Abstract
A chelator and a pro-chelator that can be activated by H2O2 and subsequently sequesters iron and attenuates the Fenton reaction have been developed; both molecules are fluorescent excitable by visible light, and H2O2-activation, as well as iron-chelation, induces remarkable changes in fluorescence.
Abnormal accumulation of redox metals (e.g., iron and copper) in certain tissues in the body and the metal-promoted Fenton reactions (eqn (1)), which yield highly reactive and deleterious oxidizing species, have been implicated in the pathogenesis of Parkinson’s disease (PD), Alzheimer’s disease (AD), atherosclerosis, hemochromatosis, liver damage, diabetes and cancer, etc.1 H2O2, one of the key reactants in the Fenton reactions, is also one of the major endogenous reactive oxygen species (ROS).1 H2O2 is essential for cellular activities (e.g., as a second messenger) but can be extremely toxic to cells at high concentrations.1 The production of H2O2 is significantly increased, but its elimination mechanisms become impaired, in patients with neurodegenerative disease.1d,e Elevated levels of H2O2 and the Fenton reactions contribute to oxidative stress and neurodegeneration. Anti-Fenton agents offer a promise in treatment and prevention of these diseases. We and others have been developing Fenton antidotes via an H2O2-triggered prochelator activation and a subsequent metal caging strategy.2,3 As the process involves consumption of H2O2 by the prochelator, subsequent sequestration of the metal and prevention Fenton reactions, we called it an ‘‘anti-Fenton reaction’’.2 The advantage of this strategy is that chelation can only be triggered by toxic levels of H2O2 and thus will not interfere with healthy metal homeostasis. However, direct measurement of the efficacy and cellular fate of these novel compounds, currently being tested in living systems, is hindered by the lack of an easily trackable marker.
| (1) |
To overcome these drawbacks, we have developed our second generation prochelator, 2-boronobenzaldehyde benzoyl-hydrazone, with an anthracene fluorophore tag (SBH-B-AN), and the corresponding active chelator with the same tag (SBH-AN). Both the fluorophore-tagged prochelator and chelator give a strong fluorescent light upon excitation by UV or visible light (400 nm), thus providing an easy trace of their cellular uptake and distribution via a fluorescent microscope. Furthermore, the activation of the prochelator by H2O2 induces a new fluorescence peak centred at 480 nm, while the iron chelation induces a marked quenching of the fluorescence, thus allowing the anti-Fenton process to be monitored in realtime by fluorescence techniques.
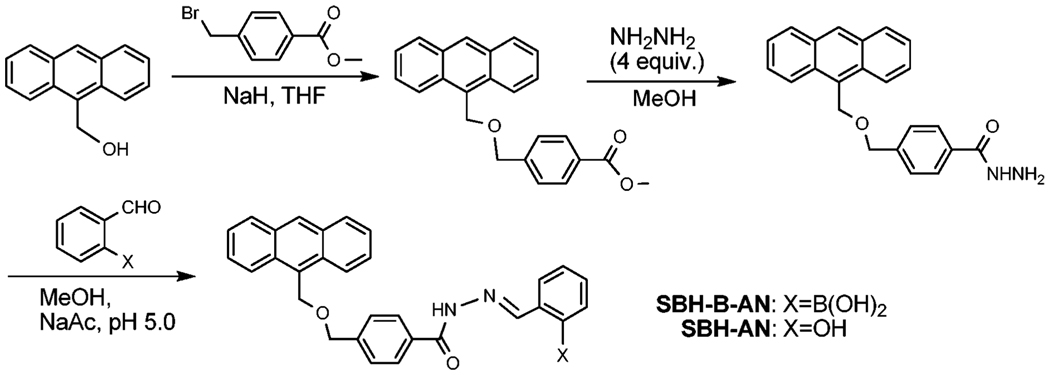
SBH-AN and prochelotor SBH-B-AN were synthesized by a 3-step procedure (Scheme 1 and ESI†). The iron-binding moiety salicylaldehyde benzoylhydrazone (SBH) was chosen because of its comparable iron-binding affinity to the SIH analogues,4 a better lipophilicity (therefore potentially higher cell penetration) and a slower hydrolysis rate under physiological relevant conditions.5
Scheme 1.
Synthesis and abbreviations of the compounds.
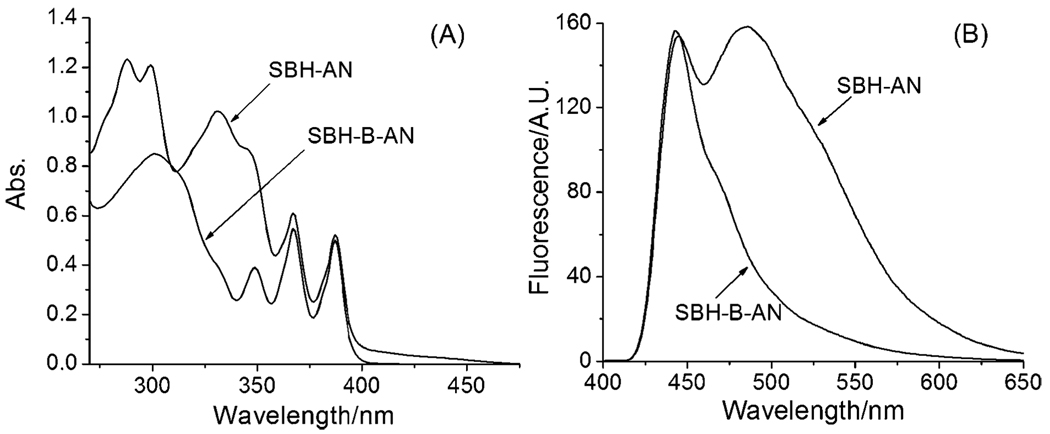
First, we examined the photophysical properties of SBH-AN and the prochelotor SBH-B-AN at pH 7.30. SBH-AN displays a spectrum similar to that of a combination of the SBH moiety and the anthracene moiety (Fig. 1(A)), assignable to the π–π* transitions.6,7 Replacing the –OH group with a boronic acid group in SBH-B-AN significantly changes the absorption of the SBH moiety in the UV region (270–330 nm) and the visible region (390–470 nm), while little change is observable for the anthracene moiety. Upon excitation by UV light (≤360 nm), both compounds showed a similar photoluminescence response and give a blue fluorescence, due to the anthracene fluorophore.7 Interestingly, upon excitation at 400 nm (the phenolate absorption), different emission profiles were observed: the prochelator SBH-B-AN showed a single fluorescence band at 440 nm while the active chelator SBH-AN displayed a strong additional fluorescence band at ~480 nm (Fig. 1(B)). This characteristic allows for realtime fluorescent monitoring the response of SBH-B-AN to H2O2.
Fig. 1.
(A) UV-vis spectra and (B) fluorescence spectra (λex = 400 nm, with a 415 nm cutoff filter) of 10 µM SBH-B-AN and SBH-AN in DMF/KPB (1 : 1), pH 7.30, at 298 K.
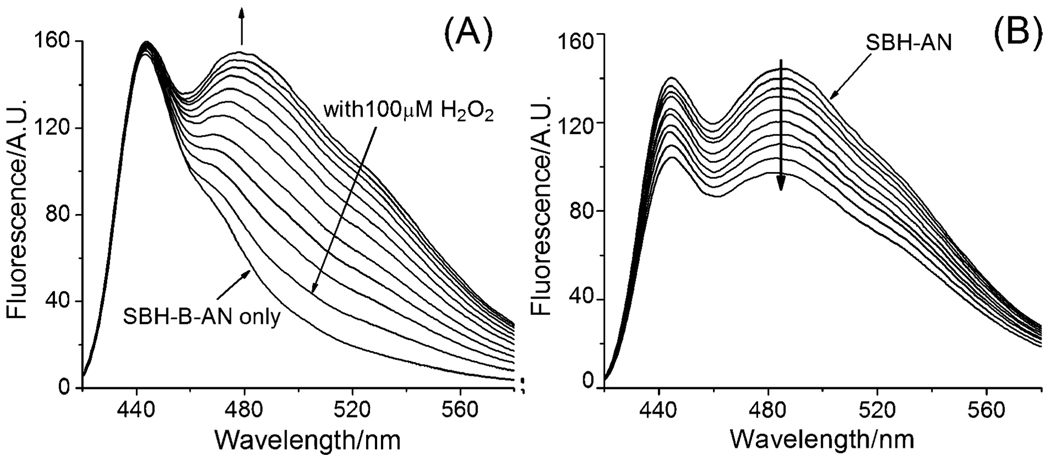
Second, we examined the spectroscopic changes when prochelotor SBH-B-AN reacted with H2O2. Upon adding H2O2 to SBH-B-AN in DMF (N,N-dimethylformide)–KPB (potassium phosphate buffer) (20 mM, pH 7.3, v/v, 1 : 1), no change occurred to the fluorescence peak at 440 nm, while a new peak emerged at 480 nm and grew over time (Fig. 2(A)). The final fluorescence spectrum of the mixture almost matches that of SBH-AN, implying a conversion of SBH-B-AN to SBH-AN. A 5.5-fold increase in intensity at 520 nm was observed.
Fig. 2.
(A) Fluorescence spectra (λex = 400 nm) of the time course of the reactions of 10 µM SBH-B-AN with 100 µM H2O2 (0 to 120 min); (B) fluorescence (λex = 400 nm) titration of 10 µM SBH-AN with FeIII in 1 µM increments in DMF–KPB (1 : 1), pH 7.30, at 298 K.
To further characterize the chemistry of the conversion, the reaction was followed by 1H NMR in dimethylsulfoxide (DMSO). After incubating SBH-B-AN with H2O2, 1H NMR peaks (Fig. S1†) for SBH-B-AN (δ11.05(s), –NH–; δ8.76(s), –CH═N–; δ8.50(s), –B(OH)2) gradually decreased in intensity, while the peaks corresponding to SBH-AN (δ12.08(s) –NH–; 11.30(s), –OH–; 8.64(s), –CH═N–) appeared simultaneously and increased in intensity with time. The peaks (δ7.44–7.37(m), 7.30(t) and 6.95–6.90(m), salicyl-aldehyde) also underwent similar conversions. Meanwhile, the peak for boric acid, the deprotection product of boronic acid by H2O2, appeared at δ6.55(s). No 1H NMR change was observed for the anthracene moiety due to its distance from the reaction site. In ~4 h, SBH-B-AN had been cleanly converted to SBH-AN with no intermediate formed, as indicated by the 1H NMR spectra. The conversion from SBH-B-AN to SBH-AN was also confirmed by UV-vis difference spectra in DMF–KPB (20 mM, pH 7.3, v/v, 1 : 1) (Fig. S2†). With a ten-fold excess of H2O2, the conversion reaction is pseudo-first order with an apparent rate constant (Kobs) of 4.37 × 10−4 s−1.
Third, we investigated whether SBH-AN and SBH-B-AN bind iron under physiological relevant conditions and whether the chelation can be detected by fluorescent techniques. Since metal binding to SBH induces characteristic changes to the absorption bands,6,8 UV-vis titration was performed first to probe the binding between SBH-AN and FeIII in potassium phosphate buffer (KPB), pH 7.30. Although the changes in spectra were largely obstructed by the strong absorption from the anthracene moiety in the UV region, evident spectroscopic changes due to iron-binding were observed (Fig. S3†): a decrease in intensity around 330 nm with simultaneous increases in intensity around 370 nm and in the visible region (400–500 nm). These changes match those reported for Fe–SBH complexation,8 suggesting the formation of specific SBH-AN–Fe complexes. However, when a similar titration was performed with the pro-chelator, no change in absorption was observed in the UV-vis region (Fig. S4†), indicating no interaction between the pro-chelator with FeIII under the conditions applied. However, upon the addition of H2O2 to the mixture, characteristic absorption bands for Fe-binding emerged and increased in intensity (Fig. S5†), suggesting the formation of SBH-AN–Fe complexes. The spectroscopic changes indicate that H2O2 “activates” SBH-B-AN to SBH-AN, which subsequently chelates iron (Fig. S6†). Interestingly, iron-binding also induces marked fluorescent changes. Titration of the chelator SBH-AN with FeIII induced a quenching of fluorescence for both the 440 nm and 480 nm bands (Fig. 2(B)), presumably due to metal-coordination-induced molecular collisional quenching. In contrast, similar titration experiments with the prochelator SBH-B-AN induced little fluorescent change (Fig. S7†).
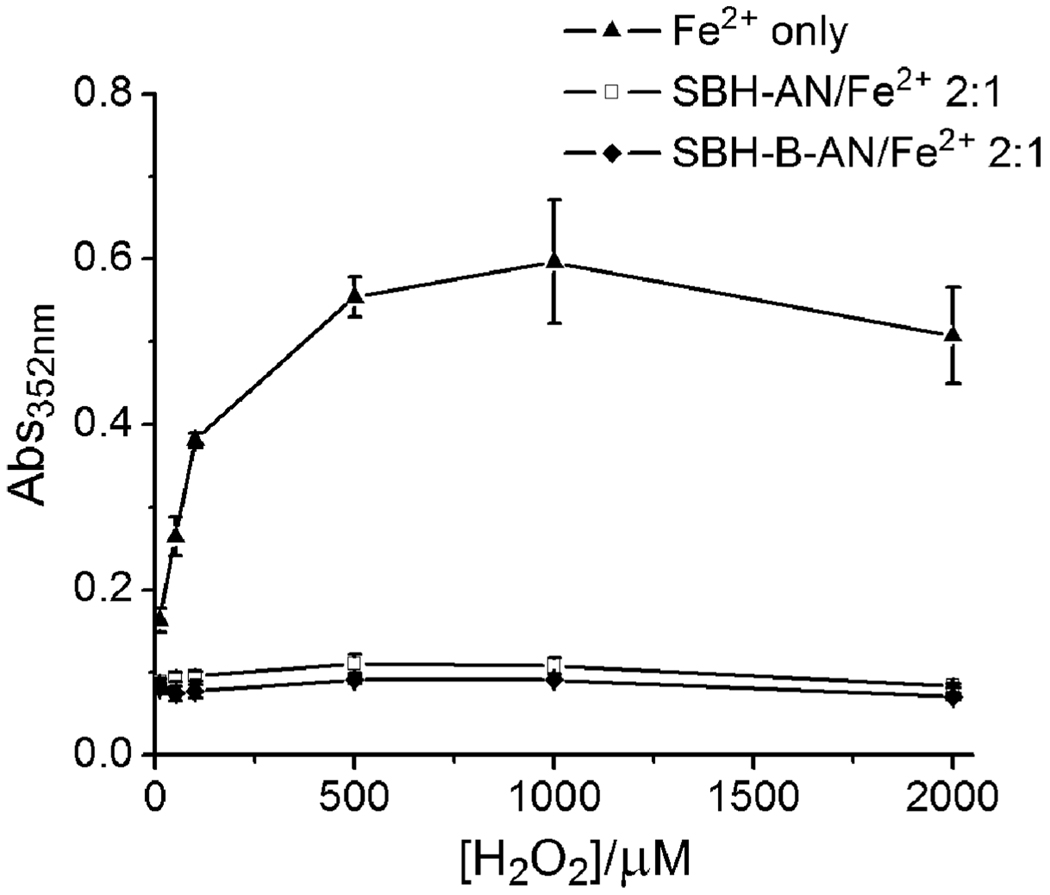
Finally, we tested the anti-Fenton activities of SBH-AN and SBH-B-AN using a 2-deoxyribose degradation assay.2 As shown in Fig. 3, both SBH-B-AN and SBH-AN at a 2 : 1 chelator :Fe ratio significantly inhibited hydroxyl radical-induced 2-deoxyribose degradation, indicating that SBH-B-AN and SBH-AN efficiently attenuate the Fenton reaction under physiologically relevant conditions.
Fig. 3.
Effect of SBH-AN and SBH-B-AN on the oxidative degradation of 2-deoxyribose promoted by FeII (20 µM) in the presence of H2O2 and hydroascorbate (5 mM) in 20 mM KPB, pH 7.20.
In summary, we have developed a novel fluorescent pro-chelator, SBH-B-AN, and an active chelator SBH-AN, both excitable by non-damaging visible light. SBH-B-AN can sense H2O2 and be converted to SBH-AN, which subsequently sequesters iron and attenuates the Fenton reaction. The H2O2-activation and the iron-chelation processes produce specific fluorescent changes. These novel properties may be utilized in the realtime monitoring of cellular oxidative stress status (H2O2 levels) and in tracking their anti-Fenton efficacy and metabolism in living systems by fluorescent techniques.
Supplementary Material
Acknowledgments
Acknowledgement is made to the University of Massachusetts, Dartmouth, the American Parkinson Disease Association, National Parkinson Foundation and the National Institutes of Health for funding. This publication was made possible by Grant Number 1 R21 AT002743-02 from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
Footnotes
Electronic supplementary information (ESI) available: Details on synthesis, absorption and fluorescence spectra and the deoxyribose assay.
Notes and references
- 1.(a) Crichton R. Inorganic Biochemistry of Iron Metabolism. West Sussex: John Wiley & Sons, Ltd; 2001. [Google Scholar]; (b) Barnham KJ, Masters CL, Bush AI. Nat. Rev. Drug Discovery. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]; (c) Perez CA, Tong Y, Guo M. Curr. Bioactive Comp. 2008;4:150–158. doi: 10.2174/157340708786305952. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M, Lubec G. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]; (e) Tabner BJ, Turnbull S, El-Agnaf OMA, Allsop D. Free Radical Biol. Med. 2002;32:1076–1083. doi: 10.1016/s0891-5849(02)00801-8. [DOI] [PubMed] [Google Scholar]
- 2.Wei Y, Guo M. Angew. Chem., Int. Ed. 2007;46:4722–4725. doi: 10.1002/anie.200604859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charkoudian LK, Pham DM, Franz KJ. J. Am. Chem. Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 4.Chaston TB, Richardson DR. Am. J. Hematol. 2003;73:200–210. doi: 10.1002/ajh.10348. [DOI] [PubMed] [Google Scholar]
- 5.Buss JL, Neuzil J, Gellert N, Weber C, Ponka P. Biochem. Pharmacol. 2003;65:161–172. doi: 10.1016/s0006-2952(02)01512-5. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y-H, Lu Y-W, Wu C-L, Shao Q, Chen X-L, Bimbong RNB. Spectrochim. Acta, Part A. 2006;65:695–701. doi: 10.1016/j.saa.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima A. J. Lumin. 1977;15:277–282. [Google Scholar]
- 8.Dubois JE, Fakhrayan H, Doucet JP, El Hage Chahine JM. Inorg. Chem. 1992;31:853–859. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.