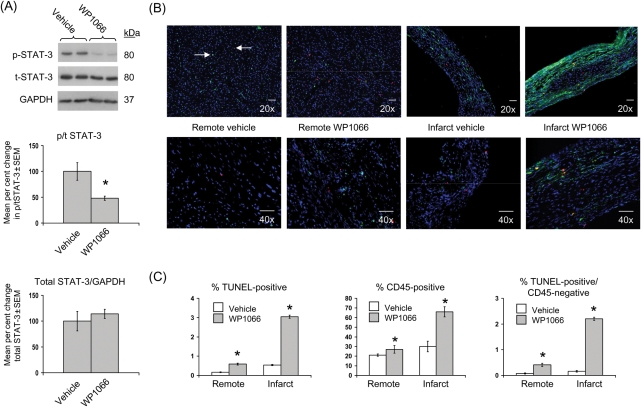
Table 2 summarizes survival and echocardiographic measurements obtained 3.5 weeks after CAL surgeries in a second group of 5- to 6-week-old male wild-type C57BL/6J mice (representative echocardiographic images in Supplementary material online, Figure S2). Prior to randomization into vehicle or WP1066 treatment groups (i.e. at 3.5 weeks post-CAL), mice demonstrated 75% survival, an FS of ∼23%, and an EDD of ∼4.5 mm. These values were unchanged after 3 days of either vehicle or WP1066 administration and are similar to those obtained at 4 weeks post-CAL in wild-type and leptin-replete Ob/Ob mouse groups (Table 1). After 3 days of WP1066 administration, the level of cardiac p/t STAT-3 protein and cardiac STAT-3 DNA-binding activity were both decreased ∼50% relative to vehicle-treated controls (Figure 5A and Table 2).
Table 2.
Post-CALa survival, echocardiographic, and relative cardiac activated STAT-3 levels in wild-type mice administered vehicle or WP1066 STAT-3 inhibitor for 3 days beginning at 3.5 weeks post-CAL
| Group | Survival (%) | End-diastolic dimension (mm) | Fractional shortening (%) | STAT-3 activity (fold change vs. vehicle) |
|---|---|---|---|---|
| All mice at 3.5 weeks post-CAL (n = 12) | 75 | 4.48 ± 0.04 | 23.4 ± 0.6 | NA |
| After 3 days of vehicle (n = 6) | 100 | 4.50 ± 0.04 | 22.9 ± 0.9 | 1.0 ± 0.15 |
| After 3 days of WP1066 (n = 6) | 100 | 4.45 ± 0.04 | 23.3 ± 0.6 | 0.49 ± 0.07# |
an = 16 prior to CAL.
#P < 0.05 vs. vehicle group.
Figure 5.
Inhibition of STAT-3 post-coronary artery ligation increases cardiac apoptosis and inflammation. Data for bar graphs are expressed as per cent changes in mean values ± SEM relative to vehicle-treated mice, which were arbitrarily assigned a value of 100% after statistical calculations were performed. *P < 0.05 relative to vehicle-treated mice. (A) Per cent change in cardiac phosphorylated (p)/total (t) STAT-3 (top graph) and total STAT-3/GAPDH (bottom graph) with representative western blots is shown. (B) Representative ×20 and ×40 power immunofluorescent staining of cardiac sections from remote and infarcted tissue in vehicle- and WP1066-treated mice. Nuclei (stained with DAPI) are shown in blue, an example of a TUNEL-positive cell (left facing arrow) is shown in red, CD45-positive cells are shown in green, an example of a TUNEL/CD45 co-stained cell (right facing arrow) is shown with both red/green staining, and accompanying scale bar (10 µM) is shown in white. (C) Mean ± SEM per cent apoptotic (left panel), CD45-positive (middle panel), and TUNEL-positive/CD45-negative cells (right panel) are shown graphically.
3.7. STAT-3 inhibition in the setting of chronic ischaemic injury increases cardiac apoptosis and inflammatory cell number similar to that observed in leptin-deficiency in both remote and infarcted myocardium
In remote myocardium, mice administered WP1066 had an apoptotic index of ∼0.6%, an inflammatory index of ∼27%, and a non-inflammatory cell apoptotic (TUNEL-positive/CD45-negative cells) index of ∼0.4% (Figure 5B and C). Infarcted tissue from these same mice demonstrated an apoptotic index of ∼3%, an inflammatory index of ∼66%, and a non-inflammatory cell apoptotic index of ∼2.2%. These indices of apoptosis and inflammation are similar to those observed in obese and lean leptin-deficient Ob/Ob mice subjected to CAL (Figure 2). In contrast, vehicle-treated mice had indices of apoptosis, inflammation, and non-inflammatory cell apoptosis (Figure 5B and C) that matched those seen in wild-type and leptin-replete Ob/Ob mice (Figure 2).
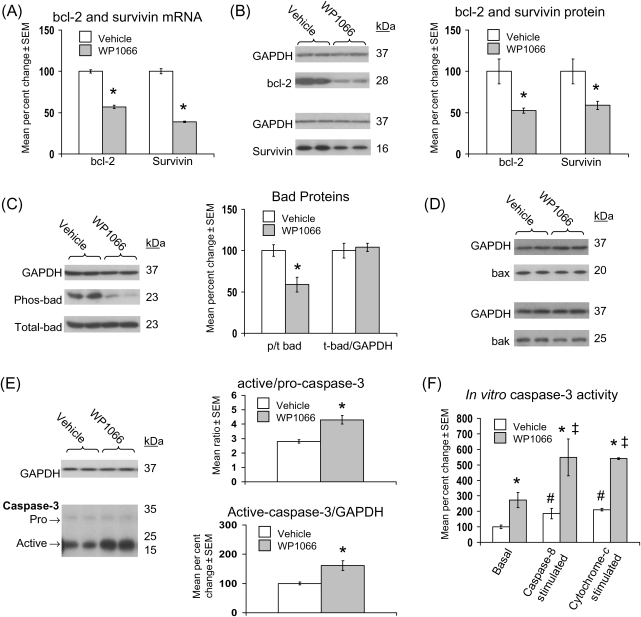
3.8. STAT-3 inhibition post-coronary artery ligation decreases anti-apoptotic bcl-2 and survivin expression, decreases phosphorylated bad, and increases caspase-3 activation
As observed in leptin-deficient mice post-CAL (Figure 3), cardiac bcl-2 and survivin mRNA (Figure 6A) and protein (Figure 6B) expression were significantly decreased after WP1066 treatment. Similarly, the ratio of cardiac p/t bad was significantly decreased after administration of WP1066 (Figure 6C), whereas the expression of total bad, bax, and bak was unchanged (Figure 6D). However, the ratio of active- to pro-caspase-3 was increased from ∼2.8 to ∼4.3, and the GAPDH normalized expression of active caspase-3 was increased to 160% of vehicle-treated controls (Figure 6E). These increases in caspase-3 expression were paralleled by a similar increase in basal, and even greater increases in caspase-8 and cytochrome c stimulated, in vitro caspase-3 activity (Figure 6F).
Figure 6.
Inhibition of STAT-3 post-coronary artery ligation decreases anti-apoptotic bcl-2 and survivin expression, decreases bad phosphorylation, and increases caspase-3 activation. Data for bar graphs are expressed as per cent changes in mean values ± SEM relative to vehicle-treated mice, which were arbitrarily assigned a value of 100% after statistical calculations were performed. *P < 0.05 relative to vehicle; #P < 0.05 relative to basal vehicle-treated; ‡P < 0.05 relative to basal WP1066-treated. (A) Per cent change in cardiac bcl-2 and survivin mRNA. (B) Per cent change in cardiac bcl-2 and survivin protein with representative western blots are shown. (C) As in (B), except that phosphorylated (p) and total (t) bad protein were measured. (D) Representative western blots for cardiac bax and bak proteins are shown. (E) As in (B), except that pro- and active-caspase-3 were measured. (F) Per cent change in cardiac basal and caspase-8 stimulated in vitro caspase-3 activity from vehicle- and WP1066-treated groups after normalizing to total protein.
4. Discussion
In our prior study,18 we showed that leptin has protective and beneficial effects in the setting of myocardial injury leading to cardiac dysfunction and eventual HF. Specifically, leptin-deficient Ob/Ob mice post-CAL, whether obese or lean, had worse survival and cardiac function relative to wild-type or leptin-replete Ob/Ob mice post-CAL (Table 1). Here, by looking at apoptosis and inflammation in hearts from these same animals, and in wild-type mice treated with a STAT-3 inhibitor, we present a potential mechanism to account for the worse morbidity and mortality in leptin-deficient mice post-CAL. Specifically, leptin-deficient sham and CAL mice demonstrated increased apoptosis and inflammation that occurred independent of weight. Although rates of apoptosis and inflammation were much higher in infarcted compared with remote (non-infarcted) tissue, examination of both areas demonstrated that the predominant apoptotic cell type in the setting of cardiac injury and leptin deficiency is non-inflammatory. The increased apoptotic and inflammation indices seen in leptin-deficient mice post-CAL were attenuated in Ob/Ob mice replete with leptin. Our data also suggest that leptin deficiency and its accompanying loss of cardiac STAT-3 activation are linked to decreased expression of the anti-apoptotic genes bcl-2 and survivin in the heart after CAL. The significance of these changes in bcl-2 and survivin expression on the apoptotic cascade is manifest by our additional finding of increased basal and caspase-8/cytochrome c stimulated caspase-3 activity in the hearts of obese and lean leptin-deficient Ob/Ob mice relative to wild-type mice. Together, these results provide evidence that intact leptin signalling limits cardiac apoptosis, especially in the setting of myocardial injury post-CAL.
A major finding in this study was an association between leptin deficiency and increased indices of cardiac inflammation and apoptosis, not only in the setting of cardiac injury, but also in non-infarcted hearts from Ob/Ob sham mice. Whereas increased inflammation and apoptosis were seen in Ob/Ob sham and Ob/Ob CAL mice relative to mice with intact leptin signalling, we found that the fraction of apoptotic cells that were also CD45-positive did not increase proportionally. This suggests that the absolute increase in cardiac apoptosis that accompanies leptin deficiency is the result of increased non-inflammatory cell death, including cardiomyocytes and fibroblasts. This increased cardiomyocyte and/or other non-inflammatory cell death likely contributes to the worse functional and survival outcomes that occur 4 weeks after experimentally induced MI,18 and that observed in leptin-deficient Ob/Ob mice with ageing20 and myocarditis.22 Thus, leptin deficiency is linked to increased cardiac inflammation and non-inflammatory cell apoptosis.
Our data are in agreement with published studies showing that leptin deficiency results in increased cardiac apoptosis due to ageing in Ob/Ob and db/db mice,20,23 and increased cardiac inflammation and apoptosis in Ob/Ob mice with viral myocarditis.22 Others have also shown that leptin deficiency is linked to greater susceptibility to lipopolysaccharide-induced lethality,24 a four-fold increase in basal levels of circulating monocytes,25 and higher basal circulating levels of C-reactive protein and interleukin-6.26 Hence, it is not unreasonable to speculate that the increased cardiac inflammatory index that we observed in both ischaemic and non-ischaemic myocardium from the Ob/Ob mouse is due in part to a baseline dysregulation of the systemic immune and inflammatory response that accompanies the leptin-deficient state.
Post-CAL, our data demonstrate that leptin deficiency results in greater amounts of cardiac apoptosis when compared with wild-type or leptin-replete Ob/Ob mice. Apoptosis is a complex process that is regulated by the activity of a number of proteins, including bcl-2, survivin, bad, bax, and bad. Whereas bax and bad demonstrated no leptin or STAT-3-dependent change post-CAL, bcl-2, survivin, and the ratio of p/t bad were significantly increased in mice with intact leptin signalling. In the heart, bcl-2 limits apoptosis and protects against cardiac ischaemia/reperfusion damage in vivo, 27 survivin decreases apoptosis in failing myocardium,14 and phosphorylated bad is associated with smaller infarct sizes after ischaemia/reperfusion injury.28 Both bcl-2 and survivin are STAT-3 inducible16,17 and both have been shown to be upregulated by leptin through leptin receptor-mediated STAT-3 signal transduction.17,29 Further, bad phosphorylation has been linked to STAT-3 activation in the setting of cardiac ischaemia/reperfusion injury independent of other classic pro-survival kinases.28 Consistent with our prior finding of a relative loss of STAT-3 expression and activity in leptin-deficient mice relative to wild-type or leptin-replete Ob/Ob mice post-CAL,18 when we examined the cardiac expression of bcl-2, survivin, and p/t bad in these same mice, and in mice treated with the STAT-3 inhibitor WP1066, we observed a similar relative loss of expression. Functionally, this blunted anti-apoptotic protein expression in leptin-deficient and WP1066-treated mice post-CAL correlates with an increased amount of cardiac apoptosis and is consistent with the reported relationships between leptin, STAT-3, bcl-2, survivin, caspase-3, and apoptosis in the heart14,19,20,22,27 and other tissues.16,17,29 Thus, leptin deficiency results in increased cardiac apoptosis post-CAL that is linked to a relative decrease in cardiac STAT-3 activation and bcl-2, survivin, and p/t bad expression.
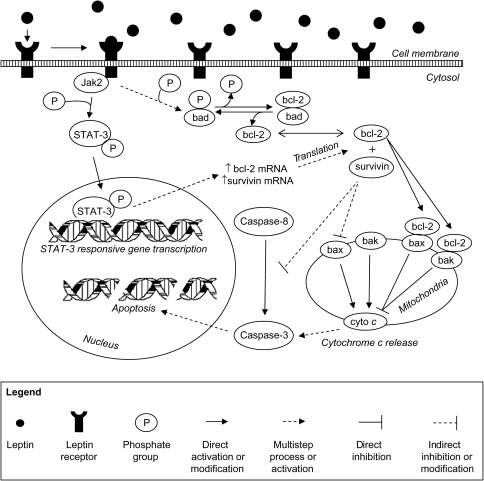
Our data links increased rates of apoptosis post-CAL in leptin-deficient mice to decreased anti-apoptotic bcl-2, survivin, and p/t bad expression. However, the effect of anti-apoptotic proteins on the amount of apoptotic cell death that ultimately occurs depends on the activity of caspase-3, a final effector molecule in both the intrinsic and extrinsic apoptotic cascades. In obese and lean leptin-deficient Ob/Ob sham and CAL mice, and in mice treated with the STAT-3 inhibitor WP1066, the expression of active caspase-3 was significantly increased relative to their respective control mice. Further, the in vitro assessment of caspase-3 activity paralleled these changes in active protein expression, and the increased caspase-8 and cytochrome c stimulated in vitro caspase-3 activity seen in leptin-deficient and WP1066-treated mice confirms the functional significance of their blunted bcl-2, survivin, and phosphorylated-bad expression post-CAL. Taken together, our data suggest that leptin deficiency is linked to increased cardiac apoptosis through greater caspase-3 activity and that restoration of leptin signalling downregulates cardiac caspase-3 activity post-CAL (Figure 7). These findings are consistent with in vitro data showing leptin-mediated downregulation of caspase-3 activity in rat cardiomyocytes exposed to oxidative stress19 and in vivo data showing that leptin-deficient mice have increased active caspase-3 expression relative to wild-type mice.20 Thus, leptin deficiency, independent of weight, results in greater caspase-3 activity and increased apoptosis that are attenuated with leptin repletion, suggesting that leptin is an important factor in limiting cardiac cell death in both infarcted and remote (non-infarcted) tissue post-MI.
Figure 7.
Schematic conceptual diagram illustrating the putative role of leptin in attenuating cardiac apoptosis after chronic myocardial ischaemic injury. See text for definition of abbreviations.
Although our data suggest a leptin- and STAT-3-mediated attenuation of cardiac apoptosis post-CAL, there are several limitations to our study that deserve comment. First, we have limited our analysis to a small number of known STAT-3 responsive anti-apoptotic proteins and a final effector molecule, caspase-3. It is possible that other non-STAT-3-mediated effects are contributing to the changes seen in cardiac apoptosis and caspase-3 activity in leptin deficiency. Indeed, our data in leptin-deficient sham vs. wild-type sham mice demonstrate that differences in apoptosis and caspase-3 activity occur in the setting of equal bcl-2 and survivin expression, suggesting that additional upstream regulatory proteins not examined here, but nevertheless important, must be involved. Secondly, we have only looked at inflammatory and apoptotic events at 4 weeks post-CAL. It is possible that even more dramatic changes in these indices would be present in the immediate 2- to 3-day period after MI. Finally, we observed that increased caspase-3 activity in leptin-deficient hearts is linked to increased apoptosis. However, other non-apoptosis-regulating targets of caspase-3 action may contribute to the worse morbidity and mortality observed in leptin-deficient mice post-CAL.18 For example, in addition to poly(ADP-ribose) polymerase, which coordinates DNA repair and decreases the activity of endogenous endonucleases, caspase-3 cleaves and inactivates key contractile proteins, including tropomyosin, cardiac troponin, and myosin heavy chains.30 Hence, while these non-apoptotic targets of caspase-3 action were not the focus of this study, their proteolysis by caspase-3 may contribute to the exacerbated indices of morbidity and mortality that occur in leptin-deficient mice post-CAL.18
In conclusion, leptin has been shown to limit apoptotic cell death in cultured cardiomyocytes19 and in the hearts of leptin-deficient Ob/Ob mice as they age.20 Our study is complementary and extends these previous findings by demonstrating that leptin also limits apoptosis in the setting of chronic ischaemic cardiac injury. We also demonstrate that regulation of cardiac apoptosis by leptin is mechanistically linked to increased STAT-3 responsive bcl-2 and survivin expression, and ultimately, to decreased caspase-3 activity. It is important to note that this regulation of apoptosis post-CAL by leptin occurred independent of weight, as lean and obese leptin-deficient Ob/Ob mice groups demonstrated equally detrimental increases in apoptosis that reverted to wild-type levels with leptin repletion. Along with increased apoptosis, leptin deficiency was also associated with increased inflammatory cell number and a decreased fraction of apoptotic cells that were CD45-positive, suggesting that leptin not only decreases inflammation post-MI, but may also serve to specifically preserve cardiomyocyte survival at the site of infarction. Cardiomyocyte survival in the face of cardiac ischaemic injury is a key factor in limiting the morbidity and mortality associated with HF.1 As such, our data suggest that reduced or absent leptin signalling is a potential mechanism that may increase cardiac apoptosis and inflammation, and worsen myocardial injury in response to chronic ischaemia.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health [HL087009-02 to K.R.M. and HL077785 to C.P.O’D.].
Acknowledgements
We thank the Center for Biologic Imaging at the University of Pittsburgh.
Conflict of interest: none declared.
References
- 1.Khoynezhad A, Ziba J, Tortolani AJ. A synopsis of research in cardiac apoptosis and its application to congestive heart failure. Tex Heart Inst J. 2007;34:352–359. [PMC free article] [PubMed] [Google Scholar]
- 2.Garg S, Naruka J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–79. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002;193:145–153. doi: 10.1002/jcp.10174. [DOI] [PubMed] [Google Scholar]
- 4.Kang P, Izumo S. Apoptosis in heart failure: a critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 5.Abbate A, DeFalco M, Morales C, Gelpi RJ, Prisco M, DeLuca A, et al. Electron microscopy characterization of cardiomyocyte apoptosis in ischemic heart disease. Int J Cardiol. 2006;114:118–120. doi: 10.1016/j.ijcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi P, Yang R, Barouch LA. Decreased p110a catalytic activity accompanies increased myocyte apoptosis and cardiac hypertrophy in leptin deficient ob/ob mice. Cell Cycle. 2008;7:560–565. doi: 10.4161/cc.7.5.5529. [DOI] [PubMed] [Google Scholar]
- 7.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf B, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 9.van Empel V, Bertrand ATA, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Azamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 11.Hengartner M. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 12.Crow M, Mani K, Nam KJ, Kitsis RN. The mitochondial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 13.Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:3001–3010. doi: 10.1186/gb-2001-2-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levkau B, Shafers M, Wohlschlaeger J, von Wnuck Lipinski K, Keul P, Hermann S, et al. Survivin determines cardiac function by controlling total cardiomyocyte number. Circulation. 2008;117:1583–1593. doi: 10.1161/CIRCULATIONAHA.107.734160. [DOI] [PubMed] [Google Scholar]
- 15.Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci USA. 2004;101:769–774. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharya S, Ray RM, Johnson LR. STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J. 2005;392:335–344. doi: 10.1042/BJ20050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin-STAT3 signaling in MCF7 cells. Biochem Biophys Res Commun. 2008;368:1–5. doi: 10.1016/j.bbrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 18.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, et al. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- 19.Equchi M, Liu Y, Shin EJ, Sweeney G. Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J. 2008;275:3136–3144. doi: 10.1111/j.1742-4658.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 20.Barouch L, Gao D, Lei C, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 21.Kong L, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda T, Takahashi T, Kudo S, Takeda T, Tsugawa H, Takekoshi N. Leptin deficiency enhances myocardial necrosis and lethality in a murine model of viral myocarditis. Life Sci. 2004;75:1435–1447. doi: 10.1016/j.lfs.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, et al. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–379. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 24.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 25.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor-a and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dube MG, Torto R, Kalra SP. Increased leptin expression selectively in the hypothalamus suppresses inflammatory markers CRP and IL-6 in leptin-deficient diabetic obese mice. Peptides. 2008;29:593–608. doi: 10.1016/j.peptides.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpressin of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 28.Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, et al. Pharmacological preconditioning with tumor necrosis factor-a activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 29.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 30.Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:6252–6256. doi: 10.1073/pnas.092022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.