Abstract
Background and Aims
Because of differences in snowmelt time, the reproductive phenologies of alpine plants are highly variable among local populations, and there is large variation in seed set across populations. Temporal variation in pollinator availability during the season may be a major factor affecting not only seed production but also outcrossing rate of alpine plants.
Methods
Among local populations of Phyllodoce aleutica that experience different snowmelt regimes, flowering phenology, pollinator availability, seed-set rate, and outcrossing rate were compared with reference to the mating system (self-compatibility or heterospecific compatibility with a co-occurring congeneric species).
Key Results
Flowering occurred sequentially among populations reflecting snowmelt time from mid-July to late August. The visit frequency of bumble-bees increased substantially in late July when workers appeared. Both seed set and outcrossing rate increased as flowering season progressed. Although flowers were self-compatible and heterospecific compatible, the mixed-pollination experiment revealed that fertilization with conspecific, outcrossing pollen took priority over selfing and hybridization, indicating a cryptic self-incompatibility. In early snowmelt populations, seed production was pollen-limited and autogamous selfing was common. However, genetic analyses revealed that selfed progenies did not contribute to the maintenance of populations due to late-acting inbreeding depression.
Conclusions
Large variations in seed-set and outcrossing rates among populations were caused by the timing of pollinator availability during the season and the cryptic self-incompatibility of this species. Despite the intensive pollen limitation in part of the early season, reproductive assurance by autogamous selfing was not evident. Under fluctuating conditions of pollinator availability and flowering structures, P. aleutica maintained the genetic composition by conspecific outcrossing.
Key words: Alpine snowbed, autogamy, bumble-bee, cryptic self-incompatibility, flowering phenology, mixed pollination, outcrossing rate, Phyllodoce aleutica, pollination success, seasonality, self-pollination
INTRODUCTION
Variation in the abundance and availability of pollinators influences the reproductive success of plants. For example, under continuous pollen limitation because of low pollinator activity or availability, autogamous selfing may be advantageous by providing reproductive assurance (Kalisz and Vogler, 2003; Moeller, 2006). In contrast, preferential fertilization with outcross-pollen in a mixed-mating system and obligate outcrossing due to self-sterility are common for plants with frequent pollinator visits. However, the effectiveness of these mating systems should vary if the activity and availability of pollinators fluctuate spatially and temporally. Even under high pollinator activity, geitonogamous pollination may result in the abundant production of inbred seeds and a decrease in siring success due to pollen discounting in mixed-mating plants (de Jong et al., 1993). Therefore, pollinator activity may cause large variations in reproductive success among populations, depending on the mating system of individual plants.
The ecological significance of variation in flowering time and pollinator activity should be interpreted over a regional scale because phenological patterns often vary among populations within a local area (Kudo, 2006). These variations may cause differences in pollination efficiency, resulting in different reproductive outcomes among populations. The alpine ecosystem could be an excellent experimental system for research on plant–pollinator interactions, because plant communities are arranged as a mosaic pattern within a local area and individual communities have specific flowering structures (Yumoto, 1986; Inouye and Pyke, 1988; Kudo, 1991). The mosaic pattern of alpine vegetation reflects spatial variations in snow accumulation and snowmelt patterns, generated by variation in local topography.
Because the species composition and flowering schedules vary among communities, diverse flowering patterns are formed along the snowmelt gradient in alpine ecosystems (Kudo, 2006). Some plant species are distributed widely along the snowmelt gradient, and because their flowering times vary substantially depending on snowmelt time, pollination situations may vary among populations with different snowmelt regimes. First, seasonal variations in pollinator activity should influence pollination efficiency (Inouye and Pyke, 1988; Totland, 1994; Aizen, 2001; Hirao et al., 2006). In early-flowering populations, the low activity of pollinators may result in a quantitative pollen limitation due to low visit frequency under cool conditions (Kudo, 1993; Molau, 1993). As the flowering season progresses, pollinator activity increases with increasing temperature, resulting in more frequent visits to flowers. However, frequent pollinator visits may accelerate geitonogamous pollination within patches because many alpine shrub species form large clonal patches producing dense flowers (e.g. Kameyama et al., 2008). In this case, a qualitative pollen limitation due to geitonogamous selfing may restrict seed production if ovule and/or seed discounting or late-acting inbreeding depression exist (Eckert, 2000; Nuortila et al., 2002; Hirao et al., 2006). Secondly, pollination interactions among plant species may vary at a local scale if changes in qualitative or quantitative species compositions occur where these species share pollinators (e.g. Rathcke, 1983; Kasagi and Kudo, 2005). For example, an increase in more attractive species may accelerate the pollen limitation of other species if exploitation competition occurs, or this may increase the frequency of heterospecific pollination if interference competition occurs (Waser, 1978, 1983). However, little is known about the effects of interspecific competition on pollination success in natural plant communities.
This study aimed to clarify the variations in flowering timing and pollination situations among local populations with different snowmelt regimes and their impacts on seed production and outcrossing rate in a snowbed shrub species, Phyllodoce aleutica (Ericaceae). Phyllodoce aleutica is a common snowbed shrub that is a widely distributed along the snowmelt gradient, and an important nectar resource for bumble-bees due its predominant distribution in Japanese alpine regions. Previous studies revealed that this species competes for bumble-bee pollination with an F1 hybrid (P. caerulea f. yesoensis) between P. aleutica and P. caerulea in the Taisetsu Mountains (Kasagi and Kudo, 2003; Kameyama et al., 2008). Although the distributions of these species largely overlap, P. caerulea f. yesoensis is the predominant species in early snowmelt places, while P. aleutica is common in late snowmelt places. These variations in distribution pattern cause differences in the preference of bumble-bee visits between the two species (Kasagi and Kudo, 2005). However, the effects of pollination context on reproductive outcome have not been assessed based on genetic data. The goal of the present study was to clarify the ecological significance of the variation in seed set and genetic quality of seeds among local populations in terms of seasonal variations in pollination context and flowering phenology.
MATERIALS AND METHODS
Study sites
This study was conducted in the central part of the Taisetsu Mountains, Hokkaido, northern Japan (43°33'N, 142°53'E) in 2006 and 2007. The Taisetsu Mountains are characterized by snowy cold winters and wet summers. Annual mean temperature at 1700 m a.s.l. is –0·9°C, and monthly air temperatures during the summer season are 8·3°C in June, 10·9°C in July, 12·6°C in August and 7·3°C in September. This site is usually covered with snow again by early to mid-October.
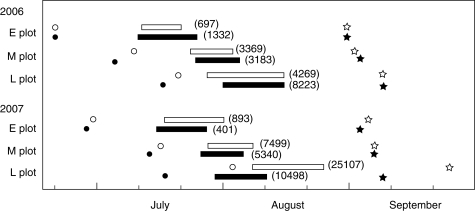
Two south-east-facing slopes between 1700 m and 1880 m a.s.l. were selected as the main research sites, located near Lake Hisago (HIS) and Mt Goshiki (GOS) and separated from each other by about 3 km. Three plots were established along the snowmelt gradient (E plot, early snowmelt plot; M plot, middle snowmelt plot; L plot, late snowmelt plot) at each site (for details, see Kasagi and Kudo, 2003). The size of each plot was 20 × 20 m. The snowmelt timing was late June at the E plot, early to mid-July at the M plot, and mid-July to early August at the L plot (Fig. 1). The timing of snowmelt at the L plot at HIS was extremely late in 2007.
Fig. 1.
Flowering periods at each plot at HIS (white bars) and GOS (black bars) in 2006 and 2007. Circles indicate the snowmelt time of individual plots. Stars indicate the first date of seed dispersal. The number of inflorescences per plot is shown in parenthesis.
Study plants
Phyllodoce aleutica (Ericaceae) is an evergreen dwarf shrub inhabiting the alpine snowbed of the North Pacific region. In the Taisetsu Mountains, P. aleutica is frequently distributed among middle to late snowmelt locations. A related species, Phyllodoce caerulea occurs in habitats with early snowmelt. A F1 hybrid of these species, Phyllodoce caerulea f. yesoensis occupies the intermediate habitat of these species along the snowmelt gradient with large overlaps with the distribution range of P. aleutica (Kameyama et al., 2008). A previous study revealed that P. aleutica is self-compatible and can interbreed with other Phyllodoce taxa (Kasagi, 2002; Kasagi and Kudo, 2003, 2005). However, these studies were not based on genetic assessment, and the phylogenetic relationships among these Phyllodoce taxa were only revealed afterwards (Kameyama et al., 2008). Therefore, a reassessment of their mating system is needed. Phyllodoce flowers are predominantly visited by bumble-bees (Bombus spp.) and they are important nectar and pollen sources for bumble-bees due to their abundant flower production and long flowering season in alpine regions (Kasagi and Kudo, 2003).
Phenology and flower production
The times of snowmelt, flowering and fruit dehiscence of P. aleutica were recorded within individual plots in 2006 and 2007. Observation of flowering phenology was performed at 3- to 7-d intervals. At the peak of flowering, the numbers of inflorescences were counted at each plot. The correlation between snowmelt and first flowering date (in day of year) was analysed using Spearman's correlation test.
Visit frequency and behaviour of bumble-bees
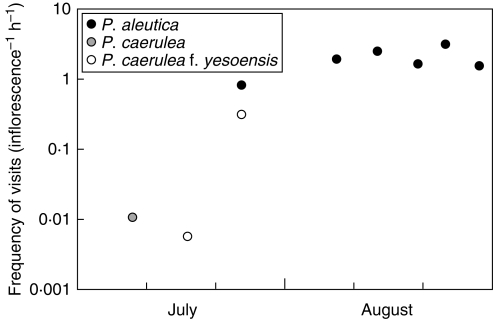
To clarify the seasonal trend of pollinator activity, the frequency and types of bumble-bee species visiting Phyllodoce flowers were assessed in 2007. Because the flowering of P. aleutica did not occur by mid-July, observations of P. caerulea and P. caerulea f. yesoensis made earlier in the season were included to expand the observation period. A quadrat (5 × 5 m) was established in each plot at the peak flowering time of the Phyllodoce taxa, and the number of inflorescences and the species and caste of bumble-bees that visited flowers in each quadrat were recorded. The observation period ranged from 2 h to 6 h depending on visit frequency. The observations of bumble-bees were conducted on calm, fine days between 9 July and 29 August; P. caerulea population on 9 July, P. caerulea f. yesoensis population on 17 July, mixed P. caerulea f. yesoensis and P. aleutica populations on 25 July, and P. aleutica populations on 8, 14, 20, 24 and 29 August.
For workers of Bombus hypocrita sapporensis (Apidae), the predominant pollinator of P. aleutica (see Results), data were recorded on foraging behaviour, i.e. foraging type (nectar or pollen), sequential inflorescence visits within a quadrat, the number of flowers visited per inflorescence, and flight distance between inflorescences for individual bees. Mean values of individual bees were used for the analyses of flower number visited and flight distance. The generalized linear model (GLM) was used for the analyses of sequential inflorescence visits considering a logarithmic link function and a Poisson error distribution. GLM assuming a Gaussian error distribution was used for the analyses of the number of flowers visited and flight distance. In the GLMs, observation date from the first appearance of workers (19 July), foraging type and inflorescence density of the quadrat were included as explanatory variables, and then model selection based on the AIC was performed.
Microsatellite analysis
Five microsatellite markers [PC24-1, PC33, PC57, PA27 (Kameyama et al., 2006) and RM9D1 (Naito et al., 1998)] were used to estimate the paternity of seeds produced by artificial pollination experiments and in natural conditions (explained later). In all analyses, genomic DNA was isolated from leaves or seeds by a cetyltrimethylammonium bromide (CTAB) extraction procedure (Stewart and Via, 1993). Polymerase chain reaction (PCR) amplification was performed with a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). The PCR products were examined using an ABI PRISM 3100 automated sequencer (Applied Biosystems) and GENESCAN analysis software (Applied Biosystems).
The allele frequencies of five microsatellite markers, which were required to conduct the assignment test (see section ‘Seed set and paternity’), were estimated from the genotypes of leaves collected from 209 P. aleutica genets and 54 P. caerulea f. yesoensis genets, which were randomly collected over plots. The number of alleles per locus and the observed heterogeneity for each locus were calculated, and Wright's fixation index (F; Weir and Cockerham, 1984) of P. aleutica was calculated using GENEPOP version 3.4 on the Web (Raymond and Rousset, 1995).
Breeding system
To assess the breeding system of P. aleutica, artificial pollination experiments were conducted at the E plot in 2007, and the M and L plots in 2006 near Lake Hisago (HIS). Experiments were not conducted at the E plot in 2006 due to bad weather conditions. Sixty inflorescences were selected at random from each plot, and ten inflorescences were used for each of the following six pollination treatments; outcross (O), self (S), hybrid (H: P. caerulea f. yesoensis), and the mixtures of O and S (OS), O and H (OH), and O, S and H (OSH).
The selected inflorescences were covered with fine-meshed nylon nets to exclude insect visitors. For each inflorescence, one target flower bud was emasculated just before opening and other flowers were removed. After opening of the target flowers, hand-pollination was conducted by covering the stigma surface completely with pollen grains. Pollen grains for each treatment were collected from a single pollen donor growing at least 5 m away from the recipient plant. All fruits derived from the pollination treatments were harvested just before dehiscence and seed set (proportion of ovules that developed into matured seeds) was measured using a microscope. To evaluate the effects of snowmelt time (E, M and L plots) and pollination treatment (pollen donor: O, S, H, OS, OH and OSH) on seed set, logistic regression analysis was applied, in which snowmelt time, pollination treatment, and their interaction were considered to be fixed factors and the maternal plant to be a random factor. Then, model selection based on the AIC was performed. In some cases, fruits were damaged accidentally by insects or human disturbance or dehisced before harvesting, which reduced the number of samples for each treatment (see Results).
The paternity of seeds produced by the mixed pollination treatments (OS, OH and OSH) was identified unambiguously by comparing the microsatellite genotypes of pollen donors used in the pollination experiment, maternal plants, and their seeds. Totals of 8, 8 and 12 seeds were analysed per fruit from each of ten fruits produced by OS, OH, and OSH, respectively. To evaluate the effects of snowmelt time (E, M and L plots) on paternity of OS and OH, logistic regression analysis was performed, in which snowmelt time was considered to be a fixed factor and the maternal plant to be a random factor. In the analysis of OSH, multinominal logistic regression was applied, where snowmelt time was considered to be a fixed factor and no random effect was considered. In all analyses, model selection based on the AIC was performed.
Seed set and paternity
To evaluate seed set under natural conditions, the following experiments were conducted along the snowmelt gradient (E, M and L plots) at two sites (HIS and GOS) over 2 years (2006 and 2007). Twenty-four inflorescences were selected at random for each plot every year, then one flower was emasculated and the other flowers were left intact. The emasculated flower and a randomly selected single intact flower were marked with fine coloured tape in each inflorescence. In 2006, 12 and none of 24 inflorescences were emasculated at the E plot at HIS and GOS, respectively, because of bad weather conditions. In addition, a small number of fruits were damaged accidentally by insects or human disturbance, which further reduced the number of samples for each treatment (see Results). Fruits were harvested just before dehiscence and their seed-set rate was measured as described above. The contribution of autogamous self-pollination to seed set was estimated, where 1 – (seed set of emasculated flowers/seed set of intact flowers) is designated as ‘autogamous impact’. The effects of year (2006 and 2007), site (HIS and GOS), snowmelt time (E, M and L plots) and treatment (control and emasculation) on seed set were estimated by logistic regression analysis for which year, site, snowmelt time, treatment and the interaction of snowmelt time and treatment were considered to be fixed factors and the maternal plant to be a random factor. Then, model selection based on the AIC was performed.
Two seeds were selected from each harvested fruit and their paternities were estimated from the genotypic information of seeds and their maternal plants along with allele frequencies of P. aleutica and P. caerulea f. yesoensis. Paternity at the E plot at HIS could not be analysed in 2006 due to the extremely small number of seeds that were produced, and a small number of seeds could not be genotyped probably because of the low quality and/or quantity of extracted DNA (see Results). Selfed seeds whose genotypes were explained by maternal alleles were identified directly. The paternal plants of the remaining seeds belong to either one of two pollen sources: P. aleutica or P. caerulea f. yesoensis. The pollen haplotype of individual seeds was estimated by subtracting the maternal alleles from those of the seeds. In many cases, highly polymorphic microsatellite markers made it possible to identify the alleles derived from paternal plants unambiguously. The most likely pollen sources were assigned for every pollen haplotype by multiplying the allele frequencies of potential sources (P. aleutica or P. caerulea f. yesoensis) and comparing their likelihoods (Paetkau et al., 1995). If the alleles derived from paternal plants were ambiguous, both alleles were retained as candidates and their average probabilities (allele frequencies) were used. High genetic differentiation between the two taxa (Kameyama et al., 2008) ensured the adequacy of this assignment test. The effects of year (2006 and 2007), site (HIS and GOS), snowmelt time (E, M and L plots), and treatment (control and emasculation) on paternity were estimated by multinominal logistic regression analysis for which year, site, snowmelt time, treatment and the interaction of snowmelt time and treatment were considered to be fixed factors and no random factor was considered. Then, model selection based on the AIC was performed.
RESULTS
Phenology and flower production
Snowmelt occurred from the E plots to the L plots in both years, while snowmelt progressed slowly in 2007 (Fig. 1). Flowering occurred from mid-July to late August depending on snowmelt time at each plot. First flowering was observed from 6 d to 21 d after snowmelt, and the period for flowering initiation from snow disappearance (y, day) was significantly shortened with the delay of snowmelt as follows: y = −0·343x + 79·06, r2 = 0·83, n = 12, P < 0·0001, where x is the day of year of snowmelt. This is because daily temperature increases as the season progresses from June to August. Flowering lasted 14·3 d (range 10–21 d, n = 12) within the plots. Seed dispersal (fruit dehiscence) usually occurred in early September except for the L plot at HIS in 2007, where seed dispersal occurred in late September due to late snowmelt and flowering.
Inflorescence production varied highly over the plots between years. The number of inflorescences within plots ranged from 401 to 25107 (Fig. 1). Inflorescence production increased at later snowmelt plots, and it was greater in 2007 than in 2006 except for E plot at GOS.
Visit frequency and behaviour of bumble-bees
Bombus hypocrita sapporensis was the dominant visitor of Phyllodoce flowers, and it accounted for 74–100 % of visitors (Table 1). In early to mid-July, only queens were observed and at very low frequency. In late July, worker bees appeared and visit frequencies increased abruptly (Fig. 2). The high frequency of visits lasted until late August. Workers of Bombus beaticola moshkarareppus, the second most frequent visitor of Phyllodoce flowers, were observed in August. New queens and male bees appeared after mid-August.
Table 1.
Changes over the season in species' compositions of bumble-bees visiting Phyllodoce flowers in the summer of 2007. Data are the proportion of Bombus species recorded
|
B. hypocrita |
B. beaticola |
B. hyponorum | ||||||
|---|---|---|---|---|---|---|---|---|
| Season | Observation hours | No. of bees observed | Queen | Worker | Male | Queen | Worker | Worker |
| Early July | 5 | 7 | 1·00 | – | – | – | – | – |
| Mid-July | 6 | 11 | 0·91 | 0·09 | – | – | – | – |
| Late July | 12 | 110 | 0·03 | 0·96 | – | – | – | 0·01 |
| Early August | 3 | 66 | – | 0·86 | – | – | 0·14 | – |
| Mid-August | 6 | 243 | 0·03 | 0·84 | – | – | 0·08 | 0·04 |
| Late August | 7 | 248 | 0·00 | 0·74 | 0·03 | 0·01 | 0·22 | – |
Fig. 2.
Visit frequencies of bumble-bees per inflorescence per hour throughout the flowering periods of Phyllodoce taxa in 2007.
Workers of B. hypocrita visited 11–25 inflorescences sequentially within plots (Table 2). GLM analysis revealed that sequential visits increased with inflorescence density (Z = 12·71, P < 0·001) and that pollen-foraging bees visited more inflorescences than nectar-foraging bees (Z = 4·67, P < 0·001), while seasonal effect was not selected by the AIC. The number of flower visits per inflorescence ranged from 2·0 to 3·0; visits decreased as the season progressed (t = −4·44, P < 0·001) and increased with inflorescence density (t = 8·58, P < 0·001), while foraging behaviour was not selected by the AIC. Flight distance between inflorescences ranged from 13 cm to 21 cm; flight distance increased as the season progressed (t = 6·87, P < 0·001) and decreased with increasing inflorescence density (t = −8·39, P < 0·001), while foraging behaviour was again not selected by the AIC. In late July, workers visited P. aleutica flowers for nectar collection, while the proportion of pollen collection increased as the season progressed.
Table 2.
Foraging behaviour of Bombus hypocrita workers in Phyllodoce aleutica patches
| Season | Inflorescence density m–2 | Behaviour | n | No. of inflorescence visits | No. of flower visits per inflorescence | Inter-inflorescence flight distance (cm) |
|---|---|---|---|---|---|---|
| Late July | 36 | Nectar | 39 | 10·6 ± 6·0 | 2·4 ± 0·7 | 14·8 ± 4·1 |
| Early August | 32 | Nectar | 22 | 12·5 ± 7·4 | 2·1 ± 0·5 | 20·8 ± 7·9 |
| Pollen | 18 | 12·6 ± 7·8 | 2·0 ± 0·4 | 20·5 ± 4·7 | ||
| Mid-August | 165 | Nectar | 26 | 18·5 ± 10·6 | 3·0 ± 0·5 | 13·0 ± 3·7 |
| Pollen | 26 | 24·9 ± 15·3 | 3·0 ± 0·6 | 12·9 ± 3·3 | ||
| Late August | 124 | Nectar | 25 | 17·6 ± 10·7 | 2·4 ± 0·7 | 17·6 ± 5·2 |
| Pollen | 26 | 18·9 ± 10·4 | 2·1 ± 0·4 | 19·7 ± 4·7 |
Sequential inflorescence visits within patches, the number of flower visits per inflorescence, and flight distance (cm) between inflorescences.
Values are mean ± s.e.
Microsatellite polymorphism
The five microsatellite markers were highly variable and the total numbers of alleles were 25, 30, 4, 28 and 32 at loci PC24-1, PC33, PC57, PA27 and RM9D1, respectively. Within P. aleutica and P. caerulea f. yesoensis, the number of alleles per locus ranged from 3 to 24 and 3 to 29, respectively. The observed heterogeneities for each locus ranged from 0·524 to 0·967 (mean 0·854) and 0·944–1·000 (mean 0·983) for P. aleutica and P. caerulea f. yesoensis, respectively. The fixation index (F) of P. aleutica was not different from zero at all loci within all plots (P > 0·05), indicating that selfed progenies do not contribute to the maintenance of P. aleutica populations.
Breeding system
Seed sets obtained by artificial pollination experiments are shown in Table 3. Seed sets at the E plot were significantly lower than those at the M and L plots (E vs. M, Z = −3·95, P < 0·001; E vs. L, Z = −4·57, P < 0·001), irrespective of pollination treatments. The interaction between plot and pollination treatment was not selected by the AIC, indicating that the effects of pollination treatment were similar among plots. Seeds sets from self (S) and hybrid (H) pollination were significantly lower than those obtained by outcross (O) pollination (O vs. S, Z = −6·63, P < 0·001; O vs. H, Z = −5·16, P < 0·001), and the self-compatibility and heterospecific compatibility over plots were 0·48 and 0·57, respectively. These results indicate that P. aleutica is partially self-compatible and can produce seed by self-pollination. It is noteworthy that the seed sets of mixed pollination (OS, OH and OSH) were not significantly different from those of outcross (O) pollination (O vs. OS, Z = 0·81, P = 0·42; O vs. OH, Z = 0·37, P = 0·71; O vs. OSH, Z = 0·34, P = 0·73). This indicates that there is no interference effect of self- and heterospecific pollen deposition on the stigmas in P. aleutica.
Table 3.
Seed set obtained by artificial pollination experiments along the snowmelt gradient (E, M and L plots)
| Pollen donor | E plot | M plot | L plot |
|---|---|---|---|
| Outcross (O) | 0·54 ± 0·05 (10) | 0·85 ± 0·05 (6) | 0·67 ± 0·06 (10) |
| Self (S) | 0·22 ± 0·06 (10) | 0·42 ± 0·08 (9) | 0·33 ± 0·06 (10) |
| Hybrid (H) | 0·32 ± 0·07 (10) | 0·31 ± 0·08 (6) | 0·48 ± 0·06 (10) |
| OS | 0·60 ± 0·06 (10) | 0·71 ± 0·05 (10) | 0·83 ± 0·01 (10) |
| OH | 0·61 ± 0·06 (10) | 0·74 ± 0·06 (10) | 0·72 ± 0·05 (10) |
| OSH | 0·61 ± 0·06 (10) | 0·65 ± 0·07 (10) | 0·82 ± 0·05 (10) |
Sample sizes are shown in parenthesis.
Values are mean ± s.e.
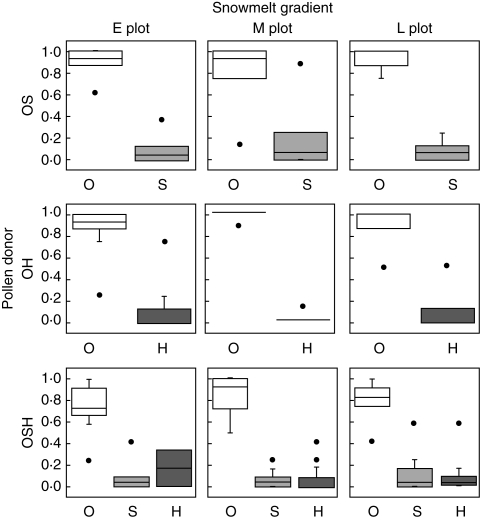
Most seeds produced by the mixed pollination treatments (OS, OH and OSH) were sired by outcross pollen (Fig. 3), and their paternities did not differ among plots (plot was not selected by the AIC). Thus, it was further examined whether the differences in paternity were solely dependent on the differences of self- and/or heterospecific compatibilities. For example, because the self-compatibility, which was obtained from the single outcrossing and selfing treatments, was 0·48, the expected probabilities of the outcrossing and selfing rates in the OS treatment were 0·68 [= 1/(1 + 0·48)] and 0·32 [= 0·48/(1 + 0·48)], respectively. In all pollination treatments, the observed outcrossing rates were significantly higher than those expected (χ2 tests: OS, χ2 = 47·8, P < 0·001; OH, χ2 = 86·9, P < 0·001; OSH, χ2 = 134·6, P < 0·001). This indicates that selective outcrossing occurs when stigmas receive pollen from multiple sources.
Fig. 3.
Paternity of seeds produced by mixed pollination treatment (OS, OH and OSH). Box plots show the median and ranges from 25th to 75th and 10th to 90th percentiles. The circles represent outliers.
Seed set and paternity
Seed-set rate under natural conditions (Table 4) varied greatly depending on year, plot, treatment, and the interaction between plot and treatment, while the site effect was not selected by the AIC. Seed-set rate was significantly lower in 2006 (Z = −5·93, P < 0·001) and at the E plots (E vs. M, Z = −8·38, P < 0·001; E vs. L, Z = −9·14, P < 0·001). Seed-set rates of intact flowers varied highly between 2006 and 2007 at the E plots (18–62 % in HIS and 25–40 % in GOS). The emasculation treatment significantly decreased seed-set rate at the E plots (Z = −4·12, P < 0·001), but no differences were observed between the M and L plots (Z = −0·32, P = 0·75). Autogamous impact, which is the contribution of autogamous pollination to seed-set rate, was apparently high at the E plots (range 0·26 to 0·95, mean 0·52) compared with that at the M and L plots (range −0·15 to 0·20, mean 0·07).
Table 4.
Seed-set rate along the snowmelt gradients (E, M and L plots) at two sites (HIS and GOS) during 2 years (2006 and 2007) under intact and emasculation treatments
| Site | Treatment | 2006 |
2007 |
||||
|---|---|---|---|---|---|---|---|
| E plot | M plot | L plot | E plot | M plot | L plot | ||
| HIS | Intact | 0·18 ± 0·04 (24) | 0·68 ± 0·03 (24) | 0·70 ± 0·04 (23) | 0·62 ± 0·04 (24) | 0·77 ± 0·03 (24) | 0·59 ± 0·05 (24) |
| Emasculated | 0·01 ± 0·01 (11) | 0·58 ± 0·05 (18) | 0·58 ± 0·05 (20) | 0·37 ± 0·05 (24) | 0·70 ± 0·04 (23) | 0·47 ± 0·06 (24) | |
| Autogamous impact | 0·95 ± 0·04 (11) | 0·20 ± 0·07 (18) | 0·12 ± 0·08 (19) | 0·35 ± 0·09 (24) | 0·07 ± 0·05 (23) | −0·04 ± 0·23 (24) | |
| GOS | Intact | 0·25 ± 0·03 (24) | 0·60 ± 0·04 (24) | 0·67 ± 0·04 (24) | 0·40 ± 0·05 (24) | 0·65 ± 0·05 (24) | 0·83 ± 0·02 (24) |
| Emasculated | No data | 0·50 ± 0·06 (22) | 0·71 ± 0·04 (21) | 0·19 ± 0·04 (24) | 0·66 ± 0·04 (23) | 0·76 ± 0·03 (24) | |
| Autogamous impact | No data | 0·20 ± 0·06 (22) | −0·15 ± 0·15 (21) | 0·26 ± 0·25 (22) | 0·05 ± 0·03 (22) | 0·09 ± 0·03 (24) | |
Autogamous impact is defined as 1 – (seed set of emasculated flowers/seed set of intact flowers).
Samples sizes are shown in the parenthesis.
Values are mean ± s.e.
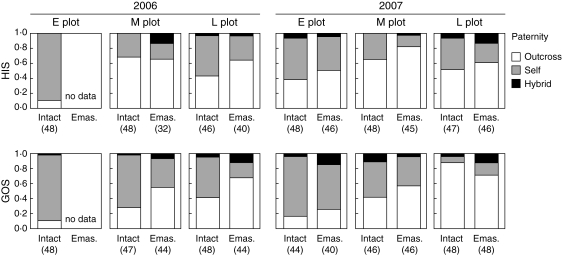
Seeds produced under natural conditions were mostly sired by conspecific (P. aleutica) pollen, and the contribution of hybrid P. caerulea f. yesoensis pollen was very small (range 0–0·15, mean 0·058; Fig. 4). The outcrossing rate under natural conditions varied greatly among sites, years, plots and treatments, while the interaction between plot and treatment was not selected by the AIC. The effect of the site was marginal but the outcrossing rate tended to be lower at GOS (O vs. S, Z = −2·59, P = 0·009; O vs. H, Z = −2·34, P = 0·020). The outcrossing rate was significantly lower at the E plot (O vs. S, Z = −8·02, P < 0·001; O vs. H, Z = −2·48, P = 0·013) but significantly higher in 2007 (O vs. S, Z = 4·46, P < 0·001; O vs. H, Z = −0·64, P = 0·53) and was significantly increased by the emasculation treatment (O vs. S, Z = 4·96, P < 0·001 and O vs. H, Z = −1·68, P = 0·094).
Fig. 4.
Paternity of seeds produced under natural conditions along the snowmelt gradients (E, M and L plots) at two sites (HIS and GOS) during 2 years (2006 and 2007) under intact and emasculation treatments (Intact and Emas.). Two seeds were collected from each fruit (see Table 4 for number of fruits analysed). The sample sizes are shown in parenthesis. The paternity of each seed was assigned based on five microsatellite genotypes (see text).
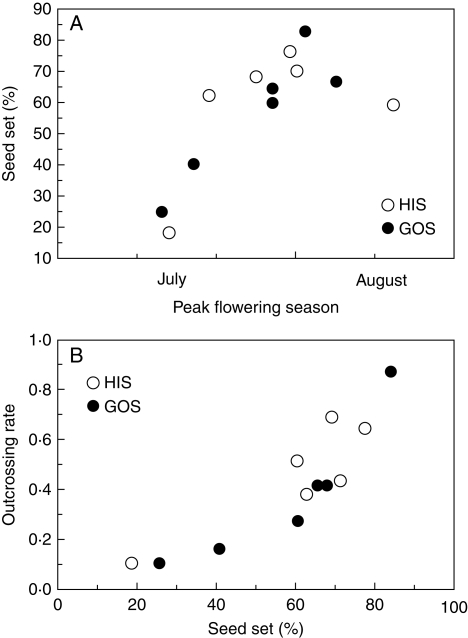
When seed-set data of intact flowers were pooled over plots, there was a significant positive correlation between peak flowering time and seed-set rate (r = 0·61, P = 0·035, Spearman's correlation; Fig. 5A). Furthermore, the outcrossing rate correlated strongly with the seed-set rate (r = 0·87, P = 0·0002), ranging from 10 % to 88 % (Fig. 5B). Therefore, both seed-set rate and outcrossing rate varied greatly both spatially and temporally.
Fig. 5.
(A) Relationships between flowering time and seed-set rate under natural pollination and (B) between seed-set rate and outcrossing rate at two sites (HIS and GOS).
DISCUSSION
Temporal variations in flowering time and bumble-bee availability
The flowering time of P. aleutica was determined by the time of snowmelt and consequent temperature conditions, as demonstrated in a previous study (Kudo and Suzuki, 1999). Although snowmelt time varies highly from year to year (Kudo and Hirao, 2006), the flowering period of P. aleutica usually lasts from mid-July to late August in this region. The flowering of P. caerulea, which inhabits early-snowmelt places, starts in early July, then P. caerulea f. yesoensis blooms at almost the same period as P. aleutica (Kameyama et al., 2008). Thus, Phyllodoce taxa can provide nectar and pollen rewards to bumble-bees over a period of 2 months.
During the flowering period, the activity and behaviour of bumble-bees changed greatly. In early to mid-July, only queen bees visited Phyllodoce flowers, occasionally for nectar foraging. In addition to the low visit frequency, bumble-bees tended to prefer P. caerulea f. yesoensis flowers to P. aleutica flowers in early snowmelt places due to the larger nectar presentation by P. caerulea f. yesoensis (Kasagi and Kudo, 2003; note that P. caerulea in that study was proved to be mostly P. caerulea f. yesoensis; see Kameyama et al., 2008). Therefore, early snowmelt populations of P. aleutica suffer from severe pollen limitation, as indicated by the previous study (Kasagi and Kudo, 2003). After the appearance of worker bees in late July, the pollinator situation changed dramatically. Visit frequency to inflorescences by worker bees was 100 times greater than by queen bees. This very clear seasonal change in pollinator activity reflects the life history of bumble-bees inhabiting alpine environments with very short growth seasons (Tomono and Sota, 1997). Worker bees visited P. aleutica flowers mainly for nectar in late July, but the proportion foraging for pollen increased later in the season. Worker bees tended to visit many inflorescences and inter-inflorescence flights were about 20 cm. Pollen-collecting bees tended to visit more inflorescences within patches than nectar-foraging bees. Furthermore, sequential inflorescence visits increased and flight distance decreased with increasing inflorescence density as reported in previous studies (Thomson, 1981; Zimmerman, 1981; Goulson, 2000). This means that the intensity of geitonogamous pollination should accelerate in the late flowering period. Therefore, pollination situations shift from quantitative pollen limitation to qualitative pollen limitation as the season progresses.
Mating system of P. aleutica
Although autogamous selfing is evident in P. aleutica, the probability of selfed seed production was significantly lower than that of seed production by outcrossing even when stigmas received enough pollen grains. Partial self-sterility is frequently reported in many ericaceous species with mixed-pollination systems including alpine shrub species, and it has been explained as early-acting inbreeding depression in which a certain number of selfed embryos are aborted (Krebs and Hancock, 1991; Rathcke and Real, 1993; Hokanson and Hancock, 2000; Raspé et al., 2004; Nuortila et al., 2006). In this case, the production of outcross seeds under mixed-pollination should be accompanied by seed discounting (e.g. Herlihy and Eckert, 2002). In contrast, mixed-pollination in P. aleutica did not decrease the seed-set rate in comparison with pure outcross-pollination. Furthermore, undeveloped embryos (or ovules) remaining in mature fruits are commonly very small in size (Y. Kameyama, pers. obs.), indicating that they might be unfertilized ovules. Because mixed-pollination resulted in a higher proportion of outcross seeds than expected from self-compatibility, P. aleutica may have cryptic self-incompatibility without ovule discounting (Cruzan and Barrett, 1993; Eckert and Barrett, 1994).
Cryptic self-incompatibility acts as a buffer against detrimental effects of geitonogamous pollination (Eckert and Barrett, 1994; Galloway et al., 2003). Because P. aleutica often forms large clonal patches in which inflorescences are produced at high densities, geitonogamous self-pollination is common, especially in late-snowmelt populations because of intensive pollen collection by worker bees. Thus, cryptic self-incompatibility is an effective mechanism for promoting outcrossing.
Autogamous selfing is often regarded as an evolutionary response to cope with pollen limitation (reproductive assurance; Kalisz and Vogler, 2003; Moeller, 2006; but see also Kubota et al., 2008). Seed production of P. aleutica was actually ensured by autogamous selfing at the E plots, where pollen availability was limited because of low pollinator activity. In addition, germination rates of selfed and outcrossed seeds were 0·91 and 0·94, respectively, with no significant difference (Y. Kameyama et al., unpubl. res.). However, the ability for selfing was similar among plots (SC = 0·48), and the Wright's fixation index (F) was not different from zero at all five loci at all plots. These results indicate that selfed progeny do not generally contribute to the established-plant component of P. aleutica populations, suggesting that there is no adaptive significance of autogamous selfing in this species. The establishment of selfed progeny should be prevented after seed germination, because of strong late-acting inbreeding depression (Michalski and Durka, 2007; see also Ishida, 2006).
This conclusion is contrary to what was proposed in previous studies (Kasagi, 2002; Kasagi and Kudo, 2003), in which it was speculated that the evolution of selfing ability in early snowmelt populations (SC = 0·44–0·61) occurred to cope with strong pollen limitation in comparison with late snowmelt populations (SC = 0·04–0·06). The extremely low self-compatibility indices in late snowmelt populations might be ascribable to the yearly variation in climate conditions. In alpine snowbeds, yearly fluctuations of snowmelt time tend to be large in late snowmelt places (Kudo and Hirao, 2006). If inter-annual variations in snowmelt time affect the level of resource limitation and if selective abortion prevents the development of selfed seeds depending on resource availability, the apparent levels of self-compatibility fluctuate especially in late snowmelt habitats. While further studies are required, it is clear that the self-compatibility of P. aleutica is not genetically different along the snowmelt gradient, and the progeny derived from selfing do not contribute to the maintenance of P. aleutica populations.
Variations in seed set rate and outcrossing rate
Seed-set rate varied greatly among populations and low seed-set rate in early-flowering plants was caused by pollen limitation due to low pollinator activity (Kudo, 1993; Molau, 1993; Kudo and Suzuki, 2002; Kudo and Hirao, 2006). The most interesting finding of this study is the large variation in outcrossing rate, which was strongly correlated with seed-set rate. In previous studies, variations in outcrossing rate among populations have mainly been reported over comparatively large geographic scales (Barrett and Husband, 1990; Herlihy and Eckert, 2005; Moeller, 2006), which may reflect geographic changes in plant–pollinator interactions. In contrast, there have been very few studies on the variations in outcrossing rate on a local scale. In the comparisons of outcrossing rates of Aquilegia coerulea among populations in the central and southern Rocky Mountains, Brunet and Sweet (2006) revealed that pollinator type and behaviour strongly influence the outcrossing rate, which ranged from 0·41 to 0·93. It was concluded that such variations are caused by geitonogamous selfing and are a non-adaptive phenomenon (see also Eckert, 2000). In the present study, the outcrossing rate of P. aleutica varied from 0·10 to 0·88 over a few kilometres. Because the outcrossing rate was low in early-snowmelt populations, where low pollinator activity restricted seed production, variation in outcrossing rate was largely caused by autogamous selfing. Because of cryptic self-incompatibility, P. aleutica might reduce the negative effects of geitonogamous pollination under frequent visits by bumble-bee workers in the late season. Nevertheless, the outcrossing rate at the M and L plots under natural pollination (Fig. 4) was lower than that of the mixed-pollination experiment (about 90 %). This indicates that intensive geitonogamous pollination within clonal patches may dilute the deposition of outcross pollen on stigmas (Eckert, 2000).
Despite the frequency of interspecific bumble-bee movements between P. aleutica and P. caerulea f. yesoensis (Kasagi and Kudo, 2005), the interference of heterospecific pollen deposition on P. aleutica stigmas was small. Although moderate heterospecific compatibility was observed with the supply of pure P. caerulea f. yesoensis pollen, the proportion of outcrossed seeds was predominant when mixed pollen from both species was supplied. This trend was very similar to the pattern of cryptic self-compatibility. Furthermore, ovule and/or seed discounting by heterospecific pollination was not evident because the seed-set rate of the mixed-pollination was similar to that of pure outcrossing pollination. Some studies have demonstrated that interspecific competition for pollination influences the quality and quantity of pollination service, resulting in a reduction in seed production and outcrossing rate (Brown et al., 2002; Bell et al., 2005). However, the mating system of P. aleutica effectively prevents hybrid formation irrespective of the evidence of mixed-pollination under natural conditions. A backcross taxon P. aleutica var. marmorata is formed by the hybridization between P. caerulea f. yesoensis (pollen donor) and P. aleutica (recipient), but its occurrence was very rare (Kameyama et al., 2008). In addition to the low production rate of the hybrid seeds under natural pollination (Fig. 4), this indicates weak interference effects on the pollination process between the species, at least in terms of seed production.
In conclusion, the large variation in outcrossing rates among local populations and between years is caused by the interaction between the spatio-temporal variation in pollinator availability and the mixed-mating system of P. aleutica. However, the selfing ability of this species does not contribute to the maintenance of populations. In a theoretical model of the mixed-mating system, Porcher and Lande (2005) predict that mixed-mating systems with low to moderate selfing rates may not have adaptive meaning but may be a by-product of unavoidable geitonogamous selfing. This may be the case in P. aleutica, regardless of altitude, because of the formation of long-lived large clonal patches with intensive inflorescence production.
ACKNOWLEDGEMENTS
The authors thank Y. Kawai for field survey support, A. S. Hirao, T. Kasagi, T. Ida and T. Kubo for helpful discussions, and M. Ohara for offering facilities for genetic analysis. This work was supported by a grant-in-aid from the Ministry of Environment of Japan from the Global Environmental Research Fund (F-052) and from the Japan Society for the Promotion of Science (16370007, 19770009).
LITERATURE CITED
- Aizen MA. Flower sex ratio, pollinator abundance, and the seasonal pollination dynamics of a protandrous plant. Ecology. 2001;82:127–144. [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86:762–771. [Google Scholar]
- Barrett SCH, Husband B. Variation in outcrossing rates in Eichhornia paniculata: the role of demographic and reproductive factors. Plant Species Biology. 1990;5:41–55. [Google Scholar]
- Brown BJ, Mitchell RJ, Graham SA. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology. 2002;83:2328–2336. [Google Scholar]
- Brunet J, Sweet HR. Impact of insect pollinator group and floral display size on outcrossing rate. Evolution. 2006;60:234–246. [PubMed] [Google Scholar]
- Cruzan MB, Barrett SCH. Contribution of cryptic incompatibility to the mating system of Eichhornia paniculata (Pontederiaceae) Evolution. 1993;47:925–934. doi: 10.1111/j.1558-5646.1993.tb01245.x. [DOI] [PubMed] [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Eckert CG, Barrett SCH. Post-pollination mechanisms and the maintenance of outcrossing in self-compatible, tristylous, Decodon verticillatus (Lythraceae) Heredity. 1994;72:396–411. [Google Scholar]
- Galloway LF, Etterson JR, Hamrick JL. Outcrossing rate and inbreeding depression in the herbaceous autotetrapoid, Campanula americana. Heredity. 2003;90:308–315. doi: 10.1038/sj.hdy.6800242. [DOI] [PubMed] [Google Scholar]
- Goulson D. Why do pollinators visit proportionally fewer flowers in large patches? Oikos. 2000;91:485–492. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Evolution of self-fertilization at geographical range margins? A comparison of demographic, floral, and mating system variables in central vs. peripheral populations of Aquilegia canadensis (Ranunculaceae) American Journal of Botany. 2005;92:744–751. doi: 10.3732/ajb.92.4.744. [DOI] [PubMed] [Google Scholar]
- Hirao AS, Kameyama Y, Ohara M, Isagi Y, Kudo G. Seasonal changes in pollinator activity influence pollen dispersal and seed production of the alpine shrub Rhododendron aureum (Ericaceae) Molecular Ecology. 2006;15:1165–1173. doi: 10.1111/j.1365-294X.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- Hokanson K, Hancock J. Early-acting inbreeding depression in three species of Vaccinium (Ericaceae) Sexual Plant Reproduction. 2000;13:145–150. [Google Scholar]
- Inouye DW, Pyke GH. Pollination biology in the Snowy Mountains of Australia: comparisons with montane Colorado, USA. Australian Journal of Ecology. 1988;13:191–210. [Google Scholar]
- Ishida K. Maintenance of inbreeding depression in a highly self-fertilizing tree, Magnolia obovata Thunb. Evolutionary Ecology. 2006;20:173–191. [Google Scholar]
- de Jong, Waser TJ, Klinkhamer PGL. Geitonogamy: the neglected side of selfing. Trends in Ecology and Evolution. 1993;8:321–325. doi: 10.1016/0169-5347(93)90239-L. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology. 2003;84:2928–2942. [Google Scholar]
- Kameyama Y, Kasagi T, Kudo G. Eight microsatellite markers for sympatric alpine shrubs, Phyllodoce aleutica and P. caerulea (Ericaceae) Molecular Ecology Notes. 2006;6:402–404. [Google Scholar]
- Kameyama Y, Kasagi T, Kudo G. A hybrid zone dominated by fertile F1s of two alpine shrub species, Phyllodoce caerulea and Phyllodoce aleutica, along a snowmelt gradient. Journal of Evolutionary Biology. 2008;21:588–597. doi: 10.1111/j.1420-9101.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- Kasagi T. Intraspecific variations in selfing ability of two sympatric alpine shrubs, Phyllodoce caerulea and Phyllodoce aleutica, along snowmelt gradients. Plant Species Biology. 2002;17:133–138. [Google Scholar]
- Kasagi T, Kudo G. Variations in bumble bee preference and pollen limitation among neighboring populations: comparisons between Phyllodoce caerulea and Phyllodoce aleutica (Ericaceae) along snowmelt gradients. American Journal of Botany. 2003;90:1321–1327. doi: 10.3732/ajb.90.9.1321. [DOI] [PubMed] [Google Scholar]
- Kasagi T, Kudo G. Interspecific pollinator movements and heterospecific incompatibility: comparisons between Phyllodoce caerulea and Phyllodoce aleutica along snowmelt gradients. Evolutionary Ecology Research. 2005;7:73–87. [Google Scholar]
- Krebs SL, Hancock JF. Embryonic genetic load in the highbush blueberry, Vaccinium corymbosum (Ericaceae) American Journal of Botany. 1991;78:1427–1437. [Google Scholar]
- Kubota S, Kameyama Y, Hirao AS, Ohara M. Adaptive significance of self-fertilization in a hermaphroditic perennial, Trillium camshatcense (Melanthiaceae) American Journal of Botany. 2008;95:482–489. doi: 10.3732/ajb.95.4.482. [DOI] [PubMed] [Google Scholar]
- Kudo G. Effects of snows-free period on the phenology of alpine plants inhabiting snow patches. Arctic and Alpine Research. 1991;23:436–443. [Google Scholar]
- Kudo G. Relationship between flowering time and fruit set of the entomophilous alpine shrub, Rhododendron aureum (Ericaceae), inhabiting snow patches. American Journal of Botany. 1993;80:1300–1304. [Google Scholar]
- Kudo G. Flowering phenologies of animal-pollinated plants: reproductive strategies and agents of selection. In: Barrett SCH, Harder LD, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 139–155. [Google Scholar]
- Kudo G, Hirao AS. Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Population Ecology. 2006;48:49–58. [Google Scholar]
- Kudo G, Suzuki S. Flowering phenology of alpine plant communities along a gradient of snowmelt timing. Polar Bioscience. 1999;12:100–113. [Google Scholar]
- Kudo G, Suzuki S. Relationships between flowering phenology and fruit-set of dwarf shrubs in alpine fellfields in northern Japan: a comparison with a subarctic heath land in northern Sweden. Arctic, Antarctic, and Alpine Research. 2002;34:185–190. [Google Scholar]
- Michalski S, Durka W. High selfing and high inbreeding depression in peripheral populations of Juncus atratus. Molecular Ecology. 2007;16:4715–4727. doi: 10.1111/j.1365-294X.2007.03547.x. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Molau U. Relationships between flowering phenology and life history strategies in tundra plants. Arctic and Alpine Research. 1993;25:391–402. [Google Scholar]
- Naito K, Isagi Y, Nakagoshi N. Isolation and characterization of microsatellites of Rhododendron metternichii Sieb. et Zucc. var. hondoense Nakai. Molecular Ecology. 1998;7:927–928. [Google Scholar]
- Nuortila C, Tuomi J, Laine K. Inter-parent distance affects reproductive success in two clonal shrubs, Vaccinium myrtillus and Vaccinium vitis-idaea (Ericaceae) Canadian Journal of Botany. 2002;80:875–884. [Google Scholar]
- Nuortila C, Tuomi J, Aspi J, Laine K. Early-acting inbreeding depression in a clonal dwarf shrub, Vaccinium myrtillus, in a northern boreal forest. Annales Botanici Fennici. 2006;43:36–48. [Google Scholar]
- Paetkau D, Calvert W, Stirling I, Strobeck C. Microsatellite analysis of population structure in Canadian polar bears. Molecular Ecology. 1995;4:347–354. doi: 10.1111/j.1365-294x.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Porcher E, Lande R. The evolution of self-fertilization and inbreeding depression under pollen discounting and pollen limitation. Journal of Evolutionary Biology. 2005;18:497–508. doi: 10.1111/j.1420-9101.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Raspé O, Guillaume P, Jacquemart AL. Inbreeding depression and biased paternity after mixed-pollination in Vaccinium myrtillus L. (Ericaceae) International Journal of Plant Sciences. 2004;165:765–771. [Google Scholar]
- Rathcke B. Competition and facilitation among plants for pollination. In: Real L, editor. Pollination biology. Orlando, FL: Academic Press; 1983. pp. 305–329. [Google Scholar]
- Rathcke B, Real I. Autogamy and inbreeding depression in mountain laurel, Kalmid latifolia (Ericaceae) American Journal of Botany. 1993;80:143–146. [Google Scholar]
- Raymond M, Rousset F. GENEPOP (version 1.2): population genetic software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Stewart CN, Jr, Via LE. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques. 1993;14:748–750. [PubMed] [Google Scholar]
- Thomson JD. Spatial and temporal components of resource assessment by flower-feeding insects. Journal of Animal Ecology. 1981;50:49–59. [Google Scholar]
- Tomono T, Sota T. The life history and pollination ecology of bumblebees in the alpine zone of central Japan. Japanese Journal of Entomology. 1997;65:237–255. [Google Scholar]
- Totland Ø. Intraseasonal variation in pollination intensity and seed set in an alpine population of Ranunculus acris in southwestern Norway. Ecography. 1994;17:159–165. [Google Scholar]
- Waser NM. Interspecific pollen transfer and competition between co-occurring plant species. Oecologia. 1978;36:223–236. doi: 10.1007/BF00349811. [DOI] [PubMed] [Google Scholar]
- Waser NM. Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones CE, Little RJ, editors. Experimental handbook. New York, NY: Scientific and Academic Editors; 1983. pp. 277–238. [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Yumoto T. The ecological pollination syndromes of insect-pollinated plants in an alpine meadow. Ecological Research. 1986;1:83–95. [Google Scholar]
- Zimmerman M. Optimal foraging, plant density and the marginal value theorem. Oecologia. 1981;49:148–153. doi: 10.1007/BF00349181. [DOI] [PubMed] [Google Scholar]