Abstract
Background
Co-flowering plant species frequently share pollinators. Pollinator sharing is often detrimental to one or more of these species, leading to competition for pollination. Perhaps because it offers an intriguing juxtaposition of ecological opposites – mutualism and competition – within one relatively tractable system, competition for pollination has captured the interest of ecologists for over a century.
Scope
Our intent is to contemplate exciting areas for further work on competition for pollination, rather than to exhaustively review past studies. After a brief historical summary, we present a conceptual framework that incorporates many aspects of competition for pollination, involving both the quantity and quality of pollination services, and both female and male sex functions of flowers. Using this framework, we contemplate a relatively subtle mechanism of competition involving pollen loss, and consider how competition might affect plant mating systems, overall reproductive success and multi-species interactions. We next consider how competition for pollination might be altered by several emerging consequences of a changing planet, including the spread of alien species, climate change and pollinator declines. Most of these topics represent new frontiers whose exploration has just begun.
Conclusions
Competition for pollination has served as a model for the integration of ecological and evolutionary perspectives in the study of species interactions. Its study has elucidated both obvious and more subtle mechanisms, and has documented a range of outcomes. However, the potential for this interaction to inform our understanding of both pure and applied aspects of pollination biology has only begun to be realized.
Key words: Alien plants, climate change, competition for pollination, facilitation, mating system, mechanism, Lythrum, Mimulus, pollinator visitation, sexual function, invasive species, pollen loss
INTRODUCTION
Pollination is a classic ecological mutualism in which plants provide floral visitors with rewards such as nectar, and pollinating animals in turn facilitate plant reproduction by dispersing pollen to conspecific plants. Yet this well-recognized mutualism may be substantially altered if co-flowering species compete for the services of shared pollinators. This intriguing counterpoint of mutualistic and competitive interactions may be one reason for a recurring interest among pollination biologists in aspects of competition for pollination (e.g. Robertson, 1895; Waser, 1978a, b, Brown et al., 2002).
Competition for pollination exemplifies the richness of questions and approaches inherent in pollination biology. Its study touches on a range of disciplines, from animal behaviour to plant morphology, and brings to the fore the diverse ecological and evolutionary perspectives that dominate modern pollination biology. The interaction casts into sharp relief the inherent conflict of interest between plants and pollinators, which must be appreciated to understand this and other mutualisms (Bronstein, 2001). Because competition for pollination, unlike other forms of competition among sessile organisms, acts at a distance that varies with and derives from the animals' perspective, it raises fascinating issues of scale and spatial or landscape context. Furthermore, unlike vegetative competition between plants, competition for pollination directly involves reproductive success.
Competition for pollination also serves as a model for theoretical and experimental dissection of mechanisms. Recognition that competition may occur, not only through reduced visitation of flowers by pollinators, but also through changes in the amount and quality of pollen dispersed, has opened new perspectives on the interaction. Indeed, some of the subtle mechanisms of competition for pollination do not easily fit within common definitions of competition that stress a limited supply of essential resources (e.g. Keddy, 1989), thus forcing us to expand our thinking about competition more generally. Because of the importance of pollination as an ecosystem service (Nabhan and Buchmann, 1997; Aizen et al., 2009; Lonsdorf et al., 2009), competition for pollination has recently resurfaced as a topic of interest in new contexts related to a changing planet.
For these and other reasons, we feel a review is in order. But in truth this is not a typical review. Although we briefly consider past work, our aim is to muse about future research direction. Rather than compile and analyse all past studies, we wish to combine our various perspectives on competition for pollination so as to identify frontiers where further research will be most exciting and profitable. Following a brief historical sketch, we present a heuristic model that delves in more detail into competition for pollination through the two types of mechanism already noted: changes in pollinator visitation and in pollen import and export. This conceptual model clarifies, we hope, how different mechanisms influence fitness by different pathways, some of which are subtle and many of which are ripe for investigation. We next turn to several aspects of competition for pollination on a changing planet that, to our minds, invite exploration. Our overall intent is to stimulate thinking and research.
SETTING THE STAGE: A BRIEF HISTORY
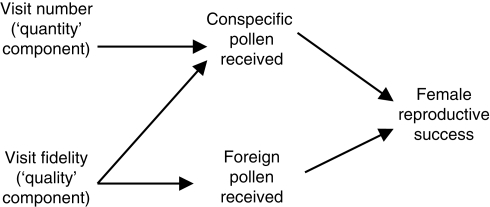
Competition via shared pollinators appears to have been recognized first by the American entomologist Charles Robertson. In formulating a Darwinian view of flowering phenology, Robertson (1895, pp. 100–101) reasoned that evolution could produce similar species that flower together at the same time, thus placing ‘nearly related forms in competition … for the aid of the same pollinating agency’. Such competition, if sufficiently severe, might make it ‘advantageous … for some of the forms to avoid competition … [by modifying] their floral characters so as to attract a different set of visitors, or [by separating] their times of blooming so they may not have to compete with a great many similar flowers for the attention of the same kinds of insects’. In this remarkably modern idea Robertson predicts an evolutionary outcome (which he calls ‘avoidance of competition’ and we might call resource partitioning), but is not explicit as to mechanism. Surely Robertson was thinking of competitors drawing away visitors, and thus of a mechanism involving a reduced number of visits (Fig. 1, top left) – an interpretation consistent with his reference (which persists in much modern literature) to ‘competition for pollinators’. At the same time, we are intrigued that Robertson (1895, p. 103) discussed wind-pollinated plants, suggesting that he was on the verge of recognizing mechanisms that do not derive from the behaviour of pollinating animals.
Fig. 1.
A starting framework for mechanisms of competition for pollination. Most early authors and many current ones focus on a reduction in number of visits to flowers in the presence of putative competitors. However, changes in visit ‘quality’ are also possible. In this conceptual model ‘quality’ refers to the degree to which pollinators restrict their visits to a focal plant species (see Thomson, 1978, 1981; Waser, 1983b), whereas Waser (1983b) and Waser and Price (1983) used the term to refer to genetic quality of conspecific pollen (one example being the degree of genetic similarity of pollen and pistil; an aspect also treated in this paper), and Herrera (1987) used it simply to refer to the per-visit deposition of conspecific pollen.
Almost three decades later, Clements and Long (1923, p. 10) echoed Robertson, reasoning that ‘competition is regarded as natural when plants of two or more species grow so close or intermingled that their flowers compete for the same group of visitors’. To extend his studies on vegetative competition among plants into the realm of reproductive competition, Frederic Clements undertook experimental studies of the phenotypic traits of flowers that induce insect visits. However, his monograph with Francis Long drew no conclusions as to the commonness of competition for pollination, and did not look beyond the most obvious mechanism involving number of pollinator visits.
Relatively little was added to this picture for several more decades. Various authors advanced ‘plausibility arguments’ about the reality of competition, based on observations of multiple plant species with morphological similarity and phenological overlap, and the expectation that they should compete because of a surplus of flowers relative to pollinators (e.g. Free, 1968; Hocking, 1968; Mosquin, 1971; Schemske et al., 1978; see also Zimmerman, 1980). Others put forward observations of apparent displacement of phenologies as evidence for resource partitioning as a response to competition (e.g. Macior, 1971; Reader, 1975; Heinrich, 1975; Lack, 1976; Stiles, 1977; Whalen, 1978). But direct evidence of competition, especially experimental demonstration of fitness cost to species in the presence of putative competitors, remained a rarity, as did consideration of mechanisms beyond those involving pollinator visitation (for a review, see Waser, 1983a).
Early signs of an expanded conceptual focus can be found. In mixed plantings of two species of Clarkia, Lewis (1961) clearly saw the possibility of fitness cost to one species (in the form of loss of ovules due to the formation of sterile hybrids) resulting from the receipt of pollen from other species. This is an aspect of competition derived from the movement of pollinators between the two species, rather than from their relative scarcity, i.e. involving a ‘quality’ rather than ‘quantity’ component of pollination (Fig. 1). Levin and Kerster (1967) and Levin (1969), reported similar findings in experimental plantings of Phlox, and speculated on phenotypic divergence of species as an evolutionary consequence. Levin and Anderson (1970) and Straw (1972) also provided theoretical models for the ecological dynamics of competition for pollination.
From such work emerged a realization that different mechanisms of competition must be distinguished. In discussing ecological, genetic and evolutionary consequences of hummingbird pollination, Feinsinger (1978) contrasted competition based on visit number with competition based on purity of pollen loads, and echoed the conclusion of Levin and Anderson (1970) that in two-species mixtures ‘mixed pollen loads reduce the effective pollination of the rare species’. Waser (1978b, 1983a) similarly distinguished ‘competition through pollinator preference’ from ‘competition through interspecific pollen transfer’. The latter mechanism includes loss of pollen deposited on foreign flowers, loss of receptive stigma surface, and loss of pollen and ovules in the formation of hybrids of low or zero fitness, all mechanisms involving visit fidelity (Fig. 1, bottom left) rather than visit number (see also Rathcke, 1983).
These advances foreshadowed further empirical progress. The cleanest way to determine whether competition occurs is to add individuals of one species to populations of other species, thus avoiding the confounding of intraspecific and interspecific effects that occurs when overall plant density is held constant as species composition of a mixture is varied (see Connolly, 1988; Keddy, 1989). Experimental addition of putative competitors for pollination soon demonstrated for several systems that the interaction exists and that it can involve interspecific pollen transfer (e.g. Waser, 1978a; Kephart, 1983; Campbell and Motten, 1985; Galen and Gregory, 1989; Jennersten and Kwak, 1991). The more exact mechanistic effects of interspecific pollen transfer were elucidated in a number of cases (Thomson et al., 1981; Waser and Fugate, 1986; Feinsinger et al., 1988; Feinsinger and Tiebout, 1991; Murphy, 1992; Murcia and Feinsinger, 1996; see the recent review by Morales and Traveset, 2008). A finding of competition was far from universal, however: a number of studies detected no effect of plant species on each others' reproductive success (e.g. Mitchell, 1987; Rathcke, 1988; Armbruster and McGuire, 1991; McGuire and Armbruster, 1991; see also the review by Feinsinger, 1987).
Indeed, we must briefly consider the opposite possibility of facilitation rather than competition. If pollinators view flowers of several species as equivalent in a sensory, cognitive, and ultimately behavioural sense, adding more flowers of another species should increase the total number of pollinators attracted to the community. Facilitation is suggested if this also increases the per-capita visitation to one or more species [Feldman et al. (2004) showed formally that a sigmoidal increase is necessary], although facilitation in visitation still might be accompanied by reduced visit quality (Fig. 1, bottom left), and so in itself does not demonstrate overall reproductive facilitation. The possibility of facilitation was raised by Macior (1971) and Watt et al. (1974), and Straw (1972) and Bobisud and Neuhaus (1975) included it in theoretical models of plants interacting via shared pollinators. Waser and Real (1979) presented evidence for ‘effective mutualism’ between early-flowering and later-flowering species, wherein the first-flowering species supports the pollinators of the next to flower (although in periods of flowering overlap these species might also compete for pollination). Soon thereafter, Thomson (1981) offered an elegant analysis of the spatial domain, explicitly considering how insect behaviour affects and is affected by plants. In the process he provided the first clear empirical demonstration of enhanced per-flower visitation with increasing density in natural plant mixtures. Simultaneously, Schemske (1981) argued that striking floral convergence in two neotropical gingers represents an adaptation derived from facilitation, although he did not present information on pollinator visitation. Rathcke (1983) reviewed the early literature on both facilitation and competition, and extended Thomson's line of thought (Thomson, 1981) to propose that increasing plant density could cause a shift from facilitation to competition.
AN UPDATED CONCEPTUAL FRAMEWORK
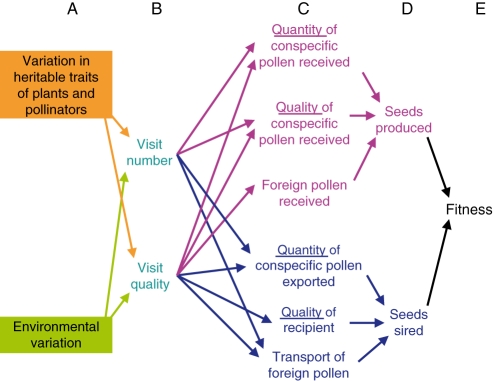
The view of competition summarized above has developed in our minds into a more complete picture (Fig. 2). This is indeed a picture, although (as with Fig. 1) we present it as a path diagram. Our goal here is to focus thinking on the issue, emphasizing the mechanistic causes and consequences of pollinator sharing.
Fig. 2.
A more complete conceptual framework for mechanisms of competition for pollination, including effects of the number of visits (their ‘quantity’) and aspects of their ‘quality’. Visit quality in turn might affect the amount of pollen received from conspecifics and competitors, as well as qualities of that pollen. To the left are drivers that determine how the system behaves in space (roughly, ecology) and time (both ecology and evolution). In equating ecology with environmental variability, both the abiotic and the biotic environments are included. The lower portion of the diagram (in blue) indicates pollination success through male sexual function. The upper (pink) indicates female function.
Our expansion of Fig. 1 suggests that the extent to which plant species affect one another's pollination is influenced by the ecological context (Fig. 2A, bottom), including pollinator abundance and the number and proximity of conspecific and foreign plants. This context is extrinsic to the focal species being considered, so it is labelled ‘environmental variation’ to stress its variation in time and space, in itself a critical thing to study. Furthermore, the interactions among plant species are influenced by the evolutionary context. This is labelled as ‘variation in heritable traits of plants and pollinators’ (Fig. 2A, top), again to acknowledge that such variation underlies phenotypic variation intrinsic to the participants such as variation in the behaviour and morphology of pollinating animals and the flowers they visit. Both central tendency and variation in the phenotype evolve via natural selection, in part imposed by the interplay of mutualism and competition, and they represent a legacy from prior generations of the plant–pollinator interaction.
As in Fig. 1, two important general components of pollination are the numbers of flower visits a plant receives, and their quality (Fig. 2B). ‘Visit quality’ reflects the amount and genetic attributes of the pollen delivered to flowers by animal pollinators. The limits of language are apparent here, because (as hinted by Robertson, 1895) even wind-pollinated plants of one or more species might compete by altering the quantity of pollen received and its genetic properties, including the identities of its conspecific sources and the degree to which it is mixed with ‘foreign’ pollen, i.e. that of other species (Waser, 1983a). Competition for pollination among wind-pollinated species is largely unstudied (but see Niklas and U, 1982; Culley et al., 2002), and should certainly not remain so, but the remainder of our comments return to animal-pollinated species.
Quantity and quality of visits are partly but not completely independent; both respond to certain aspects of pollinator behaviour, such as flower constancy (e.g. Chittka et al., 1999), the time spent probing flowers (e.g. Cresswell and Galen, 1991), foraging posture (e.g. Sigrist and Sazima, 2004), and the extent of grooming (e.g. Harder and Wilson, 1998). Our conceptual framework makes more explicit the mechanisms by which competitors might affect reproduction through changes in visit number and quality. It incorporates the idea that the number and quality of visits can affect the amounts of both conspecific and foreign pollen transferred (Fig. 2C). Additionally, visit quality has the potential to influence the fitness value of that pollen (e.g. degree of kinship of conspecific pollen to the pistil on which it arrives, intrinsic genetic quality of specific donors, diversity of donors, and the effects of foreign pollen on the transport and germination of conspecific pollen). Finally, the framework reminds us that every member of a sexual species has a father and a mother, so that sharing pollinators might affect not only success in receiving pollen, but also success in exporting it to other plants (Fig. 2D, blue portions).
This framework is a generalization; each case to which it is applied will require some tailoring to fit specific circumstances. Occasionally it might be possible and profitable to develop and fit a formal path analysis to a tailored version of the general framework, i.e. to treat it as a formal model. However, our own intent with the framework is to present a heuristic; a less-formal model intended to guide understanding. It may often serve well as a checklist of major topics to consider in studying any specific facet of competition for pollination. We hope, too, that it will stimulate fresh thinking. Indeed, generating this framework has stimulated us to consider new views on mechanisms of competition involving pollen loss, on the role of community context on competition, and on the effect of competition on plant mating systems and different components of plant fitness. We turn next to these topics.
The importance and magnitude of pollen loss in competition for pollination
A relatively subtle mechanism of competition for pollination involves the loss of pollen on stigmas or other flower parts of a competitor (Morales and Traveset, 2008; Fig. 2C). This may be an important mechanism because of the intrinsic inefficiency of pollination itself. In most animal-pollinated species, even in the absence of competitors, <1 % of pollen is exported to conspecifics (Harder and Thomson, 1989; Holsinger and Thomson, 1994; Johnson et al., 2005). This low efficiency follows from factors at several levels (Inouye et al., 1994), including limited pollen pick-up by pollinators (Sahli and Conner, 2007), passive loss during transport (Thomson, 2003), removal of pollen from the pollinator's body by active grooming or preening (Harder, 1990), moving of the pollen to corbiculae or scopae of bees (Thorp, 2000) and pollen deposition on flowers of the same plant (a form of pollen discounting; Rademaker et al., 1997). Even this partial list suggests that pollen might have little prospect of reaching stigmas of other conspecifics, but when we add in competition for pollination the opportunities for loss multiply. Interspecific movements of pollinators may amplify the factors just listed, and can add new possibilities, some of which are noted below. In our conceptual framework (Fig. 2), pollen loss is represented by a reduction in visit quality through reduced pollen receipt or export. A number of questions beg for further attention.
What circumstances encourage pollen loss?
Pollen-harvesting visitors such as bees are more likely to cause pollen loss than are non-harvesters or pollinators that groom or preen relatively infrequently (such as hummingbirds; e.g. Schemske, 1975). Likewise, a floral competitor that produces abundant pollen, or that contacts a similar area of the pollinator's body with reproductive parts, might foster more pollen loss (Waser, 1983a; Murcia and Feinsinger, 1996; Fig. 2A). All of these factors are also likely to reduce the proportion of pollen carried over to successively visited flowers, and therefore reduce the genetic diversity of pollen deposited on each conspecific stigma (one aspect of quality). It is also possible that the extent of pollinator grooming changes with the presence of a competitor. Investigating these possibilities by determining pollen fate and carry-over patterns (e.g. Thomson, 1986; Morris et al., 1994, 1995; Fenster et al., 1996; Matsumara and Washitani, 2002) would be especially rewarding for plants with a variety of shared pollinators (e.g. birds, bats, insects).
Does pollen loss occur because of co-transport?
We use the term ‘co-transport’ to indicate that pollen of several species is carried by a pollinator (Fig. 2). Limited space on the pollinator's body might restrict the load that can be carried, so that adding pollen of one species reduces the amount of pollen of another species. Likewise, pollen from conspecifics might be buried under pollen from a competitor (e.g. Lertzman, 1981). Such co-transport losses would affect receipt and export not only of conspecific but also of foreign pollen. Although there is an extensive literature on character displacement of floral parts that affect the site of pollen deposition on visitors (e.g. Waser, 1983a; Armbruster et al., 1994; Caruso 2000; Muchhala and Potts, 2007), direct exploration of such costs of co-transport seems to be rare [although Waser and Price (1984, p. 266) reported results suggesting no overall cost for one hummingbird-pollinated system].
How does the number of consecutively probed competitor flowers affect pollen loss?
Although there is evidence that interspecific movements reduce pollen receipt (e.g. Campbell, 1985; Campbell and Motten, 1985; Feinsinger et al., 1988), little is known about how the number of competitor flowers visited affects pollen receipt, and even less about pollen export (but see Murcia and Feinsinger, 1996). Visiting more competitor flowers should generally reduce the amount of pollen of the focal species which is carried, especially when the competitor's floral parts brush pollen off the visitor. But even when pollen is segregated on the pollinator's body, if foragers departing a focal species continue grooming while visiting a competitor the amount of pollen available to deposit on subsequent focal species flowers will decline rapidly, reducing pollen transport (Flanagan et al., 2009).
Effects of pollinator sharing on plant mating systems
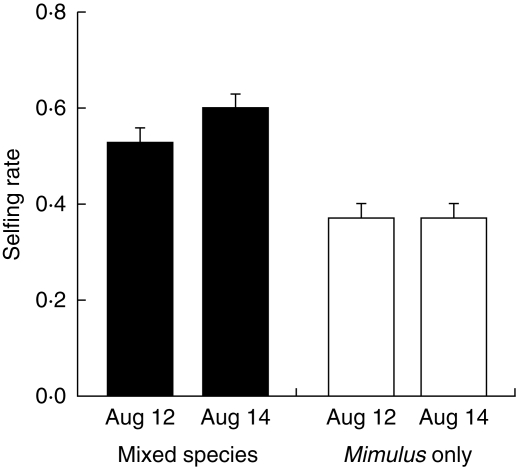
Plant mating systems vary widely within and among populations (Barrett, 2003), and competition for pollination might contribute to this variation (Campbell, 1985). This possibility arises because frequent pollinator movements between co-occurring species may lead to substantial pollen loss and reduced outcross pollen deposition (Fig. 2C). Assuming that the amount of self-pollen arriving on flowers remains unchanged, the proportion of offspring resulting from selfing should increase. Bell et al. (2005) found strong support for this hypothesis in a study of Mimulus ringens, using experimental arrays of plants with and without a co-flowering competitor, Lobelia siphilitica (Fig. 3). To our knowledge, no other studies have directly explored the effects of competition for pollination on selfing rates (but, for a related study, see Fishman and Wyatt, 1999). Additional work is needed to evaluate the generality of these effects in other taxa, and to address additional questions, as follows.
Fig. 3.
Effect of the presence of Lobelia siphilitica on selfing rate for Mimulus ringens. Selfing in Mimulus increased significantly when the competitor was present, and this pattern was consistent among days.
How does competition for pollination affect selfing?
Competition for pollination may potentially influence two components of self-fertilization: the amount of selfing within flowers and, if there are multiple flowers, the amount of selfing among flowers on a display (geitonogamous selfing). Relative changes in these two components may depend on the ways competitors influence patterns of pollinator behaviour, and this might affect the overall selfing rate. For example, if the proportion of geitonogamous moves declines in the presence of an attractive competitor, then the decrease in geitonogamous selfing may partially offset any increase in intrafloral selfing due to pollen loss. Through a simple modification of the checkerboard experimental design used by Bell et al. (2005) it would be possible to tease apart the relative contributions of competition for pollination to these two forms of selfing. A researcher could manipulate floral displays of the focal species so that half of the displays have a single open flower, and the other half have some set number greater than one.
Do competition-mediated changes in the selfing rate have important effects on plant reproductive success?
Increases in the selfing rate are less important if inbreeding depression is weak, since the fitness reduction due to increased selfing is equal to the increase in selfing rate multiplied by the magnitude of inbreeding depression under selfing (Fig. 2C – ‘pollen quality’, and 2E – ‘fitness’). For example, competition for pollination with Lobelia siphilitica increased selfing in Mimulus ringens by 20 %, and reduced Mimulus seed set 37 % (Bell et al., 2005). Since inbreeding depression in Mimulus ringens is fairly weak (21 %), the reduction in seed quality in this case had much less of an effect on reproductive success than did the reduction in offspring number. Note that greenhouse measures of inbreeding depression often underestimate field values (Dudash, 1990); if inbreeding depression is higher in the field, the mating system effect would become increasingly important. More work on mating system effects in species or populations varying in selfing rate and inbreeding depression would be informative.
How does competition for pollination influence other aspects of the mating system, such as the diversity of mates?
The number and relative abundance of mates contributing to a seed crop can affect reproductive performance, including seed production, fruit maturation, and the vigour of resulting offspring (Karron and Marshall, 1990; Paschke et al., 2002). Competition for pollination may lower mate diversity by reducing the amount of pollen delivered to stigmas, and the distance it moves (Fig. 2C). For example, the diversity of pollen donors siring seeds is strongly influenced by patterns of pollen carry-over (Campbell, 1998; R. J. Mitchell et al., unpubl. res.), and pollen loss due to competitors should reduce the extent of carry-over (Fig. 4). Thus, competition for pollination should reduce both mate diversity and gene dispersal distance (Campbell, 1985). The effects of competition for pollination on mate diversity are likely to be most pronounced in species with limited carry-over, such as those pollinated by bees, or other visitors that groom intensively. Studies that examine how mate diversity and pollen carry-over are affected by competition for pollination would provide important insights.
Fig. 4.
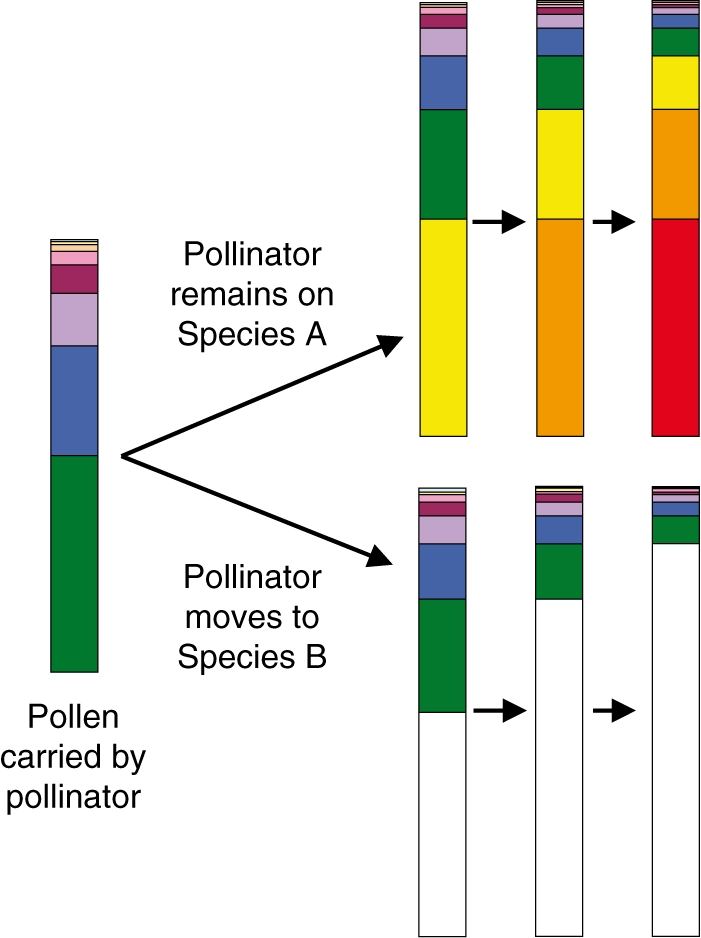
Potential effects of interspecific pollinator movements on pollen loss and mate diversity. The bar graph on the far left indicates the profile of pollen carried by a pollinator that has been visiting many individuals of Species A (each colour signifies a different donor's pollen). The upper row of pollen profiles indicates the diversity of pollen carried if the pollinator continues visiting Species A (each bar indicates the pollen profile after a successively visited flower). The colours yellow, orange and red correspond to pollen from the next three flowers in the visitation sequence. The lower row of profiles is for a pollinator that moves instead to Species B. As the pollinator visits more flowers of Species B the amount and diversity of Species A pollen should decline.
Multi-species interactions and the effect of community context
Ecological communities are often characterized by their considerable diversity of species and of interspecific interactions, but few studies investigate how this element of the ecological context (Fig. 2A) relates to competition for pollination. There is an especially strong need for manipulative experiments in this area. To date, most experimental studies of competition for pollination (including our own) have focused on pair-wise interactions (there are exceptions; e.g. Feinsinger, 1978; Rathcke, 1988). Assessment of more realistic and diverse community contexts would be valuable (Strauss and Irwin, 2004; Geber and Moeller, 2006; Sargent and Ackerly, 2008), and this suggests several pertinent questions.
Are competitive effects in assemblages of species predictable from pairwise interactions?
Few studies have explored the dynamics of competition for pollination between more than two plant species. One way to begin investigating multi-species competition would be to plant gardens with different combinations of one, two and three species at a time (e.g. Ghazoul, 2006). Measuring the effect of each species combination on pollinator visitation and reproductive success for a focal species (Fig. 2) would shed light on how the diversity of competitors influences the magnitude of competition for pollination, and its mechanisms. Such experiments might reveal additive effects, in which the result of multispecies competition on reproductive success is a linear combination of the pairwise effects, or instead non-additive or intransitive effects, in which competitive abilities form no consistent hierarchy (Petraitis, 1979). Non-additive effects could strengthen or weaken competition, or perhaps even lead to facilitation. Note that when multiple floral competitors are present, several different mechanisms of competition could occur simultaneously, which might contribute to non-additive and potentially unpredictable outcomes. One non-additive outcome of special interest would be domination of pollination by a plant species that is especially attractive to pollinators (a ‘cornucopian species’ sensu Mosquin, 1971; see also Whitney, 1984; Laverty, 1992), which then greatly reduces success of many or all other species, regardless of their identities. In North America, Lythrum salicaria may be an example of such a dominant competitor (Brown et al., 2002; R. J. Flanagan, unpubl. res.).
A limitation to experimental study of large communities is that measuring the response of several species in all combinations geometrically increases the number of sampling units required, making such studies unwieldy even for three or four species (see, for example, Naeem and Wright, 2003). One approach has been to choose one or a few focal species within a larger assemblage and concentrate on these while manipulating aspects of their competitive environment (e.g. Keddy et al., 1994; Bell et al., 2005; Larson et al., 2006; Lopezaraiza-Mikel et al., 2007). This limits the inferences one can draw about multi-species competition for pollination, but may be the only feasible way to proceed in any but the most species-poor communities. Another approach that should not be dismissed is to augment experiments with observational studies of communities of plants that share pollinators (e.g. Feinsinger, 1978; Stone et al., 1998; Larson et al., 2006).
What insights can be gained from a pollination network approach?
A distinctly different method for studying multi-species systems is presented by recent work on pollination networks (e.g. Memmott, 1999; Bascompte et al., 2003; Aizen et al., 2008; Stang et al., 2009; Vázquez et al., 2009). Pollination network studies use a form of food-web analysis to investigate the community structure of connections between plants and floral visitors (Fig. 2A). Such studies did not explicitly consider competition for pollination until the pioneering work of Lopezaraiza-Mikel et al. (2007). These authors removed flowers of the invasive Impatiens glandulifera from some field plots, leaving other plots as controls, and compared pollination networks. Removal substantially affected network structure, with invaded plots having greater pollinator species richness, more total visitors, and more foreign pollen transferred.
What is the scale at which plants affect one another's pollination?
Competitive effects involving interspecific pollinator movements surely will be influenced by the scale of individual pollinator foraging ranges, which vary dramatically both within and among species (Steffan-Dewenter et al., 2001; Knight et al., 2005; Greenleaf et al., 2007). For widely foraging pollinators this may mean that plants well-separated from one another still interact through pollinator sharing (as can be true for species separated temporally in their flowering; Waser and Real, 1979). But the factors that determine a pollinator's landscape-scale foraging decisions are not well known, and results might be contingent on idiosyncrasies of each local situation (although see Westphal et al., 2003, 2006; Ricketts et al., 2008). For these reasons, the number and identity of competitors is virtually certain to vary depending on behavioural abilities and propensities, and on ecological context (Fig. 2A). There is a rich, challenging and rewarding field open here for experimental manipulation of plant spacing and context, and comparison of the responses of different pollinator taxa (e.g. small vs. large bees; see Steffan-Dewenter et al., 2001; Kinyo, 2005).
Effects of pollinator sharing on overall reproductive success and fitness
Hermaphroditic plants achieve reproductive success by both mothering and fathering seeds (Fig. 2D). Patterns of selection through maternal and paternal success often (although not always) differ, making measurement of both sexual functions highly desirable for any evolutionary investigation (Ashman and Morgan, 2004). The effects of pollinator sharing on siring success are likely to resemble those on maternal function in many ways (Fig. 2), such as reduced export of pollen to conspecifics following from grooming-induced pollen loss. However, male function effects do involve some new possibilities. For example, co-transport losses (caused by limited space on the pollinator's body) are likely to have much stronger impacts on pollen export than on import. Also, pollen deposited on foreign stigmas or otherwise lost during visits to a competitor species may reduce the pollen available to sire seeds on conspecifics, discounting the value of that pollen. Very little is known about these or other possible effects of pollinator sharing on pollen export and siring success. One hint is provided by Flanagan et al. (2009), who found that pollinator movements between species significantly reduced conspecific pollen deposition. These losses seemed to primarily occur during transport of pollen (e.g. passively during flight or as a result of pollinator grooming) rather than during contact with structures of the competitor plant. Another detailed study (Murcia and Feinsinger, 1996) identified pollen loss to petals of the competitor as the cause of declines in pollen deposition. Distances of pollen export can also be influenced by competitors (Campbell, 1985).
Admittedly, it is much more demanding to measure pollen export and siring success than to assess comparable female measures (Snow and Lewis, 1993). Furthermore, pollination and reproduction are only components of fitness, and subsequent events such as dispersal and germination of seeds, emergence of seedlings, and growth of seedlings to sexual maturity may enhance or reduce effects at the pollination stage (e.g. Price et al., 2008; see also Feldman et al., 2004). The prospect of not only measuring seed production and siring success (Fig. 2D), but also performance through the vegetative part of the life cycle (Fig. 2E) is truly intimidating, and we are not aware of any pollination study that has yet achieved this, in any context. We have no desire to set an unachievable standard, but we do advocate further thought on how conclusions about competition for pollination might be altered by including male function and later parts of the life cycle. For some questions, luckily, a partial accounting of fitness may suffice. For example, a study of plant population dynamics in the presence of competitors might reasonably focus on seed production and ignore male sexual function, although some assessment of success in the seedling generation would seem necessary.
COMPETITION FOR POLLINATION ON A CHANGING PLANET
Anthropogenic change dominates current thinking in ecology. After a period of relative quiescence, studies of competition for pollination are appearing that focus on aspects of anthropogenic change. The apparent speed and severity of this change place an additional premium on diversity and originality of approaches.
Competition between native and alien plants
Over the last few centuries humans have transported invasive alien plant species across the globe. Most considerations of invasive plant species focus primarily on their direct vegetative effects on natives, but many of these plants rely on animal pollinators that they may share with natives. A growing body of work demonstrates that invasive aliens may also affect pollination of native species (e.g. Chittka and Schürkens, 2001; Brown et al., 2002; Moragues and Traveset, 2005; reviewed by Bjerknes et al., 2007; Bartomeus et al., 2008a, b; Munoz and Cavieres, 2008; Aizen et al., 2008). Studies examining the effects of human-introduced plants on native pollination systems represent a promising and important area for expanded research, and the following emphases occur to us.
Are the effects of invasive species context-dependent?
Although a growing body of work is accumulating, the effects of invasive plant species on native pollination systems are largely unknown. Evidence to date suggests that the effect of invasives on natives ranges from negative to neutral to positive (Moragues and Traveset, 2005; Totland et al., 2006; Bjerknes et al., 2007; Lopezaraiza-Mikel et al., 2007; Munoz and Cavieres, 2008; Bartomeus et al., 2008b). This range of response may reflect differing ecological and evolutionary contexts (Fig. 2A). These contexts vary widely among systems, and it is important to determine which ones are most likely to foster which responses (see Bjerknes et al., 2007). For example, whether invasives compete with or facilitate natives may depend on the relative abundance or density of the invasive species (Bjerknes et al., 2007; Munoz and Cavieres, 2008), on the abundance or morphology of other co-flowering species, and on the regional abundance of pollinators. It would be valuable to determine the role of competition for pollination (if any) in slowing or facilitating invasions, and whether the likely impact of an invader on pollination services can be forecast from floral morphology or the identity and behaviour of its pollinators, both within its ancestral and introduced ranges.
What are the effects of native plants on pollination of crops, and vice versa?
Although not traditionally considered invasive species, insect-pollinated crops are often aliens, and may have some of the same effects as invasives on natives with which they share pollinators. Substantial gene flow can occur from genetically modified crops to weedy relatives (Snow and Palma, 1997; Ellstrand et al., 1999), suggesting the possibility of competition through foreign pollen receipt (Fig. 2C). However, the interaction of crops and wild species has seldom been viewed through the lens of competition (but see Free, 1970). Instead, current work has taken the equally interesting, but opposite viewpoint of investigating how pollination of crops is facilitated by native plant species. These studies have demonstrated that pollinators from nearby wild areas often visit and effectively pollinate crops, providing an important and valuable ecosystem service (Kremen et al., 2002; Ricketts et al., 2004; Winfree et al., 2007). Yet, viewed as a competitive interaction, it is apparent that this subsidy may come at a cost to native plants (Fig. 2), just as natives can suffer from sharing pollinators with invasive species. On a longer time scale, crops (and invasives) may, however, support expansion of pollinator populations. This might provide a long-term benefit through greater pollinator service overall (Waser and Real, 1979; Traveset and Richardson, 2006; Tepedino et al., 2008), without removing the possibility of competition through interspecific pollen transfer. These and other conflicting effects probably depend on ecological context and scale, so untangling them will be a challenge. This challenge is exacerbated by agricultural use of large and mobile colonies of cultivated generalist pollinators such as honey bees, which may link plant species that would otherwise not share pollinators, and whose abundance may not match local floral resources. Invasive generalist pollinators may also complicate the situation.
Does competition for pollination between invasive and native species alter selection on plant traits?
Invasives that share pollinators with natives may generate new patterns of natural selection and gene flow with important evolutionary consequences (Fig. 2A). For example, competition might foster character displacement (e.g. Waser, 1978a, 1983a; Caruso 2000; Armbruster and Muchhala, 2009) or convergence (e.g. Waser and Real, 1979; Schemske, 1981). Most invasions are relatively recent, and can even be dated, so it may be feasible to document evolutionary changes in real time (see Mooney and Cleland, 2001), along with evaluation of the mechanistic basis of selection (e.g. Campbell et al., 1991). For native species with invasive relatives, hybridization may occur (Barbour et al., 2003; Morrison and Mauck, 2007; Johnson and Galloway, 2008). This raises several possibilities, ranging from genetic swamping of natives to introgression of native genes into the genome of the invasive species, which might facilitate expanded invasion.
Climate change, pollinator declines, and competition
Anthropogenic changes in climate are strengthening, and are likely to influence the occurrence and magnitude of competition for pollination by altering ecological context. These influences are virtually certain to be difficult to predict. Climate change should directly and indirectly affect the abundance, geographic range, vigour, phenology and behaviour of both plants and pollinators, all of which can influence interactions among plant species mediated through shared pollinators (Fig. 2; Hegland et al., 2009).
Climate change may most immediately affect plants by altering their resource status – directly through increases in availability of carbon (via increased atmospheric CO2), or indirectly by increases in nutrients such as nitrogen and phosphorus (mobilized via, for example, increased warm-season precipitation). No work has yet directly investigated how climate change might affect competition for pollination, and other effects on flowering communities are just beginning to be explored (e.g. Price and Waser, 1998, 2000). Immediate plastic responses of plant traits related to pollination are known in some cases, but vary across species. Examples include increased, decreased or unchanged rates of nectar production (Osborne et al., 1997; Rusterholz and Erhardt, 1998); increases in numbers of flowers produced by some but not all species (Osborne et al., 1997), and other changes that might differentially alter attractiveness to pollinators (Wookey et al., 1993). How such responses would affect any element of competition for pollination in a wild community (and with independent responses of different species of plants and pollinators to climate change) requires additional investigation.
Declines in pollinator populations have been reported around the globe (e.g. Buchmann and Nabhan, 1996; Biesmeijer et al., 2006; Colla and Packer, 2008; Goulson et al., 2008), and may in part be the result of climate change (Allen-Wardell, 1998; Committee on the Status of Pollinators in North America, 2007; Fig. 2A). In turn, declines in plant populations may be linked to those of pollinators (Biesmeijer et al., 2006). With such interdependent population dynamics, forecasting the outcome is uncertain – the future may bring a shortage of pollinators relative to plants at some times and places, and a shortage of plants relative to pollinators in others. When there is a shortage of pollinators, forms of competition for pollination derived from changes in visit number should increase (Fig. 2B; see Vamosi et al., 2006). In this situation, pollinators would face a world of relatively under-visited flowers (consequently rich in nectar and pollen), and might therefore reduce their visitation to less-rewarding species or avoid these entirely. Either option would reduce success through both female and male sex functions for the undervisited species. Conversely, if there is a shortage of flowers relative to pollinators, pollinators might broaden their diets, and therefore increase competition through mechanisms derived from a change in visit quality (Fig. 2). Pollinators facing a world depleted of flowers should be less choosy and deposit more foreign pollen. Over the longer term, as declines in populations of plants and animals lead to local extinctions (Biesmeijer et al., 2006, Colla and Packer, 2008; Goulson et al., 2008), and as novel communities of mutualists and antagonists are assembled (e.g. Pitelka et al., 1997; Memmott et al., 2004), competition for pollination is likely to change in unpredictable ways, but could well intensify. Evolutionary responses are also likely (Fig. 2A).
Finally, it seems almost certain that climate change will affect the ecological context by altering the phenological synchrony of interacting species. After all, different species of plants and pollinators respond to different environmental cues in individually differing ways (e.g. Inouye et al., 2000, Lyon et al., 2008). Changes in phenology will alter not only the extent to which different plant species overlap in flowering time, but also their synchrony with different pollinator species (Memmott et al., 2007). Experimental studies of such effects are exceptionally challenging because of the difficulty of manipulating plants on a scale that also will affect mobile pollinators.
CONCLUSIONS
We have attempted here to add our own ideas to a conceptual framework of competition for pollination that has developed over many generations of biologists, and to organize our thoughts on exciting future research avenues in part around this framework. We make no claim to encyclopedic coverage of previous work or future possibilities. We personally find competition for pollination an exciting and intriguing interaction, and our interest has grown rather than diminished with time. We hope that readers will be stimulated by the ideas we have collected, and we especially look forward to completely fresh thinking that goes beyond this work.
ACKNOWLEDGEMENTS
We thank Don Levin, Mary Price and three anonymous reviewers for constructive criticism and discussions. This work was supported in part by the National Science Foundation [grant numbers DEB 9816712 (J.D.K.) and DEB 9903308 (R.J.M.)], with further support to R.J.M. from the University of Akron's Glenny Endowment.
LITERATURE CITED
- Aizen MA, Morales CL, Morales JM. Invasive mutualists erode native pollination webs. PLoS Biology. 2008;6:e31. doi: 10.1371/journal.pbio.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production and diversity deficits. Annals of Botany. 2009;103:1579–1588. doi: 10.1093/aob/mcp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Wardell G, Bernhardt P, Bitner R, et al. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conservation Biology. 1998;12:8–17. [Google Scholar]
- Armbruster WS, McGuire AD. Experimental assessment of reproductive interactions between sympatric Aster and Erigeron (Asteraceae) in interior Alaska. American Journal of Botany. 1991;78:1449–1457. [Google Scholar]
- Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. Floral character displacement generates assemblage structure of western Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. [Google Scholar]
- Ashman T-L, Morgan MT. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London B: Biological Sciences. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE, Tibbits WN, Wiltshire RJE. Gene flow between introduced and native Eucalyptus species. New Forests. 2002;23:177–191. [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartomeus I, Bosch J, Montserrat V. High invasive pollen transfer, yet low deposition on native stigmas in a Carpobrotus-invaded community. Annals of Botany. 2008;a 102:417–424. doi: 10.1093/aob/mcn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartomeus I, Montserrat V, Santamaria L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia. 2008;b 155:761–770. doi: 10.1007/s00442-007-0946-1. [DOI] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Melian CJ, Olesen JM. The nested assembly of plant–animal mutualistic networks. Proceedings of the National Academy of Sciences of the USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86:776–785. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Bjerknes A, Totland Ø, Hegland SJ, Neilsen A. Do alien plant invasions really affect pollination success in native plant species? Biological Conservation. 2007;138:1–12. [Google Scholar]
- Bobisud L, Neuhaus R. Pollinator constancy and survival of rare species. Oecologia. 1975;21:263–272. doi: 10.1007/PL00020265. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. Mutualisms. In: Fox C, Fairbairn D, Roff D, editors. Evolutionary ecology: perspectives and synthesis. Oxford: Oxford University Press; 2001. pp. 315–330. [Google Scholar]
- Brown BJ, Mitchell R, Graham SA. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology. 2002;83:2328–2336. [Google Scholar]
- Buchmann SL, Nabhan GP. The forgotten pollinators. Washington, DC: Island Press; 1996. [Google Scholar]
- Campbell DR. Pollen and gene dispersal: the influence of competition for pollination. Evolution. 1985;39:418–431. doi: 10.1111/j.1558-5646.1985.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Multiple paternity in fruits of Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1998;85:1022–1027. [PubMed] [Google Scholar]
- Campbell DR, Motten AF. The mechanism of competition for pollination between two forest herbs. Ecology. 1985;66:554–563. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. A mechanistic analysis of phenotypic selection: pollen export and corolla width in Ipomopsis aggregata. Evolution. 1991;45:1458–1467. doi: 10.1111/j.1558-5646.1991.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution. 2000;54:1546–1557. doi: 10.1111/j.0014-3820.2000.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Chittka L, Schürkens S. Successful invasion of a floral market. Nature. 2001;411:653. doi: 10.1038/35079676. [DOI] [PubMed] [Google Scholar]
- Chittka L, Thomson JD, Waser NM. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86:361–377. [Google Scholar]
- Clements RE, Long FL. Experimental pollination: an outline of the ecology of flowers and insects. Washington, DC: Carnegie Institution of Washington, Publication #336; 1923. [Google Scholar]
- Colla SR, Packer L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation. 2008;17:1379–1391. [Google Scholar]
- Committee on the Status of Pollinators in North America. Status of pollinators in North America. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- Connolly J. What is wrong with replacement series? Trends in Ecology and Evolution. 1988;3:24–26. doi: 10.1016/0169-5347(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Cresswell JE, Galen C. Frequency-dependent selection and adaptive surfaces for foral character combinations: the pollination of Polemonium viscosum. American Naturalist. 1991;138:1342–1353. [Google Scholar]
- Culley TM, Weller SG, Sakai AK. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution. 2002;17:361–369. [Google Scholar]
- Dudash MR. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution. 1990;44:1129–1139. doi: 10.1111/j.1558-5646.1990.tb05220.x. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Prentice HC, Hancock JF. Gene flow and introgression from domesticated plants into their wild relatives. Annual Review of Ecology and Systematics. 1999;30:539–563. [Google Scholar]
- Feinsinger P. Ecological interactions between plants and hummingbirds in a successional tropical community. Ecological Monographs. 1978;48:269–287. [Google Scholar]
- Feinsinger P. Effects of plant species on each other's pollination: is community structure influenced? Trends in Ecology and Evolution. 1987;5:123–126. doi: 10.1016/0169-5347(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Feinsinger P, Tiebout HM. Competition among plants sharing hummingbird pollinators: laboratory experiments on a mechanism. Ecology. 1991;72:1946–1952. [Google Scholar]
- Feinsinger P, Busby WH, Tiebout HM. Effects of indiscriminate foraging by tropical hummingbirds on pollination and plant reproductive success: experiments with two tropical treelets (Rubiaceae) Oecologia. 1988;76:471–474. doi: 10.1007/BF00377045. [DOI] [PubMed] [Google Scholar]
- Feldman TS, Morris WF, Wilson WG. When can two plant species facilitate each other's pollination? Oikos. 2004;105:197–207. [Google Scholar]
- Fenster CB, Hassler CL, Dudash MR. Fluorescent dye particles are good pollen analogs for hummingbird-pollinated Silene virginica (Caryophyllaceae) Canadian Journal of Botany. 1996;74:189–193. [Google Scholar]
- Fishman L, Wyatt R. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 1999;53:1723–1733. doi: 10.1111/j.1558-5646.1999.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Mitchell RJ, Knutowski D, Karron JD. Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens (Phrymaceae) American Journal of Botany. 2009;96:809–815. doi: 10.3732/ajb.0800317. [DOI] [PubMed] [Google Scholar]
- Free JB. Dandelion as a competitor to fruit trees for bee visits. Journal of Applied Ecology. 1968;5:169–178. [Google Scholar]
- Free JB. Insect pollination of crops. London: Academic Press; 1970. [Google Scholar]
- Galen C, Gregory T. Interspecific pollen transfer as a mechanism of competition: consequences of foreign pollen contamination for seed set in the alpine wildflower, Polemonium viscosum. Oecologia. 1989;81:120–123. doi: 10.1007/BF00377020. [DOI] [PubMed] [Google Scholar]
- Geber MA, Moeller DA. Pollinator responses to plant communities and implications for reproductive character evolution. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 102–119. [Google Scholar]
- Ghazoul J. Floral diversity and the facilitation of pollination. Journal of Ecology. 2006;94:295–304. [Google Scholar]
- Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annual Review of Entomology. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- Greenleaf S, Williams N, Winfree R, Kremen C. Bee foraging ranges and their relationship to body size. Oecologia. 2007;153:589–596. doi: 10.1007/s00442-007-0752-9. [DOI] [PubMed] [Google Scholar]
- Harder LD. Behavioural responses by bumble bees to variation in pollen availability. Oecologia. 1990;85:41–47. doi: 10.1007/BF00317341. [DOI] [PubMed] [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal in animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Harder LD, Wilson WG. Theoretical consequences of heterogeneous transport conditions for pollen dispersal by animals. Ecology. 1998;79:2789–2807. [Google Scholar]
- Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. How does climate warming affect plant–pollinator interactions? Ecology Letters. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Bee flowers: a hypothesis on flower variety and blooming times. Evolution. 1975;29:325–334. doi: 10.1111/j.1558-5646.1975.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos. 1987;50:79–90. [Google Scholar]
- Hocking B. Insect–flower associations in the high arctic with special reference to nectar. Oikos. 1968;19:359–388. [Google Scholar]
- Holsinger KE, Thomson JD. Pollen discounting in Erythronium grandiflorum: mass-action estimates from pollen transfer dynamics. American Naturalist. 1994;144:799–812. [Google Scholar]
- Inouye DW, Gill DE, Dudash MR, Fenster CB. A model and lexicon for pollen fate. American Journal of Botany. 1994;81:1517–1530. [Google Scholar]
- Inouye DW, Barr B, Armitage KB, Inouye BD. Climate change is affecting altitudinal migrants and hibernating species. Proceedings of the National Academy of Sciences of the USA. 2000;97:1630–1633. doi: 10.1073/pnas.97.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennersten O, Kwak M. Competition for bumblebee visitation between Melampyrum pratense and Viscaria vulgaris with healthy and Ustilago-infected flowers. Oecologia. 1991;86:88–98. doi: 10.1007/BF00317394. [DOI] [PubMed] [Google Scholar]
- Johnson L, Galloway L. From horticultural plantings into wild populations: movement of pollen and genes in Lobelia cardinalis. Plant Ecology. 2008;197:55–67. [Google Scholar]
- Johnson SD, Neal PR, Harder LD. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society. 2005;86:175–190. [Google Scholar]
- Karron JD, Marshall DL. Fitness consequences of multiple paternity in wild radish, Raphanus sativus. Evolution. 1990;44:260–268. doi: 10.1111/j.1558-5646.1990.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Keddy PA. Competition. London: Chapman and Hall; 1989. [Google Scholar]
- Keddy PA, Twolan-Strutt L, Wisheu I. Competitive effect and response rankings in 20 wetland plants: are they consistent across three environments? Ecology. 1994;82:635–643. [Google Scholar]
- Kephart SR. The partitioning of pollinators among three species of Asclepias. Ecology. 1983;64:120–133. [Google Scholar]
- Kinyo AS. Department of Biology. University of Akron; 2005. Effects of distance from invasive Lythrum salicaria on pollinator visitation rate and reproductive success in native Lythrum alatum. MS Thesis. [Google Scholar]
- Knight ME, Martin AP, Bishop S, et al. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Molecular Ecology. 2005;14:1811–1820. doi: 10.1111/j.1365-294X.2005.02540.x. [DOI] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences of the USA. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack A. Competition for pollinators and evolution in Centaurea. New Phytologist. 1976;77:787–792. [Google Scholar]
- Larson DL, Royer RA, Royer MR. Insect visitation and pollen deposition in an invaded prairie plant community. Biological Conservation. 2006;130:148–159. [Google Scholar]
- Laverty TM. Plant interactions for pollinator visits: a test of the magnet species effect. Oecologia. 1992;89:502–508. doi: 10.1007/BF00317156. [DOI] [PubMed] [Google Scholar]
- Lertzman KP. Pollen transfer: processes and consequences. University of British Columbia; 1981. MS Thesis. [Google Scholar]
- Levin DA. The effect of corolla color and outline on interspecific pollen flow in Phlox. Evolution. 1969;23:444–455. doi: 10.1111/j.1558-5646.1969.tb03527.x. [DOI] [PubMed] [Google Scholar]
- Levin DA, Anderson WW. Competition for pollinators between simultaneously flowering species. American Naturalist. 1970;104:455–467. [Google Scholar]
- Levin DA, Kerster HW. An analysis of interspecific pollen exchange in Phlox. American Naturalist. 1967;101:387–399. [Google Scholar]
- Lewis H. Experimental sympatric populations of Clarkia. American Naturalist. 1961;95:155–168. [Google Scholar]
- Lonsdorf E, Kremen C, Ricketts TH, Winfree R, Williams NM, Greenleaf SS. Modeling pollination services across agricultural landscapes. Annals of Botany. 2009;103:1589–1600. doi: 10.1093/aob/mcp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J. The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecology Letters. 2007;10:539–550. doi: 10.1111/j.1461-0248.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Lyon BE, Chaine AS, Winkler DW. A matter of timing. Science. 2008;321:1051–1052. doi: 10.1126/science.1159822. [DOI] [PubMed] [Google Scholar]
- McGuire AD, Armbruster WS. An experimental test for reproductive interactions between two sequentially blooming Saxifraga species (Saxifragaceae) American Journal of Botany. 1991;78:214–219. [Google Scholar]
- Macior LW. Co-evolution of plants and animals: systematic insights from plant–insect interactions. Taxon. 1971;20:17–28. [Google Scholar]
- Matsumara C, Washitani I. Heterostylous morph differences in pollen transfer and deposition patterns in Primula sieboldii on a visitation by a queen bumblebee, measured with a semi-natural experimental system. Plant Species Biology. 2002;17:1–12. [Google Scholar]
- Memmott J. The structure of a plant–pollinator food-web. Ecology Letters. 1999;2:276–280. doi: 10.1046/j.1461-0248.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society of London B: Biological Sciences. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecology Letters. 2007;8:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. Mechanisms of competition for pollination between two Colorado wildflowers. University of California, Riverside; 1987. MA Thesis. [Google Scholar]
- Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the USA. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moragues E, Traveset A. Effect of Carpobrotus spp. on the pollination success of native plant species of the Balearic Islands. Biological Conservation. 2005;122:611–619. [Google Scholar]
- Morales CL, Traveset A. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences. 2008;27:221–238. [Google Scholar]
- Morris WF, Price MV, Waser NM, Thomson JD, Thomson B, Stratton DA. Systematic increase in pollen carryover and its consequences for geitonogamy in plant populations. Oikos. 1994;71:431–440. [Google Scholar]
- Morris WF, Mangel M, Adler FR. Mechanisms of pollen deposition by insect pollinators. Evolutionary Ecology. 1995;9:304–317. [Google Scholar]
- Morrison JA, Mauck K. Experimental field comparison of native and non-native maple seedlings: natural enemies, ecophysiology, growth and survival. Journal of Ecology. 2007;95:1036–1049. [Google Scholar]
- Mosquin T. Competition for pollinators as a stimulus for the evolution of flowering time. Oikos. 1971;22:398–402. [Google Scholar]
- Muchhala N, Potts MD. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proceedings of the Royal Society of London B, Biological Sciences. 2007;274:2731–2737. doi: 10.1098/rspb.2007.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A, Cavieres LA. The presence of a showy invasive plant disrupts pollinator service and reproductive success in native alpine species only at high density. Journal of Ecology. 2008;96:459–467. [Google Scholar]
- Murcia C, Feinsinger P. Interspecific pollen loss by hummingbirds visiting flower mixtures: effects of floral architecture. Ecology. 1996;77:550–560. [Google Scholar]
- Murphy SD. The determination of the allelopathic potential of pollen and nectar. In: Linskens HF, Jackson JF, editors. Modern methods of plant analysis. Vol. 13. Berlin: Springer; 1992. pp. 333–357. New Series Plant toxin analysis. [Google Scholar]
- Nabhan GP, Buchmann SL. Services provided by pollinators. In: Daily G, editor. Nature's services: societal dependence on natural ecosystems. Washington DC: Island Press; 1997. pp. 133–150. [Google Scholar]
- Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecology Letters. 2003;6:567–579. [Google Scholar]
- Niklas KJ, U KTP. Pollination and airflow patterns around conifer ovulate cones. Science. 1982;217:442–444. doi: 10.1126/science.217.4558.442. [DOI] [PubMed] [Google Scholar]
- Osborne JL, Awmack CS, Clark SJ, Williams IH, Mills VC. Nectar and flower production in Vicia faba L. (field bean) at ambient and elevated carbon dioxide. Apidologie. 1997;28:43–55. [Google Scholar]
- Paschke M, Abs C, Schmid B. Effects of population size and pollen diversity on reproductive success and offspring size in the narrow endemic Cochlearia bavarica (Brassicaceae) American Journal of Botany. 2002;89:1250–1259. doi: 10.3732/ajb.89.8.1250. [DOI] [PubMed] [Google Scholar]
- Petraitis PS. Competitive networks and measures of intransitivity. American Naturalist. 1979;114:921–925. [Google Scholar]
- Pitelka LF, Plant Migration Workshop Group. Plant migration and climate change. American Scientist. 1997;85:464–473. [Google Scholar]
- Price MV, Waser NM. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology. 1998;79:1261–1271. [Google Scholar]
- Price MV, Waser NM. Responses of subalpine meadow vegetation to four years of experimental warming. Ecological Applications. 2000;10:811–823. [Google Scholar]
- Price MV, Campbell DR, Waser NM, Brody AK. Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology. 2008;89:1596–1604. doi: 10.1890/07-0614.1. [DOI] [PubMed] [Google Scholar]
- Rademaker MC, de Jong TJ, Klinkhamer PGL. Pollen dynamics of bumble-bee visitation on Echium vulgare. Functional Ecology. 1997;11:554–563. [Google Scholar]
- Rathcke B. Competition and facilitation among plants for pollination. In: Real L, editor. Pollination biology. New York, NY: Academic Press; 1983. pp. 305–329. [Google Scholar]
- Rathcke B. Interactions for pollination among coflowering shrubs. Ecology. 1988;69:446–457. [Google Scholar]
- Reader RJ. Competitive relationships of some bog ericads for major insect pollinators. Canadian Journal of Botany. 1975;53:1300–1305. [Google Scholar]
- Ricketts TH. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conservation Biology. 2004;18:1262–1271. [Google Scholar]
- Ricketts TH, Regetz J, Steffan-Dewenter I, et al. Landscape effects on crop pollination services: are there general patterns? Ecology Letters. 2008;11:499–515. doi: 10.1111/j.1461-0248.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Robertson C. The philosophy of flower seasons, and the phaenological relations of the entomophilous flora and the anthophilous insect fauna. American Naturalist. 1895;29:97–117. [Google Scholar]
- Rusterholz H-P, Erhardt A. Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia. 1998;113:341–349. doi: 10.1007/s004420050385. [DOI] [PubMed] [Google Scholar]
- Sahli HF, Conner JK. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish, Raphanus raphanistrum (Brassicaceae) American Journal of Botany. 2007;94:203–209. doi: 10.3732/ajb.94.2.203. [DOI] [PubMed] [Google Scholar]
- Sargent RD, Ackerly DD. Plant–pollinator interactions and the assembly of plant communities. Trends in Ecology and Evolution. 2008;23:123–130. doi: 10.1016/j.tree.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Schemske DW. Time budget and foraging site preference of the cinnamon hummingbird in Costa Rica. The Condor. 1975;77:216–217. [Google Scholar]
- Schemske DW. Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology. 1981;62:946–954. [Google Scholar]
- Schemske DW, Willson MF, Melampy MN, et al. Flowering ecology of some spring woodland herbs. Ecology. 1978;59:351–366. [Google Scholar]
- Sigrist MR, Sazima M. Pollination and reproductive biology of twelve species of neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Annals of Botany. 2004;94:33–41. doi: 10.1093/aob/mch108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow AA, Lewis PO. Reproductive traits and male fertility in plants: empirical approaches. Annual Review of Ecology and Systematics. 1993;24:331–351. [Google Scholar]
- Snow AA, Palma PM. Commercialization of transgenic plants: potential ecological risks. Bioscience. 1997;47:86–96. [Google Scholar]
- Stang M, Klinkhamer PGL, Waser NM, Stang I, van der Meijden E. Size-specific interaction patterns and size matching in a plant pollinator interaction web. Annals of Botany. 2009;103:1459–1469. doi: 10.1093/aob/mcp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan-Dewenter I, Münzenberg U, Tscharntke T. Pollination, seed set and seed predation on a landscape scale. Proceedings of the Royal Society of London B, Biological Sciences. 2001;268:1685–1690. doi: 10.1098/rspb.2001.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles FG. Coadapted competitors: the flowering seasons of hummingbird-pollinated plants in a tropical forest. Science. 1977;198:1177–1178. doi: 10.1126/science.198.4322.1177. [DOI] [PubMed] [Google Scholar]
- Stone GN, Willmer P, Rowe JA. Partitioning of pollinators during flowering in an African Acacia community. Ecology. 1998;79:2808–2827. [Google Scholar]
- Strauss SY, Irwin RE. Ecological and evolutionary consequences of multispecies plant–animal interactions. Annual Review of Ecology, Evolution, and Systematics. 2004;35:435–466. [Google Scholar]
- Straw RM. A Markov model for pollinator constancy and competition. American Naturalist. 1972;106:597–620. [Google Scholar]
- Tepedino VJ, Bradley BA, Griswold TL. Might flowers of invasive plants increase native bee carrying capacity? Intimations from Capitol Reef National Park, Utah. Natural Areas Journal. 2008;28:44–50. [Google Scholar]
- Thomson JD. Effect of stand composition on insect visitation in two-species mixtures of Hieracium. American Midland Naturalist. 1978;100:431–440. [Google Scholar]
- Thomson JD. Spatial and temporal components of resource assessment by flower-feeding insects. Journal of Animal Ecology. 1981;50:49–59. [Google Scholar]
- Thomson JD. Pollen transport and deposition by bumble bees in Erythronium: influences of floral nectar and bee grooming. Journal of Ecology. 1986;74:329–341. [Google Scholar]
- Thomson JD. When is it mutualism? American Naturalist. 2003;162:s1–s9. doi: 10.1086/378683. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Andrews BJ, Plowright RC. The effect of a foreign pollen on ovule development in Diervilla lonicera (Caprifoliaceae) New Phytologist. 1981;90:777–783. [Google Scholar]
- Thorp RW. The collection of pollen by bees. Plant Systematics and Evolution. 2000;222:211–223. [Google Scholar]
- Totland Ø, Nielsen A, Bjerknes A-L, Ohlson M. Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. American Journal of Botany. 2006;93:868–873. doi: 10.3732/ajb.93.6.868. [DOI] [PubMed] [Google Scholar]
- Traveset A, Richardson DM. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology and Evolution. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Vamosi JC, Knight TM, Steets J, Mazer SJ, Burd M, Ashman T-L. Pollination decays in biodiversity hotspots. Proceedings of the National Academy of Sciences of the USA. 2006;103:956–961. doi: 10.1073/pnas.0507165103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez D, Blüthgen N, Cagnolo L, Chacoff NP. Uniting pattern and process in plant–animal mutualistic networks: a review. Annals of Botany. 2009;103:1445–1457. doi: 10.1093/aob/mcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser NM. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology. 1978;a 59:934–944. [Google Scholar]
- Waser NM. Interspecific pollen transfer and competition between co-occuring plant species. Oecologia. 1978;b 36:223–236. doi: 10.1007/BF00349811. [DOI] [PubMed] [Google Scholar]
- Waser NM. Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. a. New York, NY: Van Nostrand Reinhold; 1983. pp. 277–293. [Google Scholar]
- Waser NM. The adaptive nature of floral traits: ideas and evidence. In: Real L, editor. Pollination biology. b. New York, NY: Academic Press; 1983. pp. 242–285. [Google Scholar]
- Waser NM, Fugate ML. Pollen precedence and stigma closure: a mechanism of competition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia. 1986;70:573–577. doi: 10.1007/BF00379906. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. Optimal and actual outcrossing in plants, and the nature of plant–pollinator interaction. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 341–359. [Google Scholar]
- Waser NM, Price MV. Experimental studies of pollen carryover: effects of floral variability in Ipomopsis aggregata. Oecologia. 1984;62:262–268. doi: 10.1007/BF00379024. [DOI] [PubMed] [Google Scholar]
- Waser NM, Real LA. Effective mutualism between sequentially flowering plant species. Nature. 1979;281:670–672. [Google Scholar]
- Watt WB, Hoch PG, Mills SG. Nectar resource use by Colias butterflies: chemical and visual aspects. Oecologia. 1974;14:353–374. doi: 10.1007/BF00384578. [DOI] [PubMed] [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecology Letters. 2003;6:961–965. [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Bumblebees experience landscapes at different spatial scales: possible implications for coexistence. Oecologia. 2006;149:289–300. doi: 10.1007/s00442-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Whalen MD. Reproductive character displacement and floral diversity in Solanum section Androceras. Systematic Botany. 1978;3:77–86. [Google Scholar]
- Whitney GG. The reproductive biology of raspberries and plant–pollinator community structure. American Journal of Botany. 1984;71:887–894. [Google Scholar]
- Winfree R, Williams NM, Dushoff J, Kremen C. Native bees provide insurance against ongoing honey bee losses. Ecology Letters. 2007;10:1105–1113. doi: 10.1111/j.1461-0248.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Wookey PA, Parsons AN, Welker JM, et al. Comparative responses of phenology and reproductive development to simulated environmental change in sub-arctic and high arctic plants. Oikos. 1993;67:490–502. [Google Scholar]
- Zimmerman M. Reproduction in Polemonium: competition for pollinators. Ecology. 1980;61:497–501. [Google Scholar]