Abstract
Background and Aims
A deeper understanding of mutualism can be reached by studying systems with measurable costs and benefits. Most studies of this type focus on an unusual class of obligate, species-specific pollination mutualisms. The interaction between Datura wrightii (Solanaceae) and the hawkmoth Manduca sexta offers similar advantages but greater generality. Adult moths both nectar at and deposit eggs on the same plant; larvae are herbivorous. The antagonistic component of this interaction has been well studied. Here the role of M. sexta as a pollinator of D. wrightii, particularly in the context of this moth's frequent nectaring visits to the bat-pollinated plant Agave palmeri, is documented.
Methods
Hand-pollinations were used to determine breeding system and the reproductive consequences of mixed loads of A. palmeri and D. wrightii pollen. Plants and moths were caged overnight to assess whether nectaring visits led to fruit and seed set. Finally, pollen deposited on field-collected stigmas was identified, with a particular focus on documenting the presence of D. wrightii and A. palmeri grains.
Key Results
Datura wrightii is highly self-compatible, and a visit that deposits either outcross or self pollen almost doubles fruit and seed set compared with unvisited flowers. Manduca sexta transferred enough pollen to produce fruit and seed sets comparable to hand-pollination treatments. Agave palmeri did not interfere with D. wrightii success: in the field, stigmas received almost pure D. wrightii pollen, and hand-addition of large quantities of A. palmeri pollen had no measurable effect on fruit and seed set.
Conclusions
The floral visitation component of the D. wrightii–M. sexta interaction is indeed mutualistic. This finding is essential background to future development of this interaction as a model system for studying mutualism's costs and benefits. It is already proving valuable for dissecting third-species effects on the outcome of mutualism. Results indicate that M. sexta's heavy visitation to A. palmeri has no negative effect on the benefits conferred to D. wrightii. However, it can be predicted to augment M. sexta populations to the point where the costs of the interaction begin to exceed its benefits.
Key words: Datura wrightii, Agave palmeri, pollination, herbivory, mutualism
INTRODUCTION
A major advance in our growing understanding of mutualisms has been the recognition that these interactions not only confer benefits to their participants, but usually inflict costs as well. The costs of mutualism are unusually well documented in the case of biotically pollinated plants. Pollination requires investments into production of floral tissues, volatile attractants and nectar (Pyke, 1991; Galen et al., 1999; Andersson 1999, 2000). Costs are also associated with the actions of antagonists attracted to flowers and floral rewards (Shykoff and Bucheli, 1995; Adler and Bronstein, 2004; McCall and Irwin, 2006). Pollination systems clearly evolve in the context of the ratio of benefits to costs. The evolution of floral morphology (Kobayashi et al., 1999; Galen and Cuba, 2001), floral chemistry (Raguso, 2008), nectar traits (Thakar et al., 2003; Dupont et al., 2004), and even breeding systems (Johnson et al., 2004; Tang and Huang, 2007) may only be interpretable in light of the costs of pollination investments (Bronstein et al., 2006).
Weighing the costs and benefits of mutualisms can be challenging (Bronstein, 2001). It is difficult to express costs and benefits in the same currency, let alone to quantify their relative magnitudes. Some mutualisms offer unusual features that ameliorate these difficulties, however. In particular, the majority of empirical studies of the costs and benefits of mutualisms focus on pollinating seed parasite (sometimes termed nursery or brood site pollination) systems. In these interactions, the best known of which involve figs and yuccas, highly specialized insects both pollinate flowers and lay their eggs within plant reproductive structures (Holland and Fleming, 1999; Sakai, 2002; Dufaÿ and Anstett, 2003; Pellmyr, 2003; Herre et al., 2008). The benefits of pollination in terms of seeds initiated can be compared with its costs, measured as the number of seeds lost to the pollinators' offspring (e.g. Pellmyr, 1989; Bronstein, 2001; Holland, 2002; Westerbergh, 2004). However, the highly unusual natural history of these pollination systems, including their extreme specificity, unusual floral attractants and rewards, and unique nature of the cost of mutualism, have constrained the generality of conclusions that can be drawn from studying them.
Datura wrightii (Solanaceae) and the hawkmoth Manduca sexta (Sphingidae) interact in a much more stereotypical pollination mutualism. The plant displays massive, white, sweet-smelling flowers that produce copious volumes of nectar highly attractive to these nocturnal insects, which make up the majority of its floral visitors; conversely, M. sexta adults rely heavily but not exclusively on D. wrightii for nectar (Alarcón et al., 2008). Yet this interaction offers advantages similar to the more idiosyncratic pollinating seed parasite relationships for studying costs and benefits of mutualisms. After nectaring at D. wrightii flowers, M. sexta females lay eggs on the leaves. The larvae feed upon the tissue of their natal host plants. Thus, the benefits of pollination can potentially be weighed against the potentially high costs of herbivory by the pollinators' offspring (Bronstein et al., 2007), generating an excellent test case of the conditions that promote persistence in the face of costs of mutualism.
It is reasonable to predict that, given the costs that D. wrightii is likely to incur by attracting M. sexta, the pollination benefits that they confer in return must be extremely high. However, although M. sexta behaviour and neurobiology in response to D. wrightii floral traits have been well documented (Raguso et al., 2003; Raguso and Willis, 2002, 2005; Guerenstein et al., 2004; Thom et al., 2004; Riffell et al., 2008), the quality of Manduca as a pollinator is unknown. Determining that this interaction does in fact confer benefits to D. wrightii is of course essential before it can be considered further as a model system for the study of mutualism.
Here, the quality of M. sexta as a pollinator of D. wrightii in south-eastern Arizona, USA is assessed. (a) Experimental results documenting D. wrightii breeding system in south-eastern Arizona are presented. The magnitude of the difference in reproductive success between treatments that allow autonomous pollination only and treatments that involve active pollen transfer indicates the degree to which D. wrightii benefits from the services of pollinators. (b) Experiments in field cages designed to assess the ability of M. sexta to pick up and transfer pollen are described. If D. wrightii reproductive success resulting from M. sexta nectaring behaviour is comparable to the number produced from hand pollinations, this would indicate a clear pollination benefit from this moth. (c) Experimental results documenting reproductive consequences for D. wrightii of the deposition of A. palmeri pollen, the predominant pollen carried by M. sexta at the field sites, are presented. These studies indicate whether M. sexta's reliance on A. palmeri constitutes one of the costs of this mutualism from the plant perspective. Finally, to investigate whether such a cost may be inflicted in nature, (d) the identity and number of pollen grains deposited on D. wrightii stigmas in the field are documented.
STUDY SYSTEM AND SITE
Datura wrightii Regel (formerly D. meteloides) is a common perennial herb found in sandy or gravelly microsites from western Texas to California and Mexico. In Arizona, D. wrightii occurs at 300–1980 m a.s.l. (Kearney and Peebles, 1960). Plants regrow each year to sizes that vary with season; when moisture is plentiful, they may grow to 1 m tall and >2 m in diameter. The primary growing season is during the hot summer monsoonal period. Datura wrightii blooms from mid-April to early November in this region, opening low numbers of exceptionally large (up to 25 cm), white, tubular flowers each night. Flowers are open for a single night and secrete copious (65 ± 10 µL) concentrated (25 % sucrose equivalents) nectar (Elle and Hare, 2002; Raguso et al., 2003; Riffell et al., 2008). Fruits are spiny capsules containing up to several hundred seeds. Mature fruits split open and seeds drop to the ground. They possess an elaiosome, and are dispersed by ants (Ness and Bressmer, 2005).
Manduca sexta is a large, widespread North and Central American hawkmoth. Despite its broad geographic range, in any one region it has only a handful of larval host plants, almost exclusively Solanaceae. It also commonly feeds upon solanaceous crop plants, giving rise to its common name, the tobacco hornworm. In the south-western United States, where its agricultural hosts are not grown commercially, M. sexta oviposits only on Datura spp. and (more rarely) Proboscidea spp. (Martyniaceae) (Mechaber and Hildebrand, 2000; Mira and Bernays, 2002).
Before laying eggs on D. wrightii, a M. sexta female typically makes a nectaring visit to a flower on the same plant. Raguso et al. (2003) have calculated that the nectar within a single D. wrightii flower provides a 1·2 g M. sexta with 10–15 min of hovering capability; a short bout of five to ten flower visits provides a moth with enough energy to fly for 1 h or more. Yet, although over three-quarters of the visitors to D. wrightii flowers are M. sexta individuals and almost all moths examined carry at least some D. wrightii pollen, these moths clearly visit other plants for nectar as well. In particular, the majority of the pollen grains carried by the average M. sexta individual are Agave palmeri (Agavaceae; Alarcón et al., 2008). This bat-pollinated species exhibits a radically different floral syndrome, but produces copious amounts of nectar (Slauson, 2000; Scott, 2004). It has been shown that while M. sexta is innately attracted to and prefers to forage upon Datura, it easily learns to incorporate Agave in its diet (Riffell et al., 2008).
The benefit of M. sexta pollination, which is documented in the present paper, comes at the cost of herbivory. Female M. sexta can lay up to 400 eggs in their lifetime (Madden and Chamberlin, 1945) and up to 100 each night (G. Davidowitz, unpubl. res.). Provided that there is enough foliage, a larva will feed exclusively on its natal plant (primarily on leaves, but also on flowers and fruits). The few larvae that escape predation and parasitism grow to be so large that a single individual can completely defoliate its host by its last instar (McFadden, 1968). An individual M. sexta larva can process 1400–1900 cm2 of leaves (Heinrich, 1971; Casey, 1976), which is greater than the size of many D. wrightii in natural settings. Datura wrightii rapidly regrows following defoliation, however, and the fitness cost of M. sexta herbivory is still unclear.
METHODS
Study site
The work reported here was carried out in, and/or using material collected at, the Santa Rita Experimental Range (SRER), located 45 km south of Tucson, Arizona, USA at the base of the Santa Rita Mountains (31·78°, W 110·82°, 1320 m. a.s.l.). The habitat is characterized by semi-arid grasslands with seasonally flowing washes, bordered by mesquite and oak woodlands (Medina, 2003). During the summer months (July–September) SRER receives an average of 29·2 cm of precipitation and has a mean maximum temperature of 31·7 °C (NOAA Western Regional Climate).
Breeding system
Datura wrightii is known to be self-compatible. Self-pollination occurs when anthers and stigmas come into contact, both as the flowers open and as the corolla is shed the next day (Elle and Hare, 2002), as well as when visitors transfer pollen among flowers on the same plant. Hand-pollination experiments were used to quantify fruit and seed set resulting from outcross pollination, autonomous self-pollination (i.e. in unvisited flowers) and geitonogamy (i.e. due to active transfer of self pollen). Elle and Hare (2002) have presented some similar data for a moist-habitat Californian population. However, it was considered essential to document the breeding system at the same site at which moth pollination effectiveness was being studied, particularly since environmental factors are well documented to affect floral phenotype in this species (Elle and Hare, 2002).
Several hundred seeds were extracted from mature D. wrightii fruits collected at SRER in autumn of 2001. In late spring of 2002, 89 plants derived from the pooled seed collection were grown in the greenhouse in 8-in. standard pots, in a 3 : 2 : 1 Sungro Sunshine Mix #3–vermiculite–sand mixture. Plants were grown under natural daylight hours and watered three times weekly. They were fertilized once every 2 weeks, and transplanted to larger pots in late April. Plants began flowering in June and continued to open between none and five flowers each night at dusk through October. Expanded buds were tagged 2–3 d before they were expected to open and randomly assigned to one of three treatments, conducted when possible in rotating order on each plant. Flowers designated for the outcross hand-pollination treatment (n = 45 flowers) were emasculated at this point. Then, at dusk on the night each flower opened, anthers were clipped from a newly opened flower on a randomly chosen plant and swiped five times across the stigma. Two anthers were clipped from flowers assigned to the geitonogamous self-pollination (hereafter, hand self-pollination) treatment (n = 108 flowers) within several minutes of opening. These anthers were swiped five times each across the stigma of the same flower. Finally, in the autonomous self-pollination treatment, flowers were left unmanipulated (n = 168 flowers). The anther–stigma separation was measured in flowers assigned to the autonomous self-pollination treatments, as herkogamy is known to predict the rate of autonomous selfing in another D. wrightii population (Elle and Hare, 2002). Anther–stigma separation was measured as the height in millimetres from the top of the anthers to the tip of the stigma.
Tagged flowers were censused daily, and fruit initiation, development and abortion noted. Fruits were bagged with mesh netting approx. 5 weeks after the date of pollination. Once a fruit had dried, split open, and shed most of its seeds into the bag, approx. 7 weeks after pollination, the fruit and bag were removed. In the laboratory, fruits were allowed to fully dry in individual cups. They were then weighed. Seeds were extracted and weighed as a group. All seeds were then counted, noting any that were partially developed. Fruit set was compared across treatments using G2 tests, and seed set was compared across treatments using t-tests.
Pollen transfer by Manduca sexta
To determine whether M. sexta could function as a D. wrightii pollinator, during the summer of 2002, caged moths were allowed to freely visit flowering plants and the subsequent fruit and seed set measured. Moths had either been reared in the laboratory from field-collected larvae, or else were newly emerged moths from a laboratory-maintained colony (methods in Davidowitz et al., 2003, 2004). Plants came from the same greenhouse-grown population described above.
Moths were placed in large (2 × 2 × 2 m) field cages with the plants overnight; no attempt was made to control the number of visits that individual flowers received. Two treatments were used. In the moth self-pollination treatment (n = 31 flowers from 23 plants), a single focal plant with between one and three open flowers was placed in the cage with one or two moths. Any pollen transferred onto the stigmas was therefore self pollen. Moths had not previously been exposed to flowers. Therefore, the only pollen they carried was that picked up in the course of the experiment. In the moth outcross pollination treatment (n = 18 flowers from 18 plants), the following were placed in the cage: (a) a focal plant bearing a single flower, which was emasculated prior to the experiment, using the protocol described above; (b) a second plant bearing one or two open flowers; and (c) between one and four moths. Fruit and seed set were recorded for the focal plant only, which could only have received outcross pollen. On the morning following all experiments, focal plants were returned to the greenhouse. Treatment flowers were labelled, and fruit and seed set subsequently recorded using the protocol described above. Fruit set and seed set per fruit were compared among treatments using G2 tests and t-tests, respectively.
Consequences of foreign pollen deposition
Most M. sexta individuals captured in the field during 2004–2005 (n = 148 moths) carried Agave palmeri pollen. Indeed, 50–70 % of pollen grains on the average individual were A. palmeri; an additional 20–30 % were D. wrightii, with the remaining 10–20 % of pollen representing diverse other species (data shown in Alarcón et al., 2008). In light of the high abundance of A. palmeri pollen on moths, consequences of its deposition for D. wrightii reproduction were experimentally investigated in 2005.
Four experimental treatments were designed to simulate possible pollination scenarios involving A. palmeri and D. wrightii in nature. These treatments used greenhouse-grown D. wrightii and field-collected A. palmeri. Each treatment was replicated 50 times. The first pair of treatments replicated the outcross hand-pollination treatment and the autonomous self-pollination treatment conducted in 2002. The same technique was used as in the earlier experiment; however, in the outcross treatment, pollen from flowers of multiple plants rather than a single plant was applied.
The second pair of treatments simulated mixed pollination. In both of these treatments, A. palmeri anthers were taken from mature umbels collected from SRER the previous day. In the Agave + Datura outcross hand-pollination treatment, a flower was emasculated, as in the Datura outcross hand-pollination treatment. Agave palmeri pollen was then applied by swiping a single mature anther across the surface of the D. wrightii stigma five times, leading to the deposition of a thick layer of pollen grains. Outcross D. wrightii pollen was then applied as in the Datura outcross hand-pollination treatment. The Agave + Datura autonomous self-pollination treatment involved addition of A. palmeri pollen to flowers allowed to self-pollinate without intervention.
Following the experimental pollination treatment, each flower was tagged and censused daily. Fruit initiation, development and abortion were noted as in the earlier breeding system experiment. Fruit collection and measurement of fruit and seed numbers and weights were recorded as in that experiment. Fruit set and seed set per fruit were compared among treatments using G2 tests and t-tests.
Patterns of pollen deposition in the field
The identities and relative abundances of pollen that insects carry on their bodies do not necessarily reflect what those insects deposit on stigmas. Patterns of pollen deposition on D. wrightii stigmas were examined at SRER to determine if they were similar to the identities and proportions of pollen carried on the bodies of M. sexta, by far the most common visitor to these flowers at the study site (Alarcón et al., 2008; Riffell et al., 2008).
Stigmas were collected once weekly between 6 June and 21 July 2006 from 127 flowers that had been open the previous night on about 40 plants. Collections were made at two sites at SRER. One was located within 0·5 km of a large number of A. palmeri individuals, the other about 3 km distant, well within the flight range of an individual M. sexta. Agave palmeri was in flower on three of the collection dates, with its flowering peak on the final date (J. L. Bronstein et al., unpubl. res.). Datura wrightii corollas had not yet wilted at the time stigmas were collected, suggesting that relatively little autonomous pollen had been deposited (Elle and Hare, 2002). Stigmas were placed in individual labelled glassine envelopes in a cooler, and returned to the laboratory. There, the stigmas were cut and placed on a microscope slide. They were stained with an aqueous fuschin stain, mixed with lightly dyed fuschin gel, heated over an alcohol lamp, and squashed with a coverslip. Slides were then examined under a compound microscope. All pollen grains were counted and identified to species to the greatest extent possible, using reference slides prepared in 2005 from all flowers located within 3 km of the study site whose flowers had been judged accessible to hawkmoths.
RESULTS
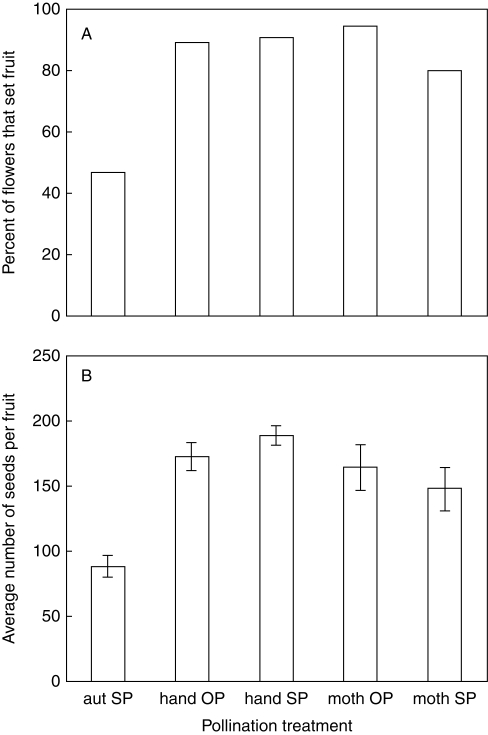
Datura wrightii was highly self-compatible (Fig. 1). Forty-seven per cent of all unmanipulated flowers (i.e. those in the autonomous self-pollination treatment) set fruit (Fig. 1A), with an average of 88·8 ± 69·0 seeds per fruit (Fig. 1B). Flowers in this treatment that did produce fruit had a significantly smaller anther–stigma separation than those that did not produce fruit (t = 2·65, P = 0·009; Table 1). In other words, flowers whose anthers were located much higher than the stigma were less likely to self-pollinate when the corolla slipped off compared with flowers in which the anthers and stigma were more similar in height (or in which the anthers were actually lower than the stigma; 1·7 % of 233 sampled flowers).
Fig. 1.
Reproductive success from hand-pollination and caged moth pollination experiments. (A) Fruit set, i.e. the percentage of flowers in that pollination treatment that set fruit. Fruit set from autonomous self-pollination was significantly lower than other treatments, which did not differ significantly from each other (see text). (B) Mean (± s.e.m.) seed set per maturing fruit. Seed set from autonomous self-pollination was significantly lower than other treatments; moth hand-pollination resulted in lower seed set than did hand self-pollination, but other treatments did not differ significantly from each other (see text). Abbreviations: aut SP, autonomous self-pollination, from unmanipulated flowers; hand OP, hand outcross-pollination, i.e. from emasculated flowers on which pollen from other plants had been actively deposited; hand SP, hand self-pollination, i.e. from flowers on which pollen from other flowers the same plant had been actively deposited; moth OP, moth outcross-pollination, i.e. from emasculated flowers exposed overnight to Manduca sexta in a cage with flowers on other plants; moth SP, moth hand-pollination, i.e. from flowers exposed overnight to M. sexta in a cage with only that plant present.
Table 1.
Anther–stigma separation and fruit set in autonomously self-pollinated flowers
| n | Mean | s.e. | Range | |
|---|---|---|---|---|
| Autonomously pollinated flowers that did set fruit | 48 | 11·5 | 7·1 | −10–25 |
| Autonomously pollinated flowers that did not set fruit | 62 | 14·9 | 6·2 | 5–31 |
Anther–stigma separation predicted the likelihood of fruit set: flowers that did not set fruit had anthers significantly more distant from (higher than) the stigma than in flowers that did set fruit (t-test, t = 2·65, P = 0·009). Values are shown in mm as either positive (anthers above stigmas) or negative (anthers below stigmas).
Flowers on which self pollen was hand-deposited experienced higher fruit set (91 %; G2 = 62·03, P < 0·0001) and produced significantly more seeds per fruit (t = 8·90, P < 0·0001) than those allowed to self-pollinate autonomously. Hand-deposition of self versus outcross pollen resulted in very similar fruit sets (G2 = 0·104, P = 0·75; Fig. 1A) and seed sets (t = 1·19, P = 0·24; Fig. 1B). Hand-outcrossed fruits produced significantly more seeds (t = 6·15, P < 0·0001) than those resulting from autonomous self-pollination, and fruit set was significantly higher as well (G2 = 79·57, P < 0·0001).
Flowers visited by caged M. sexta that received only self pollen experienced fruit set (80 %) similar to flowers in the hand self-pollination treatment (G2 = 2·41, P = 0·12, Fig. 1A), although seed set per fruit was significantly lower (t = 2·37, P = 0·02, Fig. 1B). Flowers visited by caged M. sexta in which the moths deposited outcross pollen experienced fruit sets (94 %, G2 = 0·47, P = 0·49) and seed sets (164·9 ± 70·5, t = 0·41, P = 0·68) similar to those seen in the hand outcross pollination treatment (Fig. 1).
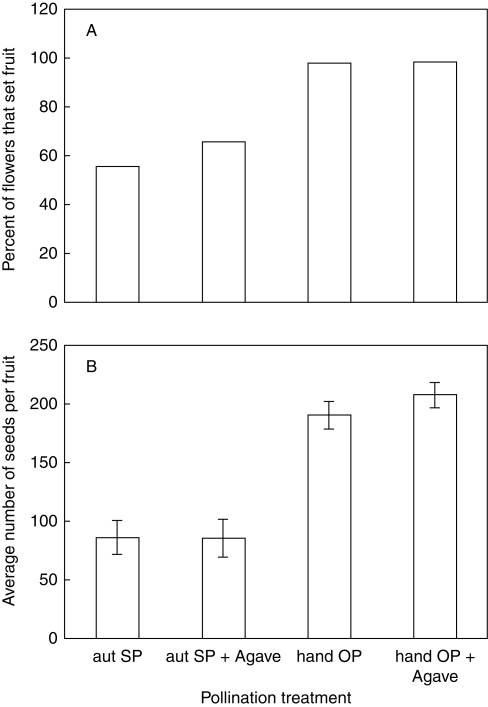
Breeding system results from the 2005 experiment designed to look at the effects of A. palmeri pollen deposition on D. wrightii reproductive success were qualitatively similar to those reported above from 2002. As in the earlier experiment, fruit set was about 40 % higher in the hand-outcross treatment (G2 = 25·397, P < 0·0001; Fig. 2A). Fruits from the hand-outcross treatment set significantly more seeds per fruit than those from the autonomous self-pollination treatment (t = 8·485, P < 0·0001; Fig. 2B).
Fig. 2.
Reproductive success of hand-pollinated flowers that were and were not augmented with Agave palmeri pollen. Addition of A. palmeri pollen had no effect on fruit set (A) or mean (± s.e.m.) seed set per fruit (B). In contrast, as in the earlier experiment whose results are shown in Fig. 1, flowers receiving outcross pollen set significantly more fruits and seeds per fruit than those that were autonomously pollinated. Abbreviations: aut SP, autonomous self-pollination, from unmanipulated flowers; hand OP, hand outcross-pollination, i.e. from flowers on which pollen from other plants had been actively deposited; aut SP + Agave, flowers on which A. palmeri pollen alone was actively deposited; hand OP + Agave, emasculated flowers to which both outcross D. wrightii and A. palmeri pollen were actively deposited.
At the study site, M. sexta carries large amounts of A. palmeri pollen (Alarcón et al., 2008; Riffell et al., 2008). What would be the consequences if this pollen were deposited on D. wrightii stigmas? In fact, hand-deposition of large amounts of A. palmeri pollen had no significant effect on D. wrightii reproductive output (Fig. 2). The number of seeds per fruit between the Datura outcross hand-pollination and Agave + Datura hand-pollination outcross treatments did not differ (t = 1·069, P = 0·2879). Nor was there a significant difference in seed numbers per fruit between the Datura autonomous self-pollination and Agave + Datura autonomous self-pollination treatments (t = 0·401, P = 0·6897). Fruit set of outcrossed flowers with and without A. palmeri pollen was indistinguishable (98 %, G2 = 0·005, P = 0·9412), as was fruit set of selfed flowers with and without A. palmeri (62 % vs. 55 %, G2 = 0·544, P = 0·4608).
Data on stigmatic pollen loads in the field (Table 2) suggest that, in spite of the large amounts of A. palmeri pollen carried by floral visitors, very little of it is deposited on D. wrightii stigmas. Stigmas received a median of 408 pollen grains, of which 95 % was D. wrightii. Only 0·2 % of all observed pollen grains were A. palmeri. All of this pollen was deposited during the period in which the A. palmeri population nearest to the study sites was flowering. The most common identifiable foreign pollen was Argemone sp. (Papaveraceae), the California prickly poppy, which was common at the study site and in flower during the study. Argemone pollen was never identified on the body of any local hawkmoth (Alarcón et al., 2008). It may have been imported by honey bees, which were occasional visitors to D. wrightii flowers and frequently observed on Argemone (J. L. Bronstein et al., unpubl. obs.).
Table 2.
Pollens represented on 127 Datura wrightii stigmas sampled in the field from approx. 40 plants from 6 June to 21 July 2006
| Species | % stigmas | % of all pollen deposited | Range deposited |
|---|---|---|---|
| Datura wrightii | 100 | 94·7 | 8–1950 |
| Argemone sp. | 69·3 | 2·7 | 1–149 |
| Misc. unidentified* | 59·0 | 2·4 | 1–289 |
| Agave palmeri | 3·9 | 0·2 | 1–26 |
| Pinaceae | 3·9 | ≪0·01 | 1 |
| Compositae† | 4·7 | ≪0·01 | 1–3 |
| Onagraceae | 3·1 | ≪0·01 | 1–2 |
Flowers open at dusk for a single night; stigmas were collected the following morning.
*The ‘Miscellaneous unidentified’ class includes a large number of unidentified species.
†‘Compositae’ are species in this family other than Argemone spp.
DISCUSSION
In the south-western United States, the life history of Manduca sexta is intertwined with that of Datura wrightii at two life-history stages: adults partially subsist on D. wrightii nectar, while larvae consume D. wrightii nearly exclusively (Bronstein et al., 2007). This linkage emerges because M. sexta females characteristically lay their eggs on D. wrightii leaves during nectaring visits to these plants. Floral visits have the potential to result in pollen transfer.
The linkage in this system between pollination by adults and consumption by offspring creates a marked ecological resemblance to the so-called pollinating seed parasite or nursery pollination systems, well studied interactions that have helped shape our current understanding of plant/animal mutualisms (Holland and Fleming, 1999; Sakai, 2002; Dufaÿ and Anstett, 2003; Pellmyr, 2003; Herre et al., 2008). In these associations, the best-known of which involve figs and yuccas, a highly specialized insect is obligately associated with (most commonly) a single plant species. It pollinates the plant associate, in many cases through deliberate actions, and then deposits its eggs on or within the reproductive tissue; pollinator offspring destroy or develop at the expense of some of the seeds. These plants have little or no ability to self-fertilize. Hence, whatever level of reproductive success achieved by these plants is due to the actions of their obligate, specific seed consumers (Bronstein, 2001).
The M. sexta–D. wrightii association is considerably more open and generalized than these pollinating seed parasite relationships. Consequently, it may be more useful as a general model for exploring how costs and benefits mediate mutualism dynamics. First, as in most plant/pollinator interactions, M. sexta visits flowers in order to obtain nectar. In contrast, most pollinating seed parasites are not seeking this reward, and their plant associates commonly produce little or no nectar (e.g. Fleming and Holland, 1998). Some, including fig wasps, lack feeding mouthparts. Since nectar production is perhaps the best-understood cost of pollination to plants (e.g. Pyke, 1991) and one of the most thoroughly studied costs of mutualism overall, it is fitting that a model system should involve it. Secondly, the pollinators' offspring feed primarily on leaves (e.g. Bernays and Woods, 2000; Kester et al., 2002), rather than seeds or gall tissue as in pollinating seed parasite mutualisms. The extensive knowledge that has accumulated in the past century on the ecology, evolution and physiology of herbivory can therefore be taken advantage of to interpret the cost of this interaction. Furthermore, M. sexta is nearly unique among insects in having the neurobiological basis of its foraging choices under intensive study (e.g. Guerenstein et al., 2004; Riffell et al., 2008). Thirdly, the M. sexta–D. wrightii interaction does not exhibit the extreme species-specificity characteristic of pollinating seed parasite systems. Pollen loads indicate that M. sexta adults at the study site regularly visit flowers of at least one other plant species, Agave palmeri, for nectar (Alarcón et al., 2008; Riffell et al., 2008). Conversely, pollen loads and field observations reveal that D. wrightii receives visits from at least two other hawkmoth species, as well as from honey bees (Alarcón et al., 2008; Riffell et al., 2008; J. L. Bronstein et al., unpubl. obs.). Although it is not known if these visits can result in pollen transfer, this seems likely, as it has been found that almost any jostling of the flower leads some pollen to be shed from the anthers, which are continuously distributed but typically located well above the stigma (Fig. 2). The interaction is also not species-specific at the larval consumption phase. Across its range, M. sexta consumes a range of solanaceous host plants, including both native and domesticated Nicotiana species, tomato, Capsicum and other Datura species (e.g. Madden, 1945; Kester et al., 2002; Mira and Bernays, 2002). It has been documented to use a single genus from a different plant family, Proboscidea (Martyniaceae) (Mechaber and Hildebrand, 2000). Conversely, D. wrightii has other herbivores (cf. Hare and Elle, 2002), although they consume considerably less tissue than does M. sexta. Finally, unlike pollinating seed parasite systems but like the majority of plant/pollinator interactions, the interaction (while close) is not obligate for either partner. A few M. sexta adults carry no D. wrightii pollen, and in fact for part of the summer appear to forage almost exclusively on A. palmeri (Alarcón et al., 2008; Riffell et al., 2008); late in the summer, Proboscidea parviflora is available as an alternative host plant (Mechaber and Hildebrand, 2000; Mira and Bernays, 2002; J. L. Bronstein et al., unpubl. res.). Furthermore, as has been shown here and which is consistent with other studies, D. wrightii is highly self-compatible (Figs 1 and 3). About half of all unvisited flowers set fruit, producing about 88 seeds per fruit. When self pollen is actively deposited, seed set and fruit set are identical to outcrossed flowers (Fig. 1). It has been found that selfed seeds germinate as readily as do seeds produced by outcross pollination, although the seedlings perform poorly when subjected to water stress (A. Tyler, University of Arizona, unpubl. res.). Thus, the easily identified and quantified antagonistic component of this relatively open association makes it a valuable general model for weighing the benefits versus costs of mutualism.
Of course, the M. sexta–D. wrightii association can only be a useful model system in this regard if it is in fact a mutualism. Surprisingly, although its antagonistic component has been relatively well studied, almost no information has been available on its potentially mutualistic component. Previous authors have documented M. sexta nectaring visits to D. wrightii (Grant and Grant, 1983; Adler and Bronstein, 2004; Guerenstein et al., 2004; Thom et al., 2004; Raguso and Willis, 2005), and its floral and nectar traits are well described (Grant and Grant, 1983; Elle and Hare, 2002; Raguso et al., 2003; Guerenstein et al., 2004). However, the reproductive consequences of these visits have not been examined previously. Here, it has been shown that this moth is a highly effective pollinator of D. wrightii. Visits from moths caged overnight with flowers led to fruit and seed sets typical of those produced in hand-pollination experiments (Fig. 1). Although these experiments may have led to the deposition of unnaturally high pollen loads, given that few flowers were available to the caged moths relative to the numbers these large-bodied moths may visit in the field (Raguso et al., 2003), field-collected stigmas themselves received a median of 388 D. wrightii pollen grains. Furthermore, almost every field-collected stigma bore at least some D. wrightii pollen, and the deposited loads were remarkably pure (Table 2). In combination, these data point to M. sexta as a highly reliable, if not exclusive visitor to D. wrightii that regularly deposits large quantities of pollen that can lead to high fruit and seed set. The degree to which M. sexta deposits self versus outcross pollen is not known. Nor is it known what the proportion of self pollen on stigmas of M. sexta-visited flowers that arrived from other flowers on the plant is relative to pollen that fell passively from the anthers during moth visits. However, in light of the high degree of self-compatibility and elevated reproduction of visited flowers regardless of pollen source, its nectaring visits clearly benefit D. wrightii.
The high quality of M. sexta as a D. wrightii pollinator is particularly intriguing in light of the moths' heavy reliance on A. palmeri as a nectar source. Agave palmeri is locally abundant in parts of the study site. Individuals bear several large panicles of flowers, each of which contains about 600 µL of nectar. In contrast, D. wrightii plants typically bear only 0–30 flowers at a time. Its flowering season stretches from April to November, but even at its midsummer flowering peak it is relatively uncommon: it made up <0·3 % of all flowers in a community-level study conducted in 2005 (Alarcón et al., 2008). Overall, the nectar standing crop of A. palmeri offers about a 6-fold higher energy content than D. wrightii and can sustain M. sexta flight for much longer durations (Riffell et al., 2008). Manduca sexta has a strong, innate preference for D. wrightii over A. palmeri, presumably linked to females' requirement to locate D. wrightii as a host plant; however, it can easily learn to incorporate A. palmeri into its diet (Riffell et al., 2008).
These data suggest that by subsidizing the flight of M. sexta when D. wrightii flowers are rare, A. palmeri, a bat-pollinated species, is facilitating the success of the M. sexta–D. wrightii pollination interaction. However, for this interpretation to be valid, it must be shown that deposition of A. palmeri pollen does not interfere with D. wrightii reproductive success. If it did, then Agave's role would likely be negative.
It has been shown here that A. palmeri pollen interferes in no detectable way with the quality of M. sexta visits to D. wrightii flowers. Remarkably little A. palmeri pollen is deposited by the moths on D. wrightii stigmas (Table 2). There is no direct evidence that the moths that visited the flowers from which stigmas were collected were in fact carrying A. palmeri. However, it has been documented in other years that the majority of M. sexta individuals do carry A. palmeri pollen; during weeks in which A. palmeri is abundant and D. wrightii scarce, pollen loads are strongly Agave-biased (Alarcón et al., 2008; Riffell et al., 2008). Furthermore, phenological censuses during this study indicated that the nearest A. palmeri stands were in full flower on the last three dates on which Datura stigmas were sampled. Thus, it is highly likely that moth visitors did indeed carry large loads of A. palmeri. In the present pollen transport studies, pollen from moth probosces has been recorded. Elsewhere it has been demonstrated that pollen loads on moths' ventral surfaces are no different (Alarcón et al., 2008). However, the precise location on the proboscis occupied by pollens of different species has not been documented. It is possible that A. palmeri and D. wrightii pollen are deposited on different parts of the proboscis, such that when moths visit subsequent Datura flowers, only Datura grains come in contact with stigmas. Similar phenomena are well documented in studies of plant species that share pollinators (Morales and Traveset, 2008); in the present case, two plant species share floral visitors but visits result in pollination for only one of them. Alternatively, A. palmeri pollen might adhere to the proboscis in a way that resists deposition. This species shows clear adaptations for pollination by bats (Slauson, 2000; Scott, 2004; Riffell et al., 2008), so it might not be surprising if the moth proboscis proved to be a poor mediator of pollen transfer. Further experiments will be necessary to distinguish among these possibilities.
Even if A. palmeri pollen did land on D. wrightii stigmas, the present experiments indicate that it would not interfere with reproductive success, a not uncommon result in studies of this type (Morales and Traveset, 2008). Adding A. palmeri pollen, even in large quantities, did not produce D. wrightii fruit sets or seed sets any different from what would have been experienced upon receipt of D. wrightii pollen alone (Fig. 2). The experimental protocol was intended to maximize the likelihood of finding pollen interference: negative effects have been more commonly found in hand-pollination experiments such as ours in which heterospecific pollen is deposited first (Morales and Traveset, 2008). These data further support the interpretation that A. palmeri facilitates the success of the M. sexta–D. wrightii pollination interaction.
It is nevertheless possible that Agave's overall role is negative from the perspective of Datura. Manduca sexta is not only a highly effective pollinator, but a specialized herbivore of D. wrightii. A single larva can consume the entire above-ground biomass of an average-sized plant (McFadden, 1968). Agave palmeri's subsidization of flight for feeding and reproductive activities potentially increases M. sexta's local population size, as well as that of the many other hawkmoths that appear to rely on it in this habitat (Alarcón et al., 2008). While this should lead to an increase in D. wrightii pollinator abundance at the local scale, plant reproductive success should plateau (or even decrease; Young and Young, 1992) above some visitation level. The cost of the association, however, should continue to rise with increasing visits by M. sexta females. Individual eggs have a very low likelihood of survival to pupation (about 1 % in studies of Mira and Bernays, 2002). The more eggs that are deposited, the greater the chance that D. wrightii will be completely defoliated by the pollinators' offspring. The fitness cost of defoliation to these perennial herbs has not yet been fully measured. However, the potential for Agave to affect the cost as much or more than the benefit of the Datura–Manduca association raises the possibility that the net effect of the association might be negative, not positive, when A. palmeri is locally abundant. Such context-dependency seems likely to be a geographically variable phenomenon. Not all south-western D. wrightii populations grow within M. sexta's flight range from A. palmeri (or similar agaves). Future work will test whether rates of pollination and herbivory in this system are more widely dependent upon the presence of additional nectar resources, be it A. palmeri or other species.
Third-species effects on the outcomes of mutualism are increasingly well documented in a wide range of mutualisms, particularly ones that are not strictly species-specific (Bronstein, 1994; Bronstein and Barbosa, 2002). Outcomes of pollination mutualisms, and in some cases their evolutionary trajectories, can be altered by nectar-robbers, predators, herbivores, seed predators and competitors (e.g. Herrera, 2000; Dedej and Delaplane, 2004; Schatz et al., 2006; Irwin and Adler, 2006; McCall and Irwin, 2006; Ferrière et al., 2007; Muñoz and Cavieres, 2008). The D. wrightii–M. sexta association will provide an ideal opportunity to document such effects in depth, due to the ability to identify, separate and measure effects of a third species on both the benefits and the costs of the interaction. This example highlights the potential value of this association as a model system for the study of mutualism.
ACKNOWLEDGEMENTS
We thank Ruben Alarcón, Jeff Riffell, Leif Abrell and John Hildebrand for their contributions to studies that form the background for this paper, and Abreeza Zegeer for greenhouse assistance. Comments of Nick Waser, the Bronstein laboratory group, and two referees greatly improved the manuscript. This work was partially supported by National Science Foundation grants to J.L.B., G.D. and T.H. (DEB 0316205 and 0522431). We also thank the National Institutes of Health-supported Arizona Biology Network for providing support to M.F.
LITERATURE CITED
- Adler LS, Bronstein JL. Attracting antagonists: does floral nectar increase leaf herbivory? Ecology. 2004;85:1519–1526. [Google Scholar]
- Alarcón R, Davidowitz G, Bronstein JL. Nectar usage in a southern Arizona hawkmoth community. Ecological Entomology. 2008;33:503–509. [Google Scholar]
- Andersson S. The cost of floral attractants in Achillea ptarmica (Asteraceae): evidence from a ray removal experiment. Plant Biology. 1999;1(5):69–572. [Google Scholar]
- Andersson S. The cost of flowers in Nigella degenii inferred from flower and perianth removal experiments. International Journal of Plant Sciences. 2000;161:903–908. [Google Scholar]
- Bernays EA, Woods HA. Foraging in nature by larvae of Manduca sexta – influenced by an endogenous oscillation. Journal of Insect Physiology. 2000;46:825–836. doi: 10.1016/s0022-1910(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. Conditional outcomes in mutualistic interactions. Trends in Ecology and Evolution. 1994;9:214–217. doi: 10.1016/0169-5347(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. The costs of mutualism. American Zoologist. 2001;41:127–141. [Google Scholar]
- Bronstein JL, Barbosa P. Multitrophic/multispecies mutualistic interactions: the role of non-mutualists in shaping and mediating mutualisms. In: Hawkins B, Tsharntke T, editors. Multitrophic level interactions. Cambridge: Cambridge University Press; 2002. pp. 44–65. [Google Scholar]
- Bronstein JL, Alarcón R, Geber M. Tansley Review: Evolution of insect/plant mutualisms. New Phytologist. 2006;172:412–428. doi: 10.1111/j.1469-8137.2006.01864.x. [DOI] [PubMed] [Google Scholar]
- Bronstein JL, Huxman TE, Davidowitz G. Plant-mediated effects linking herbivory and pollination. In: Ohgushi T, Craig TG, Price PW, editors. Ecological communities: plant mediation in indirect interaction webs. Cambridge: Cambridge University Press; 2007. pp. 79–103. [Google Scholar]
- Casey TM. Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae) Ecology. 1976;57:485–497. [Google Scholar]
- Davidowitz G, D'Amico LJ, Nijhout HF. Critical weight in the development of insect body size. Evolution & Development. 2003;5:188–197. doi: 10.1046/j.1525-142x.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- Davidowitz G, D'Amico LJ, Nijhout HF. The effects of environmental variation on a mechanism that controls insect body size. Evolutionary Ecology Research. 2004;6:49–62. [Google Scholar]
- Dedej S, Delaplane KS. Nectar-robbing carpenter bees reduce seed-setting capability of honey bees (Hymenoptera: Apidae) in rabbiteye blueberry, Vaccinium ashei, ‘Climax’. Ecological Entomology. 2004;33:100–106. [Google Scholar]
- Dufaÿ M, Anstett M-C. Conflicts between plants and pollinators that reproduce within inflorescences: evolutionary variations on a theme. Oikos. 2003;100:3–14. [Google Scholar]
- Dupont YL, Hansen DM, Valido A, Olesen JM. Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biological Conservation. 2004;118:301–311. [Google Scholar]
- Elle E, Hare JD. Environmentally-induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology. 2002;16:79–88. [Google Scholar]
- Ferrière R, Gauduchon M, Bronstein JL. Evolution and persistence of obligate mutualists and exploiters: competition for partners and evolutionary immunization. Ecology Letters. 2007;10:115–126. doi: 10.1111/j.1461-0248.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Holland JN. The evolution of obligate pollination mutualisms: senita cactus and senita moth. Oecologia. 1998;114:368–375. doi: 10.1007/s004420050459. [DOI] [PubMed] [Google Scholar]
- Galen C, Cuba J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution. 2001;55:1963–1971. doi: 10.1111/j.0014-3820.2001.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Galen C, Sherry RA, Carroll AB. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia. 1999;118:461–470. doi: 10.1007/s004420050749. [DOI] [PubMed] [Google Scholar]
- Grant V, Grant K. Behavior of hawkmoths on flowers of Datura meteloides. Botanical Gazette. 1983;144:280–284. [Google Scholar]
- Guerenstein PG, Yepez EA, van Haren J, Williams DG, Hildebrand JG. Floral CO2 emission may indicate food abundance to nectar-feeding moths. Naturwissenschaften. 2004;91:329–333. doi: 10.1007/s00114-004-0532-x. [DOI] [PubMed] [Google Scholar]
- Hare JD, Elle E. Variable impact of diverse insect herbivores on dimorphic Datura wrightii. Ecology. 2002;83:2711–2720. [Google Scholar]
- Heinrich B. The effect of leaf geometry on the feeding behavior of the caterpillar of Manduca sexta (Sphingidae) Animal Behavior. 1971;19:119–124. [Google Scholar]
- Herre EA, Jandér KC, Machado CA. Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. Annual Review of Ecology, Evolution, and Systematics. 2008;39:439–458. [Google Scholar]
- Herrera CM. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology. 2000;81:2170–2176. [Google Scholar]
- Holland JN. Benefits and costs of mutualism: demographic consequences in a pollinating seed-consumer interaction. Proceedings of the Royal Society of London Series B. 2002;269:1405–1412. doi: 10.1098/rspb.2002.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JN, Fleming TH. Mutualistic interactions between Upiga virescens (Pyralidae), a pollinating seed-consumer, and Lophocereus schottii (Cactaceae) Ecology. 1999;80:2074–2084. [Google Scholar]
- Irwin RE, Adler LS. Correlations among traits associated with herbivore resistance and pollination: implications for pollination and nectar robbing in a distylous plant. American Journal of Botany. 2006;93:64–72. [Google Scholar]
- Johnson SD, Peter CJ, Agren J. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proceedings of the Royal Society of London Series B. 2004;271:803–809. doi: 10.1098/rspb.2003.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney TH, Peebles RH. Arizona flora. Berkeley, CA: University of California Press; 1960. [Google Scholar]
- Kester KM, Peterson SC, Hanson F, Jackson DM, Severson RF. The roles of nicotine and natural enemies in determining larval feeding site distributions of Manduca sexta L. and Manduca quinquemaculata (Haworth) on tobacco. Chemoecology. 2002;12:1–10. [Google Scholar]
- Kobayashi S, Inouye K, Kato M. Mechanism of selection favoring a wide tubular corolla in Campanula punctata. Evolution. 1999;53:752–757. doi: 10.1111/j.1558-5646.1999.tb05369.x. [DOI] [PubMed] [Google Scholar]
- McCall AC, Irwin RE. Florivory: the intersection of pollination and herbivory. Ecology Letters. 2006;9:1351–1365. doi: 10.1111/j.1461-0248.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- McFadden MW. Observations on feeding and movement of tobacco hornworm larvae. Journal of Economic Entomology. 1968;61:352–356. [Google Scholar]
- Madden AH, Chamberlin FS. Biology of the tobacco hornworm in the southern cigar tobacco district. 1945 US Department of Agriculture Technical Bulletin No. 896. [Google Scholar]
- Mechaber WL, Hildebrand JG. Novel, non-solanaceous hostplant record for Manduca sexta (Lepidoptera:Sphingidae) in the southwestern United States. Annals of the Entomological Society of America. 2000;93:447–451. [Google Scholar]
- Medina A. Historical and recent flora of the Santa Rita Experimental Range. In: McClaran MP, Ffolliott PF, Edminster CB, editors. Santa Rita Experimental Range: 100 years (1903–2003) of accomplishments and contributions. Ogden, UT, Tucson, AZ: US Department of Agriculture, Forest Service, Rocky Mountain Research Station; 2003. pp. 141–148. Vol. RMRS-P-30. [Google Scholar]
- Mira A, Bernays EA. Trade-offs in host use by Manduca sexta: plant characters vs natural enemies. Oikos. 2002;97:387–397. [Google Scholar]
- Morales CL, Traveset A. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences. 2008;27:221–238. [Google Scholar]
- Muñoz AA, Cavieres LA. The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. Journal of Ecology. 2008;96:459–467. [Google Scholar]
- Ness JH, Bressmer K. Abiotic influences on the behaviour of rodents, ants, and plants affect an ant-seed mutualism. Ecoscience. 2005;12:76–81. [Google Scholar]
- Pellmyr O. The cost of mutualism: interactions between Trollius europaeus and its pollinating parasites. Oecologia. 1989;78:53–59. doi: 10.1007/BF00377197. [DOI] [PubMed] [Google Scholar]
- Pellmyr O. Yuccas, yucca moths, and coevolution: a review. Annals of the Missouri Botanical Garden. 2003;90:35–55. [Google Scholar]
- Pyke GH. What does it cost a plant to produce floral nectar? Nature. 1991;350:58–59. [Google Scholar]
- Raguso RA. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomologia Experimentalis et Applicata. 2008;128:196–207. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Animal Behaviour. 2002;64:685–695. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Animal Behaviour. 2005;69:407–418. [Google Scholar]
- Raguso RA, Henzel C, Buchmann SL, Nabhan GP. Trumpet flowers of the Sonoran Desert: floral biology of Peniocereus cacti and sacred Datura. International Journal of Plant Science. 2003;164:877–892. [Google Scholar]
- Riffell JA, Alarcón R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG. Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proceedings of the National Academy of Sciences of the USA. 2008;105:3404–3409. doi: 10.1073/pnas.0709811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S. A review of brood-site pollination mutualism: plants providing breeding sites for their pollinators. Journal of Plant Research. 2002;115:161–168. doi: 10.1007/s102650200021. [DOI] [PubMed] [Google Scholar]
- Schatz B, Proffit M, Rakhi BV, Borges RM, Hossaert-McKey M. Complex interactions on fig trees: ants capturing parasitic wasps as possible indirect mutualists of the fig–fig wasp interaction. Oikos. 2006;113:344–352. [Google Scholar]
- Scott PE. Timing of Agave palmeri flowering and nectar-feeding bat visitation in the Peloncillos and Chiricahua Mountains. Southwestern Naturalist. 2004;49:425–434. [Google Scholar]
- Shykoff JA, Bucheli E. Pollinator visitation patterns, floral rewards and the probability of transmission of Microbotryum violaceum, a venereal disease of plants. Journal of Ecology. 1995;83:189–198. [Google Scholar]
- Slauson LA. Pollination biology of two chiropterophilous agaves in Arizona. American Journal of Botany. 2000;87:825–836. [PubMed] [Google Scholar]
- Tang LL, Huang S-Q. Evidence for reductions in floral attractants with increased selfing rates in two heterandrous species. New Phytologist. 2007;175:588–595. doi: 10.1111/j.1469-8137.2007.02115.x. [DOI] [PubMed] [Google Scholar]
- Thakar JD, Kunte K, Chauhan AK, Watve AV, Watve MG. Nectarless flowers: ecological correlates and evolutionary stability. Oecologia. 2003;136:565–570. doi: 10.1007/s00442-003-1304-6. [DOI] [PubMed] [Google Scholar]
- Thom C, Guerenstein PG, Mechaber WL, Hildebrand JG. Floral CO2 reveals flower profitability to moths. Journal of Chemical Ecology. 2004;30:1285–1288. doi: 10.1023/b:joec.0000030298.77377.7d. [DOI] [PubMed] [Google Scholar]
- Westerbergh A. An interaction between a specialized seed predator moth and its dioecious host plant shifting from parasitism to mutualism. Oikos. 2004;105:564–574. [Google Scholar]
- Young HJ, Young TP. Alternative outcomes of natural and experimental high pollen loads. Ecology. 1992;73:639–647. [Google Scholar]