I. Introduction
Gene patenting has attracted intense scrutiny for decades, raising a host of ethical, legal, and economic concerns. Much of the policy debate has focused on the seemingly quantifiable and practical concerns about the effect of patents on access to useful technologies in both the context of research and the clinic.
In this paper, we summarize the dominant policy concerns and the events that have motivated these debates. We then reflect on what the evidence now says about the major concerns articulated in policy reports. We conclude by discussing what might explain some of the disparity between the empirical evidence and the policy focus.
II. Policy Concerns
While policymakers and advisory groups throughout the world have long recognized the moral and ethical concerns associated with human gene patents [1-3], such concerns have only rarely led to concrete reform proposals [4]. A systematic review of the content and timing of major policy documents highlights that policy activity has been largely stimulated by a convergence of a general social unease, the emergence of preliminary data and literature on the possible adverse practical ramifications of gene patents, and several high profile patent protection controversies.
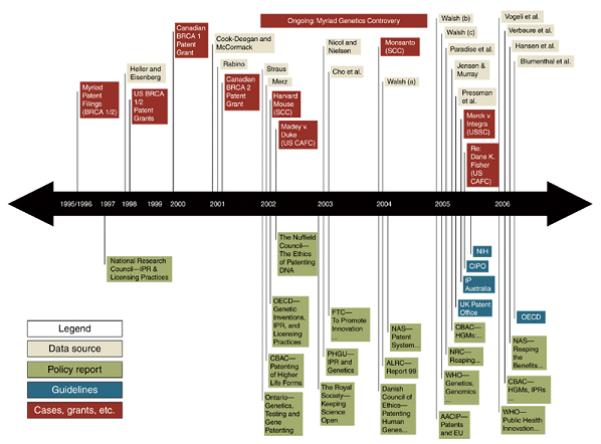
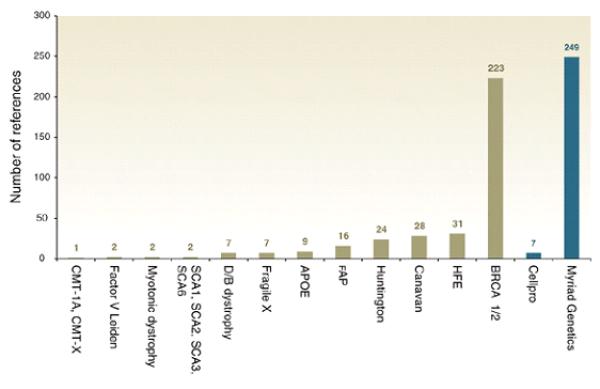
The timing of the policy activity reflects this tendency. The recommendations for diagnostic use licensing, for instance, followed the international controversy associated with Myriad Genetics’ decision to enforce the patents over the BRCA1/2 mutations [5] [see Chart I]. There have been other gene patenting controversies, such as the furor over patents related to Canavan disease, or the attempt by United States National Institutes of Health (NIH) in the early 1990s to patent over 7,000 expressed sequence tags (ESTs) [6]. The mid-1990s was also a period of rapid (roughly 50% per annum) growth in DNA-related patents in the U.S. [7]. Internationally, however, the Myriad controversy coincides with the most policy activity. Indeed, as Chart II reveals, the Myriad Genetics/BRCA 1 & 2 story is, by far, the most referenced patent controversy in the reviewed policy documents.
These controversial gene patenting stories raised several concerns in the academic and policy literature. A prominent concern was that of a “tragedy of the anti-commons”, i.e., the possibility that the large number of patents on genes and their diverse set of owners will make it difficult to acquire the rights to all necessary research inputs, which could, in turn, result in the under use of valuable technologies [8]. Second is the longstanding concern that the owners of patents on fundamental technologies will exercise their rights to exclude in ways that will prevent others from developing or accessing the technology [9-11]. The Myriad case was held out as an example and as a “harbinger” of the coming problems associated with human gene patents [5]. Such restrictions on access to patented genes were viewed as especially pernicious given a belief that such patents could not be invented around, because of the unique role that genes play in biological processes.
A closely related concern was that the strong commercial incentive built into recent policy changes, and the associated pro-commercial milieu in universities, were undermining the norms of open science [12,13], leading researchers to be more secretive about their on-going research, to delay publication of results, and to be less likely to share research materials or data. These behaviors, it was held, would retard the progress of science and technology.
Starting around 2001, this literature, together with the Myriad Genetics and similar controversies [14], began to stimulate significant policy activity [see Chart I]. In Canada, for example, the Ontario Government’s 2002 Report recommended a consideration of a variety of reforms, including strengthening the research exemption and revising the compulsory licensing provisions in the Patent Act to create an exemption for genetic diagnostic and screening tests [15]. In 2002, the UK’s Nuffield Council on Bioethics made similar recommendations [2]. The National Academy of Sciences issued two reports [7,16], both of which recommended a research exemption as a means of dealing with the anti-commons and restricted access problems. These reports were clearly influenced by emerging empirical evidence about the effects of gene patents on genetic testing services [17,18] and the Myriad controversy (the production of the Ontario Report immediately followed the eruption of controversy over Myriads’ patent in Canada and the Nuffield Council and the National Academy’s 2005 report both used Myriad as a case study) [19].
III. Reflecting on the Evidence
With the passage of time and the accumulation of more data, we can now reflect on what the available data do and do not say about the anecdotes, theories and initial evidence that spurred so much policy activity. Indeed, the policy debates around these concerns have both led to and been informed by a number of empirical studies designed to find out where and to what extent each of these concerns is manifest in the practice of biomedical research.
The results of these empirical efforts have been fairly consistent. To begin, the effects predicted by the anti-commons problem are not borne out in the available data. The effects are much less prevalent than would be expected if its hypothesized mechanisms were in fact operating. The data do show a large number of patents associated with genes. A recent study found that nearly 20% of human genes were associated with at least one U.S. patent, and many had multiple patents [20]. Another study estimated that in the U.S. over 3000 new DNA-related patents have issued every year since 1998, and more than 40,000 such patents have been granted [7]. But despite the large number of patents and numerous, heterogeneous actors — including large pharmaceutical firms, biotech startups, universities and governments [7] — studies that have examined the incidence of anti-commons problems find them relatively uncommon [21-25]. These studies span both academics and industry, and include data from the U.S., Germany, Australia and Japan.
Studies on access to upstream research tools find that while some researchers or firms are denied access to a particular technology, others have access to the same technology, suggesting that the resulting limitations have more to do with a willingness to accept the market price and access terms [28,29]. Similarly, among academic biomedical researchers in the U.S., only 1% report having to delay a project and none abandoned a project due to others’ patents, suggesting that neither anti-commons nor restrictions on access were seriously limiting academic research [22] — despite the fact that these researchers operate in a patent-dense environment, without the benefit of a clear research exemption.
One important exception is in the area of gene patents that cover a diagnostic test. Here, there are more instances of researchers and firms claiming that the patent owner is asserting exclusivity or license terms that are widely viewed as inappropriate [18,30] — thus lending some empirical evidence to support the concerns highlighted by the Myriad Genetics story. For example, Merz, et al. (2002) find that 30% of clinical labs report not developing or abandoning testing for HFE after the patent issued. Cho, et al [30] find that 25% of labs had abandoned one or more genetic test due to patents, with Myriad’s patents among the most frequently mentioned. Such unlicensed lab testing, from the perspective of the patent owner, competes with its commercial activity and hence, it is not surprising to find owners asserting their rights.
There is also substantial empirical evidence that university researchers are becoming more secretive and less willing to share research results or materials [31-36, 22]. The causes of this secrecy, however, are still in dispute. In particular, we cannot determine the impact of patents themselves on secrecy, in part because many studies of academic secrecy [31,32,36] use composite measures and, as a result, it is difficult to tease out specific causes thereof. Still, Walsh and Hong (2003) [33] and Walsh, Cho and Cohen (2005) [22] find that patents per se have little effect on discussing on-going research or on sharing of research materials. In contrast, several studies have found that commercial activity, as well as scientific competition and the cost and effort involved in sharing, all have negative effects on open science [22,31,32,36]. Industry funding is also often associated with delayed publication [31,32,37,38]. This failure to share research materials seems to have a negative impact on research [32,22]. For example, Walsh, et al. [22] find that 19% of recent requests were not fulfilled (and that failures to supply materials are increasing), and that at least 8% of respondents had a project delayed due to an inability to get timely access to research materials (compared to 1% who were delayed by an inability to get a patent license). Finally, some studies show reduced citations to publications once a corresponding patent is granted [26,27]. However, the causes and implications of such a relationship are unclear. In particular, is this a result of a change in research practices, or simply of citations practices (i.e., an unwillingness to announce infringement in print)? Even if it is the former, does this simply reflect changing incentives causing a shift by researchers (especially industry researchers) towards less encumbered research areas? The overall social welfare implications of this redirection are also uncertain, as there is both the potential loss of fewer people working on a problem, and a potential gain of a more diverse research portfolio [39].
IV. Analyzing the Concerns, Evidence and Anecdotes
The survey of policy reports reveals that the Myriad Genetics controversy was used as a primary tool for justifying patent reform — thus highlighting the potential of a single high profile controversy to mobilize both governmental and non-governmental policy makers. In Belgium, for instance, the controversy directly incited the adoption of a research exemption [40]. There were certainly other gene patenting controversies that might have been used in a similar fashion — and some of these, such as the Canavan case, are mentioned in the policy documents — but it was the Myriad case that emerged as emblematic of the fear that patents on human genetic material would have an adverse impact on access to useful technologies, both for research and clinical use [11]. This is likely because the Myriad Genetics controversy, more than any other, resonated so well with the theoretical concerns that existed in the literature. In addition, the clinical consequences were easy to understand and highly visible breast cancer constituencies were engaged.
While the available evidence suggests that the concerns associated with the Myriad Genetics case have merit in the context of diagnostic tests, the data are hardly definitive, and empirical research suggests that data about diagnostics cannot be generalized to other uses. Furthermore, five years later, there have been few similar gene patent controversies. One possibility is that the Myriad story has become a cautionary tale for the holders of similar gene patents, guiding them towards more constructive patent enforcement strategies.
The evidence regarding the “anti-commons” and restricted access concerns is clearer. The empirical research suggests that the fears of wide spread anti-commons that block the use of upstream discoveries have largely not materialized. The reasons for this reality are numerous, and are often straightforward matters of basic economics [41]. In addition to licensing being widely available [42], researchers make use of a variety strategies to develop working solutions to the problem of access, including inventing around, going offshore, challenging questionable patents, and using technology without a license [41,29]. While it has been suggested that this latter strategy is an inappropriate and unstable policy [16,43], it is important to remember that the stability of this unlicensed use is supported by a combination of the difficulty of enforcing patents due to the secrecy of research programs, costs of lost goodwill among researchers, costs of litigation, the relatively small damages to be collected from blocking research use, and the interest in the patent owner in allowing research advances in most cases [41,29]. An anti-commons or restricted access type failure requires not that any one strategy be unavailable, but that the entire suite be simultaneously ineffective, which may explain why empirically such failures are much less common than first posited.
Finally, the data concerning the increasing secrecy of university researchers seems to indicate that there may be a conflation of patenting and commercial and/or scientific competition as the cause of this trend. It appears that academic researchers are becoming more secretive, but that is not shown to be attributable to the patenting process, suggesting that the solution might not reside in modifying patent policy. Some have suggested tempering the commercial orientation of faculty and facilitating the flow of research materials [44,45]. Another approach might be recognizing the inherently competitive nature of the academic process [46] and developing additional and improved mechanisms for exchange among its members [41].
VI. Conclusion
Looking back on years of policy debates and the associated empirical work on gene patents, what lessons can be drawn? First, while there may have been good reasons for concerns, the feared problems have not widely manifested. And the problems that the data do reveal may have less to do with patents than with commercial concerns, scientific competition and frictions in sharing physical materials. Second, despite the growing acknowledgement of this empirical work, there is still a tendency to recommend policy interventions, usually including a “research exemption.” Yet, given the research noted above, a strengthened research exemption seems unlikely to address the anti-commons or restricted access problems, especially diagnostic testing. And, such reforms need to be sensitive to the incentives that patents can provide for developing and distributing research technologies.
The combination of a lack of empirical evidence of problems and a mismatch between the problems and proposed solutions may explain why there has been little actual policy change [11]. In addition, our review of the lively policy debate and the limited empirical support for the claims that are driving that debate suggest that policy makers may be responding more to a high-profile anecdote or arguments with high face validity than they do to systematic data on the issues. However, we must acknowledge that one impact of these various high-profile policy debates may have been to sensitize both administrative and funding agencies (e.g., USPTO, NIH) and patent holders to the possible adverse consequences of overly liberal patent issuing and overly restrictive licensing practices. Whether this swing of the pendulum will help, hurt or have no effect on innovation and the progress of science remains an open question. Thus, further research on the exact mechanisms underlying these effects, as well as their net impacts, should be encouraged.
Supplementary Material
Fig. 1.
Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology. Caulfield T, Cook-Deegan RM, Kieff FS, Walsh J. Evidence and anecdotes: an analysis of human gene patenting controversies. Nat Biotechnol. 2006 Sept;24:1091-94. doi:10.1038/nbt0906-1091. Copyright 2006.
Available at http://www.nature.com/nbt/journal/v24/n9/full/nbt0906-1091.html
Fig. 2.
Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology. Caulfield T, Cook-Deegan RM, Kieff FS, Walsh J. Evidence and anecdotes: an analysis of human gene patenting controversies. Nat Biotechnol. 2006 Sept;24:1091-94. doi:10.1038/nbt0906-1091. Copyright 2006.
Available at http://www.nature.com/nbt/journal/v24/n9/full/nbt0906-1091.html
Acknowledgements
We would like to thank Lori Sheremeta, Michael Sharp, CJ Murdoch, Robyn Hyde-Lay, for the invaluable research assistance and Genome Alberta, AHFMR, the Stem Cell Network and AFMNet for the funding support and the National Human Genome Research Institute and U.S. Department of Energy (RC). We would also like to thank the all of the participants of the Genome Alberta Banff Patenting Workshop (May 2006) for the insightful comments.
Contributor Information
Timothy Caulfield, Canada Research Chair in Health Law and Policy Professor, Faculty of Law and Faculty of Medicine and Dentistry Research Director, Health Law Institute, University of Alberta.
Robert M. Cook-Deegan, Director, IGSP Center for Genome Ethics, Law & Policy Research Professor of Public Policy Studies, Sanford Institute of Public Policy Research Professor, Department of Internal Medicine, School of Medicine Duke University
F. Scott Kieff, Associate Professor, School of Law, Washington University Research Fellow, Hoover Institution, Stanford University.
John P. Walsh, Associate Professor, School of Public Policy Georgia Institute of Technology
References
- 1.Danish Council of Ethics . Patenting Human Genes and Stem Cells. Danish Council of Ethics; Copenhagen: 2004. [Google Scholar]
- 2.The Nuffield Council on Bioethics . The Ethics of Patenting DNA: A Discussion Paper. Nuffield Council of Bioethics; London: 2002. [Google Scholar]
- 3.Resnik DB. DNA Patents and Human Dignity. J.L. Med. & Ethics. 2001;29:152. doi: 10.1111/j.1748-720x.2001.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 4.House of Commons, Standing Committee on Health Government of Canada; Ottawa: Assisted Human Reproduction: Building Families. 2001
- 5.Williams-Jones B. History of a gene patent: tracing the development and application of commercial BRCA testing. Health Law J. 2002;10:123–146. [PubMed] [Google Scholar]
- 6.Kevles D, Berkowitz A. The Gene Patenting Controversy: A Convergence of Law, Economic Interests, and Ethics. Brooklyn L. Rev. 2001;67:233–248. [PubMed] [Google Scholar]
- 7.National Academy of Sciences . Reaping the Benefits of Genomic and Proteomic Research: Intellectual Property Rights, Innovation, and Public Health. National Academies Press; Washington, D.C.: 2005. [PubMed] [Google Scholar]
- 8.Heller M, Eisenberg R. Can patents deter innovation? The anticommons in biomedical research. Science. 1998;280:698–701. doi: 10.1126/science.280.5364.698. [DOI] [PubMed] [Google Scholar]
- 9.Merges RP, Nelson RR. On the Complex Economics of Patent Scope. Columbia L. Rev. 1990;90:839–916. [Google Scholar]
- 10.Scotchmer S. Standing on the Shoulders of Giants: Cumulative Research and the Patent Law. J. Econ. Perspect. 1991;5:29–41. [Google Scholar]
- 11.Caulfield T. Policy Conflicts: Gene Patents and Health Care in Canada. Community Genet. 2005;8:223–227. doi: 10.1159/000087959. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RR. The Market Economy and the Scientific Commons. Research Policy. 2004;33:455–471. [Google Scholar]
- 13.David PA. Can ‘Open Science’ be Protected from the Evolving Regime of IPR Protections? Journal of Theoretical and Institutional Economics. 2004;160:1–26. [Google Scholar]
- 14.Hahn L. Owning a piece of Jonathan. Chicago Magazine. 2003 May; [Google Scholar]
- 15.Ontario Ministry of Health Government of Ontario; Toronto: Genetics, Testing and Gene Patenting: Charting New Territory in Healthcare. 2002
- 16.National Academy of Sciences . A Patent System for the 21st Century. The National Academies Press; Washington, D.C.: 2004. [Google Scholar]
- 17.Cho M. Ethical and legal issues in the 21st century in preparing for the millennium. American Association for Clinical Chemistry (AACC) Newsletter. 1998:47–53. [Google Scholar]
- 18.Merz JF, Kriss AG, Leonard DGB, Cho MK. Diagnostic testing fails the test. Nature. 2002;415:577–579. doi: 10.1038/415577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benzie R. Ontario to defy U.S. Patents on cancer genes. The National Post. 2001 Sept 20;:15. A. [Google Scholar]
- 20.Jensen K, Murray F. Enhanced: Intellectual Property Landscape of the Human Genome. Science. 2005;310:239–240. doi: 10.1126/science.1120014. [DOI] [PubMed] [Google Scholar]
- 21.Walsh JP, Cohen WM, Arora A. Working through the patent problem. Science. 2003;299:1020. doi: 10.1126/science.299.5609.1021. [DOI] [PubMed] [Google Scholar]
- 22.Walsh JP, Cho C, Cohen WM. View from the Bench: Patents and Material Transfers. Science. 2005;309:2002–2003. doi: 10.1126/science.1115813. [DOI] [PubMed] [Google Scholar]
- 23.Nicol D, Nielsen J. Patents and Medical Biotechnology: An Empirical Analysis of Issues Facing the Australian Industry - Occasional Paper No. 6. Centre for Law & Genetics; Sandy Bay, Australia: 2003. [Google Scholar]
- 24.Nagaoka S. An empirical analysis of patenting and licensing practices of research tools from three perspectives. Presentation to OECD Conference on Research Use of Patented Inventions; Madrid. May 18-19, 2006; 2006. http://www.oecd.org/dataoecd/20/54/36816178.pdf. [Google Scholar]
- 25.Straus J. Genetic Inventions and Patents — A German Empirical Study. presentation to the BMBF & OECD Workshop entitled “Genetic Inventions, Intellectual Property Rights and Licensing Practices”; Berlin. January 24-25, 2002; online: OECD <http:// http://www.oecd.org/dataoecd/36/22/1817995.pdf>. [Google Scholar]
- 26.Stern S, Murray FE. Do Formal Intellectual Property Rights Hinder the Free Flow of Scientific Knowledge? An Empirical Test of the Anti-Commons Hypothesis: NBER Working Paper No. W11465. 2005.
- 27.Sampat B. Unpublished manuscript. Columbia University, Department of Health Policy and Management; 2005. Do Academic Genomic Patents Curtail Downstream Research? [Google Scholar]
- 28.Cohen J. Chiron Stakes out its Territory. Science. 1999;285:28. [Google Scholar]
- 29.Walsh JP, Cohen WM, Arora A. Patenting and licensing of research tools and biomedical innovation. In: Cohen WM, Merrill S, editors. Patents in the Knowledge-Based Economy. NAP; Washington, DC: 2003. [Google Scholar]
- 30.Cho MK, et al. Effects of Patents and Licenses on the Provision of Clinical Genetic Testing Services. J. Mol. Diag. 2003;5:3–8. doi: 10.1016/S1525-1578(10)60444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumenthal D, et al. Withholding Research Results in Academic Life Science: Evidence From a National Survey of Faculty. JAMA. 1997;277:1224. [PubMed] [Google Scholar]
- 32.Campbell EG, et al. Data withholding in academic genetics. JAMA. 2002;287:473–480. doi: 10.1001/jama.287.4.473. [DOI] [PubMed] [Google Scholar]
- 33.Walsh JP, Hong W. Secrecy is increasing in step with competition. Nature. 2003;422:801–802. doi: 10.1038/422801c. [DOI] [PubMed] [Google Scholar]
- 34.Grushcow J. Measuring Secrecy: A Cost of the Patent System Revealed. Journal of Legal Studies. 2004;33:59–84. [Google Scholar]
- 35.Vogeli C, et al. Data Withholding and the Next Generation of Scientists: Results of a National Survey. Acad. Med. 2006;81(2):128–136. doi: 10.1097/00001888-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Blumenthal D, et al. Data Withholding in Genetics and the Other Life Sciences: Prevalences and Predictors. Acad. Med. 2006;81(2):137. doi: 10.1097/00001888-200602000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Cohen WM, Florida R, Goe R. University-Industry Research Centers in the United States. Report to the Ford Foundation. Carnegie Mellon University; Pittsburgh: 1994. [Google Scholar]
- 38.Bekelman JE, Li Y, Gross GP. Scope and Impact of Financial Conflicts of Interst in Biomedical Research. JAMA. 2003;289(4):454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta P, Maskin E. The simple economics of research portfolios. Economic Journal. 1987;97:581–595. [Google Scholar]
- 40.Van Overwalle G, Van Zimmeren E. Reshaping Belgian Patent Law: The Revision of the Research Exemption and the Introduction of a Compulsory License for Public Health. Chizaiken Forum. 2006;64:42–49. [Google Scholar]
- 41.Kieff FS. Facilitating Scientific Research: Intellectual Property Rights and the Norms of Science - A Response to Rai & Eisenberg. Northwestern University L. Rev. 2001;95:691–706. [PubMed] [Google Scholar]
- 42.Pressman, et al. Patenting and Licensing Practices for DNA-Based Patents at U.S. Academic Institutions. Nat Biotech. 2005;24:31–39. doi: 10.1038/nbt0106-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg R. Patent Swords and Shields. Science. 2003;299:5609, 1018–9. doi: 10.1126/science.1081790. [DOI] [PubMed] [Google Scholar]
- 44.Rohrbaugh MI. NIH Best Practices Guidelines for Licensing Genomic Inventions. Fed. Regist. 2005;70(68):18413–18415. [Google Scholar]
- 45.Grimm D. A Mouse for Every Gene. Science. 2006;312:1862–1866. doi: 10.1126/science.312.5782.1862. [DOI] [PubMed] [Google Scholar]
- 46.Ravetz JR. Scientific Knowledge and its Social Problems. Oxford University Press; New York: 1973. [Google Scholar]
- 47.Canadian Biotechnology Advisory Committee . Human Genetic Materials, Intellectual Property and the Health Sector. CBAC; Ottawa: 2006. [Google Scholar]
- 48.World Health Organization . Public Health Innovation and Intellectual Property Rights. WHO Press; Geneva, Switzerland: 2006. [Google Scholar]
- 49.Australian Government Advisory Committee on Intellectual Property ACIP; Sydney: Patents and Experimental Use. 2005
- 50.Canadian Biotechnology Advisory Committee Expert Working Party on Human Genetic Materials, Intellectual Property and the Health Sector . Human Genetics Materials: Making Canada’s Intellectual Property Regime Work for the Health of Canadians. CBAC; Ottawa: 2005. [Google Scholar]
- 51.National Research Council Committee on Intellectual Property Rights in Genomic and Protein Research and Innovation . Reaping the Benefits of Genomic and Proteomic Research: Intellectual Property Rights, Innovation and Public Health. National Academies Press; Washington, D.C.: 2005. [PubMed] [Google Scholar]
- 52.World Health Organization . Genetics, genomics and the patenting of DNA: Review of potential implications for health in developing countries. WHO Press; Geneva, Switzerland: 2005. [Google Scholar]
- 53.Australian Law Reform Commission . Report 99 - Genes and Ingenuity: Gene Patenting and Human Health. SOS Printing Group; Sydney: 2004. [Google Scholar]
- 54.Federal Trade Commission . To Promote Innovation: The Proper Balance of Competition and Patent Law and Policy. FTC; Washington, D.C.: 2003. [Google Scholar]
- 55.The Royal Society . Keeping Science Open: The Effects of Intellectual Property Policy on the Conduct of Science. TRS; London: 2003. [Google Scholar]
- 56.Public Health Genetics Unit, Cambridge . Intellectual Property Rights (IPRs) and Genetics. PHGU; Cambridge: 2003. [Google Scholar]
- 57.Organization for Economic Co-operation and Development . Genetic Inventions, Intellectual Property Rights & Licensing Practices. OECD Publications; Paris: 2002. [Google Scholar]
- 58.Canadian Biotechnology Advisory Committee . Patenting of Higher Life Forms and Related Issues. CBAC; Ottawa: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


