Abstract
Background
Some of the most exciting advances in pollination biology have resulted from interdisciplinary research combining ecological and evolutionary perspectives. For example, these two approaches have been essential for understanding the functional ecology of floral traits, the dynamics of pollen transport, competition for pollinator services, and patterns of specialization and generalization in plant–pollinator interactions. However, as research in these and other areas has progressed, many pollination biologists have become more specialized in their research interests, focusing their attention on either evolutionary or ecological questions. We believe that the continuing vigour of a synthetic and interdisciplinary field like pollination biology depends on renewed connections between ecological and evolutionary approaches.
Scope
In this Viewpoint paper we highlight the application of ecological and evolutionary approaches to two themes in pollination biology: (1) links between pollinator behaviour and plant mating systems, and (2) generalization and specialization in pollination systems. We also describe how mathematical models and synthetic analyses have broadened our understanding of pollination biology, especially in human-modified landscapes. We conclude with several suggestions that we hope will stimulate future research. This Viewpoint also serves as the introduction to this Special Issue on the Ecology and Evolution of Plant–Pollinator Interactions. These papers provide inspiring examples of the synergy between evolutionary and ecological approaches, and offer glimpses of great accomplishments yet to come.
Key words: Floral traits, generalization and specialization, global change, male fitness, mating systems, multiple paternity, plant–pollinator networks, pollen and gene dispersal, pollinator behaviour, pollination syndromes, pollination webs, self-fertilization
INTRODUCTION
Research on plant–pollinator interactions requires and invites a variety of viewpoints and conceptual approaches, ranging from developmental biology to community ecology, animal behaviour to floral evolution, and genetics to ecosystem studies (Chittka and Thompson, 2001; Harder and Barrett, 2006; Waser and Ollerton, 2006). These diverse approaches reflect the two historic starting points for the discipline. One approach emphasized detailed observation of floral mechanisms and the natural history of the ecological relationships between plants and pollinators, and originated with pioneering work by Sprengel (1793), Müller (1873) and Robertson (1895). The second approach focused on evolutionary processes that might affect and be affected by pollination, beginning with the insightful work of Darwin (1862, 1876, 1877). Both views have greatly expanded and matured since their origins, but remained largely separate until the mid 20th century, when experimental studies of mechanisms were increasingly used to investigate questions about the ecology and evolution of pollination within a strong theoretical framework (e.g. Bateman, 1947). The field flourished in the 1960s and 1970s with this unification of pattern and process (e.g. Baker, 1963; Grant and Grant, 1965; Macior, 1966; Levin and Kerster, 1969a, b; Levin and Anderson, 1970; Linhart, 1973; Feinsinger, 1978; Waser, 1978; Thomson and Plowright, 1980), a trend that mirrored the development of the fields of ecology and evolution at large (e.g. Connell, 1961; Grant, 1963; Paine, 1966).
During the early 1980s publication of two seminal edited volumes (Jones and Little, 1983; Real, 1983) stimulated a flurry of new research that continues today. From these works emerged several themes of continuing interest in the study of pollination biology, such as the functional ecology of floral traits, the dynamics of pollen transport, competition for pollinator services, niche relationships, and the community ecology of pollination. As research on these questions progressed, many pollination biologists became more specialized in their interests, as noted by several authors (e.g. Harder and Barrett, 1996; Holsinger, 1996). Such specialization in evolutionary and ecological perspectives can result in a separation of evolutionary and ecological approaches. However, the continuing vigour of a synthetic and interdisciplinary field like pollination biology depends on renewed connections between evolutionary and ecological perspectives.
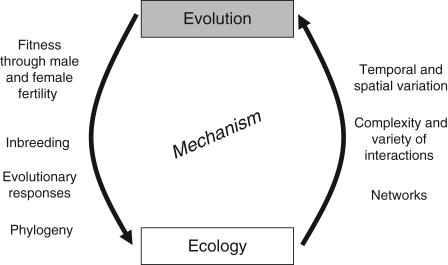
This Special Issue aims to renew the dialogue between subfields within pollination biology (Fig. 1), to draw attention to recent advances in both evolutionary and ecological approaches to the topic, and to highlight important avenues for future research. Here we discuss case studies on two themes in pollination biology at the interface of evolutionary and ecological study: (1) the link between pollinator behaviour and plant mating patterns and (2) generalization and specialization in pollination systems. We provide a historical perspective on these two themes and discuss how contributions to this Special Issue advance our understanding of the evolutionary and ecological perspectives within each of them. Throughout, we focus on pollination mediated by biotic pollinators; Friedman and Barrett (2009, this issue) provide insight into the evolutionary ecology of wind-pollinated plants. We then highlight how recent progress on mathematical models and synthetic analysis has increased our understanding of pollination biology, especially in human-modified systems. We conclude with some suggestions for future investigations that we hope will further unite research in evolutionary and ecological pollination biology.
Fig. 1.
Conceptual representation of the interplay between ecology and evolution in the study of plant–pollinator interactions. Research in pollination biology provides the opportunity to unite both ecological and evolutionary perspectives through the mechanism of pollination.
CASE STUDY I: LINKING POLLINATOR BEHAVIOUR TO PLANT MATING PATTERNS
Flowering plants cannot directly control gamete receipt or export. Instead, nearly three-quarters of Angiosperms rely on animal vectors to move pollen among flowers (National Research Council, 2007), a form of indirect control mediated through pollinators. The resulting patterns of pollen dispersal often reflect pollinator foraging behaviour, and may not optimize the quality or quantity of matings (Campbell and Dooley, 1992). For example, foraging pollinators typically move short distances between flowers, often visiting neighbouring plants (Bateman, 1947; Levin and Kerster, 1969a, b) and probing several flowers in sequence on multi-flower displays (Robertson, 1992). These foraging behaviours have important implications for plant mating. Short pollinator flights may limit the extent of pollen-mediated gene dispersal, influencing the genetic structure of populations (Wright, 1931; Turner et al., 1982), neighbourhood size (Wright, 1946; Levin and Kerster, 1968; Crawford, 1984; Levin, 1988), and the frequency of bi-parental inbreeding (Ellstrand et al., 1978; Griffin and Eckert, 2003). In self-compatible species the tendency of pollinators to visit several flowers in sequence on a single plant also increases the opportunity for geitonogamous (among-flower) self-pollination and a resulting increase in the selfing rate (Harder and Barrett, 1995, 1996; Snow et al., 1996; Karron et al., 2009, this issue). In self-incompatible species geitonogamous pollination can reduce seed production if self-pollen clogs stigmas, interferes with outcrossed pollen-tube growth, usurps ovules, or increases fruit abortion, and can reduce siring success through pollen discounting (reviewed in Snow et al., 1996).
Studies combining observations or manipulations of pollinator behaviour with measurement of pollen-mediated gene dispersal can greatly enhance our understanding of the mechanisms responsible for mating patterns (Harder and Barrett, 1996). For example, although both pollinator movements and gene movements tend to occur over short distances, comparisons in the same populations indicate that pollinator flight movements usually underestimate the extent of pollen-mediated gene dispersal (Schaal, 1980; Levin, 1981; Fenster, 1991; Karron et al., 1995b). This discrepancy is especially apparent when pollen carry-over is extensive (Broyles and Wyatt, 1991).
Research comparing the movements of different pollinator classes with the resulting patterns of pollen and gene dispersal can highlight an important mechanism for spatial and temporal variation in gene movement (Young, 2002; Adler and Irwin, 2006; J. Brunet and K. Holmquist, University of Wisconsin-Madison, pers. comm.). This integrated approach also holds promise for studies of long-distance pollinator and gene movement (e.g. Ellstrand et al., 1989; Nason et al., 1998; Sork et al., 1999; Kreyer et al., 2004). Surprisingly little is known about how these two long-tailed distributions influence each other. Quantifying landscape-scale movements is also important for understanding the factors influencing genetic differentiation among populations (Slatkin, 1985) and the potential for gene flow in genetically modified crop plants (Hayter and Cresswell, 2006).
Pollinator foraging patterns strongly influence selfing rates within and among populations (Karron et al., 1995a, 2004; Harder and Barrett, 1995), and may therefore play an important role in the evolutionary stability of mixed-mating systems, a topic of considerable recent theoretical research (e.g. Goodwillie et al., 2005; Johnston et al., 2009). Several workers have recently shown that selfing rates are influenced by spatial and temporal variation in the composition and abundance of the local pollinating fauna (Brunet and Sweet, 2006; Kameyama and Kudo, 2009, this issue; Whelan et al., 2009, this issue). Selfing rates may even vary on much finer spatial scales, due to the effects of the composition of co-flowering species competing for pollination (Campbell, 1985; Bell et al., 2005; Mitchell et al., 2009, this issue), variation in floral morphology among neighbouring plants (Karron et al., 1997; Medrano et al., 2005), and variation in the order of pollinator probes on individual floral displays (Karron et al., 2009, this issue).
Patterns of pollinator visitation are also thought to influence several other important aspects of mating systems, such as variation in male fertility (Devlin et al., 1992; Conner et al., 1996; Irwin and Brody, 2000), patterns of mate diversity at the whole-plant level (Nason et al., 1998), and patterns of multiple paternity within fruits (Dudash and Ritland, 1991; Campbell, 1998; Karron et al., 2006). These topics have received much less attention than studies of selfing rates, yet they are essential for a meaningful understanding of mating patterns. Indeed, evaluation of fitness through pollen donation is still a rarity in pollination studies (Bernasconi, 2003), even though any study that examines fitness in hermaphroditic plants is only half complete if male function is not measured. Likewise, the existence, magnitude and mechanisms of multiple paternity and mate diversity have important implications for plant evolution (Karron and Marshall, 1990; Bernasconi et al., 2004), but remain poorly understood (Bernasconi, 2003).
CASE STUDY II: GENERALIZATION AND SPECIALIZATION IN POLLINATION SYSTEMS
Plant–pollinator interactions are often viewed as mutualistic, tightly coevolved, relationships. Despite the potential for mutual benefits, these interactions also entail inherent conflicts (e.g. Waser, 1983; Pellmyr and Huth, 1994; Thomson, 2003), which may vary spatially and temporally (Thompson, 1988) and need not involve tight, pairwise coevolution (Schemske, 1983; Herrera, 1993). Recognition of these complexities has provided an important framework for research on pollination biology, and several articles in this Special Issue address these conflicts (Bronstein et al., 2009; Herrera et al., 2009; Irwin, 2009).
The shifting costs and benefits of plant–pollinator interactions may also play an important role in determining whether plant–pollinator interactions are more ‘generalized’ or ‘specialized’. The contrast between generalized and specialized interactions dates back to Faegri and van der Pijl's (1971) descriptions of ‘pollination syndromes’, and in less explicit form to Sprengel (1793), Müller (1873), Robertson (1895) and Darwin (1862). If a plant species has many different visitor taxa that provide similar pollination services, and if costs of the interaction are comparable, the net benefits to plants should also be similar and there is little incentive for plants to specialize on attracting a particular group of pollinators. On the other hand, if some floral visitors are more effective in the quantity or quality of pollen transfer (see Muchhala et al., 2009, this issue), selection should favour traits promoting these effective pollinators (Aigner, 2001; Whittall and Hodges, 2007; Brunet, 2009, this issue; Schlumpberger et al., 2009, this issue). Such selection favouring specialization on particular pollinator species or functional groups would provide a useful mechanism for the evolution of ‘pollination syndromes’, and might explain why some plants have traits that appear to restrict the suite of visitors and pollinators.
Two developments in the mid-1990s led to an expansion of research on generalization and specialization. First, an infusion of ideas from community ecology and social science network theory opened new avenues for research on pollination-based food webs and networks (e.g. Memmott, 1999; Olesen et al., 2007; Stang et al., 2009, this issue; Vázquez et al., 2009, this issue). This work has promoted a detailed understanding of the complex web of interactions between plants and pollinators, and provided a necessary counterweight to the understandable (and perhaps even necessary) simplifications of earlier work that emphasized one or a few pollinators of one or a few plant species. These methods have brought to light several new observations that bear on the topic of generalization and specialization. For example, the findings that specialized plant species tend to have generalized pollinators, and specialized pollinators tend to visit generalized plant species (see Vázquez et al., 2009, this issue, and references therein), has forced many to rethink just what is meant by the terms generalization and specialization, and to more consciously recognize the distinction between the viewpoints of plants and pollinators. Furthermore, the nested structure of plant–pollinator networks (meaning that specialists interact with subsets of the interaction partners of generalists; Bascompte and Jordano, 2007; Vázquez et al., 2009, this issue) has important implications for the conservation and stability of pollination interactions. For example, nestedness confers stability in plant–pollinator networks in simulated pollinator extinctions (Memmott et al., 2004). The degree to which these simulations mirror the natural world is not yet known.
Second, researchers began to re-examine the concept of ‘pollination syndromes’ (Waser et al., 1996), and this helped renew interest in how ecological interactions between plants and pollinators affect evolutionary patterns. This vigorous discussion largely revolves around two contradictory observations: (a) plants show remarkable diversity in morphology, scent and reward, and are often recognized as being clustered in phenotype-space around some of the classic ‘syndromes’ (Ollerton, 1996); and (b) flowers are often visited by a wide array of potential pollinators that do not fit the traditional ‘syndromes’. This disconnect between (a) pattern and (b) process has sparked a healthy and wide-ranging discussion about many facets of the pollination-syndrome concept, and about the ecology of species' interactions and the evolution of adaptations in general (Fenster et al., 2004; Wilson et al., 2004; Armbruster and Muchhala, 2009; Ollerton et al., 2009a, b, this issue). Many of the papers in this Special Issue contribute to this topic, often using new tools to re-examine these ideas (e.g. Armbruster et al., 2009; Ollerton et al., 2009a, b). Progress has been most rapid when both ecological and evolutionary approaches are combined, for example by documenting both patterns of diversity and the pollination services provided by different visitor taxa (Castellanos et al., 2003; Wilson et al., 2007), or uncovering the molecular basis of species' differences and their ecological effects (Schemske and Bradshaw, 1999; Bradshaw and Schemske, 2003).
THEORIES, MODELS AND SYNTHESES IN POLLINATION SYSTEMS
As the field of pollination ecology has grown and expanded, integration of theory, modelling and synthesis with field observations and experiments (Kareiva, 1989; Pickett et al., 1994; Werner, 1998) has provided opportunities to generalize and move beyond system-specific studies. Research in pollination biology has been at the forefront of theory-testing and model-building. In evolutionary biology, pollination systems have provided some of the best tests of theories of evolution by natural selection and the adaptive nature of floral traits (Levin, 1985; Nilsson, 1988; Hodges, 1995; Campbell et al., 1996; Galen, 1996; Schemske and Bradshaw, 1999; Campbell, 2009, this issue; Conner et al., 2009, this issue, and references therein). In ecology and animal behaviour, pollinators have been used as models in tests of optimal foraging theory (e.g. Pyke, 1984). Foraging models and simulations of pollinator flight movements are now being extended to understand the consequences for pollen movement, gene flow and patterns of mating (Cresswell, 2005; Ohashi and Thomson, 2009, this issue).
Models and syntheses are also being employed to study pollination biology in human-dominated systems, which are the fastest growing habitats worldwide (Turner et al., 1990; Vitousek, 1994; McKinney, 2002). Plants and their pollination systems are embedded in these human-modified landscapes (e.g. McFrederick and LeBuhn, 2006; Cheptou and Avendaño, 2006; Winfree et al., 2007) and pollination, especially in agricultural landscapes, confers billions of dollars annually as an ecosystem service (Losey and Vaughan, 2006). Models are being developed to predict the relative abundance of pollinators in agricultural habitats based on landscape-level field parameters, such as pollinator nesting resources, floral resources and pollinator foraging distances (Lonsdorf et al., 2009, this issue). Models and syntheses are also being used to predict the consequences of loss of pollinators on crop yield (Aizen et al., 2009, this issue). These models have become essential for developing land-use management practices and policies that promote pollinator conservation and pollination services. Moreover, both comparative studies and quantitative syntheses are proving integral in the study of pollination in disturbed landscapes. Pollination of invasive versus native congeners is only beginning to be examined (e.g. Brown et al., 2002; Kandori et al., 2009; Mitchell et al., 2009, this issue; T. Knight, Washington University St. Louis, pers. comm.), and quantitative syntheses are starting to provide an enhanced understanding of levels of pollen and pollinator limitation (Ashman et al., 2004; Hegland et al., 2009). The next step is to link the ecology of human-modified systems to the evolution of plant–pollinator interactions, potentially through changes in patterns of natural selection.
AVENUES FOR FUTURE RESEARCH
Many challenges remain in linking the evolution and ecology of plant–pollinator interactions. In this section we identify some of the areas that would benefit from additional dialogue and research, and highlight some important unanswered questions. While this is by no means an exhaustive list, we hope these questions – as well as those identified in the preceding sections – will inspire future research.
What factors influence male reproductive success in plant populations?
Equipped with powerful molecular genetic tools and new analytical methods for paternity assignment, pollination biologists have begun to tackle important questions concerning the causes and consequences of variation in paternal success (Conner et al., 1996; Cruzan, 1998; Barrett, 2003; Bernasconi, 2003; Burczyk et al., 2006). This aspect of plant–pollinator interactions can be technically challenging to study, but is critically needed because it both addresses an essential component of fitness and highlights the mechanisms of pollen transfer. Despite well-developed theory concerning the dynamics of pollen transport (Harder and Barrett, 1996; Harder and Wilson, 1998), there are no studies documenting the genetic composition of pollen on a pollinator's body (Fig. 2). However, such investigations are now possible through direct microsatellite genotyping of individual pollen grains (Matsuki et al., 2008). Genetic analysis of pollen sampled from different locations on a pollinator will help refine models of pollen dispersal (Harder and Barrett, 1996; Harder and Wilson, 1998), since researchers will be able to explore whether sites exposed to pollinator grooming differ in pollen donor composition from ‘safe’ sites not exposed to grooming (Harder and Wilson, 1998). Reseachers will also be able to test whether layers of pollen on a pollinator's body differ in pollen-donor composition (Harder and Wilson, 1998).
Fig. 2.
One important question to be addressed in the coming decade is how the genetic composition of pollen on a pollinator's body compares to the genetic composition of pollen deposited on the next conspecific stigma probed by that pollinator. In this figure two very different floral visitors (Bombus vagans and a Halictid bee) are shown visiting Dalea purpurea (Fabaceae). These visitors handle flowers differently, carry pollen on different parts of the body, have different foraging movement patterns, groom differently, contact different floral parts, carry different amounts of pollen, and probably cause very different patterns of pollen carry-over. Each of these differences might affect the genetic composition of pollen carried on the body and deposited on stigmas. Image by J. Karron.
Some of the most informative studies will combine detailed genetic analyses with rigorous field experimentation (Barrett, 2003). For example, experiments comparing sire profiles and effective mate number for flowers receiving a single pollinator probe and flowers receiving multiple pollinator probes can provide important insights into the mechanisms of multiple paternity and the opportunity for competition among pollen grains (Karron et al., 2006). Recent theoretical work suggests that variation in the number of effective mates in a fruit may also reflect patterns of pollen carry-over (Mitchell et al., unpubl. res.), which often differ markedly for grooming pollinators, such as bumble-bees, vs. non-grooming pollinators, such as hummingbirds (Waser, 1988; Castellanos et al., 2003). Direct genetic tests comparing the effects of different pollinator classes on progeny genetic composition are rare, especially within populations (Brunet and Sweet, 2006), and have not yet quantified effects of pollinator class on the diversity of mates siring progeny within fruits.
Experimental studies will also enhance our understanding of the effects of floral design and display on patterns of male fertility (Campbell, 1998; Elle and Meagher, 2000; Barrett, 2003). How do floral traits influence male fitness and functional gender? Are male and female reproductive success positively correlated or is there a trade-off between these fitness components (Conner et al., 1996; Ashman and Morgan, 2004; Hodgins and Barrett, 2008)? Few paternity studies have explored the role of ecological context, such as the effects of habitat fragmentation (Trapnell and Hamrick, 2006) or the role of competitors for pollination (Mitchell et al., 2009, this issue), and we believe such studies will further advance our understanding of the complex factors influencing mating patterns in flowering plant populations. Finally, while studies on multispecies' interactions involving plants, pollinators and antagonists (such as herbivores and nectar robbers) often provide the caveat that male function should be measured, surprisingly few experimental studies have done so (but see Irwin and Brody, 2000; Paige et al., 2001). Most studies (including many of our own) either don't measure male reproductive success, or still rely on indirect estimates such as flower production, pollen removal or pollen (fluorescent dye) donation; these estimates of male plant function may or may not be tightly correlated with realized seeds sired (Campbell, 1991). Although there is also a strong need for more studies on selection through female reproductive success, incorporating estimates of male reproductive success into studies of multispecies' plant–pollinator and plant–herbivore interactions still represents a relatively unexplored frontier. Study of selection through both female and male reproductive success, perhaps using path analysis to separate direct and indirect effects, should yield novel insights into how pollinators and herbivores affect whole-plant fitness.
How does spatial and temporal variation affect webs of plant–pollinator interactions?
Like classic food webs, pollination webs can be imposingly complex. To understand how such webs are affected by internal and external drivers, we must account for changing ecological contexts at a variety of spatial and temporal scales (Alarcón et al., 2008; Petanidou et al., 2008). Whether such effects are non-linear, stochastic or consistent, and whether different pollinators or plants are substitutable, additive or non-additive in effect is not known, but such insights are essential for further empirical and theoretical progress. Moreover, the webs produced to date either treat species' interactions as binary (present/absent) or represent the magnitude of their interaction as a function of visitation rate or pollen transport (e.g. Memmott, 1999; Olesen and Jordano, 2002; Lopezaraiza-Mikel et al., 2007). These networks have provided great insight into the complexity, community structure and evolutionary ecology of species' interactions (e.g. Bascompte et al., 2006; Petanidou et al., 2008; Vázquez et al., 2009, this issue); however, no pollination webs have been produced that estimate the magnitude of the interactions (i.e. no studies measure interaction strength; Paine, 1992), in part because the experimental manipulations required to make such estimates would be intractable in a full web. In food-web studies there is recognition that networks of species' interactions may not predict the dynamics of these systems (Paine, 1988). There is similar recognition of this limitation in pollination networks, although there is some suggestion that rates of visitation may be sufficient as estimates of the strength of interactions (Vázquez et al., 2005). Nonetheless, developing experimental methods to estimate the strength of interactions in pollination webs from both the plants' and the pollinators' perspective is critical for understanding the dynamics of native plant–pollinator communities and the response of these communities to environmental perturbations. These experimental manipulations would be most tractable if developed for a subset of species in the plant–pollinator webs; such an approach focusing on experiments in subsets of interactors has provided valuable insight into food webs (Paine, 1992).
What are the ecological and evolutionary consequences of global environmental change for plant–pollinator interactions?
There is growing recognition that plant–pollinator interactions can be drastically influenced by anthropogenic changes to ecosystems. Climate change, habitat fragmentation, agricultural intensification, urbanization, pollution, pesticides and species' invasions all have the potential to affect plant–pollinator interactions directly and indirectly (Aizen and Feinsinger, 1994; Kearns et al., 1998; Kremen et al., 2002; Memmott et al., 2007; Winfree et al., 2007; Hegland et al., 2009). While research has documented responses of plants, pollinators and interactions to these anthropogenic changes, most studies have been observational in nature, and few experimental studies tease apart the mechanisms and pathways of interactions.
In order to make predictions about when and how anthropogenic change alters plant–pollinator interactions we need to elucidate underlying mechanisms, especially those most directly linked to the disturbance. The most extensive work detailing both pattern and mechanism comes from research addressing the effects of invasive plants on native plant–pollinator interactions and on native plant reproductive success (Chittka and Schurkens, 2001; Brown et al., 2002; Traveset and Richardson, 2006; Bjerknes et al., 2007; Flanagan et al., 2009). Despite recent advances on this topic, there are still no studies that address the degree to which invasive plants affect native pollinator populations (Traveset and Richardson, 2006; Tepedino et al., 2008) nor whether changes in plant–pollinator interactions due to species' invasion affect patterns of natural selection on native species. Studies of natural selection on traits of native species could be coupled with experimental manipulation of the presence or abundance of invasive species to provide new insights on selection through pollination, and the influence of invasive species. Likewise, hand-pollination experiments that isolate the effects of invasive plants on native-plant pollination and reproduction are also still lacking. In particular, if studies find differences in native plant-pollinator visitation and seed production in the presence vs. absence of an invasive plant, hand-pollination experiments are needed to ensure that differences in seed production are actually being driven by differences in pollination and not some unmeasured mechanism (such as competition for light or nutrient resources). Studies that manipulate both invader presence and hand-pollination (pollen supplemented or control) in a factorial design would provide the greatest ecological insight. If differences in seed production were driven by differences in pollination, these studies would find a statistical interaction between invasion and hand-pollination. Similar successes and shortfalls are also apparent in research on the impacts of agricultural intensification on crop and wild plant–pollinator interactions (Kremen et al., 2002).
Ecology and evolutionary biology form the foundation of pollination biology, and some of the most exciting advances in this discipline have resulted from research combining these two perspectives. This Special Issue provides inspiring examples of the advances that result from these combined perspectives, and offers glimpses of great accomplishments yet to come. Here we have tried to highlight some of the strengths of this field, and a sample of new and important questions that are emerging. These research areas provide important avenues for potentially transformative advances in understanding the interactions between plants and pollinators.
ACKNOWLEDGEMENTS
We are grateful to Annals of Botany Editors Don Levin, Pat Heslop-Harrison and Mike Jackson, and Managing Editor David Frost, for their extraordinary efforts in editing this Special Issue. We would also like to thank each of the authors for contributing stimulating papers that strengthen the links between ecological and evolutionary approaches to the study of plant–pollinator interactions. Finally, we acknowledge with appreciation nearly 40 reviewers who provided exceptionally insightful comments on the manuscripts.
LITERATURE CITED
- Adler LS, Irwin RE. Comparison of pollen transfer dynamics by multiple floral visitors: experiments with pollen and fluorescent dye. Annals of Botany. 2006;97:141–150. doi: 10.1093/aob/mcj012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner PA. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos. 2001;95:177–184. [Google Scholar]
- Aizen MA, Feinsinger P. Habitat fragmentation, native insect pollinators, and feral honeybees in Argentine ‘Chaco Serrano. Ecological Applications. 1994;4:378–392. [Google Scholar]
- Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Annals of Botany. 2009;103:1579–1588. doi: 10.1093/aob/mcp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón R, Waser NM, Ollerton J. Year-to-year variation in the topology of a plant–pollinator network. Oikos. 2008;117:1796–1807. [Google Scholar]
- Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Armbruster WS, Hansen TF, Pélabon C, Pérez-Barrales R, Maad J. The adaptive accuracy of flowers: measurement and microevolutionary patterns. Annals of Botany. 2009;103:1529–1545. doi: 10.1093/aob/mcp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L, Morgan MT. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London B: Biological Sciences. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Baker HG. Evolutionary mechanisms in pollination biology. Science. 1963;139:877–883. doi: 10.1126/science.139.3558.877. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J, Jordano P. Plant–animal mutualistic networks: the architecture of biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2007;38:567–593. [Google Scholar]
- Bascompte J, Jordano P, Olesen JM. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Contamination of seed crops. III. Relation with isolation and distance. Heredity. 1947;1:303–336. [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing rate in Mimulus ringens. Ecology. 2005;86:762–771. [Google Scholar]
- Bernasconi G. Seed paternity in flowering plants: an evolutionary perspective. Perspectives in Plant Ecology, Evolution and Systematics. 2003;6:149–158. [Google Scholar]
- Bernasconi G, Ashman T-L, Birkhead TR, et al. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Bjerknes A, Totland Ø, Hegland SJ, Neilsen A. Do alien plant invasions really affect pollination success in native plant species? Biological Conservation. 2007;138:1–12. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower color locus produces a pollinator shift in two monkeyflower species (Mimulus) Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Bronstein JL, Huxman T, Horvath B, Farabee M, Davidowitz G. Reproductive biology of Datura wrightii: the benefits of a herbivorous pollinator. Annals of Botany. 2009;103:1435–1443. doi: 10.1093/aob/mcp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BJ, Mitchell R, Graham SA. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology. 2002;83:2328–2336. [Google Scholar]
- Broyles SB, Wyatt R. Effective pollen dispersal in a natural population of Asclepias exaltata: the influence of pollinator behaviour, genetic similarity, and mating success. American Naturalist. 1991;138:1239–1249. [Google Scholar]
- Brunet J. Pollinators of the Rocky Mountain columbine: temporal variation, functional groups and associations with floral traits. Annals of Botany. 2009;103:1567–1578. doi: 10.1093/aob/mcp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J, Sweet HR. Impact of insect pollinator group and floral display size on outcrossing rate. Evolution. 2006;60:234–246. [PubMed] [Google Scholar]
- Burczyk J, Adams WT, Birkes DS, Chybicki IJ. Using genetic markers to directly estimate gene flow and reproductive success parameters in plants on the basis of naturally regenerated seedlings. Genetics. 2006;173:363–372. doi: 10.1534/genetics.105.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR. Pollen and gene dispersal: the influences of competition for pollination. Evolution. 1985;39:418–431. doi: 10.1111/j.1558-5646.1985.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Comparing pollen dispersal and gene flow in a natural population. Evolution. 1991;45:1965–1968. doi: 10.1111/j.1558-5646.1991.tb02702.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Multiple paternity in fruits of Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1998;85:1022–1027. [PubMed] [Google Scholar]
- Campbell DR. Using phenotypic manipulations to study multivariate selection of floral trait associations. Annals of Botany. 2009;103:1577–1566. doi: 10.1093/aob/mcp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR, Dooley JL. The spatial scale of genetic differentiation in a hummingbird-pollinated plant: comparison with models of isolation by distance. American Naturalist. 1992;139:735–748. [Google Scholar]
- Campbell DR, Waser NM, Price MV. Mechanisms of hummingbird-mediated selection for flower width in Ipomopsis aggregata. Ecology. 1996;77:1463–1472. [Google Scholar]
- Castellanos M, Wilson P, Thomson J. Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon. Evolution. 2003;57:2742–2752. doi: 10.1111/j.0014-3820.2003.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Avendaño VLG. Pollination processes and the allee effect in highly fragmented populations: consequences for the mating system in urban environments. New Phytologist. 2006;172:774–783. doi: 10.1111/j.1469-8137.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- Chittka L, Schürkens S. Successful invasion of a floral market. Nature. 2001;411:653. doi: 10.1038/35079676. [DOI] [PubMed] [Google Scholar]
- Chittka L, Thomson JD. Cognitive ecology of pollination: animal behaviour and floral evolution. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Connell JH. Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecological Monographs. 1961;31:61–104. [Google Scholar]
- Conner JK, Rush S, Kercher S, Jennetten P. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). II. Selection through lifetime male and total fitness. Evolution. 1996;50:1137–1146. doi: 10.1111/j.1558-5646.1996.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Sahli HF, Karoly K. Tests of adaptation: functional studies of pollen removal and estimates of natural selection on anther position in wild radish. Annals of Botany. 2009;103:1547–1556. doi: 10.1093/aob/mcp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TR. The estimation of neighbourhood parameters for plant populations. Heredity. 1984;52:273–283. [Google Scholar]
- Cresswell JE. Accurate theoretical prediction of pollinator-mediated gene dispersal. Ecology. 2005;86:574–578. [Google Scholar]
- Cruzan MB. Genetic markers in plant evolutionary ecology. Ecology. 1998;79:400–412. [Google Scholar]
- Darwin CR. The various contrivances by which orchids are fertilized by insects. London: John Murray; 1862. [Google Scholar]
- Darwin CR. The effects of cross and self fertilization in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Devlin B, Clegg J, Ellstrand NC. The effect of flower production on male reproductive success in wild radish populations. Evolution. 1992;46:1030–1042. doi: 10.1111/j.1558-5646.1992.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Dudash MR, Ritland K. Multiple paternity and self-fertilization in relation to floral age in Mimulus guttatus (Scrophulariaceae) American Journal of Botany. 1991;78:1746–1753. [Google Scholar]
- Elle E, Meagher TR. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist. 2000;156:622–636. doi: 10.1086/316997. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Torres AM, Levin DA. Density and the rate of apparent outcrossing in Helianthus annuus (Asteraceae) Systematic Botany. 1978;3:403–407. [Google Scholar]
- Ellstrand NC, Devlin B, Marshall DL. Gene flow by pollen into small populations: data from experimental and natural stands of wild radish. Proceedings of the National Academy of Sciences of the USA. 1989;86:9044–9047. doi: 10.1073/pnas.86.22.9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. 2nd edn. New York: Pergamon Press; 1971. [Google Scholar]
- Feinsinger P. Ecological interactions between plants and hummingbirds in a successional tropical community. Ecological Monographs. 1978;48:269–287. [Google Scholar]
- Fenster CB. Gene flow in Chamaecrista fasciculata (Leguminosae). I. Gene dispersal. Evolution. 1991;45:398–409. doi: 10.1111/j.1558-5646.1991.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Thomson JD, Dudash MR. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Flanagan RJ, Mitchell RJ, Knutowski D, Karron JD. Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens (Phrymaceae) American Journal of Botany. 2009;96:809–815. doi: 10.3732/ajb.0800317. [DOI] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany. 2009;103:1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. Rates of floral evolution: adaptation to bumblebee pollination in an alpine wildflower. Polemonium viscosum. Evolution. 1996;50:120–125. doi: 10.1111/j.1558-5646.1996.tb04478.x. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics. 2005;36:47–79. [Google Scholar]
- Grant V. The origin of adaptations. New York: Columbia University Press; 1963. [Google Scholar]
- Grant V, Grant KA. Flower pollination in the phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Griffin CAM, Eckert CG. Experimental analysis of biparental inbreeding in a self fertilizing plant. Evolution. 2003;57:1513–1519. doi: 10.1111/j.0014-3820.2003.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Mating costs of large floral displays in hermaphrodite plants. Nature. 1995;373:512–514. [Google Scholar]
- Harder LD, Barrett SCH. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH., editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall; 1996. pp. 140–190. [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. New York: Oxford University Press; 2006. [Google Scholar]
- Harder L, Wilson WG. Theoretical consequences of heterogeneous transport conditions for pollen dispersal by animals. Ecology. 1998;79:2789–2807. [Google Scholar]
- Hayter KE, Cresswell JE. The influence of pollinator abundance on the dynamics and efficiency of pollination in agricultural Brassica napus: implications for landscape-scale gene dispersal. Journal of Applied Ecology. 2006;43:1196–1202. [Google Scholar]
- Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø. How does climate warming affect plant–pollinator interactions? Ecology Letters. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs. 1993;63:251–275. [Google Scholar]
- Herrera CM, de Vega C, Canto A, Pozo M. Yeasts in floral nectar: a quantitative survey. Annals of Botany. 2009;103:1415–1423. doi: 10.1093/aob/mcp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SA. The influence of nectar production on hawkmoth behaviour, self-pollination, and seed production in Mirabilis multiflora (Nyctaginaceae) American Journal of Botany. 1995;82:197–204. [Google Scholar]
- Hodgins KA, Barrett SCH. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution. 2008;62:1751–1763. doi: 10.1111/j.1558-5646.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. Pollination biology and the evolution of mating systems in flowering plants. Evolutionary Biology. 1996;29:107–149. [Google Scholar]
- Irwin RE. Realized tolerance to nectar robbing: compensation to floral enemies in Ipomopsis aggregata. Annals of Botany. 2009;103:1425–1433. doi: 10.1093/aob/mcp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RE, Brody AK. Consequences of nectar robbing for realized male function in a hummingbird-pollinated plant. Ecology. 2000;81:2637–2643. [Google Scholar]
- Johnston MO, Porcher E, Cheptou PO, et al. Correlations among fertility components can maintain mixed mating in plants. American Naturalist. 2009;173:1–11. doi: 10.1086/593705. [DOI] [PubMed] [Google Scholar]
- Jones CE, Little RJ. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. [Google Scholar]
- Kameyama Y, Kudo G. Flowering phenology influences seed production and outcrossing rate in populations of an alpine snowbed shrub, Phyllodoce aleutica: effects of pollinators and self-incompatibility. Annals of Botany. 2009;103:1385–1394. doi: 10.1093/aob/mcp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori I, Hirao T, Matsunaga S, Kurosaki T. An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia. 2009;159:559–569. doi: 10.1007/s00442-008-1250-4. [DOI] [PubMed] [Google Scholar]
- Kareiva P. Renewing the dialogue between theory and experiments in population ecology. In: Roughgarden J, May RM, Levin SA., editors. Perspectives in ecological theory. Princeton: Princeton University Press; 1989. pp. 68–88. [Google Scholar]
- Karron JD, Marshall DL. Fitness consequences of multiple paternity in wild radish, Raphanus sativus. Evolution. 1990;44:260–268. doi: 10.1111/j.1558-5646.1990.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Karron JD, Thumser NN, Tucker R, Hessenauer AJ. The influence of population density on outcrossing rates in Mimulus ringens. Heredity. 1995a;75:175–180. [Google Scholar]
- Karron JD, Tucker R, Thumser NN, Reinartz JA. Comparison of pollinator flight movements and gene dispersal patterns in Mimulus ringens. Heredity. 1995b;75:612–617. [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity. 1997;79:365–370. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Bell JM. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. American Journal of Botany. 2006;93:1306–1312. doi: 10.3732/ajb.93.9.1306. [DOI] [PubMed] [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Annals of Botany. 2009;103:1379–1383. doi: 10.1093/aob/mcp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: the conservation of plant–pollinator interactions. Annual Review of Ecology and Systematics. 1998;29:83–112. [Google Scholar]
- Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences of the USA. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyer D, Oed A, Walther-Hellwig K, Frankl R. Are forests potential landscape barriers for foraging bumblebees? Landscape scale experiments with Bombus terrestris agg. and Bombus pascuorum (Hymenoptera, Apidae) Biological Conservation. 2004;116:111–118. [Google Scholar]
- Levin DA. Dispersal versus gene flow in plants. Annals of the Missouri Botanical Garden. 1981;68:233–253. [Google Scholar]
- Levin DA. Reproductive character displacement in Phlox. Evolution. 1985;39:1275–1281. doi: 10.1111/j.1558-5646.1985.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Levin DA. The paternity pools of plants. American Naturalist. 1988;132:309–317. [Google Scholar]
- Levin DA, Anderson WW. Competition for pollinators between simultaneously flowering species. American Naturalist. 1970;104:455–467. [Google Scholar]
- Levin DA, Kerster HW. Local gene dispersal in Phlox. Evolution. 1968;22:130–139. doi: 10.1111/j.1558-5646.1968.tb03457.x. [DOI] [PubMed] [Google Scholar]
- Levin DA, Kerster HW. The dependence of bee-mediated pollen and gene dispersal upon plant density. Evolution. 1969a;23:560–571. doi: 10.1111/j.1558-5646.1969.tb03541.x. [DOI] [PubMed] [Google Scholar]
- Levin DA, Kerster HW. Density-dependent gene dispersal in Liatris. American Naturalist. 1969b;103:61–74. [Google Scholar]
- Linhart YB. Ecological and behavioural determinants of pollen dispersal in hummingbird pollinated Heliconia. American Naturalist. 1973;107:511–523. [Google Scholar]
- Lonsdorf E, Kremen C, Ricketts T, Winfree R, Williams N, Greenleaf S. Modelling pollination services across agricultural landscapes. Annals of Botany. 2009;103:1589–1600. doi: 10.1093/aob/mcp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J. The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecology Letters. 2007;10:539–550. doi: 10.1111/j.1461-0248.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Losey JE, Vaughan M. The economic value of ecological services provided by insects. Bioscience. 2006;56:311–323. [Google Scholar]
- Macior LW. Foraging behaviour of Bombus (Hymenoptera: Apidae) in relation to Aquilegia pollination. American Journal of Botany. 1966;53:302–309. [Google Scholar]
- Matsuki Y, Tateno R, Shibata M, Isagi Y. Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. American Journal of Botany. 2008;95:925–930. doi: 10.3732/ajb.0800036. [DOI] [PubMed] [Google Scholar]
- McFrederick QS, LeBuhn G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation. 2006;129:372–382. [Google Scholar]
- McKinney ML. Urbanization, biodiversity, and conservation. Bioscience. 2002;52:883–890. [Google Scholar]
- Medrano M, Herrera CM, Barrett SCH. Herkogamy and mating patterns in the self-compatible daffodil Narcissus longispathus. Annals of Botany. 2005;95:1105–1111. doi: 10.1093/aob/mci129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J. The structure of a plant–pollinator food web. Ecology Letters. 1999;2:276–280. doi: 10.1046/j.1461-0248.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society of London B: Biological Sciences. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecology Letters. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. New frontiers in competition for pollination. Annals of Botany. 2009;103:1403–1413. doi: 10.1093/aob/mcp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhala N, Caiza A, Vizuete JC, Thomson J. A generalized pollination system in the tropics: bats, birds, and Aphelandra acanthus. Annals of Botany. 2009;103:1481–1487. doi: 10.1093/aob/mcn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. Die Befruchtung der Blumen durch Insekten und die gegenseitigen Anpassungen beider: ein Beitrag zur Erkenntniss des ursächlichen Zusammenhanges in der organischen Natur. Leipzig: Wilhelm Engelmann; 1873. [Google Scholar]
- Nason JD, Herre EA, Hamrick JL. The breeding structure of a tropical keystone plant resource. Nature. 1998;391:685–687. [Google Scholar]
- National Research Council. Status of pollinators in North America. Washington, DC: National Academies Press; 2007. [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Ohashi K, Thomson JD. Trapline foraging by pollinators: its ontogeny, economics, and possible consequences for plants. Annals of Botany. 2009;103:1365–1378. doi: 10.1093/aob/mcp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Jordano P. Geographic patterns in plant–pollinator mutualistic networks. Ecology. 2002;83:2416–2424. [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. Proceedings of the National Academy of Sciences of the USA. 2007;104:19891–19896. doi: 10.1073/pnas.0706375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. Journal of Ecology. 1996;84:767–769. [Google Scholar]
- Ollerton J, Alarcón R, Waser NM, et al. A global test of the pollination syndrome hypothesis. Annals of Botany. 2009a;103:1471–1480. doi: 10.1093/aob/mcp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Masinde S, Meve U, Picker M, Whittington A. Fly pollination in Ceropegia (Apocynaceae: Asclepiadoideae): biogeographic and phylogenetic perspectives. Annals of Botany. 2009b;103:1501–1514. doi: 10.1093/aob/mcp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige KN, Williams B, Hickox T. Overcompensation through the paternal component of fitness in Ipomopsis arizonica. Oecologia. 2001;128:72–76. doi: 10.1007/s004420100647. [DOI] [PubMed] [Google Scholar]
- Paine RT. Food web complexity and species diversity. American Naturalist. 1966;100:65–75. [Google Scholar]
- Paine RT. Road maps of interactions or grist for theoretical development? Ecology. 1988;69:1648–1654. [Google Scholar]
- Paine RT. Food-web analysis through field measurement of per capita interaction strength. Nature. 1992;355:73–75. [Google Scholar]
- Pellmyr O, Huth CJ. Evolutionary stability of mutualism between yuccas and yucca moths. Nature. 1994;372:257–260. [Google Scholar]
- Petanidou T, Kallimanis AS, Tzanopoulos J, Sgardelis SP, Pantis JD. Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications of estimates of specialization. Ecology Letters. 2008;11:564–575. doi: 10.1111/j.1461-0248.2008.01170.x. [DOI] [PubMed] [Google Scholar]
- Pickett STA, Kolasa J, Jones CG. Ecological understanding: the nature of theory and the theory of nature. New York: Academic Press; 1994. [Google Scholar]
- Pyke GH. Optimal foraging theory: a critical review. Annual Review of Ecology and Systematics. 1984;15:523–575. [Google Scholar]
- Real L. Pollination biology. New York: Academic Press; 1983. [Google Scholar]
- Robertson AW. The relationship between floral display size, pollen carryover and geitonogamy in Myosotis colensoi (Kirk) Macbride (Boraginaceae) Biological Journal of the Linnean Society. 1992;46:333–349. [Google Scholar]
- Robertson C. The philosophy of flower seasons, and the phaenological relations of the entomophilous flora and the anthophilous insect fauna. American Naturalist. 1895;29:97–117. [Google Scholar]
- Schaal BA. Measurement of gene flow in Lupinus texensis. Nature. 1980;284:450–451. [Google Scholar]
- Schemske DW. Limits to specialization and coevolution in plant-animal mutualisms. In: Nitecki MH., editor. Coevolution. Chicago: University of Chicago Press; 1983. pp. 67–109. [Google Scholar]
- Schemske DW, Bradshaw HD. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences of the USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpberger BO, Cocucci AA, Moré M, Sérsic AN, Raguso RA. Extreme variation in floral characters and its consequences for pollinator attraction among populations of an Andean cactus. Annals of Botany. 2009;103:1489–1500. doi: 10.1093/aob/mcp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M. Gene flow in natural populations. Annual Review of Ecology and Systematics. 1985;16:393–430. [Google Scholar]
- Snow AA, Spira TP, Simpson R, Klips RA. The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH., editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall; 1996. pp. 191–216. [Google Scholar]
- Sork VL, Nason J, Campbell DR, Fernandez JF. Landscape approaches to historical and contemporary gene flow in plants. Trends in Ecology and Evolution. 1999;14:219–224. doi: 10.1016/s0169-5347(98)01585-7. [DOI] [PubMed] [Google Scholar]
- Sprengel CK. Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. Berlin: Friedrich Vieweg dem aeltern; 1793. [Google Scholar]
- Stang M, Klinkhamer PGL, Waser NM, Stang I, van der Meijden E. Size-specific interaction patterns and size matching in a plant–pollinator interaction web. Annals of Botany. 2009;103:1459–1469. doi: 10.1093/aob/mcp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepedino VJ, Bradley BA, Griswold TL. Might flowers of invasive plants increase native bee carrying capacity? Intimations from Capitol Reef National Park, Utah. Natural Areas Journal. 2008;28:44–50. [Google Scholar]
- Thompson JN. Coevolution and alternative hypotheses on insect plant interactions. Ecology. 1988;69:893–895. [Google Scholar]
- Thomson JD. When is it mutualism? 2001 Presidential Address, American Society of Naturalists. American Naturalist. 2003;162:S1–S9. doi: 10.1086/378683. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Plowright RC. Pollen carryover, nectar rewards, and pollinator behaviour with special reference to Diervitta tonicera. Oecologia. 1980;46:68–74. doi: 10.1007/BF00346968. [DOI] [PubMed] [Google Scholar]
- Trapnell DW, Hamrick JL. Floral display and mating patterns within populations of the neotropical epiphytic orchid, Laelia rubescens. American Journal of Botany. 2006;93:1010–1018. doi: 10.3732/ajb.93.7.1010. [DOI] [PubMed] [Google Scholar]
- Traveset A, Richardson DM. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology and Evolution. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Turner BL, II, Clark WC, Kates RW, Richards JF, Mathews JT, Meyer WB. The Earth as transformed by human actions: global and regional changes in the biosphere over the past 300 years. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Turner ME, Stephens JC, Anderson WW. Homozygosity and patch structure in plant populations as a result of nearest-neighbor pollination. Proceedings of the National Academy of Sciences of the USA. 1982;79:203–207. doi: 10.1073/pnas.79.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez DP, Morris WF, Jordano P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecology Letters. 2005;8:1088–1094. [Google Scholar]
- Vázquez DP, Blüthgen N, Cagnolo L, Chacoff NP. Uniting pattern and process in plant-animal mutualistic networks: a review. Annals of Botany. 2009;103:1445–1457. doi: 10.1093/aob/mcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM. Beyond global warming: ecology and global change. Ecology. 1994;75:1861–1876. [Google Scholar]
- Waser NM. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology. 1978;59:934–944. [Google Scholar]
- Waser NM. The adaptive nature of floral traits: ideas and evidence. In: Real L., editor. Pollination biology. New York: Academic Press; 1983. pp. 242–285. [Google Scholar]
- Waser NM. Comparative pollen and dye transfer by pollinators of Delphinium nelsonii. Functional Ecology. 1988;2:41–48. [Google Scholar]
- Waser NM, Ollerton J. Plant–pollinator interactions: from specialization to generalization. Chicago: University of Chicago Press; 2006. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams N, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Werner EE. Ecological experiments and a research program in community ecology. In: Resetarits WJ, Bernardo J., editors. Experimental ecology: issues and perspectives. Oxford: Oxford University Press; 1998. pp. 3–26. [Google Scholar]
- Whelan RJ, Ayre DJ, Beynon FM. The birds and the bees: pollinator behaviour and variation in the mating system of the rare shrub Grevillea macleayana. Annals of Botany. 2009;103:1395–1401. doi: 10.1093/aob/mcp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Wilson P, Wolfe AD, Armbruster WS, Thomson JD. Constrained lability in floral evolution: counting convergent origins of hummingbird pollination in Penstemon and Keckiella. New Phytologist. 2007;176:883–890. doi: 10.1111/j.1469-8137.2007.02219.x. [DOI] [PubMed] [Google Scholar]
- Winfree R, Griswold T, Kremen C. Effect of human disturbance on bee communities in a forested ecosystem. Conservation Biology. 2007;21:213–223. doi: 10.1111/j.1523-1739.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution in mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. Isolation by distance under diverse systems of mating. Genetics. 1946;31:39–59. doi: 10.1093/genetics/31.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HJ. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae) American Journal of Botany. 2002;89:433–440. doi: 10.3732/ajb.89.3.433. [DOI] [PubMed] [Google Scholar]