Abstract
The ‘mitochondrial permeability transition', characterized by a sudden induced change of the inner mitochondrial membrane permeability for water as well as for small substances (≤1.5 kDa), has been known for three decades. Research interest in the entity responsible for this phenomenon, the ‘mitochondrial permeability transition pore’ (mPTP), has dramatically increased after demonstration that it plays a key role in the life and death decision in cells. Therefore, a better understanding of this phenomenon and its regulation by environmental stresses, kinase signalling, and pharmacological intervention is vital. The characterization of the molecular identity of the mPTP will allow identification of possible pharmacological targets and assist in drug design for its precise regulation. However, despite extensive research efforts, at this point the pore-forming core component(s) of the mPTP remain unidentified. Pivotal new genetic evidence has shown that components once believed to be core elements of the mPTP (namely mitochondrial adenine nucleotide translocator and cyclophilin D) are instead only mPTP regulators (or in the case of voltage-dependent anion channels, probably entirely dispensable). This review provides an update on the current state of knowledge regarding the regulation of the mPTP.
KEYWORDS: Adenine nucleotide translocator, Cyclophilin D, Mitochondrial voltage-dependent anion channel, Hexokinase, Creatine kinase, Mitochondrial peripheral benzodiazepine receptor, Bcl-2, Glycogen synthase kinase-3β
1. Introduction
The outer and inner mitochondrial membranes (OMM and IMM), being in many ways significantly different from each other, are each composed of phospholipid bilayers that contain numerous integral proteins. The OMM is much more permeable as a result of an abundance of the principal OMM protein, the voltage-dependent anion channel (VDAC), which forms relatively large internal channels (about 2–3 nm) and allows ions, metabolites, and certain small molecules to move between the cytoplasm and mitochondria. In contrast, the IMM is highly impermeable, and the transport of ions, molecules, and metabolites across it is supported by specialized membrane transporters and exchangers; it is also rich in cardiolipin, an unusual phospholipid which is typically found in bacterial plasma membranes. The IMM is a very good electric insulator: at a thickness of about 10 nm, this membrane can keep a potential difference of ∼180 mV, thereby creating an electric field strength of about 1.8 × 105 V/cm.1 Proton pumping driven by substrate oxidation constantly supports mitochondrial membrane potential (ΔΨm) and compensates for proton and other ion leakage, due to ATP synthesis and other regulated and unregulated ion transport.
The abrupt change of the IMM permeability, characterized by the sudden loss of ΔΨm, is known as the ‘permeability transition’ and the entity responsible for this phenomenon was named the ‘mitochondrial permeability transition pore (mPTP)’. mPTP induction causes the non-selective traffic between the mitochondrial matrix and cytosol of not only small-charged particles but also of water and substances up to 1.5 kDa in size, a phenomenon that was discovered by Haworth and Hunter.2–4 Owing to the progress of patch-clamping studies applied to the IMM, the term ‘mitochondrial megachannel’ has also been utilized for probably the same phenomenon.5,6
The molecular structure and identity of the mPTP is not yet known. A complicating factor in the characterization of the mPTP relates to the circumstances in which the permeability transition phenomenon was discovered and initially studied, e.g. in isolated mitochondria,2–4 and that it was not studied in intact cells until techniques became available almost two decades later.7,8 These two experimental systems have often failed to exhibit the expected parallelism in terms of behaviour, e.g. there are apparent differences in the role and sensitivity to Ca2+ as an mPTP trigger, and in the effects of mPTP inhibitors under specific conditions, suggesting potential differences in the mPTP size, its thresholds, and architecture.9–13 It has been estimated that a single mPTP per mitochondrion can discharge the ΔΨm very quickly.14 Yet unresolved difficulties with isolation and identification of the mPTP may be related to the fact that (i) the assembly of the mPTP from its constituent components, and its induction, is likely a rare event and (ii) it may be assembled from components that, while relatively abundant, generate only a single macromolecular cluster that exists only transiently. There may even be several different regulatory entities assembled around the main core components, yielding somewhat different behaviours in different cells or under different conditions. This may partially explain the use of the terms ‘regulated’ and ‘unregulated’ by different authors to describe mPTP, or the in vivo vs. in vitro differences in Ca2+ sensitivities, demonstrating the inherent complexities in observing the obscure nature of this phenomenon.15 Furthermore, a number of recent genetic knockout studies have convincingly shown the irrelevance of certain previously ‘indisputable’ elements as belonging to the core architecture of the mPTP complex since the mPTP phenomenon persists even in their absence,16–20 as will be discussed later in this review.
The relevance of the permeability transition to the ‘life or death’ decision of the cell21,22 underlies the necessity to understand the detailed structure and behaviour of this phenomenon. Crompton and Costi23 implicated, for the first time, the mPTP-induction as a critical contributing factor to ischaemia/reperfusion injury in a cell-based model. They hypothesized that reoxygenation after prolonged ischaemia could result in excessive mitochondrial damage due to the mPTP induction and that ATP depletion and oxidative stress upon reperfusion would provide additional stimuli to promote further mPTP activation and consequently exacerbate the damage. Proof that mPTP occurs after ischaemia/reperfusion was experimentally obtained shortly thereafter in an in vivo setting using isolated rat hearts.24 Importantly, under normal circumstances, oxidative phosphorylation in mitochondria results in both the majority of cellular ATP production and relatively low levels of background reactive oxygen species (ROS) production; however, once the mPTP is induced, the situation is drastically reversed with the mitochondria becoming not only the producers of high levels of cytotoxic ROS but also ATP consumers due to the futile cycle in an effort to restore the Δψm, thereby further contributing to cellular damage. In intact cardiomyocytes we have demonstrated that in individual mitochondria the mPTP induced by an elevated level of ‘triggering ROS’ is followed by an additional ROS burst that occurs simultaneously with mitochondrial Δψm dissipation, a phenomenon which we named, ROS-induced ROS release (RIRR). Furthermore, RIRR can propagate and thus can be responsible for a large fraction of the oxidative damage detected in cells after reperfusion.25 It has also been found that ROS rather than Ca2+ appears to be the more important mPTP trigger in excitable cells such as cardiomyocytes and neurons; additionally, cell survival has negatively been correlated to the fraction of cellular mitochondria that undergo mPTP induction.9,10 Finally, it should be emphasized that ageing is also a critical factor contributing to the ability of tissues to resist damaging stress (see Juhaszova et al.26 for review, and citations therein). Specifically, there is an age-related impairment of the intrinsic capacity of the heart to resist damaging stress, such as ischaemia/reperfusion injury (an effect aggravated by co-morbidities such as diabetes, but potentially attenuated by caloric restriction and physical conditioning/exercise). Indeed, cardiomyocytes from aged hearts have a markedly lower mPTP–ROS threshold and consequently a higher probability of mPTP induction when compared with those of young animals.27 It also appears that the stimulus threshold for triggering endogenous protection signalling mechanisms increases with age, e.g. the ability to induce ischaemic preconditioning is significantly blunted in the old heart (an effect potentially aggravated by commonly used medications that can block certain key steps in protection signalling, including sulfonylureas, antioxidants, partial fatty acid oxidation (PFOX) inhibitors, and COX2 inhibitors, but also likely attenuated by caloric restriction and exercise) (see Juhaszova et al.26 for review, and citations therein). Additionally, mutations in mitochondrial transfer RNA have been correlated with hypertension and hypercholesterolemia, major age-related cardiovascular risk factors.28
The characterization of the molecular identity of mPTP should allow identification of potential pharmacological targets and assist in rational drug design for therapeutic aims requiring precise mPTP regulation. When solved, this could provide tools for either preventing unwanted cell death when appropriate or for promoting the elimination of unwanted cells, for example in the situation when a small, damaged, but still viable region of the heart, if not eliminated, becomes a focus of abnormal automaticity leading to pathological disturbances in cardiac rhythm (including ventricular tachycardia and ventricular fibrillation). The direct link between mPTP induction and oxidative stress-related cell death in cardiomyocytes and other vital organs during ischaemia/reperfusion injury requires developing better strategies to prevent or limit the possibility of damage.
In this review, we will present what is known about certain key elements of the permeability transition phenomenon and possible targets for pharmacological intervention. We will discuss what is known about the essential (core) pore-forming elements of the mPTP and other regulatory components that control the activity of the mPTP or its sensitivity to modulating factors (such as Ca2+, proteins, lipids, etc.) (Figure 1). Some of the components that are known or have been suspected to be involved in the formation and/or regulation of the mPTP include adenine nucleotide translocator (ANT) (also known as ATP/ADP carrier, AAC), cyclophilin D (Cyp-D, encoded by the Ppif gene), VDAC (also known as mitochondrial porin), hexokinase, creatine kinase, mitochondrial peripheral benzodiazepine receptor (PBR; translocator protein, TSPO), Bcl-2, glycogen synthase kinase-3β (GSK-3β), and cytochrome c.9,29–31 Since some of the tentative elements of the mPTP complex belong to the OMM, some reside in the intermembrane space and some are located in the IMM, it has been hypothesized that the mPTP complex could be organized by a structure previously known as the mitochondrial ‘contact site’.32,33 However, this hypothesis has never been experimentally validated and remains only a speculation (comprehensively discussed by Bernardi et al.34). The important role of a principal lipidic counter-partner of the mPTP, cardiolipin,35 will also be discussed.
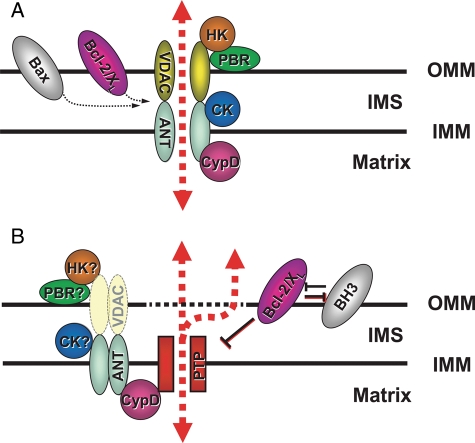
Figure 1.
Proposed mPTP complex architecture: (A) Classical view. The mPTP structure is formed by the VDAC–ANT–CyP-D complex, which is located at the ‘contact sites’. Hexokinase II (HKII), mitochondrial creatine kinase (CK), benzodiazepine receptor (PBR), and Bcl-2-family members (Bcl-2, Bcl-xL, and Bax) are included as putative regulatory components. (B) Current view. The core elements comprising the mPTP itself (denoted ‘PTP’ for permeability transition pore) are presently unidentified, but are probably regulated by the adjacent elements as indicated. Note that VDAC, portrayed as a ‘shadow,’ is no longer seen as an essential pore component or even a regulator based on recent genetic evidence. Question mark symbols signify where important open questions remain as far as the participation as a regulator of the mPTP (see text; modified from Juhaszova et al.9).
2. Adenine nucleotide translocator
ANT, a relatively small protein (about 300 AA residues) and the most abundant protein of the IMM, catalyses the selective, electrogenic, and reversible exchange of ADP for ATP with a 1:1 stoichiometry. ANT transports nucleotides at their full ionization state, i.e. the antiport is accomplished by the exchange of ATP4− for ADP3−.36 The direction of nucleotide transport is determined by cell conditions (under normal physiological conditions ATP4− is transported out of the mitochondria and ADP3− is transported in, while the opposite is true under anoxic/ischaemic conditions). This nucleotide exchange is limited by the availability of free nucleotides that are typically at much lower concentration than their Mg2+-bound forms. ANT also transports other solutes such as phosphoenolpyruvate,37 pyrophosphate,38 and creatine phosphate,39 although with very low efficiency.
Substantial evidence supports the involvement of ANT in fatty acid-induced mitochondrial uncoupling. It has been suggested that ANT can transport anionic forms of fatty acids, which is typically the rate limiting step in the mitochondrial uncoupling process.40 Similarly, ANT may facilitate the transport of anionic forms of weak uncouplers (such as 2,4-dinitrophenol) across the IMM.41 This highlights the role of ANT (as well as other mitochondrial carriers like the glutamate/aspartate carrier or UCPs) in the regulation of ΔΨm and ROS production, the latter being tightly correlated with the ΔΨm level.42
Known inhibitors of ANT are highly specific for the inhibition of the nucleotide transport. Atractyloside and its derivative carboxyatractyloside are competitive inhibitors of nucleotide transport by ANT.43,44 The binding of these inhibitors on the cytosolic side causes the transition of ANT into its c-conformation, while binding of another competitive inhibitor, bongkrekic acid,45 causes a transition into the m-conformation when applied from mitochondrial matrix side.
Among other ANT inhibitors, acyl-CoA, particularly long-chain derivatives such as palmytoyl- and oleyl-CoA,46 and rhodamine 6G47 are well known (consequently, the rhodamine-based ΔΨm probes should be used with proper precautions). The search for natural modulators of ANT led to discovery of the small water-soluble protein CyP-D, which has a binding site on the matrix side of ANT.48
Bcl-2 family proteins are also known ANT modulators.49 Viruses have evolved complex pro- and anti-apoptotic strategies (regarding the host cells they infect) for their own benefit, often acting specifically at the mitochondrial level. The interaction of viral proteins with different members of Bcl-2 family proteins as well as mPTP regulators has been demonstrated. For example, the interaction of HIV-1 viral protein R (Vpr) with ANT has been implicated in mPTP induction and cell death.50 Some viruses encode products resembling Bcl-2 protein family domains that may operate via interaction with mPTP structures (reviewed in Galluzzi et al.51).
In reconstituted systems, purified ANT formed leaky channels and pore-like structures.52 Single channel current measurements in these systems demonstrated Ca2+-dependent high conductances with multiple sublevels.35 On the basis of the similarity of the ANT-derived channel activity to the tentative mitochondrial multiconductance-level channel and regulation of the mPTP phenomenon by ANT ligands,2,53 it was originally suggested that ANT might be a core element of the mPTP complex.48,54 Later studies, however, cast serious doubt on this conclusion because in an ANT double knockout mouse (ANT1−/− and ANT2−/−) typical Ca2+- and cyclosporin A (CsA)-sensitive mPTP-like behaviour of the IMM was observed, though with a higher Ca2+ threshold.19 However, considering the importance of mitochondrial ATP/ADP exchange in the cell, other as-yet undetermined compensatory system(s) may exist, such as other unrecognized ANT isoforms or another carrier taking charge of mitochondrial nucleotide transport. On the other hand, unless new evidence comes to light that such compensatory mechanisms exist and lead to the expression of a protein that mimics all the functions of the ANT, it should become a settled matter that the ANT is a regulatory element of the mPTP rather than (part of) its core structure. Figure 2 summarizes the current view of the mPTP regulation by ANT and CyP-D.
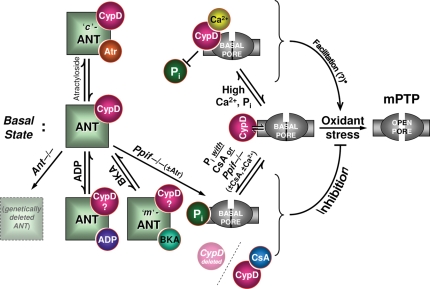
Figure 2.
Regulatory roles of ANT, CyP-D, and Pi. The right side of the figure indicates symbolically the threshold for mPTP induction by oxidant stress, whereas the left side indicates mPTP regulatory elements. The middle row (i.e. examined horizontally) indicates the basal state; the regulatory mechanisms shown symbolically in the upper row lead to facilitation of mPTP-induction, while those in the lower row indicate inhibition. Middle row depicts the basal state of ANT and CyP-D as they relate to the basal threshold for mPTP induction by oxidant stress. Top row reflects factors that facilitate mPTP induction: atractyloside, Ca2+, and indirect effects of Pi. Bottom row includes factors that are known to inhibit mPTP induction: genetic deletion of ANT (ANT is dispensable for mPTP formation per se; inhibition of CyP-D by CsA remains protective), ADP or bongkrekic acid (requirement/role of CyP-D under these conditions is unknown), CsA and genetic deletion of CyP-D in the presence of Pi (atractyloside, CsA and Ca2+ are no longer effective when compared with WT). Note the opposing mechanisms of Pi in mPTP-induction: (i) Pi as a direct mPTP desensitizer (bottom row) is opposed by CyP-D binding (top row), whereas (ii) Pi may also act as an indirect mPTP sensitizer (through regulation of Mg2+ and/or polyphosphate levels; top row; see text). Note also that Ca2+ is not a major factor in mPTP induction in cardiomyocytes and neurons. Ppif is the gene encoding CyP-D in mouse.
Additionally, during ischaemia and hypoxia, intracellular concentrations of ADP and Pi rise as ATP is used up. This results in a marked increase in mPTP opening probability, likely caused by the prevalence of ANT in c-conformation under these conditions. Consequently, factors restoring intracellular ATP level are considered protective, opposing mPTP induction.
3. Cyclophilin D and phosphate
Experiments employing a Cyp-D affinity column demonstrated a direct interaction between Cyp-D and ANT. Furthermore, pretreatment of the column with CsA prevented the binding of ANT to the column, thereby confirming that Cyp-D is in fact the target of CsA.55
After the sensitivity of the mPTP to CsA was recognized,56 there were attempts to establish a case that the mPTP is always blocked by CsA. Currently, it is believed that rather than completely blocking the mPTP, CsA desensitizes and delays its opening. Originally, a simplified scheme proposed that CyP-D, residing in the mitochondrial matrix, undergoes Ca2+-dependent binding to the matrix site of ANT, thereby favouring the ‘conversion’ of ANT into the mPTP.48 The relevance of the peptidyl–prolyl cis–trans isomerase (PPIase or rotamase) activity of CyP-D (essential for CyP-D chaperone-like activity) to mPTP induction is not yet clear. One of the earlier hypotheses suggested that the mPTP is formed by aggregation of misfolded membrane proteins.15 According to this scheme, chaperone-like proteins blocked mPTP conductance by binding and refolding these proteins. CsA interaction with the hydrophobic pocket of Cyp-D resulted in inhibition of both mPTP opening and PPIase activity. It has also been proposed that CsA may compete for Cyp-D with some unknown substrate necessary for mPTP induction.57 Once the effects of CsA and diethylpyrocarbonate on PPIase activity and mPTP-induction, respectively, were compared—although both inhibited the PPIase activity of Cyp-D—the first agent inhibited mPTP, while the second one induced it—it was noted that PPIase activity may be irrelevant to mPTP-induction.58 In contrast, it has been reported that PPIase activity is essential for protection against apoptotic stimuli in HEK293 and rat glioma C6 cell systems overexpressing CyP-D.59 This observation suggests that CyP-D may play a differential role in mitochondrial injury triggered by mPTP induction and by apoptotic stimuli, respectively (see below).
Similar to the situation with ANT, recent genetic studies have demonstrated that CyP-D is also not an essential component of the mPTP but rather plays a regulatory role18 (Figure 2). CyP-D deletion renders the mPTP insensitive to CsA, an observation which has confirmed that the target of this drug in modulating the mPTP is indeed CyP-D. Two other studies demonstrated that cells missing CyP-D are resistant to oxidative stress and Ca2+ overload,16 and that although they remain quite sensitive to apoptotic stimuli, they become resistant to necrotic death stimuli.20 This lead to the overall conclusion that mPTP induction may be important for necrosis but not necessarily for apoptosis.
The unexpectedly complex role of inorganic phosphate (Pi) in the modulation of the mPTP was recently highlighted. Careful observations now suggest that Pi may apparently exert dual actions on the mPTP depending on the functional state presence of CyP-D. As pointed out by Bernardi,60 Pi can either be an important mPTP sensitizer, probably acting by decreasing matrix-free Mg2+ 61 and/or by the formation of polyphosphates,62 or play a role in desensitizing the mPTP to Ca2+ (in the presence of CsA, or under circumstances of CyP-D ablation). Accordingly, in this model the presence of CyP-D tends to prevent the intrinsic inhibitory action of Pi by blocking the Pi regulatory site on the mPTP (thus, CsA or genetic deletion of CyP-D act by unmasking the direct mPTP-desensitizing action of Pi; Figure 2).60
The therapeutic application of CsA as a desensitizer of the mPTP has been hindered for many years because of a variety of factors, including a very narrow optimal concentration range24 (possibly related to the Pi levels, as discussed above), the potential occurrence of cancer,63 and nephrotoxicity.64 Nevertheless, CsA was found to be effective in limiting infarct size during acute myocardial infarction,65 and in correcting mitochondrial dysfunction in collagen VI myopathies.66 The protective properties of CsA in different pathologies have recently been comprehensively reviewed.34 Besides CsA, more specific CyP-D inhibitors have also been investigated that do not exhibit undesirable side effects (such as the inhibition of the protein phosphatase calcineurin). In particular, non-immunosuppressive CsA analogues, e.g. methylAla(3)ethylVal(4)-cyclosporine (Debio 025)67 and (N-methyl-4-isoleucine cyclosporine) NIM811,68 and Sanglifehrin A (a compound unrelated to CsA),69 have shown promise as potent mPTP desensitizers with less adverse side effects in vivo when compared with CsA.
4. Voltage-dependent anion channel
Both the high permeability of the OMM and the channel properties of VDAC have been known for many decades.70,71 Based on purified protein reconstitution experiments, the major regulator of VDAC was thought to be ΔΨm which when increased results in the closed state.71,72 The observed voltage dependence of VDAC was surprising, because the OMM does not contain voltage generators. Two alternative explanations have been investigated to explain this fact: (i) it has been suggested that the close proximity of the VDAC in ‘contact sites’ to the IMM may result in sensing of the high voltage existing across the IMM and consequent VDAC closure. In isolated mitochondria the number of ‘contact sites’ depends on the mitochondrial functional state (in state 3 the number of ‘contact sites’ is higher than in state 473). Thus, depending on the metabolic state, the total VDAC conductance may vary. (ii) Although, in the reconstituted system, the voltage gating of VDAC is apparent, in living cells, other regulators of its conductance, e.g. regulatory proteins, may be involved (discussed below). It was suggested that VDAC is the first check-point responsible for control of the transport through the OMM and that its conductance is the major factor in regulation of the mitochondrial inward and outward traffic of oxidative substrates, nucleotides, amino acids (AA), small peptides, signalling molecules, etc.74
Specific VDAC inhibitors or modulators have not been described yet. VDAC readily interacts with anionic rather than with cationic structures and in the open state VDAC preferentially conducts anions. One of the first described artificial regulators of VDAC is the Konig's synthetic polyanion.75 In an in vitro system, this construct induced VDAC closure, resulting in impermeability of the OMM, for example, to ATP and ADP, and consequent block of mitochondrial metabolism. Another very effective group of compounds that induce VDAC closure are phosphorothioate oligonucleotides.76 Additionally, it was suggested that protection against apoptosis elicited by the BH4 domain of Bcl-xL may be partially mediated by VDAC closure.77
The well-known VDAC modulator, NADH, causes a steep voltage dependence of VDAC behaviour.78 A similar steep voltage-dependent mode of action was also induced by an unidentified ‘soluble protein modulator’ that behaves as a physiological controller of VDAC gating, and thus its involvement in the governing of metabolic flux across the OMM has been suggested.79 It has been proposed that this modulator may be responsible for matching VDAC conductance to changes in the cell's environment. The identity of this modulator was unknown then but the molecular mass (around 90 kDa)80 closely matches that of a cytosolic, VDAC interacting protein, the hexokinase (HK).
VDAC possesses a GSK-3β phosphorylation consensus motif at amino acids 51–55. Two protective mechanisms triggered by GSK-3β-inhibition-mediated VDAC dephosphorylation were suggested: (i) a resulting increase in HKII binding to mitochondria,81 although additional experiments found that insulin did not protect against the effects of forced HKII detachment caused by the peptide, HKII-VBD,82 and (ii) VDAC dephosphorylation-induced VDAC closure and consequent inhibition of the ATP influx through VDAC during ischaemia.83 However, the direct relationship of VDAC phosphorylation status, and the role played by HKII (if any), to the mPTP has yet to be unambiguously demonstrated.
Although VDAC had originally been recognized as a key component of the mPTP,29–31 recently, after demonstration that the main features of the permeability transition are still preserved in mitochondria isolated from the VDAC1–VDAC3 knock-out mouse and in VDAC 1/3−/− cells in which VDAC2 has been silenced,17 the necessity of VDAC in the mPTP has also been thoroughly disputed. Indeed, fibroblasts lacking all three VDAC isoforms exhibited similar stress-induced mPTP induction and cell death in response to the ‘pro-death’ Bcl-2 family members as did wild-type (WT) cells, suggesting not only the complete dispensability of VDAC as an element of mPTP but also its irrelevance to the Bax- and tBid-induced cell death.17 Furthermore, recent experiments in cells lacking VDAC1 and 3 (and without detectable binding between HK II and residual VDAC2) demonstrated also the dispensability of VDAC for cell death elicited by HKII detachment from mitochondria by a HKII N-terminal peptide (HKII-VBD, see below).82 Thus, VDAC is not an mPTP element and currently, beyond speculation there is no compelling direct evidence for its mPTP-regulatory role either (Figure 3).
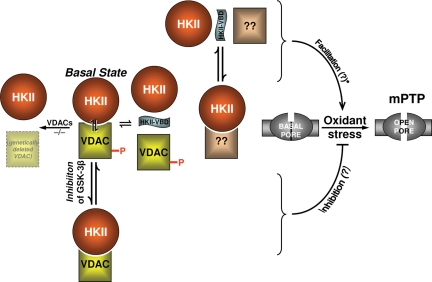
Figure 3.
Regulatory roles of VDAC and HKII. Figure layout scheme is the same as in Figure 2. Middle row of the scheme depicts the basal state of VDACs (WT and genetically deleted) and HKII, and their relationship to the basal state and oxidant stress-induced mPTP. mPTP is similar in WT controls as well as in mitochondria lacking VDACs, thus VDAC is dispensable for mPTP-induction. Top row represents facilitation of the ROS-induced mPTP by HKII-VBD peptide: however, because VDAC is dispensable, it is possible that HKII dissociation from some other site may be relevant. Note that this still does not prove that the HKII dissociation from any site by HKII-VBD peptide is casually related to facilitation of mPTP-induction. Bottom row: GSK-3β inhibition results in VDAC dephosphorylation which has been linked with cell protection,81,83 so it remains possible that VDAC phosphorylation may be involved in mPTP regulation.
5. Hexokinases
As a key glycolytic enzyme, HK provides a functional link between mitochondria and cytosol by governing the preferential utilization of mitochondrial ATP for glucose phosphorylation. In the late 1970s and early 1980s it was demonstrated that the unknown VDAC-binding protein is indeed the HK84,85 and since then this glycolytic enzyme has been recognized as the main VDAC modulator. The current standing regarding the role of HKII, one of the mitochondrial-bound isoforms, in mPTP regulation is summarized in Figure 3.
Mammalian cells express four isoforms of HK: types I, II, III, and IV (glucokinase),86 all of which exhibit different affinities for ATP and glucose. Multiple isoforms often coexist in a given tissue. The N-terminal sequence of the mitochondrial HKI and II has high affinity to VDAC,87 whereas HKIII and IV (cytosolic, preferentially expressed in non-muscle cells) lack affinity for VDAC. Mitochondria-bound isoform I is the ‘housekeeping’ enzyme expressed in all mammalian tissues. HKII, highly expressed in cardiac and skeletal muscle, appears to represent the principal regulated (e.g. insulin-responsive) isoform in many cell types. HKII expression is significantly increased in rapidly growing cancer cells where it is involved in the increased rate of glycolysis. It has been proposed that HKI is tightly bound not only to VDAC, but also specifically to VDAC located in the ‘contact sites’.88 While HKI exists essentially in a mitochondria-bound form, HKII has been found both in mitochondria-bound as well as in soluble cytosolic form. Wilson speculated that HKII may also play an anabolic role by generating glucose-6-phosphate that is utilized in the pentose phosphate pathway to supply NADPH for lipid synthesis.89
Glucose and ATP act both as catalytic substrates and modulators of HK-VDAC binding. Considering the ability of the mitochondrial ‘contact sites’ to modulate the amount of bound HK in response to mitochondrial metabolic state73 manipulations of the metabolic state (for instance, via applying deoxyglucose) can have a dramatic effect on both the HK catalytic reaction rate and overall cell metabolism.
Three of the HK isoforms (I, II, and III) are inhibited by the product of their catalysis, glucose-6-phosphate, while type IV is insensitive to this product.90 The reaction product of the first three isoforms is also capable of releasing HK from mitochondria. The ‘solubilizing’ activity of glucose-6-phosphate is highly variable and seems to be species dependent, which may reflect the evolution of multiple VDAC isoforms with different affinities for HK binding.91
In addition, different drugs may facilitate the release of HK from mitochondria, thereby mimicking the natural effect of glucose-6-phosphate. Antifungal drugs such as clotrimazol and bifonazol are effective hexokinase-releasing agents.92 Similar properties were assigned to the peptides corresponding to the N-terminal 29 AA residue domain common for both the HKI and HKII (HKII-VDB; VDAC-binding domain).93,94 Recent findings indicate that forced release of HKII by this peptide invokes a cell death signal that engages mPTP opening.82 Surprisingly, this peptide was also fully effective in VDAC1/3−/− fibroblasts (where the VDAC2 isoform was also found not to be bound to HKII) proving the complete dispensability of VDACs for the induction of apoptosis in these cells (see previous section). Thus, HKII detachment from another site might be the signal to initiate apoptosis by relay of a conformational change from OMM to IMM and to components/modulators of the mPTP, independently of VDAC's presence.
6. Benzodiazepine receptor
Attempts to identify the nature of benzodiazepine binding to the OMM resulted in the discovery of a mitochondrial peripheral-type benzodiazepine receptor which is also known as the 18 kDa translocator protein (TspO).95 TspO binds a number of ligands of varying nature,96 but its physiological role remains obscure. Some speculative functions assigned to this receptor include, for example, steroid transport,97 porphyrin transport,98 and oxygen (redox) sensing.99 TspO can form a multimeric complex with VDAC and ANT95 in the OMM. Additionally, patch clamping studies have revealed that a Ca2+-dependent mitochondrial multi-conductance channel, presumably the mPTP, was equally sensitive to CsA6 and to the TspO ligand, Ro5-4864,100 thereby demonstrating that TspO may be relevant to the mPTP entity or its regulation. Indeed, a proteinaceous supercomplex isolated from mitochondrial ‘contact sites’ demonstrated catalytic activity sensitive to this TspO ligand.31 In light of these findings, several tentative models of the mPTP complex have included TspO/PBR as a module attached to VDAC (Figure 1).
Among many natural TspO ligands, the putative endogenous diazepam-binding inhibitor DBI, an 11 kDa polypeptide of 86 AA, is well known.101 DBI could be cleaved into several biologically active fragments, including triakontatetra-neuropeptide (TTN; AA 17–50), octadeca-neuropeptide (ODN; AA 33–50), and octapeptide (OP; AA 43–50) that also act as TspO ligands.102,103 The beneficial effect of TspO ligands in the prevention of ischaemia/reperfusion-induced damage is well established.104,105 In a recent report, the TspO ligand, Ro5-4864, administered at the onset of reperfusion, protected against post-ischaemic contractile impairment, including reperfusion arrhythmias, probably by acting on a target which is located upstream of mPTP106 thus suggesting that TspO is probably a regulator, but likely not an mPTP component.
7. Mitochondrial creatine kinase
Mitochondrial creatine kinase (mtCK), located in the mitochondrial intermembrane space, catalyses reversible transfer of the phosphoryl group from phospho-creatine to ADP. mtCK is encoded by two different genes that are expressed in a tissue-specific manner: the sarcomeric isoform is expressed in heart and skeletal muscles, whereas the ubiquitous isoform is expressed in smooth muscle, the brain, kidney, and other tissues; however, no expression was detected for liver and lung.107
Isolated mtCK exhibits an association/dissociation equilibrium of preferentially octameric and dimeric structures.108 mtCK has been studied mostly in in vitro systems that typically rely on the biophysical examination of purified mtCK and its interaction with model membranes. In these experiments the cube-like octameric mtCK formed close contacts between the two membranes.109 Indeed, it has been demonstrated that octameric mtCK was enriched in isolated ‘contact sites’.108 mtCK forms high-molecular-weight complexes also containing VDAC and ANT, and may provide the structural basis for functional interaction between the components of the two membranes. Because of these factors, it has been proposed that mtCK may be involved in mPTP regulation.29
This enzyme is very sensitive due to the low tolerance of its sulfhydryl groups to oxidative stress.110 This sensitivity is thought to be the mechanism by which ROS induces a shift of the equilibrium between the two mtCK oligomer structures towards the dimeric form.111 Correspondingly, in cells containing mtCK, it has been postulated that the mPTP threshold diminished by oxygen radicals is possibly caused at least in part by the vulnerability of mtCK to oxidant stress. Thus, under these circumstances, a shift towards the octameric structure of mtCK could result in an acquisition of a high tolerance to mPTP induction, whereas a shift to the opposite mode (towards the mtCK dimeric structure) may favour mPTP-induction.112 Mitochondria isolated from transgenic mice expressing ubiquitous mtCK in liver (which expresses no CK in WT) were not different vs. WT controls in their ability to undergo (Ca2++atractylate)-induced mPTP; however, creatine or cyclocreatine, stabilizing mtCK in an ANT-bound octameric form, inhibited mPTP induction only in transgenic mice.112 Thus, the mtCK could also potentially be involved in the mPTP regulation.
8. Essential lipids
Boundary lipids are also known to play an important role in mPTP regulation. The essential role of cardiolipin, a hallmark phospholipid of the IMM and mitochondrial ‘contact sites’, has long been recognized. Cardiolipin is tightly bound to ANT and is required for the ATP/ADP exchange activity of ANT reconstituted into bilayer membranes.113 Ca2+ exhibits a very high affinity for cardiolipin, and displaces this lipid from ANT upon binding, thereby promoting its conversion into a ‘pore-forming’ m-conformation. The relevance of Ca2+ to mPTP induction in situ seems to depend on cell type: although strict Ca2+ dependence of the mPTP is characteristic of isolated mitochondria and probably many types of non-excitable cells, it is essentially absent in cardiomyocytes and neurons (see below).
It has been shown that the proapoptotic factor, tBid, which contains a BH3 domain, induces cardiolipin-dependent release of cyctochrome c after translocation to mitochondria.114 tBid-induced changes in cardiolipin within the mitochondrial membranes result in modulation of the ANT activity.115 The data also suggest that inhibitors of peroxidase activity could be protective against cell death stimuli via the conservation of intact mitochondrial cardiolipin,116 and the control of ROS levels.
9. Other regulatory mechanisms
9.1. Calcium ions
Based on experiments with isolated mitochondria, Ca2+ was thought to be the most important factor in mPTP induction. Recently though, experiments in intact excitable cells such as cardiomyocytes and neurons demonstrated that the mPTP is largely insensitive to increased cytosolic Ca2+ (to levels exceeding an order of magnitude above basal), but becomes apparently very Ca2+ sensitive when these same mitochondria are separated from the normal cytoplasm. Thus, Ca2+ has probably been inappropriately ‘blamed’ for mPTP induction and subsequent cell death in cells subjected to injury-producing stresses such as hypoxia/reoxygenation in cardiomyocytes or exitotoxic stress in neurons9,25 and discussed in online Supplementary material.10 Rather, oxidant stress mechanisms appear to be the dominant factors responsible for mPTP induction due to stress injury in excitable cells. While Ca2+ might be of little relevance as far as mPTP induction in excitable cells, the role of Ca2+ in the mPTP induction remains important in a variety of intact non-excitable cells. Indeed, recently, it has been demonstrated that the antibiotic minocycline protected against mPTP induction and mitochondrial injury during rat liver transplantation, which in turn preserved graft function by inhibition of mitochondrial Ca2+ uptake.117 Interestingly, the difference in Ca2+ sensitivity of the mPTP between these two categories of cells is abolished by gentle cell permeabilization procedures10—all cell types demonstrated Ca2+ sensitivity of the mPTP after permeabilization. The Ca2+ dependence of the mPTP after cell permeabilization or isolation of mitochondria may be explained by the existence of a yet-to-be defined soluble factor(s) involved in regulating Ca2+ sensitivity which is lost during cell permeabilization/mitochondrial isolation.9,10
9.2. Mitochondrial phosphate carrier
Recently, a new model for the mPTP has been suggested in which the mitochondrial phosphate carrier (PiC, which would provide the pore-forming component instead of the ANT), facilitated by CyP-D and possibly the interaction with the ANT and it ligands, initiates the Ca2+-induced conformational change that triggers the mPTP. This conclusion was based, in part, on findings that PiC binds Cyp-D in a CsA-sensitive manner,118 and was supported by the observation that PiC silencing dramatically delays cytochrome c mobilization and apoptosis.119 Although it is tempting to make the association, the complex effects of Pi on mPTP induction (see above) do not necessarily implicate the PiC as a constituent of the mPTP (as discussed by Basso et al.60). This issue of the PiC remains a subject of active debate and deserves further attention and research.
9.3. Acidosis
In vitro experiments with isolated mitochondria demonstrated for the first time that a decrease in pH delays mPTP opening.2 The protective effect of acidosis, which seems to act directly or in proximity to the mPTP rather than through upstream (e.g. kinase) signalling, was later confirmed in vivo in intact cells9 and in situ in hearts exposed to ischaemia/reoxygenation applied at reperfusion.120 These data suggest that careful, catheter-based approaches directing controlled acidosis to the infarct zone at the point of reperfusion could have therapeutic promise as a postconditioning strategy in the treatment of patients with acute myocardial infarction.
9.4. Protein kinases and phosphatases
Ischemic pre- and postconditioning activate endogenous signalling mechanisms resulting in the most potent forms of protection capable of reducing cell death following prolonged periods of ischaemia (e.g. as a consequence of arterial occlusion). Activation of these endogenous mechanisms can be triggered by brief episodes of transient ischaemia and reperfusion preceding a prolonged ischaemic insult (i.e. preconditioning; originally demonstrated by Murry et al.121) or repetitive ischaemia applied during early reperfusion (i.e. postconditioning (see Zhao et al.,122 for comprehensive reviews, and also Skyschally et al.123 and Yellon and Downey125). Similar levels of protection could be achieved by pharmacological activation of variety of surface receptors and their downstream signalling pathways including the mitochondrial ATP-dependent K+ channel (mitoKATP) (see below) and kinases such as PKA, PKB/Akt, PKC, PKG, and glycogen synthase kinase-3β (GSK-3β) (reviewed in Juhaszova et al.26,124 and Yellon and Downey125). GSK-3β was found to serve as a critical convergence point of these signalling pathways, and in turn was responsible for conveying the signals downstream to mPTP components/modulators;10 reviewed in Juhaszova et al.124 The role of GSK-3β in cell protection was found to be mediated by the mPTP.10,126 Inhibition of GSK-3β by phosphorylation on Ser9 resulted in an increased mPTP–ROS threshold and consequent cell protection. Therefore, the application of reversible GSK-3 inhibitors has the potential to be a useful cell protection strategy (Figure 4).
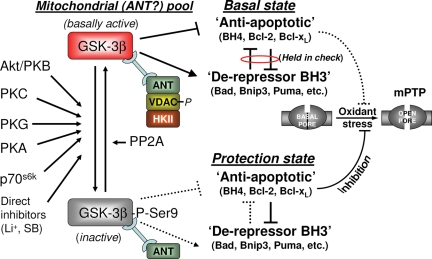
Figure 4.
Proposed model of mPTP modulation by GSK-3β. The phosphorylation state of the mitochondrial-(ANT)-associated pool of GSK-3β contributes to the balance of Bcl-2 family protein effects, the result of which determines the resistance of the mPTP to oxidant stress. Basal state (top): the local GSK-3β pool is active, binds ANT in a complex with phosphorylated VDAC, CyP-D, and possibly other mPTP regulatory elements. ‘Anti-apoptotic’ BH4- and the ‘de-repressor’ BH3-only domain Bcl-2 protein family members are held in mutual check. Protection state (bottom): induced by phosphorylation/inactivation of this mitochondrial GSK-3β subdomain pool resulting in a shift in the balance between Bcl-2 family members toward unmasking the activity of ‘anti-apoptotic’ Bcl-2 family members (and possibly a shift in the interaction between the mPTP and other of its regulators) and consequently in protection of the mPTP against oxidant stress injury (modified from Juhaszova et al.9).
An in silico search for GSK-3β phosphorylation motifs within proteins involved in protection yielded several potential candidates, including Bcl-2, Bis, and protein phosphatase 2A.9,10 Experimental data demonstrated that GSK-3β also phosphorylates VDAC, which leads to a decrease in HKII binding to mitochondria, and inhibition of the ATP influx into mitochondria81,83 (Figures 3 and 4).
The putative mitoKATP has also been proposed to play a role as a mediator or a possible end-effector of protection signalling (we think the latter role is unlikely). Ischaemic pre/postconditioning and pharmacological activation of the mitoKATP has been shown to induce a protective state sensitive to inhibition by mitoKATP blockers such as 5-hydroxydecanoate (5-HD) and glybenclamide.127–129 In isolated cardiomyocytes mitoKATP activation-induced mitochondrial K+ influx has been associated with regulatory mitochondrial swelling (seen also in isolated mitochondrial suspension130), an increase in oxygen consumption, and the production of a redox signal; furthermore, these actions of diazoxide were abolished not only by 5-HD but also by the ROS scavenger N-acetylcysteine, implicating redox signal in certain types of conditioning processes.10,131 Subsequent redox activation of PKC in mitochondria has been demonstrated.10,132 This PKC signal, together with the mitochondrial volume activated respiration and resulting redox signals, has been implicated in maintaining the memory of the conditioning process in a positive feedback loop by sustaining the activation state of the mitoKATP.10 Preconditioning-induced translocation of PKCϵ into the mitochondria (and/or activation of resident PKCϵ) of cardiomyocytes resulted in cell protection.10,133,134 The opposing roles of protein kinase C (PKC) isoforms, specifically PKCϵ and PKCδ, in cell protection are relatively well defined for heart tissue.133 PKCϵ has been demonstrated to physically associate with the mPTP modulators ANT and VDAC (the latter's status as an mPTP modulator being questionable), and to phosphorylate VDAC in heart mitochondria.135
The general role of kinases in mPTP protection has even been questioned recently.136 Based on the analysis of the phosphoprotein levels in the mitochondria isolated from preconditioned hearts, it was concluded that protein kinase-mediated changes in mitochondrial phosphoprotein levels may not be required for the preconditioning-induced mPTP inhibition, but rather that preconditioning-induced reduction in oxidative stress is responsible for mPTP protection. It has been noted, however, that experimental conditions strongly influence the detection of protein phosphorylation status, so real changes may be artifactually lost.83 Therefore, in light of the extensive existing evidence to the contrary, it would seem that kinases should continue to be recognized as important regulatory elements in cell protection (until and unless considerable new evidence to the contrary could be obtained).
9.5. Bcl-2 family proteins
Early on, in vitro studies of the interaction between purified Bcl-2 or BAX, and ANT in artificial lipid bilayers suggested that Bcl-2 and Bax may form with ANT channels with different behaviours. In contrast to Bax, Bcl-2 does not form active channels when incorporated into membranes with ANT. Bcl-2 also blocks the ability of BAX to form channels in reconstitution experiments with ANT. These data suggested that Bcl-2 family proteins may play a regulatory role in ‘mPTP induction’.49 Indeed, it has been demonstrated that a membrane permeable peptide carrying the BH4 domain of Bcl-xL (TAT–BH4 fusion peptide) increases the threshold for mPTP induction in cardiomyocytes9 and reduced infarct size in the heart after ischaemia/reperfusion137 while HA14-1, a small non-peptidic inhibitor of Bcl-2, abolished the protection afforded by TAT–BH4 and sensitized the mPTP to opening.9,138 In addition, the BH3-domain peptide of Bad, which has a high affinity for Bcl-2, completely abolished protective effects of TAT-BH4, Li+, insulin, and an inhibitor of the Na+/H+ exchange without significantly affecting the basal mPTP–ROS threshold.9 These data suggest that specific functional groups of the Bcl-2 family proteins, the ‘de-repressor’ BH3-domain-only proteins (e.g. Bad, Bnip3, Puma, etc.) together with the so-called ‘anti-apoptotic’ BH4-domain carrying proteins (e.g. Bcl-2, Bcl-xL, etc.), acting downstream of GSK-3β, appear to be able to mediate (or at least regulate) cell-protective or protection-inhibiting functions via interaction directly with the mPTP (and/or its other regulatory elements). Specifically, in the basal state, the so-called ‘anti-apoptotic’ members of the Bcl-2 family of proteins would be held in check in a functional sense by ‘de-repressors’. According to this scheme, GSK-3β in the active (basal) state may participate by modulating the interaction between regulatory components of the mPTP, i.e. ANT and CyP-D, as well as the functional interaction and binding of various Bcl-2 family proteins. These interactions could be important in maintaining a balance between opposing influences of these members of the Bcl-2 protein family, thus producing the basal state of resistance to mPTP induction. The protection state would be associated with the phosphorylation and therefore inactivation of GSK-3β, which would lead to changes in interaction between mPTP components/regulatory elements, inducing a shift in the balance within the Bcl-2 family of proteins in favour of ‘anti-apoptotic’ proteins, resulting in an increase in the mPTP–ROS threshold (see Figure 4).
9.6. Ubiquinone 0
Fontaine et al.139 demonstrated the existence of a quinone-binding site on the mPTP and initially proposed that mitochondrial complex I, which has two quinone-binding sites, might be a structural element of the mPTP. They also demonstrated that ubiquinone 0 is a potent inhibitor of the mPTP, although the quinone-binding site(s) involved in mPTP protection remains a subject of debate. However, the potential application of ubiquinone 0 as mPTP inhibitor in an in vivo setting is limited due to its unspecific toxic effects on mitochondrial respiration.140
10. Concluding remarks: mPTP with no defined core?
Despite extensive research efforts over several decades, at this point the essential core component(s) of the mPTP responsible for IMM permeabilization remain unidentified. It is likely that none of the elements discussed above form the principal structure of the mPTP; they most likely act only as modulators/regulators. In view of the new genetic data, the models of the physical entity of the mPTP complex have been forced to undergo extensive revision and correction. Therefore, based on careful re-evaluation of available data, it is possible to conclude that components once recognized as the core structural (pore-forming) elements of the mPTP (namely, mitochondrial ANT, cyclophilin D and TspO) are instead only mPTP modulators/regulators (or, in the case of VDAC, probably entirely dispensable). Additional important protein regulators include the Bcl-2 family and certain kinase signalling cascades, including those converging and acting via a mitochondrial-associated pool of GSK-3β. Recently, a novel preliminary model for the mPTP has been hypothesized in which the PiC would provide the pore-forming component that deserves further consideration. Finally, important small molecule/ion regulation is obtained from species requiring CyP-D (such as CsA, Ca2+ and Pi), those requiring the ANT (e.g. ADP, BKA, and atractyloside), or from those acting at sites that remain to be defined (e.g. accompanying acidosis).
Currently, the minimum number of components involved in the mPTP phenomenon is not known. Under specific circumstances it is possible that different mPTPs, formed by different clusters of proteins, may exist. It is not known whether just one type of mPTP can be formed in a particular cell type, or whether diverse triggers result in the same or various mPTPs, or if recruitment of different mPTP elements result in different behaviours, e.g. resulting in different thresholds to Ca2+, ROS, and exhibiting sensitivities to CsA, etc. This phenomenon may even involve different states with very subtle differences in the overall behaviour. Additionally, since a single mPTP can likely discharge the ΔΨm, it is possible if not likely that the assembly of the mPTP, from its constituent components and its induction, is a rare event, and that it could be assembled from components, which while relatively abundant, generate only a single macromolecular cluster and that exists only transiently. Multiple regulatory entities assembled around the main core components resulting in somewhat different behaviours in different cells may exist. This may partially explain the appearance of the terms ‘regulated’ and ‘unregulated’ to describe mPTP, or the in vivo vs. in vitro differences in Ca2+ sensitivities, and thereby the inherent complexities in observing the obscure nature of this phenomenon.
What is clear, however, is that the mPTP phenomenon plays a key role in the ‘life or death’ decision of the cell. In addition to the well-established role of the mPTP induction in ischaemia/reperfusion injury, recently the mPTP has also been shown to play an important role in pathologies such as Ulrich congenital muscular dystrophy, Bethlem myopathy,66 etc. (reviewed in Rasola and Bernardi141) and is likely to be discovered as playing fundamental roles in many other diseases, in the future. Finally, as we have discussed, the ageing process impairs the intrinsic capacity of the heart (and probably other vital organs)—and specifically that of the mPTP—to resist damaging stress such as excess oxidants and ischaemia/reperfusion injury; these effects are likely aggravated by comorbidities such as diabetes, but may be ameliorated somewhat by caloric restriction and physical conditioning/exercise. There are also considerable data that the ability to induce ischaemic preconditioning in aged animals and humans is impaired, and in particular, that the stimulus threshold for triggering protection increases with age.27 Thus, there needs to be an appreciation that clinical studies on elderly subjects have been hampered by the added complications arising due to the fact that cardioprotection signalling is impaired in the old heart and that the design of future clinical trials testing these questions must take this into consideration (specifically, that it may take a stronger stimulus to activate protection in aging individuals compared to young subjects). These problems with endogenous protection signalling are also potentially aggravated by commonly used medications including sulfonylureas, antioxidants, PFOX inhibitors, and COX2 inhibitors (but also potentially improved by caloric restriction and physical conditioning/exercise), so it would be prudent for future studies to critically examine drugs taken by the elderly for potential interference with endogenous protection signalling (see Juhaszova et al.26 for review, and citation therein). Postconditioning (both by means of the balloon catheter as well as by pharmacology) to reduce the size of myocardial infarction appears to be promising in small cohorts of carefully chosen patients,65,142 but it would be important to examine if efficacy could be demonstrated in the elderly. Some of the age-related impairments in protection signalling could remain unresponsive to lifestyle/diet measures. Pharmacological approaches aimed at downstream targets (such as GSK-3 and the mPTP), to bypass potentially defective upstream signalling components, might provide a rational strategy to further restore protection. Therefore, a better understanding of the mPTP and its modulation will allow identification of possible pharmacological targets and assist in drug design for its precise control.
Conflict of interest: none declared.
Funding
This research was entirely supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
References
- 1.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 2.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 5.Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991;176:1183–1188. doi: 10.1016/0006-291x(91)90410-9. [DOI] [PubMed] [Google Scholar]
- 6.Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 7.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, et al. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann N Y Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 10.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushnareva YE, Campo ML, Kinnally KW, Sokolove PM. Signal presequences increase mitochondrial permeability and open the multiple conductance channel. Arch Biochem Biophys. 1999;366:107–115. doi: 10.1006/abbi.1999.1190. [DOI] [PubMed] [Google Scholar]
- 12.Malkevitch NV, Dedukhova VI, Simonian RA, Skulachev VP, Starkov AA. Thyroxine induces cyclosporin A-insensitive, Ca2+-dependent reversible permeability transition pore in rat liver mitochondria. FEBS Lett. 1997;412:173–178. doi: 10.1016/s0014-5793(97)00666-2. [DOI] [PubMed] [Google Scholar]
- 13.Sokolove PM, Kinnally KW. A mitochondrial signal peptide from Neurospora crassa increases the permeability of isolated rat liver mitochondria. Arch Biochem Biophys. 1996;336:69–76. doi: 10.1006/abbi.1996.0533. [DOI] [PubMed] [Google Scholar]
- 14.Krasnikov BF, Zorov DB, Antonenko YN, Zaspa AA, Kulikov IV, Kristal BS, et al. Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim Biophys Acta. 2005;1708:375–392. doi: 10.1016/j.bbabio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 16.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 17.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 19.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 21.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 22.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crompton M, Costi A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J. 1990;266:33–39. doi: 10.1042/bj2660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 25.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Lakatta EG, Sollott SJ. The ‘heartbreak’ of older age. Mol Interv. 2002;2:431–446. doi: 10.1124/mi.2.7.431. [DOI] [PubMed] [Google Scholar]
- 28.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 30.Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, et al. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyssokikh MY, Goncharova NY, Zhuravlyova AV, Zorova LD, Kirichenko VV, Krasnikov BF, et al. Proteinaceous complexes from mitochondrial contact sites. Biochemistry (Mosc) 1999;64:390–398. [PubMed] [Google Scholar]
- 32.Hackenbrock CR. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci USA. 1968;61:598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohlendieck K, Riesinger I, Adams V, Krause J, Brdiczka D. Enrichment and biochemical characterization of boundary membrane contact sites from rat-liver mitochondria. Biochim Biophys Acta. 1986;860:672–689. doi: 10.1016/0005-2736(86)90567-5. [DOI] [PubMed] [Google Scholar]
- 34.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 35.Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- 36.Pfaff E, Heldt HW, Klingenberg M. Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem. 1969;10:484–493. doi: 10.1111/j.1432-1033.1969.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 37.Shug AL, Shrago E. Inhibition of phosphoenolpyruvate transport via the tricarboxylate and adenine nucleotide carrier systems of rat liver mitochondria. Biochem Biophys Res Commun. 1973;53:659–665. doi: 10.1016/0006-291x(73)90712-2. [DOI] [PubMed] [Google Scholar]
- 38.Asimakis GK, Aprille JR. In vitro alteration of the size of the liver mitochondrial adenine nucleotide pool: correlation with respiratory functions. Arch Biochem Biophys. 1980;203:307–316. doi: 10.1016/0003-9861(80)90181-2. [DOI] [PubMed] [Google Scholar]
- 39.Soboll S, Conrad A, Eistert A, Herick K, Kramer R. Uptake of creatine phosphate into heart mitochondria: a leak in the creatine shuttle. Biochim Biophys Acta. 1997;1320:27–33. doi: 10.1016/s0005-2728(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 40.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 41.Andreyev A, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Tsofina LM, et al. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem. 1989;182:585–592. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
- 42.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 43.Luciani S, Martini N, Santi R. Effects of carboxyatractyloside a structural analogue of atractyloside on mitochondrial oxidative phosphorylation. Life Sci II. 1971;10:961–968. doi: 10.1016/0024-3205(71)90099-3. [DOI] [PubMed] [Google Scholar]
- 44.Vignais PV, Vignais PM, Stanislas E. Action of potassium atractylate on oxidative phosphorylation in mitochondria and in submitochondrial particles. Biochim Biophys Acta. 1962;60:284–300. doi: 10.1016/0006-3002(62)90404-3. [DOI] [PubMed] [Google Scholar]
- 45.Henderson PJ, Lardy HA. Bongkrekic acid. An inhibitor of the adenine nucleotide translocase of mitochondria. J Biol Chem. 1970;245:1319–1326. [PubMed] [Google Scholar]
- 46.Pande SV, Blanchaer MC. Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters. J Biol Chem. 1971;246:402–411. [PubMed] [Google Scholar]
- 47.Gear AR, Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974;249:3628–3637. [PubMed] [Google Scholar]
- 48.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl–prolyl cis–trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, et al. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- 50.Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J, et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tikhonova IM, Andreyev A, Antonenko Yu N, Kaulen AD, Komrakov A, Skulachev VP. Ion permeability induced in artificial membranes by the ATP/ADP antiporter. FEBS Lett. 1994;337:231–234. doi: 10.1016/0014-5793(94)80197-5. [DOI] [PubMed] [Google Scholar]
- 53.Novgorodov SA, Gudz TI, Kushnareva YE, Zorov DB, Kudrjashov YB. Effect of ADP/ATP antiporter conformational state on the suppression of the nonspecific permeability of the inner mitochondrial membrane by cyclosporine A. FEBS Lett. 1990;277:123–126. doi: 10.1016/0014-5793(90)80824-3. [DOI] [PubMed] [Google Scholar]
- 54.Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, et al. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7:1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- 55.Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 57.Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Curr Med Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 58.Scorrano L, Nicolli A, Basso E, Petronilli V, Bernardi P. Two modes of activation of the permeability transition pore: the role of mitochondrial cyclophilin. Mol Cell Biochem. 1997;174:181–184. [PubMed] [Google Scholar]
- 59.Lin DT, Lechleiter JD. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem. 2002;277:31134–31141. doi: 10.1074/jbc.M112035200. [DOI] [PubMed] [Google Scholar]
- 60.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–2939. [PubMed] [Google Scholar]
- 62.Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, et al. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc Natl Acad Sci USA. 2007;104:18091–18096. doi: 10.1073/pnas.0708959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andre N, Roquelaure B, Conrath J. Molecular effects of cyclosporine and oncogenesis: a new model. Med Hypotheses. 2004;63:647–652. doi: 10.1016/j.mehy.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 64.Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 65.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 66.Merlini L, Angelin A, Tiepolo T, Braghetta P, Sabatelli P, Zamparelli A, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci USA. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angelin A, Tiepolo T, Sabatelli P, Grumati P, Bergamin N, Golfieri C, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci USA. 2007;104:991–996. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 70.Parsons DF. Recent advances correlating structure and function in mitochondria. Int Rev Exp Pathol. 1965;4:1–54. [PubMed] [Google Scholar]
- 71.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 72.Ermishkin LN, Mirzabekov TA. Redistribution of the electric field within the pore contributes to the voltage-dependence of mitochondrial porin channel. Biochim Biophys Acta. 1990;1021:161–168. doi: 10.1016/0005-2736(90)90029-n. [DOI] [PubMed] [Google Scholar]
- 73.Knoll G, Brdiczka D. Changes in freeze-fractured mitochondrial membranes correlated to their energetic state. Dynamic interactions of the boundary membranes. Biochim Biophys Acta. 1983;733:102–110. doi: 10.1016/0005-2736(83)90095-0. [DOI] [PubMed] [Google Scholar]
- 74.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Colombini M, Yeung CL, Tung J, Konig T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987;905:279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- 76.Stein CA, Colombini M. Specific VDAC inhibitors: phosphorothioate oligonucleotides. J Bioenerg Biomembr. 2008;40:157–162. doi: 10.1007/s10863-008-9139-9. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zizi M, Forte M, Blachly-Dyson E, Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994;269:1614–1616. [PubMed] [Google Scholar]
- 79.Liu MY, Colombini M. A soluble mitochondrial protein increases the voltage dependence of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992;24:41–46. doi: 10.1007/BF00769529. [DOI] [PubMed] [Google Scholar]
- 80.Holden MJ, Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988;241:105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- 81.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 82.Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, et al. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Felgner PL, Messer JL, Wilson JE. Purification of a hexokinase-binding protein from the outer mitochondrial membrane. J Biol Chem. 1979;254:4946–4949. [PubMed] [Google Scholar]
- 85.Linden M, Gellerfors P, Nelson BD. Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982;141:189–192. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- 86.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 87.Arora KK, Filburn CR, Pedersen PL. Structure/function relationships in hexokinase. Site-directed mutational analyses and characterization of overexpressed fragments implicate different functions for the N- and C-terminal halves of the enzyme. J Biol Chem. 1993;268:18259–18266. [PubMed] [Google Scholar]
- 88.Kottke M, Adam V, Riesinger I, Bremm G, Bosch W, Brdiczka D, et al. Mitochondrial boundary membrane contact sites in brain: points of hexokinase and creatine kinase location, and control of Ca2+ transport. Biochim Biophys Acta. 1988;935:87–102. doi: 10.1016/0005-2728(88)90111-9. [DOI] [PubMed] [Google Scholar]
- 89.Sebastian S, Horton JD, Wilson JE. Anabolic function of the type II isozyme of hexokinase in hepatic lipid synthesis. Biochem Biophys Res Commun. 2000;270:886–891. doi: 10.1006/bbrc.2000.2527. [DOI] [PubMed] [Google Scholar]
- 90.Wilson JE. Hexokinases. Rev Physiol Biochem Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 91.Kabir F, Wilson JE. Mitochondrial hexokinase in brain: coexistence of forms differing in sensitivity to solubilization by glucose-6-phosphate on the same mitochondria. Arch Biochem Biophys. 1994;310:410–416. doi: 10.1006/abbi.1994.1186. [DOI] [PubMed] [Google Scholar]
- 92.Penso J, Beitner R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur J Pharmacol. 1998;342:113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- 93.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 94.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 95.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirsch JD, Beyer CF, Malkowitz L, Loullis CC, Blume AJ. Characterization of igand binding to mitochondrial benzodiazepine receptors. Mol Pharmacol. 1989;35:164–172. [PubMed] [Google Scholar]
- 97.Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci USA. 1989;86:9813–9816. doi: 10.1073/pnas.86.24.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verma A, Nye JS, Snyder SH. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc Natl Acad Sci USA. 1987;84:2256–2260. doi: 10.1073/pnas.84.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeliseev AA, Krueger KE, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial ‘oxygen’ sensor. Proc Natl Acad Sci USA. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci USA. 1993;90:1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD, Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci USA. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferrero P, Santi MR, Conti-Tronconi B, Costa E, Guidotti A. Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci USA. 1986;83:827–831. doi: 10.1073/pnas.83.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Slobodyansky E, Guidotti A, Wambebe C, Berkovich A, Costa E. Isolation and characterization of a rat brain triakontatetraneuropeptide, a posttranslational product of diazepam binding inhibitor: specific action at the Ro 5–4864 recognition site. J Neurochem. 1989;53:1276–1284. doi: 10.1111/j.1471-4159.1989.tb07425.x. [DOI] [PubMed] [Google Scholar]
- 104.Kunduzova OR, Escourrou G, De La Farge F, Salvayre R, Seguelas MH, Leducq N, et al. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2152–2160. doi: 10.1097/01.ASN.0000133563.41148.74. [DOI] [PubMed] [Google Scholar]
- 105.Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, et al. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia–reperfusion. J Pharmacol Exp Ther. 2003;306:828–837. doi: 10.1124/jpet.103.052068. [DOI] [PubMed] [Google Scholar]
- 106.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4'-chlorodiazepam on cellular excitation–contraction coupling and ischaemia–reperfusion injury in rabbit heart. Cardiovasc Res. 2008;79:141–149. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Payne RM, Strauss AW. Expression of the mitochondrial creatine kinase genes. Mol Cell Biochem. 1994;133–134:235–243. doi: 10.1007/BF01267957. [DOI] [PubMed] [Google Scholar]
- 108.Kottke M, Adams V, Wallimann T, Nalam VK, Brdiczka D. Location and regulation of octameric mitochondrial creatine kinase in the contact sites. Biochim Biophys Acta. 1991;1061:215–225. doi: 10.1016/0005-2736(91)90287-i. [DOI] [PubMed] [Google Scholar]
- 109.Nicolay K, Rojo M, Wallimann T, Demel R, Hovius R. The role of contact sites between inner and outer mitochondrial membrane in energy transfer. Biochim Biophys Acta. 1990;1018:229–233. doi: 10.1016/0005-2728(90)90255-3. [DOI] [PubMed] [Google Scholar]
- 110.Yuan G, Kaneko M, Masuda H, Hon RB, Kobayashi A, Yamazaki N. Decrease in heart mitochondrial creatine kinase activity due to oxygen free radicals. Biochim Biophys Acta. 1992;1140:78–84. doi: 10.1016/0005-2728(92)90022-t. [DOI] [PubMed] [Google Scholar]
- 111.Koufen P, Ruck A, Brdiczka D, Wendt S, Wallimann T, Stark G. Free radical-induced inactivation of creatine kinase: influence on the octameric and dimeric states of the mitochondrial enzyme (Mib-CK) Biochem J. 1999;344:413–417. [PMC free article] [PubMed] [Google Scholar]
- 112.O'Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- 113.Hoffmann B, Stockl A, Schlame M, Beyer K, Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J Biol Chem. 1994;269:1940–1944. [PubMed] [Google Scholar]
- 114.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 115.Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 116.Borisenko GG, Kapralov AA, Tyurin VA, Maeda A, Stoyanovsky DA, Kagan VE. Molecular design of new inhibitors of peroxidase activity of cytochrome c/cardiolipin complexes: fluorescent oxadiazole-derivatized cardiolipin. Biochemistry. 2008;47:13699–13710. doi: 10.1021/bi801507s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alcala S, Klee M, Fernandez J, Fleischer A, Pimentel-Muinos FX. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene. 2008;27:44–54. doi: 10.1038/sj.onc.1210600. [DOI] [PubMed] [Google Scholar]
- 120.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning's success. Basic Res Cardiol. 2008;103:464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 122.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 123.Skyschally A, Schulz R, Heusch G. Pathophysiology of myocardial infarction: protection by ischemic pre- and postconditioning. Herz. 2008;33:88–100. doi: 10.1007/s00059-008-3101-9. [DOI] [PubMed] [Google Scholar]
- 124.Juhaszova M, Zorov D, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3β in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 126.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 127.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 128.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 129.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 131.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y, Yang XM, Iliodromitis EK, Kremastinos DT, Dost T, Cohen MV, et al. Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol. 2008;103:54–59. doi: 10.1007/s00395-007-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2008 doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 138.Milanesi E, Costantini P, Gambalunga A, Colonna R, Petronilli V, Cabrelle A, et al. The mitochondrial effects of small organic ligands of BCL-2: sensitization of BCL-2-overexpressing cells to apoptosis by a pyrimidine-2,4,6-trione derivative. J Biol Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 139.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex i. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 140.Fontaine E, Ichas F, Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J Biol Chem. 1998;273:25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- 141.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 142.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]