Abstract
Background
Epidemiological studies have shown gender differences in the incidence of congestive heart failure (CHF); however, the role of estrogen in CHF is not known. We hypothesize that estrogen prevents cardiomyocyte apoptosis and the development of CHF.
Methods and Results
17β-Estradiol (E2, 0.5 mg/60-day release) or placebo pellet was implanted subcutaneously into male Gαq transgenic (Gq) mice. After 8 weeks, E2 treatment decreased the extent of cardiac hypertrophy and dilation and improved contractility in Gq mice. E2 treatment also attenuated nicotinamide adenine dinucleotide phosphate oxidase activity and superoxide anion production via downregulation of Rac1. This correlated with reduced apoptosis in cardiomyocytes of Gq mice. The antioxidative properties of E2 were also associated with increased expression of thioredoxin (Trx), Trx reductases, and Trx reductase activity in the hearts of Gq mice. Furthermore, the activation of apoptosis signal-regulating kinase 1 and its downstream effectors, c-Jun N-terminal kinase and p38 mitogen-activated protein kinase, in the hearts of Gq mice was reduced by long-term E2 treatment. Indeed, E2 (10 nmol/L)-treated cardiomyocytes were much more resistant to angiotensin II–induced apoptosis. These antiapoptotic and cardioprotective effects of E2 were blocked by an estrogen receptor antagonist (ICI 182,780) and by a Trx reductase inhibitor (azelaic acid).
Conclusions
These findings indicate that long-term E2 treatment improves CHF by antioxidative mechanisms that involve the upregulation of Trx and inhibition of Rac1-mediated attenuated nicotinamide adenine dinucleotide phosphate oxidase activity and apoptosis signal-regulating kinase 1 /c-Jun N-terminal kinase/p38 mitogen-activated protein kinase–mediated apoptosis. These results suggest that estrogen may be a useful adjunctive therapy for patients with CHF.
Keywords: apoptosis, antioxidants, heart failure, hormones
Most of the beneficial effects of estrogen in the cardiovascular system are attributed to its vascular protective effects.1 Estrogen improves endothelial function, inhibits smooth muscle proliferation, and prevents the development of atherosclerosis.2–4 The effect of estrogen on the heart, however, is not well understood. Although estrogen has been shown to inhibit cardiac hypertrophy,5 its effect on the subsequent development of congestive heart failure (CHF) remains to be determined.
The development of CHF is marked by increased cardiomyocyte oxidative stress.6 The production of reactive oxygen species (ROS) is increased in the failing heart and correlates with progression to heart failure.7 The source of ROS remains controversial, because some studies suggest they originate from the mitochondria,8 whereas others suggest they are generated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.9 Regardless of the source, increased myocardial oxidative stress is correlated with cardiac hypertrophy10 and worsening heart failure.11 Although estrogen improves the defense against oxidative stress in endothelial cells and neurons, it is not known whether estrogen could prevent the development of CHF through an antioxidative mechanism in cardiomyocytes.12,13
The thioredoxin (Trx) system regulates the levels of intracellular ROS and modulates intracellular oxidative states, which may be important for cellular function, survival, and death.14 Trx exists either in a reduced form, Trx-(SH)2, or in an oxidized form, Trx-S2. Trx-S2 is inactive but is converted to the active intracellular antioxidant, Trx-(SH)2, by Trx reductase (TrxRD). The Trx system is functional in several subcellular compartments, including the cytosol (Trx1 and TrxRD1), mitochondria (Trx2 and TrxRD2), and endoplasmic reticulum (TrxRD3), thus making it one of the most important intracellular antioxidant defense mechanisms. Although the Trx system is upregulated by 17β-estradiol (E2) in the uterus and endothelial cells,15,16 its role in CHF is not known. To determine the effects of estrogen on CHF, we studied the effects of E2 in Gαq transgenic (Gq) mice, which have been previously shown to spontaneously develop CHF.17
Methods
Animal Protocol
We used male Gq mice (FVB background)17 containing 40 copies of the transgene and male FVB littermates (wild type). Animals were subcutaneously implanted with 60-day release pellets (Innovative Research of America, Sarasota, Fla) containing either vehicle (placebo) or E2 (0.5 mg) at the age of 6 to 7 weeks and euthanized at the age of 14 to 15 weeks (n=12 in each group). In separate experiments, 8-week-old wild-type male mice were infused with vehicle or angiotensin II (Ang II, 400 ng · kg−1 · min−1) with a miniosmotic pump (ALZET model 2004; DURECT Corp, Cupertino, Calif) with placebo or E2 tablets (0.5 mg). All experimental procedures on animals were performed with protocols approved by the Standing Committee on Animal Welfare and Protection of Harvard Medical School.
Blood Pressure and Echocardiographic Measurements
Before and after 2, 4, and 8 weeks of pellet implantation, systolic blood pressure and heart rate were measured by the tail-cuff method. Transthoracic echocardiography was performed with 15-MHz pulsed-wave Doppler echocardiography (SONOS 4500; Hewlett-Packard, Andover, Mass) as described previously.18
Histopathological Analysis
Eight weeks after pellet implantation, the heart was obtained for histopathological analysis. Ten sections or 40 vessels were examined in each heart, and results obtained from 8 hearts in each group were averaged (8 to 10 regions per heart). See supplementary materials in the online-only Data Supplement.
Western, Northern, and RNA Dot-Blot Analysis
Western, Northern, and RNA dot blot analysis were performed as described previously.10 See the online-only Data Supplement for supplementary materials.
Apoptosis Signal-Regulating Kinase 1 Immune Complex Kinase Assay
Apoptosis signal-regulating kinase 1 (ASK1) immune complex kinase assay was performed as described previously.10 See the online-only Data Supplement for supplementary materials.
Measurements of NADPH Oxidase Activation
NADPH oxidase activity in the left ventricle was determined with lucigenin chemiluminescence as described previously.19 See the online-only Data Supplement for supplementary materials.
Measurement of Superoxide (O2 −) Production
The production of O2 − in the left ventricle was measured by superoxide dismutase–inhibitable reduction of ferricytochrome c as described previously.10 To evaluate the production of tissue ROS, fresh frozen left ventricular myocardium (10-µm slices) was incubated for 1 hour at 37°C with dihydroethidium (Molecular Probes, Carlsbad, Calif; 2 µmol/L) as described previously.20 See the online-only Data Supplement for supplementary materials.
TrxRD Activity Assay
A Trx reductase assay kit was used to measure TrxRD activity in the mitochondrial or cytosolic fraction (Sigma-Aldrich, St. Louis, Mo). See the online-only Data Supplement for supplementary materials.
In Vivo Terminal dUTP Nick End-Labeling Assay
A kit (In Situ Cell Death Detection Kit, Fluorescein; Roche Applied Science, Indianapolis, Ind) was used to assess apoptotic cells in the heart. See online-only Data Supplement for supplementary materials.
Isolation of Cardiomyocytes and In Vitro Assay
Rat neonatal cardiomyocytes were prepared as described previously. 19 The number of apoptotic cardiomyocytes was counted by the terminal dUTP nick end-labeling (TUNEL) method. At least 2000 cells per each group were counted. See the online-only Data Supplement for supplementary materials.
Statistical Analysis
Values are shown as mean±SD. All parameters were evaluated with the Mann-Whitney U test or Kruskal-Wallis test when multiple mean comparisons were required. A probability value <0.05 was considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written
Results
E2 Inhibits the Progression of Heart Failure in Genetic Hypertrophy
Pathological examination of the hearts of Gq mice showed substantial enlargement and dilation of all 4 cardiac chambers (Figure 1A, top). Cardiac histology (left ventricular free wall) showed mild fibrosis and hypertrophied myocytes in hearts from Gq mice (Figure 1A, middle). Myocyte dimensions as determined by fluorescein-tagged, wheat-germ agglutinin–labeled Gq left ventricles were larger than those of nontransgenic FVB siblings (Figure 1A, bottom), which indicates cardiomyocyte hypertrophy. Treatment with E2 prevented these morphological changes and cardiomyocyte hypertrophy in hearts from Gq mice. In Gq mice treated with placebo pellets, the expression of cardiac fetal genes such as brain natriuretic peptide, α-skeletal actin, and myosin light chain-2 ventricular isoform were elevated compared with basal levels (Figure 1B). Treatment with E2 decreased the expression of these fetal genes to basal levels, which suggests an antihypertrophic effect of E2.
Figure 1. E2 inhibits the progression of heart failure in Gq transgenic mice.
A, Representative photomicrographs of hearts from FVB and Gq mice at 8 weeks after placebo or E2 pellet implantation (top; bar=2 mm). Elastica-van Gieson stain of myocardial cross sections (middle; bar=100 µm). Wheat-germ agglutinin staining of myocardial cross sections (bottom; bar=100 µm). B, RNA dot blot analysis of cardiac gene fetal expression. BNP indicates brain natriuretic factor; MLC-2v, myosin light chain-2 ventricular isoform; and α-SK, α-skeletal actin. Quantitative analysis of expression of each gene was normalized to GAPDH expression (n=8 in each group). Results are presented as mean±SD. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice.
The degree of cardiac hypertrophy in Gq mice was also assessed by the ratio of heart weight to tibial length. Although cardiomyocytes of Gq mice were larger than those of FVB mice, the ratio of heart weight to tibial length of Gq mice was not different from that of FVB mice (Table). The extent of cardiac fibrosis and perivascular fibrosis in Gq mice was substantially reduced by estrogen. The left ventricular end-diastolic dimension as measured by echocardiography was larger in Gq mice than in FVB mice. Treatment of Gq mice with E2 decreased left ventricular end-diastolic dimension by 13% compared with placebo treatment. The percent fractional shortening was also reduced in Gq mice compared with FVB mice, consistent with impaired cardiac contractility in Gq mice. Treatment of Gq mice with E2 improved percent fractional shortening, which indicates that E2 attenuates the impaired contractility and dilation of hearts from Gq mice. Similar findings were observed with estrogen on Ang II–induced cardiac fibrosis, although in contrast to Gq mice, heart weight/tibial length ratio was reduced by estrogen (see online-only Data Supplement).
Histopathological Parameters and Echocardiographic Data
| FVB |
Gq |
|||
|---|---|---|---|---|
| Mouse and Treatment |
Placebo (n=9) |
Estrogen (n=8) |
Placebo (n=8) |
Estrogen (n=9) |
| Body weight, g | 35.4±2.7 | 31.7±1.9* | 34.4±2.7 | 31.2±2.8† |
| HW, mg | 156±11 | 130±11* | 158±21 | 140±19† |
| HW/TL, mg/mm | 6.6±0.5 | 5.8±0.5* | 6.8±0.8 | 6.3±0.5 |
| CSA, µm2 | 225±60 | 196±48 | 255±77* | 234±45† |
| Fibrosis, % | 0.8±0.1 | 0.8±0.2 | 4.5±1.1* | 1.7±0.4† |
| Echocardiography | ||||
| LVEDD, mm | 3.49±0.06 | 3.19±0.04* | 4.41±0.27* | 3.85±0.11† |
| LVESD, mm | 1.64±0.20 | 1.51±0.16* | 3.29±0.29* | 2.35±0.06† |
| IVS, mm | 0.47±0.05 | 0.45±0.04 | 0.38±0.03* | 0.47±0.05† |
| LV mass, mg | 81.5±12.1 | 66.1±4.0* | 102.7±13.6* | 97.6±12.5 |
| FS, % | 52.9±5.5 | 52.6±5.4 | 25.5±2.6* | 38.9±1.5† |
HW indicates heart weight; TL, tibial length; CSA, myocardial cross-sectional area; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; IVS, intraventricular septal thickness; LV, left ventricular; and FS, fractional shortening.
HW and TL were measured, and the ratio of HW/TL was calculated. The percentage of LV fractional shortening (FS, %) was calculated as [(LVEDD–LVESD)/LVEDD]×100 (%). LV mass was calculated as 1.855×[(LVEDD+PW+ IVS)3–(LVEDD)] (mg), where PW indicates posterior wall thickness. Results are mean±SD; n=8 or 9 mice in each group.
P<0.05 compared with placebo-treated FVB mice.
P<0.05 compared with placebo-treated Gq mice.
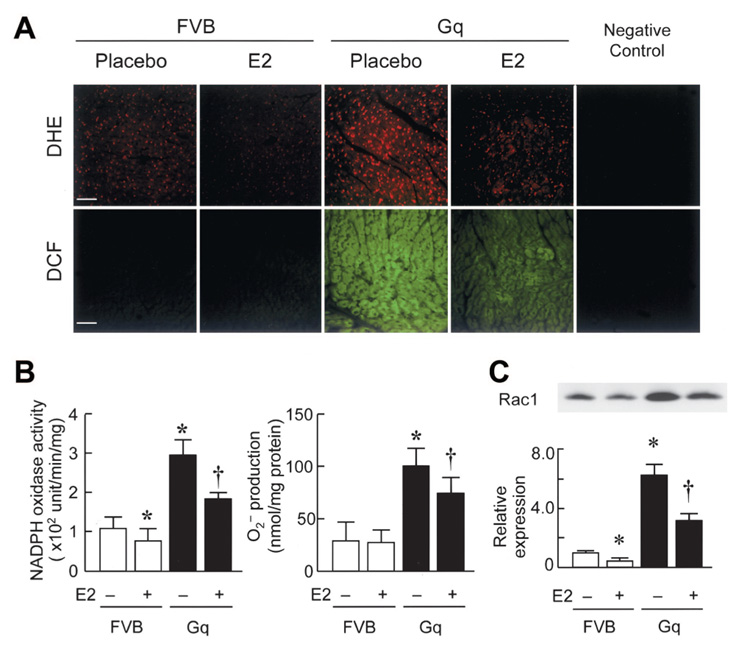
E2 Inhibits Myocardial Oxidative Stress in Gq Mice
The generation of ROS and peroxide-derived oxygen radicals was determined qualitatively in fresh frozen heart sections by histochemical staining with dihydroethidium and 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF), respectively. Compared with hearts from litter mates (control FVB mice), hearts from Gq mice showed greater dihydroethidium and DCF staining (Figure 2A). This increased staining was greatly attenuated in the hearts of Gq mice treated with E2, which suggests that estrogen inhibits the generation of myocardial ROS. To quantify the level of myocardial oxidative stress, we performed a lucigenin chemiluminescence assay for NADPH oxidase activity and superoxide dismutase–inhibitable ferricytochrome c reduction assay for superoxide anion (O2 ⨪) production on hearts from placebo- and E2-treated Gq mice. Compared with FVB mice, Gq mice showed a 3.5-fold increase in myocardial NADPH oxidase activity and O2 ⨪ production, which was reduced by E2 treatment (NADPH oxidase: 184±17 versus 290±39 U · min−1 · mg−1; O2⨪ production: 74±16 versus 101±15 nmol/mg; P<0.05 for both, n=10 in each group; Figure 2B). Because Rac1 GTPase is essential for activation of the NADPH oxidase complex, we examined whether Rac1 expression is altered in Gq mice. Rac1 expression was higher in placebo-treated Gq mice than in FVB mice (Figure 2C). Treatment with E2 decreased Rac1 expression in hearts from both FVB and Gq mice. These results indicate that E2 attenuates myocardial oxidative stress in part by inhibiting NADPH oxidase activity and O2 ⨪ production.
Figure 2. E2 inhibits myocardial oxidative stress in Gq transgenic mice.
A, Fresh frozen heart tissues were stained with dihydroethidium (DHE, red fluorescence; upper panel) or 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF, green fluorescence; lower panel). Bar=50 µm. B, NADPH oxidase activity as determined by lucigenin chemiluminescence assay and O2 − production as determined by superoxide anion dismutase–inhibitable ferricytochrome c reduction assay in heart extracts of FVB and Gq mice at 8 weeks after placebo or E2 pellet implantation. Results are mean±SD values; n=10 mice in each group. C, Expression of Rac1 in FVB and Gq mice with or without E2 treatment. Expression levels were expressed relative to those of placebo-treated FVB mice. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice.
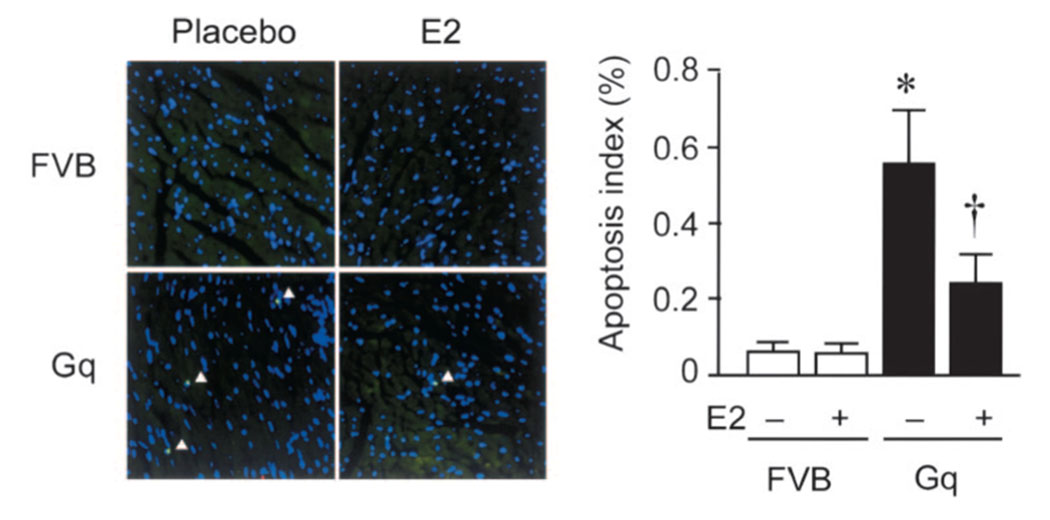
E2 Prevents Cardiomyocyte Apoptosis
Apoptosis of cardiomyocytes was quantified by TUNEL staining (Figure 3). The mean number of TUNEL-positive cardiomyocytes per total number of cardiomyocytes (Hoechst-stained nuclei) in placebo-treated FVB mice was 0.06±0.02%. In contrast, the percentage of TUNEL-positive cardiomyocytes was substantially higher in placebo-treated Gq mice (0.57±0.15%, P<0.05 versus FVB). Treatment of Gq mice with E2 resulted in a reduction in the percentage of TUNEL-positive cardiomyocytes (0.28±0.08%, P<0.05 versus Gq mice). These results indicate that E2 prevents cardiomyocyte apoptosis in Gq mice.
Figure 3. E2 prevents cardiomyocyte apoptosis in Gq transgenic mice.
Cardiomyocyte apoptosis was assessed in hearts from placebo- or E2-treated FVB and Gq mice. Apoptotic cells were detected by TUNEL staining and counterstained with Hoechst 33258 to detect nuclei in the same fields. Arrowheads indicate apoptotic cells. Results are mean±SD values; n=10 mice in each group. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice.
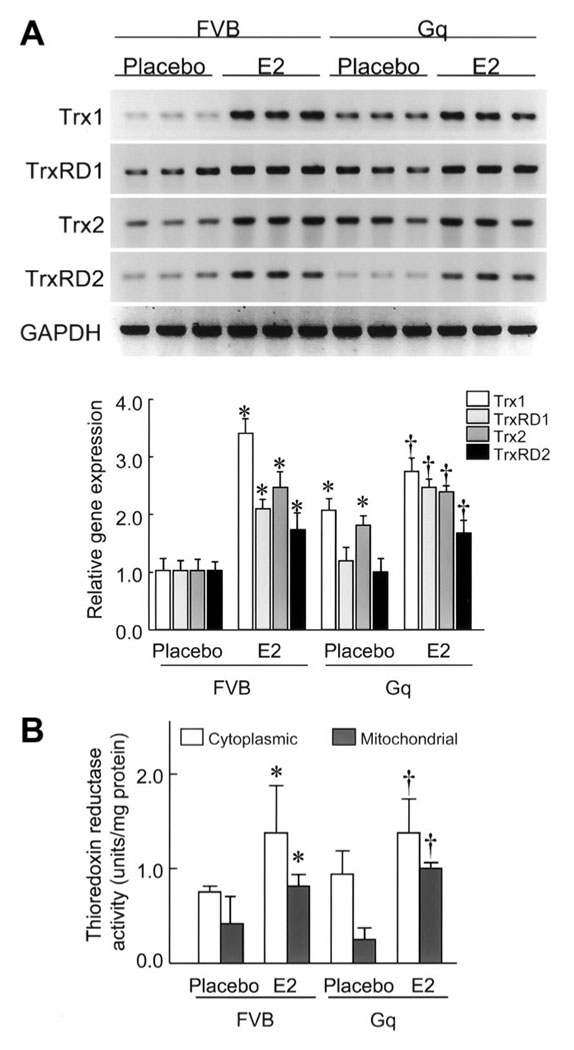
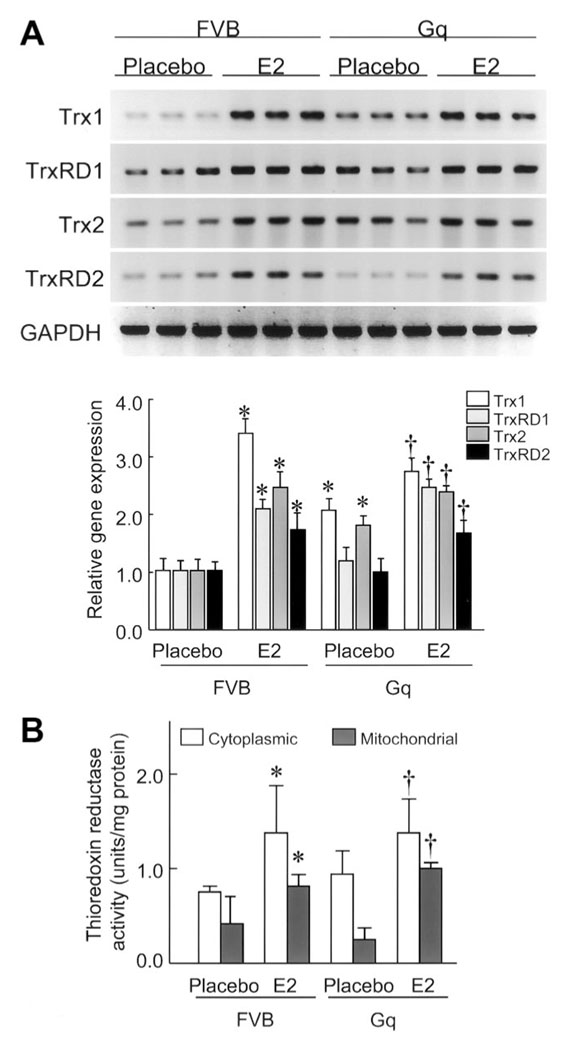
E2 Upregulates the Myocardial Trx System
To determine whether the increased myocardial oxidative stress could be due to changes in the Trx system, we investigated mRNA expression levels of Trx1, TrxRD1, Trx2, and TrxRD2 in the hearts of FVB and Gq mice with or without E2 treatment. Compared with hearts of placebo-treated FVB mice, the expression of Trx1, TrxRD1, Trx2, and TrxRD2 was increased by 3.5-, 2.1-, 2.5-, and 1.8-fold, respectively, in the hearts of E2-treated FVB mice (Figure 4A). Treatment with E2 increased the expression of Trx1, TrxRD1, Trx2, and TrxRD2 in the hearts of Gq mice by 2.7-, 2.5-, 2.5-, and 1.8-fold, respectively, compared with the hearts of placebo-treated Gq mice. These findings indicate that E2 increases the expression of components of the Trx system in the hearts of both FVB and Gq mice.
Figure 4. E2 upregulates the myocardial Trx system.
A, Trx1, TrxRD1, Trx2, and TrxRD2 mRNA expression levels in FVB and Gq mice at 8 weeks after placebo or E2 pellet implantation, analyzed by Northern blot. Expression levels of each mRNA were relative to those of GAPDH mRNA (n=9 in each group). Results are presented as mean±SD. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice. B, TrxRD activity in mitochondrial and cytosolic fraction samples of hearts of FVB and Gq mice at 8 weeks after placebo or E2 pellet implantation (n=10 in each group). Results are presented as mean±SD. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice.
To determine whether estrogen differentially modulates TrxRD activities, we measured TrxRD activity in the cytoplasm and mitochondria of heart tissues from FVB and Gq mice with or without E2 treatment. No differences were observed between the placebo-treated FVB and Gq mice with respect to cytosolic and mitochondrial TrxRD activities (Figure 4B). Treatment with E2 increased cytosolic TrxRD activities in the hearts of both FVB and Gq mice (FVB: 0.67±0.13 to 1.49±0.52 U/mg, n=10; Gq: 0.84±0.40 to 1.37±0.58 U/mg, n=10; P<0.05 for both). E2 treatment also increased mitochondrial TrxRD activities in the hearts of both FVB and Gq mice (FVB: 0.52±0.31 to 0.71±0.21 U/mg; Gq: 0.25±0.25 to 0.92±0.31 U/mg, n=10; P<0.05 for both). These results indicate that E2 upregulates the cytoplasmic and mitochondrial Trx system in both normal (FVB) and abnormal (Gq) hearts.
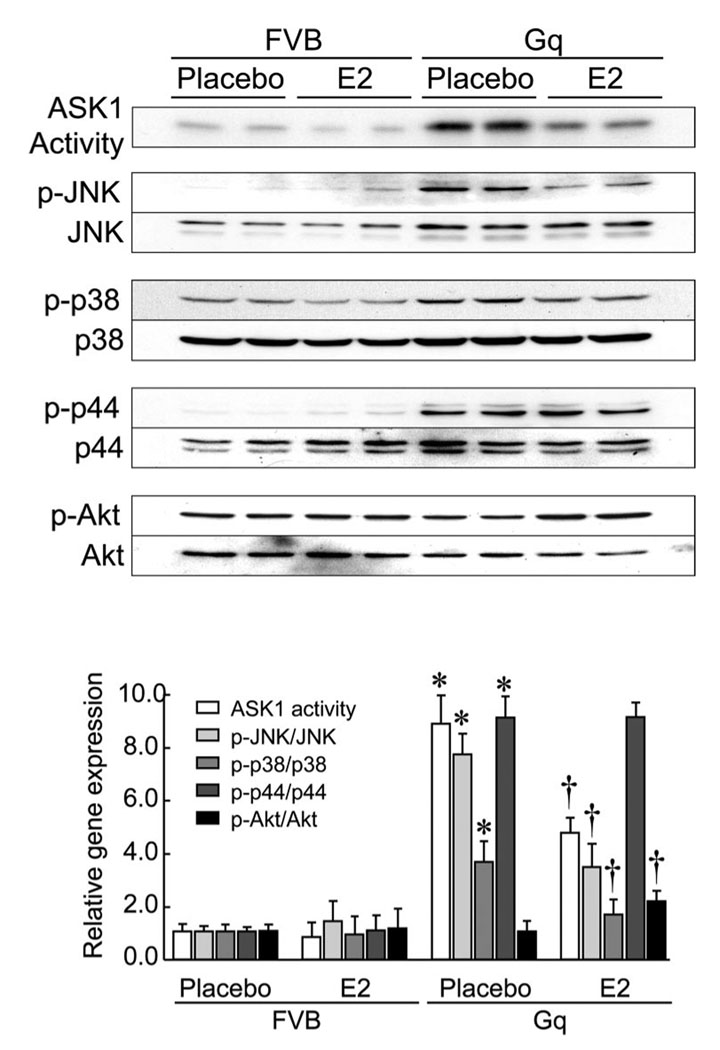
E2 Inhibits ASK1 Activity
Because estrogen prevents cardiomyocyte apoptosis, we investigated whether E2 treatment can inhibit ASK1 activity and its downstream targets, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), via up-regulation of the Trx system in the heart. Compared with the hearts of placebo-treated FVB mice, ASK1 activity and phospho-JNK, phospho-p38, and phospho-p44/p42, but not phospho-Akt, were increased in the hearts of placebo-treated Gq mice (Figure 5). Treatment with E2 decreased activation of ASK1, JNK, and p38 MAPK but not p44/p22 MAPK, in the hearts of Gq mice. Interestingly, E2 increased Akt activation in hearts of Gq mice. These findings indicate that estrogen inhibits apoptotic signaling pathways (ie, ASK1) while inducing cell-survival pathways (ie, Akt).
Figure 5. E2 inhibits ASK1 activity.
Proteins were extracted from hearts of FVB and Gq mice at 8 weeks after placebo or E2 pellet implantation and analyzed by ASK1 kinase assay and Western blots with anti-phospho-JNK (p-JNK), JNK, phosphop38 MAPK (p-p38), p38 MAPK (p38), phospho-p44/42 MAPK (p-p44), p44/42 MAPK (p44), phospho-Akt (p-Akt), and Akt antibodies. Expression levels of each phospho-protein are expressed relative to those of each total protein (n=10 in each group). Results are presented as mean±SD. *P<0.05 compared with placebo-treated FVB mice. †P<0.05 compared with placebo-treated Gq mice.
Trx System Mediates Inhibition of Peroxide-Induced Cardiomyocyte Apoptosis by E2
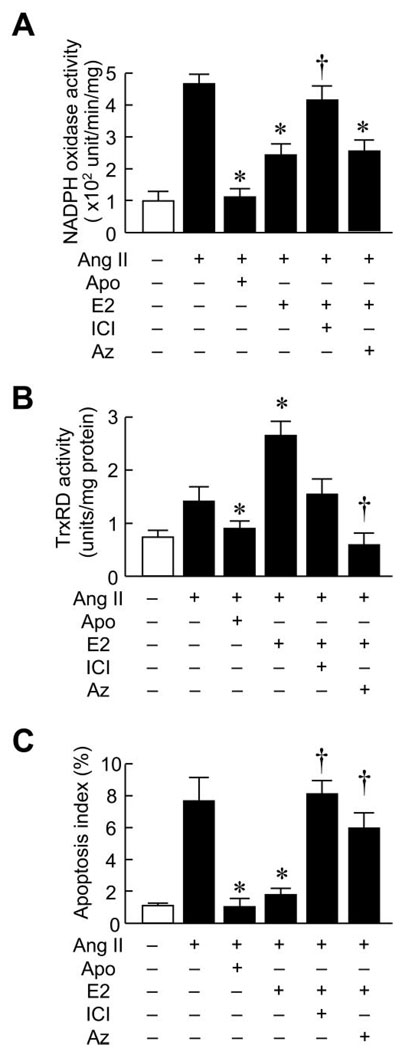
To determine whether the upregulation of Trx system by estrogen mediates the antiapoptotic effects of E2, we investigated the effects of E2 on cardiomyocyte apoptosis induced by Ang II. Treatment with Ang II (100 nmol/L) increased NADPH oxidase activity (Figure 6A). The activation of NADPH oxidase was markedly inhibited by cotreatment of apocynin and E2. The effect of E2 was blocked by the estrogen receptor antagonist ICI 182,780. TrxRD activity was increased by Ang II treatment, and this effect was abolished by apocynin treatment, which indicates that this Ang II–induced TrxRD activity was ROS-dependent. Cotreatment with E2 enhanced TrxRD activity, and this increase was diminished by ICI cotreatment. Using TUNEL staining to assess cardiomyocyte apoptosis, we found that treatment of cardiomyocytes with Ang II increased the percentage of TUNEL-positive cardiomyocytes from 1.1±0.1% to 7.8±1.4% (Figure 6C). Cotreatment with apocynin and E2 reduced the TUNEL-positive cells to 1.3±0.3% and 1.8±0.3% (P<0.05 versus Ang II treatment). The antiapoptotic effect of E2 was blocked by ICI and azelaic acid.
Figure 6. E2 prevents Ang II–induced cardiomyocyte apoptosis.
Effects of E2 on Ang II–induced apoptosis of rat neonatal cardiomyocytes in vitro. Results are expressed as (A) NADPH oxidase activity, (B) TrxRD activity, and (C) apoptosis index ratio of cells pretreated with vehicle or E2 (10 nmol/L) for 24 hours followed by the addition of 100 µmol/L Ang II for 6 hours. In some experiments, cells were cotreated with the NADPH oxidase inhibitor apocynin (Apo; 100 µmol/L), the estrogen receptor antagonist ICI 182,780 (ICI; 1 µmol/L), or the TrxRD inhibitor azelaic acid (Az; 10 µmol/L). Results are presented as mean±SD. *P<0.05 compared with Ang II plus vehicle control. †P<0.05 compared with Ang II plus E2 treatment.
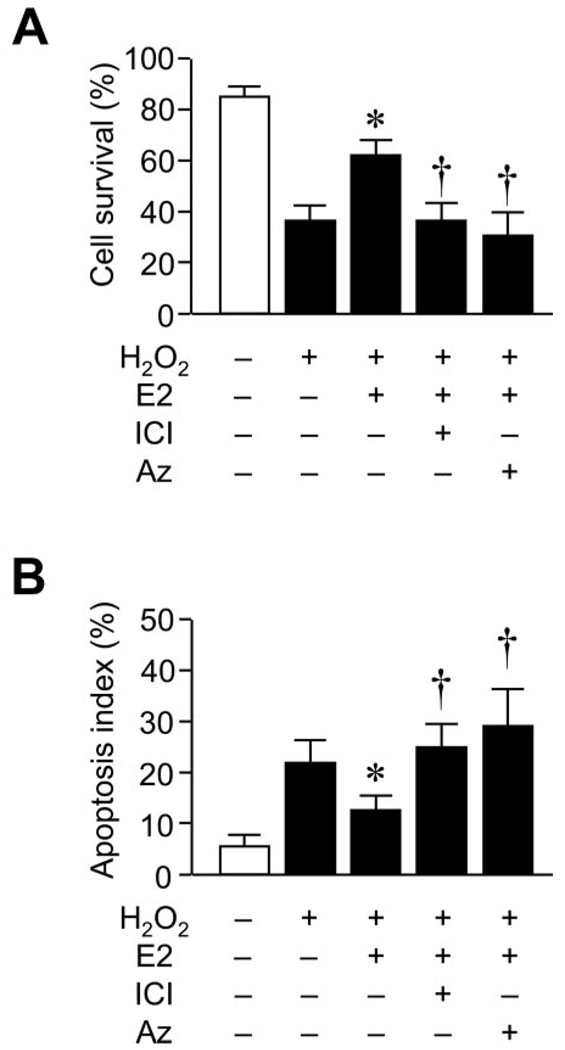
To determine whether E2 can protect cardiomyocytes from an exogenous source of oxidative stress, we investigated the effects of E2 on cardiomyocyte apoptosis induced by hydrogen peroxide (H2O2). Treatment with H2O2 decreased the percentage of surviving cardiomyocytes from 82±4% to 34±6% after 8 hours (Figure 7A). Cotreatment with E2 prevented H2O2-induced cardiomyocyte cell death and increased the percentage of surviving cells to 64±4% (P<0.05). The cell-survival effect of E2 was blocked by the estrogen receptor antagonist ICI and by the Trx inhibitor azelaic acid (Figure 7A). Similar to Ang II, treatment of cardiomyocytes with H2O2 increased the percentage of TUNEL-positive cardiomyocytes from 8±2% to 23±4% (P<0.05; Figure 7B). Cotreatment with E2 reduced the TUNEL-positive cells from 23±4% to 12±4% (P<0.05). The antiapoptotic effect of E2 was again blocked by ICI and azelaic acid. These results indicate that estrogen, acting through the estrogen receptor, increases cell survival and prevents cardiomyocyte apoptosis by upregulating the Trx system in the heart.
Figure 7. E2 prevents hydrogen peroxide–induced cardiomyocyte apoptosis.
Effects of E2 on hydrogen peroxide (H2O2)–induced apoptosis of primary mouse cardiomyocytes in vitro. Results are expressed as (A) cell-survival ratio and (B) apoptosis index ratio of cells pretreated with vehicle or E2 (10 nmol/L) for 24 hours followed by the addition of 100 µmol/L H2O2 for 8 hours. In some experiments, cells were cotreated with the estrogen receptor antagonist ICI-182,780 (ICI; 1 µmol/L) or the TrxRD inhibitor azelaic acid (Az; 10 µmol/L). Results are presented as mean±SD. *P<0.05 compared with H2O2 plus vehicle control. †P<0.05 compared with H2O2 plus E2 treatment.
Discussion
Previous studies have shown that estrogen exerts myocardial protective effects in models of pressure overload–induced cardiac hypertrophy5 and myocardial infarction.21 However, the effect of estrogen in preventing the development of CHF has not been investigated. In the present study, we extend the effects of estrogen to the development of CHF in a genetic model of dilated cardiomyopathy. The Gq transgenic mice exhibit differing phenotypes depending on the number of copies of the Gq transgene. For example, with 4 copies of the Gq transgene, mutant Gq mice develop progressive cardiac hypertrophy without left ventricular dilatation or CHF.17 However, with increased Gq transgene copy number, such as the mice used in the present study that had 40 copies of the transgene, the mutant male Gq mice spontaneously develop mild cardiac hypertrophy, with rapid progression to dilated cardiomyopathy. Using these male Gq transgenic mice, we found that treatment with estrogen attenuated the development of CHF through antioxidative and antiapoptotic mechanisms. Specifically, we found that estrogen decreased myocardial oxidative stress by inhibiting NADPH oxidase activity, reducing ROS generation, and increasing TrxRD activity in the hearts of Gq mice. The antiapoptotic effect of estrogen was blocked by an inhibitor of TrxRD, azelaic acid, which suggests that TrxRD is an important mediator of cardiomyocyte survival in CHF. These findings suggest that estrogen therapy may have benefits in patients with CHF through upregulation of TrxRD. However, to determine the relative contribution of TrxRD to the protective effects of estrogen on CHF, further studies will need to be performed with estrogen in mice lacking TrxRD.
Myocardial oxidative stress contributes to the rapid and progressive deterioration of cardiac function in patients with CHF.7 For example, NADPH oxidase–linked ROS activity has been found to be elevated in failing hearts of patients with either ischemic or dilated cardiomyopathy.6 Indeed, increased oxidative stress from pressure overload or Ang II is associated with increased cardiac remodeling and fibrosis,22,23 and antioxidant therapy has been shown to decrease cardiac fibrosis.24 The present results are consistent with these findings, because NADPH oxidase activity and ROS generation were both increased in the hearts of Gq transgenic mice. Treatment with estrogen reduced NADPH oxidase activity and ROS production and attenuated the severity of CHF in Gq transgenic mice. These findings are similar to previous studies showing that estrogen attenuates the development of cardiac hypertrophy by inhibiting the expression of NADPH oxidase25 and downregulating Rac1 GTPase.26 In addition, estrogen inhibits cardiac hypertrophy by decreasing ROS production not only through inhibition of NADPH oxidase activity but also by increasing antioxidants, such as superoxide dismutase.27 Thus, the findings of previous studies with regard to estrogen and cardiac hypertrophy are somewhat similar to the present findings with estrogen and CHF. A consequence of increased oxidative stress is that ROS may trigger apoptotic cell death.28 Because increased cardiomyocyte apoptosis is an important contributor to the loss of cardiomyocytes after maladaptive hypertrophy29 and heart failure,30 factors that regulate cellular apoptosis may affect cardiac size and contractility in CHF. Estrogen has been shown to inhibit cellular apoptosis in cardiomyocytes.31 In the present study, this was associated with estrogen’s ability to prevent the development of CHF in Gq transgenic mice. Although decreasing myocardial oxidative stress could contribute to the antiapoptotic effect of estrogen, we also found that estrogen increased the activity of protein kinase Akt in the hearts of Gq transgenic mice. The phosphatidylinositol 3-kinase/Akt pathway prevents cellular apoptosis by activating downstream cell-survival pathways.32 Indeed, activation of Akt in the heart by estrogen has also been shown to prevent cardiomyocyte apoptosis.21
The Trx system is an important cellular antioxidant defense mechanism. In mice with cardiac-specific overexpression of a dominant-negative mutant of the Trx1 gene, oxidative stress is increased in the pressure-overloaded hypertrophic heart.33 In contrast, overexpression of Trx in transgenic mice attenuates doxorubicin-induced cardiotoxicity by reducing myocardial oxidative stress.34 In a similar fashion, we found that upregulation of the Trx system by estrogen inhibited myocardial oxidative stress and attenuated the severity of CHF in Gq transgenic mice. Indeed, Trx and TrxRD are upregulated by estrogen in a variety of other tissues.15,16 Interestingly, we showed that E2 upregulates the cardiac Trx system in several different cellular compartments (ie, cytoplasm and mitochondria), which suggests that Trx may mediate multiple effects of estrogen on the heart. It remains to be determined by what mechanism the estrogen regulates Trx and TrxRD expression, given that neither the Trx nor the TrxRD promoter contains a canonical estrogen response element; however, these gene promoters contain half-estrogen response elements (GGTCA) and several SP1 sites, which have been shown to synergize with the estrogen receptor on various promoters.35 Thus, it would be genomic effects that induced Trx and TrxRD expression by estrogen. More detailed promoter studies will be required to examine these mechanisms.
Trx interacts with ASK1 through its direct binding to the N-terminal noncatalytic region of ASK1 and regulates ASK1 activity through Trx.36 ASK1 is inactivated by binding with Trx-(SH)2, which leads to ubiquitination and degradation of ASK1. Conversely, oxidization of Trx results in dimerization and activation of ASK1.37 In the present study, upregulation of TrxRD activity by estrogen increased Trx-(SH)2 and reduced ASK1 activity. ASK1 is a member of the MAPK kinase kinase that activated the JNK and p38 MAPK pathways. 38 Prolonged activation of JNK and p38 MAPK may aggravate the pathological changes in the heart through the proapoptotic action of these MAPKs. Therefore, inhibition of ASK1 activity and its downstream targets, JNK and p38 MAPK, by estrogen may contribute to some of the antiapoptotic effects of estrogen in CHF.
In the present study, we used a high dose of estrogen that maintains E2 serum concentrations 20-fold higher than the physiological range.39 Female Gq mice have been reported to exhibit a particularly severe phenotype of peripartum cardiomyopathy with the first pregnancy,40 so the physiological range of estrogen may have no cardioprotective effect in this Gq mouse model. Accordingly, we chose high-dose E2 treatment. High-dose E2-treated Gq mice fail to gain weight. This might reduce the susceptibility to heart failure in Gq mice and lead to a beneficial effect on the development of CHF. Recently, Beer et al41 reported that high-dose E2 treatment almost completely prevents development of post–myocardial infraction remodeling. However, the use of high-dose E2 is limited by adverse side effects such as an increased risk of thrombosis and promotion of breast and endometrial cancer and, in males, by a feminizing effect. Thus, at present, long-term treatment with high-dose E2 is not feasible for prevention of CHF in humans. Further studies will be needed to address the effects of estrogen-related compounds such as selective estrogen receptor modulators. Alternatively, targeting TrxRD or ASK1 may be a more feasible approach than estrogen therapy in terms of preventing CHF.
In summary, estrogen improves cardiac contractility and prevents progressive cardiac enlargement in a genetic mouse model of CHF by activating TrxRD, inhibiting NADPH oxidase activity, and reducing oxidative stress in the heart. The upregulation of the Trx system by estrogen leads to inhibition of ASK1-mediated cardiomyocyte apoptosis. Although we have demonstrated that estrogen is beneficial in a mouse model of CHF, it remains to be determined whether estrogens are useful adjunctive therapy in patients with CHF. Further clinical trials with estrogen therapy in heart failure are needed to address this issue.
Acknowledgments
Sources of Funding
This study was supported by grants from the National Institutes of Health (HL052233 and HL080187), the American Heart Association, Northeast Affiliate (to Dr Satoh), the Swiss National Science Foundation (31-114094/1 to Dr Matter), the Japan Heart Foundation (to Dr Takeshita), and Chung Gung Memorial Hospital (to Dr Wang).
Footnotes
The online-only Data Supplement, consisting of figures, tables, and an expanded Methods section, is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.106.657981/DC1.
Disclosures
None.
References
- 1.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 2.Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105:1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- 3.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento CA, Kauser K, Rubanyi GM. Effect of 17beta-estradiol in hypercholesterolemic rabbits with severe endothelial dysfunction. Am J Physiol. 1999;276:H1788–H1794. doi: 10.1152/ajpheart.1999.276.5.H1788. [DOI] [PubMed] [Google Scholar]
- 5.van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17beta-Estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 6.Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 7.Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J. Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation. 2000;101:33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 9.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 10.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vaiss. 2003;96:214–221. [PubMed] [Google Scholar]
- 12.Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation. 2001;103:724–729. doi: 10.1161/01.cir.103.5.724. [DOI] [PubMed] [Google Scholar]
- 13.Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 14.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 15.Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology. 2004;145:5485–5492. doi: 10.1210/en.2004-0471. [DOI] [PubMed] [Google Scholar]
- 16.Ejima K, Nanri H, Araki M, Uchida K, Kashimura M, Ikeda M. 17beta-Estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol. 1999;140:608–613. doi: 10.1530/eje.0.1400608. [DOI] [PubMed] [Google Scholar]
- 17.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., II Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 20.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-Estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 22.Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res. 2006;29:711–718. doi: 10.1291/hypres.29.711. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827–834. doi: 10.1016/j.jacc.2005.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol. 2004;287:H165–H171. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]
- 26.Laufs U, Adam O, Strehlow K, Wassmann S, Konkol C, Laufs K, Schmidt W, Bohm M, Nickenig G. Down-regulation of Rac-1 GTPase by estrogen. J Biol Chem. 2003;278:5956–5962. doi: 10.1074/jbc.M209813200. [DOI] [PubMed] [Google Scholar]
- 27.Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 28.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 29.Narula J, Kolodgie FD, Virmani R. Apoptosis and cardiomyopathy. Curr Opin Cardiol. 2000;15:183–188. doi: 10.1097/00001573-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 31.Pelzer T, Schumann M, Neumann M, deJager T, Stimpel M, Serfling E, Neyses L. 17beta-Estradiol prevents programmed cell death in cardiacmyocytes. Biochem Biophys Res Commun. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S, Yodoi J. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation. 2002;106:1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 35.O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 39.Geisler JG, Zawalich W, Zawalich K, Lakey JR, Stukenbrok H, Milici AJ, Soeller WC. Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide. Diabetes. 2002;51:2158–2169. doi: 10.2337/diabetes.51.7.2158. [DOI] [PubMed] [Google Scholar]
- 40.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., II Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beer S, Reincke M, Kral M, Callies F, Stromer H, Dienesch C, Steinhauer S, Ertl G, Allolio B, Neubauer S. High-dose 17beta-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Res Cardiol. 2007;102:9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]