Abstract
Background and Aims
In Australia, honey-bees have invaded systems that evolved without social insect pollinators, where many plants are adapted to vertebrate pollination. Behavioural differences between pollinators are likely to influence mating patterns, but few studies have examined this empirically in long-lived, woody, perennials. It was shown previously that outcrossing rates in Grevillea macleayana vary among populations. Here tests were conducted to determine whether the behaviour of birds and honey-bees differed between a population previously found to be highly outcrossed and two inbreeding populations.
Methods
Visit frequencies and movement patterns of the visitors to inflorescences at three sites over two seasons were compared. A caging experiment was used to test the effects of excluding birds on pollen removal from newly opened flowers and on pollen deposition on stigmas that had been washed clean.
Key Results
Honey-bees were the most frequent visitors overall, but honeyeaters were more frequent visitors in the population previously found to have a high outcrossing rate than they were in either of the other populations. More visits by honeyeaters were from distant plants. Pollen removal did not vary greatly among sites, and was not affected by bird exclusion; however, more pollen was deposited on the stigmas of cleaned pollen presenters in the population previously observed to be highly outcrossing than in the other two. This high level of pollen deposition was reduced by experimental bird exclusion.
Conclusions
Honey-bees were the most frequent visitors, by an order of magnitude, and excluding vertebrates revealed that bees were removing most of the pollen but deposited fewer pollen grains on stigmas. Birds were more frequent visitors at the site previously found to be outcrossing than the other two sites, and they moved further between plants and visited fewer inflorescences on a plant during a foraging bout than bees did. These characteristics of bird visits to G. macleayana would be sufficient to produce significant variation in outcrossing rates among sites.
Key words: Grevillea macleayana, Apis mellifera, honey-bees, honeyeaters, pollinator behaviour, pollen removal, pollen deposition, outcrossing rate
INTRODUCTION
Many plant species are apparently adapted to attract specific pollinators, but most possess more generalized floral structures that allow a variety of animals to visit and facilitate pollination (Faegri and van der Pijl, 1979; Waser and Ollerton, 2006). Fruit set in the field can be associated with the source of pollen; some elegant studies have revealed that optimal levels of fruit set can be achieved by matings with plants at a particular distance (optimal outcrossing distance) that matches the typical interplant movement patterns of the effective and abundant pollinator species (Price and Waser, 1979; Waser and Price, 1983), while others have found a mismatch between pollinator movement distances and optimal outcrossing distance (e.g. Marr et al., 2000; C. Forrest, University of Wollongong, Australia, unpubl. res.).
The determinants of a plant's mating system and the resultant genotypic quality of its seed will be an interaction between the plant's level of self-compatibility, and the quantity and quality of pollen transferred within and among plants (Schemske and Horvitz, 1984; Ramsey, 1988). These processes are expected to be determined by the frequency of pollinator visits to flowers, their shape and size in relation to flower morphology, and their foraging behaviour. As flower-visitors differ in these characteristics, they can be expected to differ in the quality of the pollination provided.
For self-compatible plants with mixed mating systems, especially those capable of autonomous self-pollination, pollinator movements would be expected to have a direct effect on the level of outcrossing. For example, high levels of selfing would be expected to result from flower-visitors that remove pollen or nectar but fail to contact receptive stigmas, thus acting as pollen ‘thieves’ (Taylor and Whelan, 1988; Butz Huryn, 1997), and from pollinators that forage mostly within individual plants – making few interplant movements. Patterns of movement of pollinators are particularly important in this regard, because increased inbreeding has been shown to be associated with reduced fitness in many species (e.g. Charlesworth and Charlesworth 1987).
Many plant species in the family Proteaceae in Australia have generalist flowers on dense flowering spikes (inflorescences) (George, 1984; Olde and Marriott, 1994, 1995). Flowers of most species are visited frequently by the introduced honey-bee, Apis mellifera, although they are generally considered to be adapted to vertebrate pollination – because of their large size, colour, scent and prolific nectar production (Collins and Rebelo, 1987; Ayre and Whelan, 1989). Honey-bees vary greatly in their efficiency as pollinators of Australian plants (Ramsey, 1988; Taylor and Whelan, 1988; Paton, 1993; Celebrezze and Paton, 2004) and, although they may collect both pollen and nectar, they are considered to be potential pollen thieves for many plant species (Paton and Ford, 1983; Gross and Mackay, 1998), including the Proteaceae (Paton and Turner, 1985; Vaughton, 1992, 1995). Grevillea macleayana, for example, is a self-compatible shrub that is considered to be vertebrate pollinated yet is highly attractive to honey-bees (Vaughton 1996). Mate choice experiments have revealed that selfing is as likely as outcrossing to produce fruits, even when flowers are bagged to exclude all animals (Harriss and Whelan, 1993; Vaughton, 1995).
In an electrophoretic study of natural progeny arrays (Ayre et al., 1994), the realized mating system of G. macleayana was tested in a range of populations. Several were highly inbred whereas one (‘Honeymoon Bay’) was highly outcrossed. We have also observed that plants in all populations receive frequent visits from honey-bees (Ayre et al., 1994; Vaughton, 1995). In this study, the aim was to examine differences in the foraging behaviours of honeyeaters and honey-bees. In particular, we wished to test whether their behaviour and effectiveness in pollen removal and deposition varied among the three populations that we had studied previously, in ways that might explain large variations in outcrossing rates among populations. As it is difficult to apply an experimental manipulation that excludes honey-bees while admitting vertebrates, the amount of pollen removed from pollen presenters and deposited on stigmas was measured in two treatments: caging to exclude vertebrates but admit honey-bees vs. open to allow access to all flower visitors.
MATERIALS AND METHODS
Grevillea macleayana currently occurs as a series of small populations within the Jervis Bay region of New South Wales, Australia, and several outlier populations (Makinson, 1999). Plants bear large numbers of 30–80 mm long terminal inflorescences. Each inflorescence is ‘toothbrush like’ and has approx. 50 flowers that open sequentially over 6–14 d (McGillivray, 1993; Makinson, 1999). Flowers are hermaphroditic and protandrous. As in many other groups within the Proteaceae, pollen is shed from the anthers on to the modified style-end, the pollen presenter, before the flower opens (Ayre and Whelan, 1989).
All aspects of this study were conducted in three sites. Two of these sites (Abraham's Bosom – 35°01′S, 150°50′E; and Elmoos Road – 35°08′S, 150°41′E) supported populations that we had previously found to be highly inbred (t = 0·10 ± 0·03 s.e. and 0·16 ± 0·04, respectively), and plants in the third site (Honeymoon Bay – 35°04′S, 150°46′E) were highly outcrossed (t = 0·85 ± 0·15). The Elmoos Road and Abraham's Bosom sites supported small populations (n < 40 plants) in well-used and partly disturbed reserves, whereas the larger Honeymoon Bay population (n > 60) occurs within a tall Eucalyptus racemosa woodland. At Elmoos Road and Abraham's Bosom, individual plants were typically small dense shrubs (1–2·5 m tall, 2–3 m diameter) occurring within shrubby heathland. In contrast, plants within the Honeymoon Bay population were relatively large and open (up to 4 m tall), and formed a midstorey (Ayre et al. 1994).
Grevillea macleayana has an extended flowering season, mostly from May to February, with a peak flowering period occurring between September and November (spring). Herein we refer to the non-peak and peak flowering periods as winter and spring, respectively.
Pollinator visits and behaviour
The number of visits by honey-bees and honeyeaters to unmanipulated inflorescences was monitored on each of six randomly chosen, non-rainy days during spring and six during the following winter. On each day, visits were observed for four, 15 min periods spaced evenly throughout the day (0630–1630 h). For bee visits, a set of open inflorescences on a branch (a different plant in each time period) was observed. For each bee visit, we recorded whether the bee was removing pollen or nectar or both. For bird visits, which were much less frequent than bee visits, all open inflorescences on all plants within a 15 m radius of a ‘target’ plant were counted. We then counted the bird visits to these plants over each of the four, 15 min periods on each day. Visit frequency to inflorescences was calculated by dividing the total number of visits recorded in a 15 min observation period by the number of inflorescences being observed for this time. This measure was averaged over the four observation periods and converted to the number of visits per inflorescence per hour over the day.
Detailed maps of each site were used to plot the visits of each bird, thus estimating, for each foraging bout, the relative numbers of inter-inflorescence movements that were within-plant vs. between-plant. This information was collected only in spring, during peak flowering, because the frequency of visits was low in winter.
Pollen removal from pollen presenters
Differences between sites in the rate of pollen removal from newly opened flowers were examined only in spring, when there were sufficiently large numbers of inflorescences per plant. We first tested the hypothesis that pollen removal was mostly diurnal, which would support the assumption that birds and honey-bees, rather than nocturnal mammals, are the most important pollinators. To do this, 126 randomly selected, newly opened, flowers (distributed among inflorescences on several plants) were tagged at dusk, after honeyeater and bee activity had finished. The flowers were examined at 0630 h the following morning. Flowers from which pollen had been removed overnight were readily detected because disturbed pollen bundles were easily seen with a ×20 magnifying glass. Twelve hours later, those tagged flowers that had been undisturbed at 0630 h were re-examined, to quantify the proportion of flowers from which pollen had been removed during the day. This was done on three separate days at each of the three sites, and the data were pooled across days for contingency analysis.
An exclosure experiment was used to distinguish the contributions of birds and honey-bees to pollen removal. Because of the logistical difficulties of caging individual inflorescences in sufficient numbers, six plants in each site were randomly selected and a portion of each of three plants was enclosed in netting (restricting access only to insects), such that many inflorescences were contained within the caged portion. A similar portion of the other three plants was tagged as an ‘open pollination’ treatment (access to both vertebrates and insects). In one site, there were insufficient plants with large numbers of inflorescences at the same stage of flowering so we used a separate portion of a plant with a cage as the open pollination treatment, but treated these as if they were separate plants. Counts of bee visits to inflorescences showed that the netting did not restrict honey-bee access. Half-opened inflorescences were selected for observation, because it had previously been determined that they were at their peak of nectar production at this stage of opening. On each inflorescence, newly opened flowers with intact pollen bundles were tagged at 0630 h. About 6 h later, pollen bundles on these flowers were scored as either ‘intact’ or ‘pollen-bundle removed’.
Pollen deposition
The relative importance of honeyeaters and honey-bees as pollinators was assessed using another caging experiment, with the same sampling design as for the pollen removal experiment (i.e. three plants per treatment per site; in both winter and spring). At 0630 h, suitable open inflorescences in the caged and open-pollination portions of each plant were selected, and the pollen presenters (including the stigmatic area) of open flowers on each inflorescence were cleaned using moistened cotton buds and water. At the end of each day, two of the washed flowers on each inflorescence were randomly selected and the numbers of pollen grains that were in contact with the stigmas were counted.
The effectiveness of the cleaning treatment was tested by removing two flowers from each inflorescence immediately after cleaning and gently dabbing the stigmatic surface of each against sticky tape (Scotch Tape™), and then the pollen grains were counted, using a light microscope. This test of the procedure revealed that it was very effective, because no flowers had pollen grains lodged in the stigmatic hairs and <5 % had pollen grains remaining on the plate of the pollen presenter.
The numbers of washed flowers examined varied among sites for each treatment, ranging from 22 to 56 for the open treatment and from 27 to 48 for the caged treatment. We treated each flower as an independent sample unit. By the end of the day, many flowers had received no pollen, creating a significantly zero-inflated data set, with two processes potentially contributing to zeros: a low rate of visits to flowers by either bees or birds, and no pollen deposition despite a flower visit. We therefore used the methods described in Martin et al. (2005) to confirm that the distribution of counts was well described by a zero-inflated Poisson model and used this to estimate the numbers of zeros from each season/site/treatment combination that were not attributable to the Poisson distribution; logistic regression was then used to compare this frequency between treatments. For the remainder of the pollen count data, three-factor analysis of variance (ANOVA), after square root transformation, was used to test for the effects of season, site and caging treatment on pollen deposition.
RESULTS
Honey-bee visits and behaviour
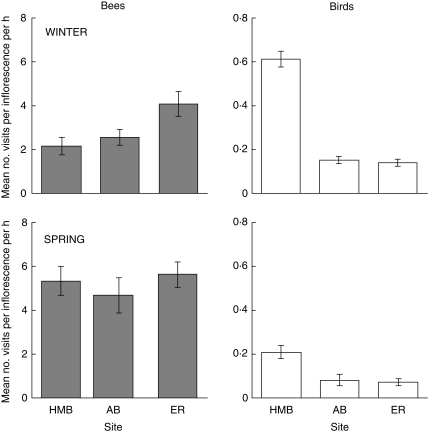
Honey-bees were the most frequent visitors to G. macleayana inflorescences. The only other insect visitors observed during the study, ants, failed to contact the stigmatic regions of flowers, suggesting that they did not pollinate. The number of bee visits per inflorescence per hour varied significantly among sites in winter (F2,17 = 9·04, 0·01 > P > 0·001), with the lowest frequency of visits at Honeymoon Bay (2·1) approximately half that at Elmoos Road (4·1). In spring, however, visit frequencies were similar among sites and considerably greater than in winter (Fig. 1).
Fig. 1.
Differences among study sites and season in the mean numbers of visits per inflorescence per hour (±s.e.) for honey-bees and birds, based on a total of 1 h of observations per plant on six plants in winter (top) and spring (bottom).
Individual honey-bees usually harvested either pollen or nectar during a foraging bout, although occasionally both were collected in a single visit to an inflorescence. Bees that were collecting pollen sometimes contacted the stigmatic regions of both newly opened flowers and flowers that had been open for several days. Bees that were collecting nectar burrowed between the open flowers to reach nectaries at the base of the flowers and rarely touched the pollen presenters. Honey-bees at Honeymoon Bay contacted the stigmatic region significantly less frequently (4·4 % of visits involved stigmatic touches) than at Abraham's Bosom (11·4 % of visits) and Elmoos Road (20·5 % of visits; G2 = 52·63; P < 0·001).
Honeyeater visits and behaviour
A variety of honeyeaters visited the flowers of G. macleayana. These were the Eastern Spinebill (Acanthorhynchus tenuirostris), New Holland Honeyeater (Phylidonyris novaehollandiae), White-cheeked Honeyeater (Phylidonyris nigra), Red Wattlebird (Anthochaera carunculata), Brush Wattlebird (Anthochaera chrysoptera) and Noisy Miner (Manorina melanocephala). Noisy Miners were only observed at Honeymoon Bay. The honeyeaters almost always contacted pollen presenters while feeding at inflorescences.
The numbers of visits per inflorescence per hour by honeyeaters were at least an order of magnitude less than those of bees, ranging from 0·07 (Elmoos Road in spring) to 0·61 visits (Honeymoon Bay in winter; Fig. 1), compared with approx. 2–6 for bees. The frequency of visits by honeyeaters varied both among sites and between seasons. During winter, the frequency of bird visits at Honeymoon Bay was more than three times greater than that at either Elmoos Road or Abraham's Bosom, and this difference was highly significant (F2,17 = 198·4, P < 0·001). The same pattern remained for the spring sampling period (F2,17 = 8·2, 0·01> P > 0·001), though the magnitude of the difference was not as great.
Bird movements were quantified only in spring. Between-plant flights were more common at Honeymoon Bay than either Elmoos Road or Abraham's Bosom (G1 = 17·6; P < 0·001). Mean flight distances between inflorescences were significantly longer at Honeymoon Bay than at Elmoos Road or Abraham's Bosom (F2,253 = 24·6; P < 0·001).
Pollen removal
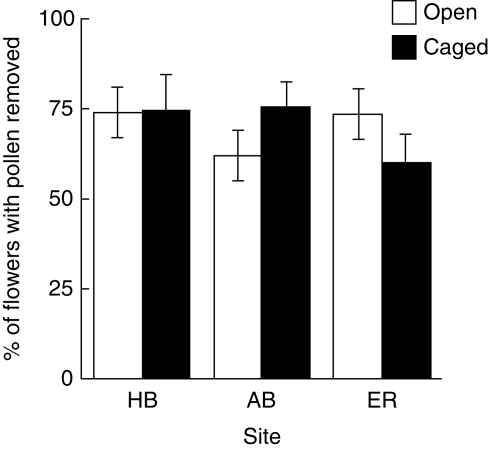
More of the 126 flowers observed had pollen bundles disturbed during the day than overnight at all sites: 99 % vs. 19 % at Honeymoon Bay, 90 % vs. 21 % at Elmoos Road and 95 % vs. 8 % at Abraham's Bosom. When we tested for the effects of bird exclusion, there was only slight, and non-significant variation among sites and between treatments in the mean percentage of flowers (two-factor ANOVA after angular transformation, with three plants as replicates for each treatment in each site) that had pollen removed from pollen presenters over a 12 h period, ranging from 59·8 % for the bird exclusion treatment at Elmoos Road to 75·4 % in the same treatment at Abraham's Bosom (Fig. 2).
Fig. 2.
Differences among sites and caging treatment in the percentage of flowers with pollen removed from pollen-presenters in each site and treatment over a 12 h day-time period in spring. Pollen removal was counted on all flowers at the right stage of opening on three plants in each treatment in each site. The uncaged treatment gave access to both birds and bees; the caged treatment excluded birds.
Pollen deposition
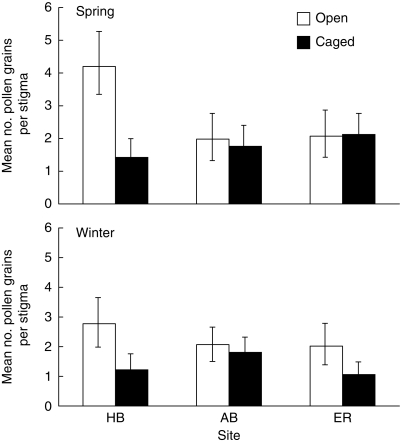
The zero-inflated Poisson model was a much better fit to the pollen count data than the Poisson distribution (see Martin et al., 2005) so we used a mixed approach, examining both the numbers of flowers with zero pollen grains that represented the ‘zero inflation’ and also the mean numbers of pollen grains per flower for the remainder. The proportion of flowers with a ‘non-Poisson’ zero pollen count did not vary significantly among seasons, sites or treatments, averaging 37·6 % and ranging from 33·6 to 35·2 % for the open treatment and from 40·5 to 40·7 % for the caged treatment. However, for the remainder of flowers, ANOVA revealed a statistically significant effect of treatment on the mean numbers of pollen grains deposited (Table 1; Fig. 3) in which caging reduced the numbers of pollen grains per flower. Overall, there were more pollen grains per flower in the open treatment at Honeymoon Bay than in any other.
Table 1.
ANOVA table for three-factor analysis of the effects of caging, site and season on the mean numbers of pollen grains per stigma, after removal of zero counts that were not explained by the Poisson model (see text)
| Source | Sums of squares | d.f. | Mean square | F | P |
|---|---|---|---|---|---|
| Site | 1·79 | 2 | 0·90 | 0·54 | 0·586 |
| Season | 1·80 | 1 | 1·80 | 1·07 | 0·301 |
| Treatment | 9·07 | 1 | 9·07 | 5·41 | 0·021 |
| Site × season | 1·23 | 2 | 0·61 | 0·37 | 0·694 |
| Site × treatment | 6·71 | 2 | 3·36 | 2·00 | 0·137 |
| Season × treatment | 0·04 | 1 | 0·04 | 0·02 | 0·883 |
| Site × season × treatment | 1·83 | 2 | 0·91 | 0·55 | 0·580 |
| Error | 588·87 | 351 | 1·68 | ||
| Total | 1387·0 | 363 |
Data were square-root transformed for analysis.
Fig. 3.
Differences among caging treatments at each site and in both seasons in the mean numbers of pollen grains deposited per stigma, after zero inflation was removed. Data are back-transformed after square-root transformation (see Table 1) and error bars represent s.e. The uncaged treatment gave access to both birds and bees; the caged treatment excluded birds but not bees.
DISCUSSION
There are many expressions of concern, in the pollination ecology literature, that introduced honey-bees may have detrimental effects on native pollination systems. There is some circumstantial evidence for reduced reproductive success in the presence of honey-bees, but few studies have manipulated pollinators and very few have focused on impacts on mating systems. Here we investigated a system in which outcrossing rates were previously found to vary markedly across three populations of G. macleayana. Experimental exclusion of birds did not reduce the high rate of day-time pollen removal from flowers, but did reduce the amount of pollen deposition on stigmas at Honeymoon Bay, the site in which honeyeaters were most active – which is also the site previously displaying high outcrossing rates.
No significant differences were found among sites in the frequency of bee visits to inflorescences, and honey-bee visits to inflorescences clearly outnumbered bird visits. The frequency of bee visits was substantially lower in winter than in spring, whereas bird visit frequency was greater in winter. In a review of other systems, Paton (1995) found that visits to flowers by honey-bees frequently outnumber those by birds, and honey-bees are often the first to visit recently opened flowers. Where honey-bees are foraging for pollen as well as nectar, it is highly likely that they could rapidly deplete pollen loads on flowers, before significant pollen movement by birds had occurred, especially at times in which bird abundance or activity was low.
Bird visit rates were highly variable within and between sites. Comparing ratios of bird with bee visits, proportionally fewer bees visited flowers at Honeymoon Bay than at Abraham's Bosom and Elmoos Road. In addition, honeyeaters made significantly more among-plant flights (relative to within-plant flights) at Honeymoon Bay than at Elmoos Road or Abraham's Bosom. Mean flight distances were significantly greater at Honeymoon Bay than at the other sites.
Honey-bees could not be observed to move between plants during this study. However, previous pollination studies (Paton, 1993) have observed that honey-bees rarely move between plants of Callistemon rugulosa (even though some plants were <3 m apart): in >9 h of observations on 4600 flowers, not once was a honey-bee observed to fly to an adjacent plant, whereas New Holland Honeyeaters moved between plants 7·3 times per hour (equivalent to one interplant move every 400 bill probes; Paton, 1993).
We observed that honey-bees contacted stigmas significantly less often at Honeymoon Bay than at the other two sites (and flower and inflorescence morphology did not appear to vary among sites), suggesting that they may have been less involved in pollen removal (and deposition) at this site. However, the bird exclusion treatment revealed a similar pattern of pollen removal between open and caged treatments. This finding may suggest that honey-bees may forage more thoroughly for pollen in the absence of birds and that high levels of bird activity, resulting in removal of pollen from many pollen presenters, makes this a less attractive resource for bees. In any event, bees were responsible for removing a substantial amount of pollen, supporting Vaughton's (1996) interpretation that pollen removal by bees was the explanation for a reduction in seed set in G. macleayana.
Although pollen may be available to pollinators throughout the day, most of the available pollen in all treatments and all sites was removed by 1200 h in the present study. This finding is comparable with other studies (e.g. Vaughton, 1992). Paton (1993) found honey-bees are often the first to visit recently opened flowers and are responsible for dislodging up to 87 % of pollen on their first visit. In comparison, several species of honeyeaters only dislodged an average of 34–35 %.
More pollen was deposited on stigmas at Honeymoon Bay than at Elmoos Road or Abraham's Bosom (see Fig. 3). Significantly more pollen was deposited in the open treatment (when compared with caged) at all sites, and this difference was most pronounced at Honeymoon Bay. This suggests that bird visits contribute substantially more to pollen deposition at Honeymoon Bay than they do at the other sites.
Generally, studies of predominantly bird-pollinated species have found that more pollen grains (per stigma) and more fruits are produced following visits by birds than by insects (Carpenter, 1976; Waser, 1978; Bertin, 1982; Collins et al., 1984), including for G. macleayana (Vaughton, 1995). Caging experiments have shown that honey-bees can be capable of pollinating Banksia ericifolia, Callistemon rugulosus, Correa reflexa and Banksia ornata, although the quantity of seed produced may be lower than when birds also had access (Paton and Turner, 1985; Paton, 1993, 1995). In the present study, honey-bees were indeed responsible for a substantial amount of pollen deposition on stigmas (when birds were excluded from flowers), although the number of pollen grains deposited per flower was greater on uncaged flowers in the site in which birds were more frequent visitors (Honeymoon Bay).
Variation in pollinator activity (rate of visits and types of movements) can account not only for differences in pollen deposition and fruit production, but also for the genetic outcome of seed (Levin, 1981). In the year of our study, birds at Honeymoon Bay exhibited significantly more among-plant flights than in the other sites. Such pollinator behaviour may have significant consequences for pollen flow. Frequent short visits to a large number of plants will maximize interplant pollen (gene) flow and may explain high outcrossing rates for Honeymoon Bay (Ayre et al., 1994), recorded in the year prior to our study. However, pollinator activities may be expected to fluctuate from one flowering season to the next, as well as between populations. In a study using microsatellite markers, England et al. (2002) found that the Honeymoon Bay population had an outcrossing rate at least as low as the other two populations, but that bird exclusion nevertheless caused a reduction in outcrossing rate. The long-term consequences of increased inbreeding depend on the relative fitness of inbred progeny. We are currently working on comparisons of performance of outcrossed and selfed seeds and seedlings.
We conclude that the characteristics of bird visits to G. macleayana are sufficient to produce significant variation in outcrossing rates among sites, especially in the presence of honey-bees, which are likely to be responsible for rapid pollen removal from flowers and little pollen deposition on flowers of distant plants.
ACKNOWLEDGEMENTS
The NSW Department of Environment and Conservation, Booderee National Park and the Department of Defence gave permission to work in the three study sites. Thanks to Ken Russell for advice and considerable help with the statistical analysis, and to Abdellatif Thcantchane for help with the ZIP modelling. Matthew O'Mullane contributed encouragement and field help during the course of this study. Financial support came from an Australian Research Council Grant to R.J.W. and D.J.A., an Australian Postgraduate Award to F.B. and the Institute for Conservation Biology (University of Wollongong).
LITERATURE CITED
- Ayre DJ, Whelan RJ. Factors controlling fruit set in hermaphroditic plants: studies with the Australian Proteaceae. Trends in Ecology and Evolution. 1989;4:267–272. doi: 10.1016/0169-5347(89)90197-3. [DOI] [PubMed] [Google Scholar]
- Ayre DJ, Whelan RJ, Reid A. Unexpectedly high levels of selfing in the Australian shrub Grevillea barklyana (Proteaceae) Heredity. 1994;72:168–174. [Google Scholar]
- Bertin RI. Floral biology, hummingbird pollination and fruit production of trumpet creeper (Campsis radicans, Bignoniaceae) American Journal of Botany. 1982;69:122–134. [Google Scholar]
- Butz Huryn VM. Ecological impacts of introduced honey-bees. Quarterly Review of Biology. 1997;72:275–297. [Google Scholar]
- Carpenter FL. Hooks for mammal pollination? Oecologia. 1976;35:123–132. doi: 10.1007/BF00344725. [DOI] [PubMed] [Google Scholar]
- Celebrezze T, Paton DC. Do introduced honey-bees (Apis mellifera, Hymenoptera) provide full pollination service to bird-adapted Australian plants with small flowers? An experimental study of Brachyloma ericoides (Epacridaceae) Austral Ecology. 2004;29:129–136. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Collins BG, Newland C, Briffa P. Nectar utilization and pollination by Australian honeyeaters and insects visiting Calothamnus quadrifidus (Myrtaceae) Australian Journal of Ecology. 1984;9:353–365. [Google Scholar]
- Collins BG, Rebelo T. Pollination biology of the Proteaceae in Australia and southern Africa. Australian Journal of Ecology. 1987;12:387–421. [Google Scholar]
- England PR, Beynon F, Ayre DJ, Whelan RJ. A molecular genetic assessment of mating-system variation in a naturally bird-pollinated shrub: contributions from birds and introduced honey-bees. Conservation Biology. 2002;15:1645–1655. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. 3rd edn. Oxford: Pergamon; 1979. [Google Scholar]
- George AS. The Banksia book. Kenthurst: Kangaroo Press; 1984. [Google Scholar]
- Gross C, Mackay D. Honey-bees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae) Biological Conservation. 1998;86:169–178. [Google Scholar]
- Harriss F, Whelan RJ. Selective fruit abortion in Grevillea barklyana (Proteaceae) Australian Journal of Botany. 1993;41:499–509. [Google Scholar]
- Levin DA. Dispersal versus gene flow in plants. Annals of the Missouri Botanic Gardens. 1981;68:233–253. [Google Scholar]
- Makinson RO. Flora of Australia Vol. 17a. Melbourne: CSIRO Publishing; 1999. Grevillea. [Google Scholar]
- Marr DL, Leebens-Mack J, Elms L, Pellmyr O. Pollen dispersal in Yucca filamentosa (Agavaceae): the paradox of self-pollination behavior by Tegeticula yuccasella (Prodoxidae) American Journal of Botany. 2000;87:670–677. [PubMed] [Google Scholar]
- Martin TG, Wintle BA, Rhodes JR, et al. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecology Letters. 2005;8:1235–1246. doi: 10.1111/j.1461-0248.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- McGillivray DJ. Grevillea. Melbourne: Melbourne University Press; 1993. [Google Scholar]
- Olde PM, Marriott NR. The Grevillea book (Vol. 1). Kenthurst, NSW: Kangaroo Press; 1994. [Google Scholar]
- Olde PM, Marriott NR. The Grevillea book (Vols 2 and 3). Kenthurst, NSW: Kangaroo Press; 1995. [Google Scholar]
- Paton DC. Honey-bees in the Australian environment. Does Apis mellifera disrupt or benefit the native biota? BioScience. 1993;43:95–103. [Google Scholar]
- Paton DC. Overview of feral and managed honey bees in Australia: distribution, abundance, extent of interactions with native biota, evidence of impacts and future research. Canberra: Australian Government; 1995. Report to the Australian Nature Conservation Agency. [Google Scholar]
- Paton DC, Ford HA. The influence of plant characteristics and honeyeater size on levels of pollination in Australian plants. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 235–248. [Google Scholar]
- Paton DC, Turner V. Pollination of Banksia ericifolia Smith: birds, mammals and insects as pollen vectors. Australian Journal of Botany. 1985;33:271–286. [Google Scholar]
- Price MV, Waser NM. Pollen dispersal and optimal outcrossing in Delphinium nelsonii. Nature. 1979;277:294–297. [Google Scholar]
- Ramsey M. Differences in pollinator effectiveness of birds and insects visiting Banksia menziesii (Proteaceae) Oecologia. 1988;76:119–124. doi: 10.1007/BF00379609. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Horvitz CC. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science. 1984;225:519–521. doi: 10.1126/science.225.4661.519. [DOI] [PubMed] [Google Scholar]
- Taylor G, Whelan RJ. Can honey-bees pollinate Grevillea? Australian Zoologist. 1988;24:193–196. [Google Scholar]
- Vaughton G. Effectiveness of nectarivorous birds and honey-bees as pollinators of Banksia spinulosa (Proteaceae) Australian Journal of Ecology. 1992;17:43–50. [Google Scholar]
- Vaughton G. No evidence for selective fruit abortion in the Australian shrub Grevillea barklyana (Proteaceae) International Journal of Plant Sciences. 1995;156:417–424. [Google Scholar]
- Vaughton G. Pollination disruption by European Honey-bees in the Australian bird-pollinated shrub Grevillea barklyana (Proteaceae) Plant Systematics and Evolution. 1996;200:89–100. [Google Scholar]
- Waser NM. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology. 1978;59:934–944. [Google Scholar]
- Waser NM, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. Chicago: University of Chicago Press; 2006. [Google Scholar]
- Waser NM, Price MV. Optimal and actual outcrossing in plants and the nature of the plant–pollinator interaction. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 341–359. [Google Scholar]