Abstract
Background and Aims
Ceropegia (Apocynaceae subfamily Asclepiadoideae) is a large, Old World genus of >180 species, all of which possess distinctive flask-shaped flowers that temporarily trap pollinators. The taxonomic diversity of pollinators, biogeographic and phylogenetic patterns of pollinator exploitation, and the level of specificity of interactions were assessed in order to begin to understand the role of pollinators in promoting diversification within the genus.
Methods
Flower visitor and pollinator data for approx. 60 Ceropegia taxa were analysed with reference to the main centres of diversity of the genus and to a cpDNA–nrDNA molecular phylogeny of the genus.
Key Results
Ceropegia spp. interact with flower-visiting Diptera from at least 26 genera in 20 families, of which 11 genera and 11 families are pollinators. Size range of flies was 0·5–4·0 mm and approx. 94 % were females. Ceropegia from particular regions do not use specific fly genera or families, though Arabian Peninsula species are pollinated by a wider range of Diptera families than those in other regions. The basal-most clade interacts with the highest diversity of Diptera families and genera, largely due to one hyper-generalist taxon, C. aristolochioides subsp. deflersiana. Species in the more-derived clades interact with a smaller diversity of Diptera. Approximately 60 % of taxa are so far recorded as interacting with only a single genus of pollinators, the remaining 40 % being less conservative in their interactions. Ceropegia spp. can therefore be ecological specialists or generalists.
Conclusions
The genus Ceropegia has largely radiated without evolutionary shifts in pollinator functional specialization, maintaining its interactions with small Diptera. Intriguing biogeographic and phylogenetic patterns may reflect processes of regional dispersal, diversification and subsequent specialization onto a narrower range of pollinators, though some of the findings may be caused by inconsistent sampling. Comparisons are made with other plant genera in the Aristolochiaceae and Araceae that have evolved flask-shaped flowers that trap female flies seeking oviposition sites.
Key words: Apocynaceae, Asclepiadoideae, Brachystelma, Ceropegia, Diptera, flower evolution, generalization, mutualism, pollination, Riocreuxia, specialization, Stapeliinae
INTRODUCTION
Species-rich genera of biotically pollinated flowering plants are often considered to have diversified via shifts in pollination niche, brought about by reproductive isolation from sister taxa (e.g. Kay and Schemske, 2008) following adaptation to novel pollinators (Grant, 1949; Stebbins, 1970; Waser, 1998). Several models can account for this (Armbruster, 2009) and reproductive isolation without pollinator shifts (i.e. in relation to the abiotic environment) is also a common mechanism (Stebbins, 1970; Johnson, 1996). Plant–pollinator interactions are therefore an important component of the biodiversity of terrestrial communities. However, the diversity of pollination systems has been studied in relatively few large genera (with >100 species) principally because of the logistical difficulties of observing pollinators for any but a small fraction of the species in a genus. Rarer still are studies that have employed a molecular phylogenetic approach to understanding floral diversification (e.g. Armbruster, 1993; Bruneau, 1996, 1997; Goldblatt et al., 2002; Wilson et al., 2004; Ronsted et al., 2005, 2007; Marussich and Machado, 2007).
The scarcity of phylogenetic studies of the pollination biology of large genera over broad geographical scales is a significant, if understandable, limitation of the literature on plant-species diversification. Significant because it constrains our ability to understand species radiations and the diversification of floral traits in relation to shifts in pollination systems; and understandable because observing plant–pollinator interactions for many species over a wide range is time consuming; e.g. Armbruster's ground breaking, pantropical research on Dalechampia spans three decades and is still ongoing.
The present study focuses on the genus Ceropegia (Apocynaceae: Asclepiadoideae, Ceropegieae), a large taxon of over 180 accepted species, with new species being regularly discovered (e.g. Dold, 2006; Malpure et al., 2006). The tribe Ceropegieae is restricted to the Old World, and Ceropegia has a distribution from the Canary Islands, across most of sub-Saharan Africa, Madagascar, the Arabian Peninsula, south-east Asia (including the Indian Subcontinent, Laos, Thailand and China), to the south-western Pacific Region (including Indonesia, Philippines, Papua New Guinea and north-east Australia). Within this range there are six recognized centres of diversity: East Africa, West Africa, southern Africa, the Arabian Peninsula, the Indian Subcontinent and Madagascar (Table 1). The genus is keenly collected by plant growers, and the functional details and floral Bauplan of the trap-flower ‘Kesselfallen-Blüten’ have been known for some time (Knuth, 1909; Vogel, 1961, 1993; Endress, 1996; Masinde, 2004). However, there are relatively few published data on the flower visitors and pollinators of Ceropegia proportional to the size of the genus (Vogel, 1961; Bhatnagar, 1986; Sabrosky, 1987; van der Meutter, 1988; Ollerton and Forster, 1995; Ollerton, 1999; Masinde, 2004).
Table 1.
The centres of diversity for Ceropegia, their terminal taxon richness (including species, subspecies and natural varieties) and the sample sizes for this study
| No. of described taxa in the region | No. of Ceropegia taxa with pollinator/flower visitor data | Percentage of described taxa | No. of accessions* for those taxa | |
|---|---|---|---|---|
| East Africa | 62 | 30 | 48 | 67 |
| Asia | 60 | 3 | 5 | 5 |
| Southern Africa | 52 | 16 | 31 | 25 |
| West Africa | 25 | 11 | 44 | 14 |
| Madagascar | 20 | 4 | 20 | 5 |
| Arabian Peninsula | 13 | 8 | 62 | 24 |
Pollinator/flower visitor totals exclude data from cultivated plants. In the second column, 20 taxa have been counted more than once because they occur in more than one biogeographic region. Regions are ranked in order of their Ceropegia taxon richness.
*‘Accessions’ refers to records of Ceropegia flowers at a particular locality that have been found to contain insects.
The present study analyses data on flower visitors and pollinators for 59 species, subspecies and natural varieties of Ceropegia (approx. one-third of known terminal taxa in the genus; Table S1 in Supplementary data available online) across the whole distribution of the genus and for all major clades (Meve and Liede-Schumann, 2007). It has been possible to assemble this large data set because of two unusual features of the pollination biology of these plants. First, being asclepiads, Ceropegia species present pollen as discrete masses (pollinia) with mechanical clips that attach to the pollinator; identifying pollinators amongst all of the flower visitors is therefore straightforward (Wyatt and Broyles, 1994; Ollerton and Liede, 1997). Secondly, as far as is known, all species of Ceropegia temporarily trap their pollinators, releasing them after a period of about 24 h, during which time pollinaria have often been picked up and/or deposited by the pollinators. Botanists who collect Ceropegia specimens therefore often also collect the flower occupants and, in spirit-preserved material, these can be removed with minimum damage to the flowers (Ollerton and Forster, 1995). The pollinaria are mechanically persistent and therefore the status of flower visitors as pollinators can be confirmed. These two biological factors provide an opportunity to accumulate plant–pollinator interaction data much more rapidly than would normally be the case for such a large genus. The Kew Herbarium holds some 650 spirit specimens of Ceropegia and these have been systematically examined for the presence of insects. In addition, published and unpublished field data collected by ourselves and others have been used to assemble a data set of 136 records in which Ceropegia flowers at a particular locality have been found to contain insects. These records are hereafter collectively referred to as ‘accessions’. This data set has been used to provide a first assessment of the diversity of pollination systems within the genus Ceropegia, across its whole range, and in both biogeographic and phylogenetic contexts. The present study addressed the following questions. (1) How diverse are pollinator-plant interactions within Ceropegia, given that all described species of Ceropegia have an identical floral Bauplan (sensu Endress, 1996), though flowers are highly variable among taxa (Fig. 1)? (2) Ceropegia has several regional centres of diversity; are there distinct biogeographic patterns of pollinator exploitation within the genus? (3) Do the different clades of Ceropegia exploit particular pollinator taxa, or are the same groups of pollinators used throughout the genus? (4) Within the context of a strict Bauplan but variable floral detail (question 1), how specific are the pollination systems of Ceropegia at the levels of insect order, family and genus?
Fig. 1.
Representative taxa of Ceropegia illustrating floral diversity in the genus: (A) C. ampliata; (B) C. imbricata; (C) C. aristolochioides subsp. aristolochioides; (D) C. crassifolia var. copleyae; (E) C. affinis; (F) C. sankuruensis; (G) C. meyeri-johannis; (H) C. denticulata; (I) C. cufodontii; and (J) C. variegata (basal inflation opened, exposing corona). Photographs: U. Meve.
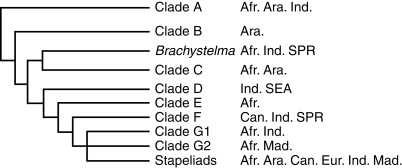
Fig. 2.
Summary phylogeny of Ceropegia, showing the major clades and their relationship to the genus Brachystelma and the stapeliads, and the distribution of those clades (adapted from Meve and Liede-Schumann, 2007). Distribution abbreviations: Afr. = Africa; Ara. = Arabian Peninsula; Can. = Canary Islands; Eur. = Europe; Ind. = Indian Subcontinent; Mad. = Madagascar; SEA = south-east Asia; SPR = south-western Pacific Region.
Addressing these questions should inform our understanding of the biogeography and phylogeny of plant–pollinator interactions in a moderately large genus of mainly tropical plants across most of its native range.
MATERIALS AND METHODS
Insects removed from the Kew Herbarium accessions were predominantly Diptera (see Results) and were initially identified to family or genus by one of us (A.W.); especially critical families were sent to experts for further determination (see Acknowledgements). Using a dissecting or stage microscope, the body length of all insects was measured and each was examined for the presence of persistent pollinaria, which was taken as confirmation that these fly taxa were legitimate pollinators; in asclepiads, removal of pollinaria is spatially and behaviourally correlated with pollinia insertion and therefore insects that are large enough, and in the correct position and orientation on the flower, are likely to both remove and deposit pollen.
Samples of Ceropegia flowers collected in the field by four of us (J.O., U.M., M.P., S.M.) and colleagues were similarly dissected and flies measured, assessed and sent to A.W. for initial determination. Field-collected South African material was identified by B. Stuckenberg (Natal Museum, South Africa). In addition, all of the available data on Ceropegia pollinators from the published literature were collated. The resulting data of Ceropegia pollinators and flower visitors were included in the ASCLEPOL database and is available online at http://www.uni-bayreuth.de/departments/planta2/research/pollina/as_pol_t.html.
Data analysis
Some of the flies in the published literature and from the authors' own collections were identified to species level. However, the taxonomy of tropical small-sized Diptera is so poorly studied that it was decided to limit the analyses to the level of genus and above. The data on Diptera–Ceropegia interactions were therefore classified into four levels of taxonomic–functional resolution:
Flies that have been identified to family level and which may or may not have been carrying pollinaria (i.e. all flower visitors).
Flies that have been identified to family level and which carried pollinaria (i.e. pollinators only).
Flies that have been identified to genus level and which may or may not have been carrying pollinaria (i.e. all flower visitors).
Flies that have been identified to genus level and which carried pollinaria (i.e. pollinators only).
The largest of these four categories is I, encompassing virtually all of the records that we possess, because most flies could be determined to family level at least. The most restrictive category is IV – relatively few of the flies determined to genus level carried pollinaria. Whilst it is important to work with as large a sample size as possible, it is categories II and IV that are of most interest from the perspective of pollination biology. Nonetheless, flies that did not carry pollinaria also represent species interactions, between Ceropegia and flies that may be legitimate pollinators, but which had not yet picked up pollinaria; or flies which may be parasitic on Ceropegia, e.g. gall-forming Cecidomyiidae (see Results). An important determinant of whether or not a fly acts as a pollinator of Ceropegia is likely to be its size: the fly must be small enough to manoeuvre down the narrowed corolla tube and within the flower chamber, but also large (and strong) enough to be able to remove pollinaria (see Results). The results are therefore presented with appropriate qualifiers as to the status of the Diptera as flower visitors or confirmed pollinators.
Phylogenetic analysis
Ceropegia, with some 180 species, represents the largest taxon within the tribe Ceropegieae, followed by Brachystelma R.Br. (approx. 100 species). The tribe consists of about 770 species altogether, distributed over four subtribes and 46 genera (Meve and Liede, 2004; Endress et al., 2007). The largest subtribe, the Stapeliinae (the stem-succulent stapeliads) corresponds to the terminal clade in the tribal phylogeny, with all genera separated by rather short genetic distances (Meve and Liede, 2004). In a recent molecular analysis of Ceropegia by Meve and Liede-Schumann (2007), based on non-coding markers of cpDNA (trnT-L and trnL-F spacers and the trnL intron) and nrDNA (ITS), the taxa investigated scatter over a grade of seven clades (labelled A–G, Fig. 2). The taxa that group together on these clades often lack morphological similarity, and show different distribution areas (e.g. Africa and Asia), pointing to a complex biogeographical history of the whole genus. On the contrary, of the 13 investigated Ceropegia taxa of East Africa, ten belong to clade C, and all the species endemic to Madagascar belong to clade G2 (Fig. 2). Asian taxa, however, are underrepresented in this study. The phylogenetic analysis suggests that Ceropegia, despite its morphological conformity, is twice paraphyletic. One of the seven clades retrieved is shared by some Ceropegia and all Brachystelma species investigated (Fig. 2), making Ceropegia without Brachystelma paraphyletic. Furthermore, all endemic Madagascan Ceropegia taxa investigated, plus the East African C. robynsiana and a subclade comprising C. bulbosa and C. konasita, share a terminal (but in most analyses unresolved) clade with the stapeliads (Fig. 2). Thus again, Ceropegia without the stapeliads is paraphyletic; both Brachystelma and the stapeliad group emerged from within Ceropegia as traditionally circumscribed. In the absence of adequate morphological characters supporting taxonomical reclassification of the groups involved into monophyletic units, Meve and Liede-Schumann (2007) proposed to hold on to the current taxonomy, since the genus Ceropegia is convincingly characterized by its pitfall flowers. Consequently, the phylogeny of Ceropegia and the Stapeliinae remains not as fully understood and more complex than that of other tribes of Asclepiadoideae (cf. Liede-Schumann et al., 2005; Rapini et al., 2006). In this study, for the sake of brevity, those clades which split earliest from the phylogeny (i.e. clades A, B and C in Fig. 2) are referred to as ‘basal’ or ‘basal-most’ and the remaining clades as ‘derived’, with the understanding that this makes no value judgement on their ‘primitiveness’ or otherwise.
RESULTS
Flower visitors and pollinators of Ceropegia
The vast majority of visitors to Ceropegia flowers, and all of the confirmed pollinators, are small species of Diptera (Table S1 in Supplementary data available online; ASCLEPOL database). Those Diptera for which the gender could be determined were overwhelmingly female (550 of 584 insects = 94·2 %). Of the flies that carried pollinaria, 81 of 83 specimens (97·6 %) carried them on their mouthparts; the remaining two flies carried them on a foreleg and the prothorax. The non-dipteran flower occupants included sap-sucking Aphididae and Cixiidae (Homoptera), Cicadellidae (Heteroptera), Collembola, herbivorous micro-Lepidoptera, and various families of parasitoidal Hymenoptera, including Chalcidoidea and Mymaridae (the latter frequently laying their eggs on the eggs of Homoptera; Noyes, 2003). Ants (Hymenoptera: Formicidae) were also occasionally found (Ollerton, 1999).
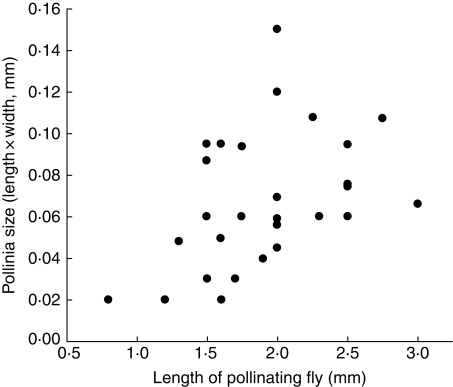
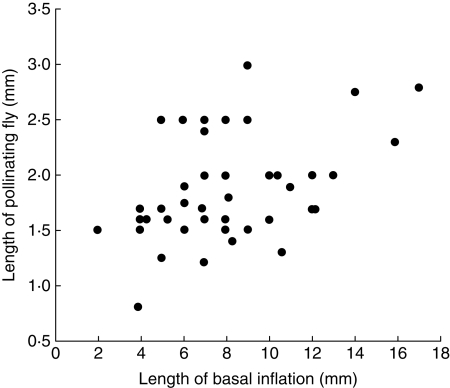
The size range of the Diptera was 0·5–4·0 mm in length; however, on average, the flies that were found with pollinaria attached were significantly larger than flies without pollinaria [mean ± s.d. (n) = 1·8 ± 0·6 mm (67) versus 1·6 ± 0·6 mm (153); z-test: z = 29·8, P≪0·01]. This suggests that some of the smallest Diptera (those less than approx. 0·8 mm, the lower range of pollinaria-carrying flies) are unlikely to be large enough to remove pollinaria. There was a significant positive correlation between fly size and the size of the pollinia being carried (Spearman's r = 0·46, n = 30, P = 0·01; Fig. 3) and between fly size and the length of the basal inflation of the corolla tube (Spearman's r = 0·39, n = 48, P≪0·006; Fig. 4), supporting the idea that the function of flies as pollinators is constrained by their size, as both basal inflation and pollinia size are correlated with overall flower size (Spearman Rank Correlations: flower length vs. size of basal inflation: r = 0·71, n = 47, P≪0·0001; flower length vs. pollinia length × width: r = 0·35, n = 25, P = 0·08). Thus flies that are too small cannot remove pollinaria, whereas flies that are too large would not be able to manoeuvre within the basal inflation.
Fig. 3.
Relationship between fly size (length) and size of the pollinia carried (pollinia length × width) for Diptera pollinating Ceropegia spp.
Fig. 4.
Relationship between the size of the basal inflation of Ceropegia flowers and the size of the flies trapped within them.
Taxonomic diversity and functional ecology of Ceropegia pollinators
Based on the data available so far, Ceropegia taxa are known to interact with at least 26 genera of Diptera belonging to 20 families (Table S1 in Supplementary data available online; Table 2). Of these flower visitors, 11 genera and 11 families are known to act as pollinators. This high taxonomic diversity of pollinators will undoubtedly increase as more data become available for Ceropegia spp. included in this study (confirming flower visitors as pollinators) and for the remaining species of Ceropegia that have not been studied. Some of the species removed from the Kew specimens are new to science (Disney, 2009).
Table 2.
Records of Diptera families and genera as flower visitors and pollinators of Ceropegia spp. in the main regions of diversity of the genus
| Genera* |
Families* |
|||
|---|---|---|---|---|
| Region | Flower visitors | Pollinators | Flower visitors | Pollinators |
| Arabian Peninsula | Allotrichoma | Allotrichoma | Agromyzidae | Calliphoridae |
| Amauromyza | Atrichopodon | Asteiidae | Ceratopogonidae | |
| Asteia | Chlororhinia (?) | Calliphoridae | Chloropidae | |
| Atrichopodon | Desmometopa | Carnidae | Drosophilidae | |
| Chaetosciara (?) | Forcipomyia | Cecidomyiidae | Ephydridae | |
| Chlororhinia (?) | Leptometopa | Ceratopogonidae | Milichiidae | |
| Desmometopa | Phytomyptera | Chironomidae | Sciaridae | |
| Diclasiopa | Chloropidae | Tachinidae | ||
| Forcipomyia | Drosophilidae | |||
| Goniurella | Ephydridae | |||
| Hecamedoides | Lygistorrhinidae | |||
| Leptometopa | Milichiidae | |||
| Lygistorrhina | Phoridae | |||
| Megaselia | Sciaridae | |||
| Meoneura | Tachinidae | |||
| Phyllomyza | Tephritidae | |||
| Phytomyptera | ||||
| n = 6 (11)† | n = 6 (9) | n = 8 (22) | n = 7 (13) | |
| East Africa | Asteia | Desmometopa | Asteiidae | Ceratopogonidae |
| Bradysia (?) | Forcipomyia | Cecidomyiidae | Chloropidae | |
| Dasyhelea | Leptometopa | Ceratopogonidae | Drosophilidae | |
| Desmometopa | Megaselia | Chloropidae | Milichiidae | |
| Forcipomyia | Drosophilidae | Sciaridae | ||
| Leptometopa | Milichiidae | |||
| Megaselia | Psychodidae | |||
| Milichiela | Phoridae | |||
| Phyllomyza | Scatopsidae | |||
| Rhexoza | Sciaridae | |||
| Stomosis | ||||
| n = 20 (42) | n = 6 (8) | n = 26 (67) | n = 13 (19) | |
| Asia | Desmometopa | Desmometopa | Ceratopogonidae | Ceratopogonidae |
| Forcipomyia | Forcipomyia | Chloropodae | Milichiidae | |
| Milichiidae | ||||
| n = 2 (3) | n = 2 (3) | n = 3 (4) | n = 2 (3) | |
| Madagascar | Leptometopa | Desmometopa | Cecidomyiidae | Milichiidae |
| Stomosis | Leptometopa | Chloropidae | ||
| Desmometopa | Stomosis | Milichiidae | ||
| n = 2 (3) | n = 2 (3) | n = 4 (6) | n = 2 (3) | |
| Southern Africa | Brachypogon | Desmometopa | Cecidomyiidae | Ceratopogonidae |
| Desmometopa | Forcipomyia | Ceratopogonidae | Chloropidae | |
| Drapetis | Megaselia | Chironomidae | Drosophilidae | |
| Forcipomyia | Rhexoza | Chloropidae | Milichiidae | |
| Leptometopa | Swammerdamella | Drosophilidae | Scatopsidae | |
| Megaselia | Hybotidae | |||
| Neophyllomyza | Milichiidae | |||
| Rhexoza | Phoridae | |||
| Swammerdamella | Scatopsidae | |||
| Sciaridae | ||||
| n = 5 (8) | n = 5 (8) | n = 16 (29) | n = 7 (9) | |
| West Africa | Desmometopa | Desmometopa | Ceratopogonidae | Ceratopogonidae |
| Forcipomyia | Forcipomyia | Chloropidae | Chloropidae | |
| Leptometopa | Leptometopa | Milichiidae | Milichiidae | |
| Mycetophilidae | Phoridae | |||
| Phoridae | ||||
| Megaselia | Megaselia | Sciaridae | ||
| n = 4 (5) | n = 4 (5) | n = 11 (14) | n = 7 (9) | |
*Families and genera were considered pollinators if pollinaria were found attached to the flies. The names are arranged in alphabetical order within cells.
†n is the number of Ceropegia taxa contributing to that cell; the number following it in parenthesis is the number of accessions of those taxa, i.e. records of Ceropegia flowers at a particular locality that have been found to contain insects.
Of the non-pollinating Diptera in Table 2, some families are repeatedly found in Ceropegia flowers but never carry pollinaria and may never be part of the pollinating fauna of these taxa. For example, Cecidomyiidae (found in six taxa) may be visiting flowers to lay eggs because their larvae are gall-making parasites, or predators of mites or of other insects (see Ollerton, 1996).
Given the wide phylogenetic range of Diptera families it is no surprise that the functional ecologies of these flies are similarly diverse and range from taxa that feed on living plant and fungus material, to carrion, dung and rotting vegetation feeders, to commensals and parasitoids of other insects (Table S2 in Supplementary data available online). However, the larvae of most taxa feed on decaying organic material of some description.
The biogeographic perspective
The broadest diversity of flower-visiting and pollinating families and genera of Diptera is found in the Arabian Peninsula region, where Ceropegia taxa are known to interact with 16 families (eight known to pollinate) and 16 genera (seven known to pollinate) of flies (Table 2). However, a significant amount of that diversity is attributable to a single taxon, Ceropegia aristolochioides ssp. deflersiana, that interacts with at least 15 families of flies in this region (Table S1 in Supplementary data available online). A closely related taxon, C. aristolochioides ssp. aristolochioides similarly interacts with six families in East Africa, so this may be a feature of this particular species (see also ‘The phylogenetic perspective’ below), which correlates with the unusually large variability of flower morphology present in both subspecies of C. aristolochioides (Meve et al., 2001). Within the ranges of the two subspecies there exist numerous distinct variants which apparently freely interbreed and for which there is no clear geographical pattern of distribution (Masinde, 2006).
East Africa and southern Africa have similar levels of diversity of Diptera that interact with Ceropegia flowers, while the remaining regions (West Africa, Madagascar and Asia) are all relatively depauperate in terms of the diversity of Ceropegia–Diptera interactions (Table 2 – though see caveats regarding sampling effort below). Some Diptera families and genera repeatedly interact with Ceropegia as pollinators across all or most regions; e.g. Ceratopogonidae (Forcipomyia), Milichiidae (Desmometopa), the hyper-diverse genus Megaselia (Phoridae), and Chloropidae. Other families and genera are restricted to only one or two regions. Apart from the high diversity in the Arabian Peninsula discussed above, however, there is no distinct pattern of biogeographic occurrence of Ceropegia taxa interacting with particular families or genera in different biogeographic regions (Table 2).
The phylogenetic perspective
The basal-most clade A of Ceropegia (Fig. 2; Meve and Liede-Schumann, 2007) interacts with the highest diversity of Diptera families (at least 16) and genera (at least 24) as flower visitors (Table 3). Fewer taxa are confirmed pollinators (seven families and six genera) but this diversity is nonetheless greater than for any of the other ‘Ceropegia’ clades labelled in Fig. 2. Uneven sampling between clades (see below) means that comparisons must be tentatively made; however, comparing clade A with the more abundantly sampled, but more-derived clade C suggests that the higher diversity found in the basal-most clade is not an artefact of sampling (Table 3). The two most-derived clades (G1 and G2; Fig. 2) differ in their patterns of Diptera diversity; despite very similar sample sizes, the largely Madagascan clade G2 interacts with a diversity of flower-visiting families and genera, whereas all of the African and Indian Subcontinent clade G1 species so far sampled are pollinated by flies of the genus Forcipomyia (Ceratopogonidae; Table 3).
Table 3.
Records of Diptera families and genera as flower visitors to the major clades of Ceropegia
| Clade | Families* | Genera* |
|---|---|---|
| A | Agromyzidae | Amauromyza |
| Asteiidae | Asteia | |
| Calliphoridae | Chlororhinia? | |
| Carnidae | Meoneura | |
| Cecidomyiidae | Undetermined genus | |
| Ceratopogonidae | Atrichopodon, Forcipomyia | |
| Chironomidae | Undetermined genus | |
| Chloropidae | Undetermined – possibly >1 genus | |
| Drosophilidae | Undetermined – possibly >1 genus | |
| Ephydridae | Hecamedoides, Diclasiopa, Allotrichoma | |
| Lygistorrhinidae | Lygistorrhina | |
| Milichiidae | Phyllomyza, Stomosis, undetermined genus | |
| Phoridae | Megaselia | |
| Sciaridae | Chaetosciara?, undetermined genus | |
| Tachinidae | Phytomyptera | |
| Tephritidae | Goniurella | |
| (7/22)† | ||
| B‡ | (0/0) | |
| C | Cecidomyiidae | Undetermined genus |
| Ceratopogonidae | Forcipomyia, Dasyhelea, Brachypogon | |
| Chironomidae | Undetermined genus | |
| Chloropidae | Undetermined – possibly >1 genus | |
| Drosophilidae | Undetermined genus | |
| Hybotidae | Drapetis | |
| Milichiidae | Desmometopa, Milichiela, Leptometopa, Neophyllomyza | |
| Phoridae | Megaselia | |
| Scatopsidae | Rhexoza, Swammerdamella | |
| Sciaridae | Undetermined genus | |
| (23/60) | ||
| D | Milichiidae | Desmometopa |
| (1/1) | ||
| E | Psychodidae | Undetermined genus |
| Phoridae | Megaselia | |
| (1/2) | ||
| F | Chloropidae | Undetermined genus |
| Mycetophilidae | Undetermined genus | |
| Sciaridae | Undetermined genus | |
| (3/4) | ||
| G1 | Ceratopogonidae | Forcipomyia |
| (4/9) | ||
| G2 | Cecidomyiidae | Undetermined genus |
| Chloropidae | Undetermined – possibly >1 genus | |
| Milichiidae | Desmometopa, Leptometopa, Stomosis | |
| Sciaridae | Bradysia?, undetermined – possibly >1 genus | |
| (6/12) |
*Families and genera which are confirmed as pollinators are presented in bold. The names are arranged in alphabetical order by family within cells.
†The numbers in parentheses are sample sizes: number of taxa of Ceropegia (species and subspecies) followed by number of accessions (see text for details). Only taxa that could be firmly assigned to clades have been included.
‡No flower visitor data are available for clade B which is included only for completeness.
Specificity of Ceropegia–pollinator interactions
The specificity of interactions between Ceropegia and its flower visitors was examined by using three approaches: (1) Ceropegia taxa that had multiple samples were assessed to look at specificity within and between regions (Table 4); (2) the frequency with which more than one Diptera family or genus was found in an individual Ceropegia flower was analysed to look at specificity at particular sites on specific dates (Fig. 5); and (3) the few cases where pollinators had been found in species sampled from the wild and in cultivation were compared (Table 5).
Table 4.
Comparisons of Ceropegia flower visitors and pollinators (Diptera genera) for taxa with three or more accessions, within and between biogeographic regions
| Clade | Ceropegia taxon | Accession* | Region | Genera† |
|---|---|---|---|---|
| A | C. aristolochioides ssp. deflersiana | K44108 | AP | Chlororhinia?, Atrichopodon, Allotrichoma, Amauromyza, Chaetosciara?, Hecamedoides, Diclasiopa, Meoneura |
| K53946 | AP | Asteia, Phyllomyza | ||
| K45745 | AP | Phytomyptera, Diclasiopa | ||
| K39277 | AP | Lygistorrhina, Megaselia | ||
| K44111 | AP | Goniurella | ||
| C. lugardiae | F604 | EA | Stomosis | |
| K58573 | EA | Forcipomyia | ||
| C | C. arabica var. abbreviata | K47780 | AP | Leptometopa |
| K49831 | AP | Leptometopa, Desmometopa | ||
| C. arabica var. superba | K47779 | AP | Desmometopa | |
| K49406 | AP | Desmometopa | ||
| K47779 | AP | Desmometopa | ||
| C. arabica var. powysii | SM686 | EA | Forcipomyia | |
| SM828 | EA | Leptometopa, Milichiela | ||
| F727 | EA | Desmometopa | ||
| K40459 | EA | Desmometopa | ||
| C. nilotica | K1930 | EA | Desmometopa | |
| K3989 | EA | Desmometopa | ||
| K56450 | EA | Desmometopa | ||
| K51019 | EA | Desmometopa | ||
| K22867 | WA | Desmometopa | ||
| K23284 | WA | Desmometopa | ||
| K50488 | SA | Desmometopa, Neophyllomyza, Forcipomyia | ||
| C. denticulata | K58558 | EA | Desmometopa | |
| K5303 | EA | Desmometopa | ||
| K58533 | EA | Desmometopa | ||
| K47158 | EA | Desmometopa | ||
| C. stenantha | K1920 | SA | Desmometopa, Swammerdamella, Drapetis | |
| K1921 | SA | Swammerdamella | ||
| K3983 | SA | Rhexoza, Brachypogon | ||
| K3982 | SA | Rhexoza | ||
| K1045 | EA | Rhexoza | ||
| K53328 | EA | Dasyhelea | ||
| K40179 | EA | Dasyhelea | ||
| C. linearis ssp. tenuis | K49196 | SA | Forcipomyia | |
| C. linearis ssp. woodii | K49209 | SA | Forcipomyia | |
| C. linearis | SM389/2 | EA | Forcipomyia | |
| K58544 | EA | Forcipomyia | ||
| K6715 | EA | Forcipomyia | ||
| C. meyeri-johannis | K3975 | EA | Forcipomyia | |
| M&L3368 | EA | Forcipomyia | ||
| B872 | EA | Forcipomyia | ||
| G1 | C. bulbosa | Bhatnagar (1986) | A | Forcipomyia |
| Ali (1994) | A | Forcipomyia | ||
| K54293 | AP | Forcipomyia | ||
| C. imbricata | K54293 | EA | Forcipomyia | |
| A404 | EA | Forcipomyia | ||
| G2 | C. albisepta | Sabrosky (1987) | M | Leptometopa |
| RM31 | M | Stomosis | ||
| C. ballyana | K47775 | EA | Leptometopa | |
| K58598 | EA | Leptometopa | ||
| K29852 | EA | Bradysia? | ||
| ? | C. papillata | K1037 | EA | Forcipomyia |
| K3975 | EA | Forcipomyia | ||
| K3403 | EA | Forcipomyia | ||
| K1039 | EA | Forcipomyia | ||
| K3405 | EA | Leptometopa | ||
| K1922 | SA | Forcipomyia | ||
| K3985 | SA | Forcipomyia | ||
| C. filipendula | K3400 | EA | Phyllomyza | |
| K3401 | EA | Leptometopa | ||
| C. claviloba | K1040 | EA | Forcipomyia | |
| K1033 | SA | Forcipomyia |
*Accessions from the Kew Herbarium are prefixed K, other prefixes refer to collectors, and references refer to previously published data.
†Confirmed pollinators are shown in bold. Species which cannot presently be assigned to a clade are indicated by a ‘?’.
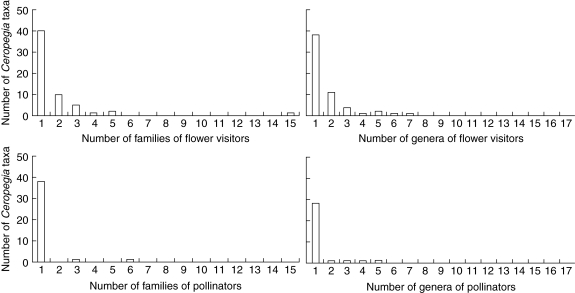
Fig. 5.
Frequency distributions of the number of genera (right-hand graphs) and families (left-hand graphs) of Diptera known to interact as flower visitors (top graphs) and confirmed as pollinators (bottom graphs) of Ceropegia species, subspecies and natural varieties (‘taxa’ on the y-axes).
Table 5.
Examples of Ceropegia taxa interacting with pollinating Diptera within compared with outside (in cultivation) their native ranges
| Ceropegia taxon | Region | Families of pollinators | Genera of pollinators |
|---|---|---|---|
| C. linearis | East Africa | Ceratopogonidae | Forcipomyia |
| C. linearis | East Africa | Ceratopogonidae | Forcipomyia |
| C. linearis | East Africa | Ceratopogonidae | Forcipomyia |
| C. linearis | East Africa | Ceratopogonidae | Forcipomyia |
| C. linearis ssp. tenuis | Southern Africa | Ceratopogonidae | Forcipomyia |
| C. linearis ssp. woodii | Southern Africa | Ceratopogonidae | Forcipomyia |
| C. linearis | Europe | Ceratopogonidae | Forcipomyia |
| C. linearis ssp. woodii | Europe | Ceratopogonidae | Forcipomyia |
| C. stapeliiformis var. serpentina | Southern Africa | Milichiidae | Leptometopa |
| C. stapeliiformis var. serpentina | Southern Africa | Milichiidae | Leptometopa |
| C. stapeliiformis var. serpentina | Southern Africa (cultivation) | Milichiidae | Leptometopa |
| C. stapeliiformis | Europe | Chloropidae | Conioscinella |
| C. stapeliiformis | Europe | Milichiidae | Madiza, Tricimba |
Details of the references and accessions are to be found in the ASCLEPOL database.
From Table 4, the proportion of the 17 terminal taxa (counting the C. linearis complex as a single taxon) that show evidence of conservatism in their interactions with particular fly genera as flower visitors or pollinators is approx. 60 % (10 of 17 taxa). For example, Ceropegia nilotica is so far known to interact only with flies of the genus Desmometopa (Milichiidae) across multiple samples from West Africa and East Africa, whilst C. bulbosa interacts with flies only in the genus Forcipomyia (Ceratopogonidae) in Asia and the Arabian Peninsula (Table 4). The remaining 40 % (7 of 17 taxa) appear to be rather less conservative in their interactions, such as C. arabica var. powysii, which interacts with flies belonging to four genera within samples from East Africa, and C. aristolochioides ssp. deflersiana which is known to be highly variable in its interactions in the Arabian Peninsula (Table 4).
From a sub-sample of 46 Ceropegia flowers from the Kew collection, over half (54 %) contained only a single individual fly. The frequency distribution was strongly right skewed with over one-third (37 %) of flowers containing two to six flies and only single examples of flowers that when opened contained 9, 10, 11 and 21 flies. Of the 135 Ceropegia accessions, 25 (18·5 %) contained more than one genus of fly, either in the same flower or from a different flower in that collection. Therefore most species blooming at a particular locality in a given season interacted with and were pollinated by only a single genus of Diptera (Fig. 5).
Ceropegia is a genus that is actively collected and grown by horticultural enthusiasts and botanical gardens. In the ASCLEPOL database, there are data on flies trapped in 20 Ceropegia taxa in cultivation from 26 accessions. For two of these taxa (the C. linearis complex and varieties of C. stapeliiformis) there are enough data to compare patterns of interaction with Diptera within their native range of southern Africa and in cultivation in Europe (Table 5). Taxa within the C. linearis complex are remarkably conservative in their exploitation of pollinators and are known only to interact with Diptera of the genus Forcipomyia (Ceratopogonidae), reinforcing the results in Table 4. In contrast, varieties of C. stapeliiformis in the wild and in cultivation in southern Africa are pollinated by flies belonging to Leptometopa (Milichiidae), whereas in Europe they are known to be pollinated by at least three genera in the families Chloropidae and Milichiidae (Table 5; Vogel, 1961). The limited evidence from these cultivated examples strengthens the conclusion that some taxa of Ceropegia are strict specialists, and conservative in their use of pollinators at least at the genus level, whereas other taxa are much less conservative and attract a range of different genera and families of Diptera that serve as pollinators.
The effect of sampling effort on the results
The number of Diptera families and genera known to be flower visitors and pollinators of Ceropegia taxa in different regions is strongly affected by sampling effort (i.e. the number of Ceropegia taxa for which there are data and number of accessions per taxon). For example, across the biogeographic zones, the number of pollinating genera per region is marginally significantly correlated with number of Ceropegia taxa studied from that region (Spearman Rank Correlation: r = 0·77, n = 6, P = 0·07) and significantly correlated with the number of accessions from each region (r = 0·84, n = 6, P = 0·04). Similar results were found for number of genera as flower visitors and for numbers of families of visitors and pollinators (results not shown). In short, variation in the number of taxa or accessions in these analyses makes it difficult to compare between taxa and regions, a long-standing problem in ecological sampling generally and studies of pollination ecology specifically (Ollerton and Cranmer, 2002; Herrera, 2005). This limits our ability to say definitively whether, for example, Ceropegia taxa in the Arabian Peninsula really do interact with a wider diversity of fly families than Ceropegia taxa in East Africa (Table 2) and whether basal clades interact with a broader range of Diptera than more-derived clades (Table 3), or, alternatively, if these patterns are due to the larger number of taxa and accessions of Arabian Peninsula and basal-most Ceropegia in the present data set. One way to control for sampling artefacts is to compare pairs of Ceropegia taxa that have a similar number of accessions in the Arabian Peninsula with other regions. Similarly, one can compare Ceropegia taxa from different clades that have similar levels of sampling. These comparisons should give an indication of whether the observed patterns of relative diversity of pollinators have a real biological basis or are an artefact of different sampling efforts in different regions and across clades (Tables 6 and 7).
Table 6.
Flower visitor diversity (Diptera families and genera) of Ceropegia taxa in the Arabian Peninsula contrasted with other biogeographic regions
| Ceropegia taxa | Region | No. of accessions | No. of families | No. of genera |
|---|---|---|---|---|
| C. aristolochioides ssp. deflersiana | Arabian Peninsula | 9 | 15 | 18 |
| C. aristolochioides ssp. aristolochioides | East Africa | 7 | 6 | 6 |
| C. arabica var. abbreviata | Arabian Peninsula | 2 | 1 | 1 |
| C. bulbosa | Asia | 2 | 1 | 1 |
| C. arabica var. arabica | Arabian Peninsula | 3 | 2 | 2 |
| C. arabica var. superba | Arabian Peninsula | 3 | 1 | 1 |
| C. arabica var. powysii | East Africa | 4 | 3 | 5 |
| C. variegata | Arabian Peninsula | 1 | 1 | 1 |
| C. variegata | East Africa | 1 | 1 | 1 |
Table 7.
Comparison of the effect of sampling effort (number of accessions) on the diversity of Diptera families and genera recorded for relatively basal and more-derived clades of Ceropegia (see Fig. 2)
| Ceropegia taxa | Clades | No. of accessions | No. of families | No. of genera |
|---|---|---|---|---|
| Basal clades | ||||
| C. denticulata | C | 4 | 1 | 1 |
| C. meyeri-johannis | C | 3 | 1 | 1 |
| C. arabica var. arabica | C | 3 | 2 | ≥2 |
| C. arabica var. superba | C | 3 | 1 | 1 |
| C. arabica var. powysii | C | 4 | 3 | 5 |
| C. variegata | C | 3 | 3 | ≥3 |
| C. linearis | C | 3 | 1 | 1 |
| C. denticulata | C | 4 | 1 | 1 |
| Derived clades | ||||
| C. imbricata | G1 | 3 | 1 | 1 |
| C. bulbosa | G1 | 3 | 1 | 1 |
| C. ballyana | G2 | 3 | 3 | ≥3 |
| C. robynsiana | G2 | 4 | 2 | ≥2 |
| C. albisepta | G2 | 2 | 3 | 4 |
For the biogeographic comparison, the results show that the higher diversity of Arabian Peninsula Ceropegia pollinators is largely an artefact, not of sampling per se, but of the influence on the data set of the hyper-generalist taxon Ceropegia aristolochioides ssp. deflersiana (Table 6). On a per-accession basis, other taxa have very similar diversities of interacting Diptera families and genera in the Arabian Peninsula compared with other regions.
For the clade contrasts, given a similar level of sampling effort, taxa in derived clades can be as specialized or generalist in their interactions with Diptera as the basal clades (Table 7). In all cases, the sampling effort in these examples is three or four accessions per taxon, except for C. albisepta that is included as an example of a member of a derived clade (G2) that interacts with four genera of Diptera despite low sampling effort (n = 2 accessions).
DISCUSSION
The floral Bauplan of Ceropegia comprises a fused corolla that is more or less tubular, often partially closed at the mouth to form a cage, and frequently with a basally inflated region. However, there is enormous variation on this theme, with species and subspecies varying considerably in size, colour, shape, odour and ornamentation (Fig. 1). Despite this variation, as far as is known the large majority of flower visitors and all confirmed pollinators are small Diptera, usually female, and typically <3 mm in length. This confirms earlier work on pollination within a much smaller subset of the genus (Vogel, 1961; Bhatnagar, 1986; Sabrosky, 1987; van der Meutter, 1988; Ollerton and Forster, 1995; Masinde, 2004) but does not preclude discovering pollination by other insect orders (e.g. Coleoptera) in Ceropegia spp. that have not yet been studied. Insects such as aphids (Hemiptera) and small wasps (Hymenoptera) that are occasionally found in Ceropegia flowers probably feed on floral tissues or developing seeds. Other infrequent flower occupants are ants (Hymenoptera: Formicidae) whose scavenging, omnivorous habits suggests that their presence in Ceropegia flowers may be to steal nectar or to scout possible food sources (indeed one individual was preserved in the act of consuming a trapped fly; see Ollerton, 1999).
Ceropegia is therefore an example of a large genus that has diversified despite an apparently functionally specialized (sensu Fenster et al., 2004; Ollerton et al., 2007), conservative pollination system which exploits only small Diptera. However, the Diptera that are known to interact with Ceropegia spp. have a taxonomic span of at least 26 genera belonging to 20 families, with a large range of life histories and ecologies (Tables S1 and S2 in Supplementary data available online). The overwhelming majority of these flies were female and it seems reasonable to infer that Ceropegia pollination systems are essentially brood site deception systems in which the flowers mimic substrates for egg laying, as is found in other ‘trap flower’ genera such as Arisaema (Vogel and Martens, 2000). Identifying the models that different Ceropegia taxa are mimicking is not straightforward because human sensory modes differ from those of Diptera. Larval feeding substrates of some Ceropegia-associated fly families includes carrion and dung (Calliphoridae), and rotting plant and fungus material (Ceratopogonidae, Drosophilidae, Milichiidae, Scatopsidae, Sciaridae). The occurrence of the predominantly aquatic Ephydridae and parasitoidal Tachinidae as pollinators of C. aristolochioides subsp. deflersiana deserves mention and may be due to the uncharacteristically ecologically generalized (sensu Fenster et al., 2004; Ollerton et al., 2007) nature of this taxon's interactions with its pollinators (Table 4 and Table S2 in Supplementary data available online). Why this should be the case is not known and deserves further study, given its variable flower morphology (Meve et al., 2001).
Pollinaria placement was in almost all cases on the mouthparts of the flies, a phylogenetically conservative trait within Ceropegia (e.g. Vogel, 1961; Masinde, 2004), and the Ceropegieae as a whole (Meve and Liede, 1994; Ollerton and Liede, 1997; Ollerton et al., 2003) although it does occur in other clades (e.g. Ollerton and Liede, 2003; Ollerton et al., 2003; Yamashiro et al., 2008).
Floral scent has been suggested to be the most important cue for distant attraction of the pollinators in Ceropegia (Vogel, 1961) and is produced by specialized epithelia (‘osmophores’) located on the upper side of the tips of the corolla lobes. In some species of Ceropegia, flower scents can be detected by the human nose, while other species are apparently unscented. Ongoing analyses of floral scent in Ceropegia species should improve our understanding of the role of scent in the pollination biology of the genus (S. Dötterl, A. Jürgens and U. Meve, unpubl. res.). For example, flower morphology and colour varies much between Ceropegia species visited by the same fly species (J. Ollerton et al., unpubl. res.), and it may be that scent is the main mode of attraction for at least some members of this genus. In which case, how have these complex flower (non-scent) phenotypes evolved? What is the role of specific corolla colours, vibratile hairs, and so forth? Are they effective attractive cues in Ceropegia? Or are they just ‘accessory attractants’ for short-distance attraction as Vogel (1961) presumed?
The proportion of Ceropegia taxa for which there are flower visitor or pollinator data varies considerably from region to region (Table 1). The African regions and the Arabian Peninsula are strongly represented in terms of proportion of described Ceropegia taxa. In contrast there are fewer data available for Asia and Madagascar. Therefore any biogeographic conclusions that are drawn regarding the use of particular genera and families as pollinators of Ceropegia must perforce be preliminary. In fact, across the whole of the distributional range of Ceropegia, a rather similar suite of flies is exploited as pollinators and several fly genera and families re-occur as Ceropegia pollinators from region to region (Table 2). This suggests some level of evolutionary conservatism in the use of a phylogenetically varied group of Diptera as pollinators, and may be due to the fact that these fly genera are typically widespread and common, mainly occurring as larvae on decaying plant and animal material (Table S2 in Supplementary Data, available online). At the moment there is no strong evidence that Ceropegia in particular regions have specialized on particular fly genera or families. The one exception is that Arabian Peninsula Ceropegia exploit a much wider range of Diptera families and genera than those in other regions (Table 2). However, this result is due largely to the influence of a single taxon, C. aristolochioides spp. deflersiana that interacts with and is pollinated by a wider range of Diptera families than any other taxon (Table S1 and Table 5). A closely related subspecies, C. aristolochioides spp. aristolochioides occurs in East Africa and is not visited by such a range of families, despite a similar level of sampling effort (Table S1); this is unlikely to be due to biogeographic differences in fly faunas as East Africa is adjacent to the Arabian Peninsula and none of these Diptera families (and genera where known) are endemic to the regions. The most likely explanation is therefore that C. aristolochioides spp. deflersiana has locally adapted to being pollinated by a diverse range of Diptera, possibly by losing the ‘private signals’ to specific pollinators that may characterize other Ceropegia (see below).
The two most basal clades for which there are data (clades A and C) interact with a broader range of Diptera families and genera than do the more-derived clades D to G2 (Table 3). If confirmed, this would be an intriguing finding as it suggests that the evolution of the more-derived groups of Ceropegia has been driven in part by specialization to particular fly families or genera. However, there are three caveats to that suggestion. First of all, if the basal-most clades are older than the more-derived clades and they could possibly occupy a wider range of ecological communities (though it is noted that basal clades are not more widely distributed than derived clades; see Fig. 2). Secondly, for some clades the diversity of pollinators is either unknown (clade B) or under-sampled (e.g. clades D and E), making any conclusions regarding phylogenetic shifts in specialization preliminary. Finally, sampling effort (numbers of taxa and accessions with pollinator data) is significantly greater in the basal-most clades compared with the more-derived clades (Table 1) and that has almost certainly affected the present findings. In fact, detailed consideration of these results in relation to sampling effort implies that species in the derived clades are no more specialized in their interactions, on average, than the basal clades (Table 7). On the other hand, the basal clades contain both very specialized and hyper-generalized Ceropegia taxa, and so it is possible that the variance in diversity of pollinators per taxon may be greater in the basal clades compared with the derived clades (Table S1 in Supplementary Data, available online).
It is clear from the molecular phylogenetic evidence that trap flower pollination has evolved and been lost several times within the tribe Ceropegieae. The genus Riocreuxia, once synonymized with Ceropegia but now known to be a member of subtribe Anisotominae and therefore only distantly related (Meve, 1998; Meve and Liede, 2004; Masinde, 2005) also possesses trap flowers that are very similar to those of Ceropegia; small Diptera, as yet unidentified, have been recovered from flowers (Masinde, 2005; J. Ollerton, unpubl. res.). In addition, the paraphyletic relationship of Ceropegia to Brachystelma and the stapeliads (Meve and Liede-Schumann, 2007) suggests that trap flowers have been lost at least twice. However, within Brachystelma and the stapeliads, there are species that possess at least some of the features of trap flowers such as a tubular corolla that is longer than wide or corolla tips fused into a cage, e.g. Brachystelma stenophyllum amongst others, and stapeliads such as some Echidnopsis spp. and Stapeliopsis urniflora. Trap-flower pollination therefore appears to be an evolutionarily labile strategy. The transition from Diptera pollination of trap flowers to open flowers has evidently been accompanied by a shift in pollinator identity. Although not as yet evaluated using gas chromatographic analyses, few if any Ceropegia species produce the fetid floral odours produced by many of the stapeliads (Jurgens et al., 2006) and some Brachystelma species. This correlates with the utilization primarily of carrion- and faeces-breeding Calliphoridae and Muscidae by the stapeliads (Meve and Liede, 1994) compared with the more varied and generally smaller Diptera employed by Ceropegia taxa. Interestingly there are no examples of New World fly-pollinated asclepiads with trap flowers (e.g. within the Gonolobinae; Ollerton and Liede, 1997), making this an example of convergent evolution that is both phylogenetically and biogeographically constrained within the Apocynaceae.
Pollination systems similar to that of Ceropegia, which utilize temporarily trapped Diptera as pollen vectors, have evolved several times in unrelated plant families such as Araceae and Aristolochiaceae. These taxa apparently have highly convergent pollination systems, with flowers or functional floral units which share traits such as flask-shaped corollas (or their analogues in the Araceae) that retain the Diptera, ‘light windows’ that affect the behaviour of the flies, dull or sombre colouration, complex corolline appendages or hairs, and odours that are often fetid or fermented. Although there is compelling evidence that these pollination systems are examples of convergent evolution, in fact the Diptera that are used as pollen vectors differ significantly across some of these genera (Table 8). In the Araceae, Arisaema mainly exploits fungus-breeding flies (Vogel and Martens, 2000) whilst Arum species mimic a range of larval food sites including dung and rotting fruit, with some species using beetles (Coleoptera) as pollen vectors (Gibernau et al., 2004). It is the genus Aristolochia (Aristolochiaceae) that is most similar to Ceropegia in the fly families it uses as pollen vectors (Table 8); there is an extraordinary similarity in floral morphology and behaviour between some members of these genera that strongly suggests convergent evolution to attract similar flies.
Table 8.
Comparison of the most commonly encountered pollen-carrying Diptera families of Ceropegia with those of other well studied trap flower genera (in addition to the major pollen vectors, other fly families are less frequently encountered in all plant genera)
| Family | Genus | Major pollen vectors | References |
|---|---|---|---|
| Apocynaceae | Ceropegia | Ceratopogonidae | This study |
| Chloropidae | |||
| Milichiidae | |||
| Araceae | Arisaema | Mycetophilidae | Vogel and Martens (2000) |
| Sciaridae | |||
| Arum | Ceratopogonidae | Gibernau et al. (2004) | |
| Drosophilidae | |||
| Psychodidae | |||
| Sphaeroceridae | |||
| Aristolochiaceae | Aristolochia | Calliphoridae | Wolda and Sabrosky (1986), Hall and Brown (1993), Trujillo and Sersic (2006), Sakai (2002), Burgess et al. (2004), Razzak et al. (1992), Bänziger and Disney (2006), J. Ollerton (unpubl. res.) |
| Ceratopogonidae | |||
| Chloropidae | |||
| Drosophilidae | |||
| Milichiidae | |||
| Phoridae |
Specializing on small flies as pollinators might be considered to be a risky and possibly ineffectual strategy for a plant. However, in temperate communities at least, small dipteran pollinators can be very abundant and active for the entire flowering season (e.g. Ollerton and Diaz, 1999). In contrast much less is known about tropical dipteran pollinators, largely due to the tendency for entomologists and insect ecologists to neglect such small, cryptic taxa (Kearns, 2001; Larson et al., 2001). Concerns about pollinator extinctions have for the most part focussed on large, obvious species such as birds, bees and moths (Kearns, 2001). However, the role of small Diptera as pollinators for some significantly diverse clades of flowering plants underscores the conservation value of understanding these flies and their interactions with genera such as Ceropegia, which is far from being an exceptional case. Within other major clades of the asclepiads, flies belonging to diverse families and genera are important pollinators (Meve and Liede, 1994; Ollerton and Liede, 1997) and recent studies have reinforced these findings. For example, Diptera are significant pollinators within the Tylophora–Vincetoxicum complex in Japan and appear to be responsible for some speciation within the group (Yamashiro et al., 2008), whilst Medeiros et al. (2008) have identified disease-carrying black flies (Simuliidae) as pollinators of Tassadia spp. in Brazil. The asclepiads are not unique in this respect and research continually identifies ‘unusual’ Diptera as pollinators (and agents of selection on floral phenotype) of diverse genera; for example, fungus gnat (Mycetophilidae)-pollinated Mitella spp. (Saxifragaceae) (Okuyama et al., 2008). The biodiversity of plant–pollinator interactions continues to surprise us in its breadth and novelty.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org/ and consist of the following. Table S1: Ceropegia taxa and their Diptera flower visitors. Table S2: Synopsis of the higher-level phylogenetic positions of Diptera families known to be flower visitors to Ceropegia, together with their associated genera, and information about the feeding ecology of these flies (where known).
ACKNOWLEDGEMENTS
We are grateful to Dr David Goyder and Prof. Simon Owens for allowing us to remove insects from the Ceropegia accessions at The Herbarium, Royal Botanic Gardens, Kew; to Dr Henry Disney and Dr Art Borkent for Diptera identification; for the generous assistance of Dr Brian Stuckenberg (Natal Museum) in the identification of South African Diptera; to the many individuals who contributed flower material and fly specimens to our project, either directly or via the Kew Herbarium; to numerous students who helped J.O. at Kew; and to Dr Regino Berjano for comments on the manuscript. Dr Paul Wilson and two anonymous referees improved an earlier draft of this paper with their insightful comments. Finally, we are very much indebted to Dr Stefan Dötterl (Bayreuth) and Dr Andreas Jürgens (Pietermaritzburg) for discussion. Funding for this research came from the University of Northampton.
This paper is dedicated to Prof. Stefan Vogel in honour of his pioneering work on pollination in Ceropegia.
LITERATURE CITED
- Ali T. Pollination ecology of some asclepiads (Asclepiadaceae) from Pakistan. University of Karachi; 1994. PhD Thesis. [Google Scholar]
- Armbruster WS. Evolution of plant pollination systems – hypotheses and tests with the neotropical vine Dalechampia. Evolution. 1993;47:1480–1505. doi: 10.1111/j.1558-5646.1993.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Bänziger H, Disney RHL. Scuttle flies (Diptera: Phoridae) imprisoned by Aristolochia baenzigeri (Aristolochiaceae) in Thailand. Bulletin de la Société Entomologique Suisse. 2006;79:29–61. [Google Scholar]
- Bhatnagar S. On insect adaptations for pollination in some asclepiads of Central India. In: Kapil RP, editor. Pollination biology – an analysis. New Dehli: Inter-India Publications; 1986. pp. 37–57. [Google Scholar]
- Bruneau A. Phylogenetic and biogeographical patterns in Erythrina (Leguminosae: Phaseoleae) as inferred from morphological and chloroplast DNA characters. Systematic Botany. 1996;21:587–605. [Google Scholar]
- Bruneau A. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae) American Journal of Botany. 1997;84:54–71. [Google Scholar]
- Burgess KS, Singfield J, Melendez V, Kevan PG. Pollination biology of Aristolochia grandiflora (Aristolochiaceae) in Veracruz, Mexico. Annals of the Missouri Botanical Garden. 2004;91:346–356. [Google Scholar]
- Disney RHL. Insects of Arabia scuttle flies (Diptera: Phoridae) Part II: the genus Megaselia. Fauna of Arabia. 2009 (in press). [Google Scholar]
- Dold AP. Ceropegia macmasteri (Apocynaceae-Asclepiadoideae-Ceropegieae), a new species from Eastern Cape, South Africa. South African Journal of Botany. 2006;72:144–146. [Google Scholar]
- Endress P. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Endress ME, Liede-Schumann S, Meve U. Advances in Apocynaceae: the enlightenment, an introduction. Annals of the Missouri Botanical Garden. 2007;94:259–267. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Gibernau M, Macquart D, Przetak G. Pollination in the genus Arum – a review. Aroideana. 2004;27:148–166. [Google Scholar]
- Goldblatt P, Savolainen V, Porteous O, et al. Radiation in the Cape flora and the phylogeny of peacock irises Moraea (Iridaceae) based on four plastid DNA regions. Molecular Phylogenetics and Evolution. 2002;25:341–360. doi: 10.1016/s1055-7903(02)00235-x. [DOI] [PubMed] [Google Scholar]
- Grant V. Pollination systems as isolating mechanisms in angiosperms. Evolution. 1949;3:82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Hall DW, Brown BV. Pollination of Aristolochia littoralis (Aristoloachiales) by males of Megaselia spp. (Diptera, Phoridae) Annals of the Entomological Society of America. 1993;86:609–613. [Google Scholar]
- Herrera CM. Plant generalization on pollinators: species property or local phenomenon? American Journal of Botany. 2005;92:13–20. doi: 10.3732/ajb.92.1.13. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Pollination, adaptation and speciation models in the Cape Flora of South Africa. Taxon. 1996;45:59–66. [Google Scholar]
- Jurgens A, Dotterl S, Meve U. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae) New Phytologist. 2006;172:452–468. doi: 10.1111/j.1469-8137.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- Kay KM, Schemske DW. Natural selection reinforces speciation in a radiation of Neotropical rainforest plants. Evolution. 2008;62:2628–2642. doi: 10.1111/j.1558-5646.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kearns CA. North American dipteran pollinators: assessing their value and conservation status. Conservation Ecology. 2001;5:5. (URL: http://www.consecol.org/vol5/iss1/art5/ ) [Google Scholar]
- Knuth P. Handbook of flower pollination. Vol. 3. Oxford: Clarendon Press; 1909. [Google Scholar]
- Larson BMH, Kevan PG, Inouye DW. Flies and flowers: taxonomic diversity of anthophiles and pollinators. The Canadian Entomologist. 2001;133:439–465. [Google Scholar]
- Liede-Schumann S, Rapini A, Goyder DJ, Chase MW. Phylogenetics of the New World subtribes of Asclepiadeae (Apocynaceae-Asclepiadoideae): Metastelmatinae, Oxypetalinae, and Gonolobinae. Systematic Botany. 2005;30:184–195. [Google Scholar]
- Malpure NV, Kamble MY, Yadav SR. A new species of Ceropegia L. (Asclepiadaceae) from the Western Ghats of India with a note on series Attenuatae Huber. Current Science. 2006;91:1140–1142. [Google Scholar]
- Marussich WA, Machado CA. Host-specificity and coevolution among pollinating and nonpollinating New World fig wasps. Molecular Ecology. 2007;16:1925–1946. doi: 10.1111/j.1365-294X.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- Masinde PS. Trap-flower fly pollination in East African Ceropegia L. (Apocynaceae) International Journal of Tropical Insect Science. 2004;24:55–72. [Google Scholar]
- Masinde PS. A revision of the African genus Riocreuxia Decne. (Apocynaceae: Asclepiadoideae-Ceropegieae) Kew Bulletin. 2005;60:401–434. [Google Scholar]
- Masinde PS. Species concepts in Ceropegia L. (Apocynaceae–Ceropegieae): a case example of Ceropegia aristolochioides Decne. In: Ghazanfarand S, Beentje HJ, editors. Taxonomy and ecology of African plants, their conservation and sustainable use. London: Royal Botanic Gardens, Kew; 2006. pp. 583–589. [Google Scholar]
- Medeiros JF, Rapini A, Barbosa UC, Py-Daniel V, Braga PIS. First record of Simuliidae (Diptera) with pollinaria of Asclepiadoideae (Apocynaceae) attached. Neotropical Entomology. 2008;37:338–341. doi: 10.1590/s1519-566x2008000300015. [DOI] [PubMed] [Google Scholar]
- van der Meutter L. Notes and photographs: fly visitors to flowers – possible pollinators in cultivation. Asklepios. 1988;44:19–25. [Google Scholar]
- Meve U. Emplectanthus N.E.Br.: a close relative of Riocreuxia Decne. in the Asclepiadaceae-Stapelieae. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1998;120:123–130. [Google Scholar]
- Meve U, Liede S. Floral biology and pollination in stapeliads – new results and a literature review. Plant Systematics and Evolution. 1994;192:99–116. [Google Scholar]
- Meve U, Liede S. Subtribal division of Ceropegieae (Apocynaceae-Asclepiadoideae) Taxon. 2004;53:61–72. [Google Scholar]
- Meve U, Liede-Schumann S. Ceropegia (Apocynaceae, Ceropegieae, Stapeliinae): paraphyletic but still taxonomically sound. Annals of the Missouri Botanical Garden. 2007;94:392–406. [Google Scholar]
- Meve U, Masinde PS, Sentner U, Liede S. RAPD analysis and taxonomic reconsideration of the Ceropegia aristolochioides complex (Apocynaceae-Ceropegieae) Plant Biology. 2001;3:622–628. [Google Scholar]
- Noyes JS. Universal Chalcidoidea Database, British Museum of Natural History. 2003. [http://www.nhm.ac.uk/research-curation/projects/chalcidoids. ] accessed 11 March 2007.
- Okuyama Y, Pellmyr O, Kato M. Parallel floral adaptations to pollination by fungus gnats within the genus Mitella (Saxifragaceae) Molecular Phylogenetics and Evolution. 2008;46:560–575. doi: 10.1016/j.ympev.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Ollerton J. Interactions between gall midges (Diptera: Cecidomyiidae) and inflorescences of Piper novae-hollandiae (Piperaceae) in Australia. The Entomologist. 1996;115:181–184. [Google Scholar]
- Ollerton J. Fly trapping in Ceropegia flowers – evidence of ant predation of pollinators. Asklepios. 1999;77:31–32. [Google Scholar]
- Ollerton J, Cranmer L. Latitudinal trends in plant–pollinator interactions: are tropical plants more specialised? Oikos. 2002;98:340–350. [Google Scholar]
- Ollerton J, Diaz A. Evidence for stabilising selection acting on flowering time in Arum maculatum (Araceae): the influence of phylogeny on adaptation. Oecologia. 1999;119:340–348. doi: 10.1007/s004420050794. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Forster P. Diptera associated with flowers of Ceropegia cumingiana in Australia. Asklepios. 1995;66:21–22. [Google Scholar]
- Ollerton J, Liede S. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. Biological Journal of the Linnean Society. 1997;62:593–610. [Google Scholar]
- Ollerton J, Liede S. Corona structure in Cynanchum: linking morphology to function. Ecotropica. 2003;9:107–112. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany. 2003;92:807–834. doi: 10.1093/aob/mcg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon. 2007;56:717–728. [Google Scholar]
- Rapini A, Chase MW, Konno TUP. Phylogenetics of South American Asclepiadoideae (Apocynaceae) Taxon. 2006;55:119–124. [Google Scholar]
- Razzak MA, Ali T, Ali SI. The pollination biology of Aristolochia bracteolata Lamk. (Aristolochiaceae) Pakistan Journal of Botany. 1992;24:79–87. [Google Scholar]
- Ronsted N, Weiblen GD, Cook JM, Salamin N, Machado CA, Savolainen V. 60 million years of co-divergence in the fig-wasp symbiosis. Proceedings of the Royal Society series B–Biological Sciences. 2005;272:2593–2599. doi: 10.1098/rspb.2005.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsted N, Salvo G, Savolainen V. Biogeographical and phylogenetic origins of African fig species (Ficus section Galoglychia) Molecular Phylogenetics and Evolution. 2007;43:190–201. doi: 10.1016/j.ympev.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Sabrosky CW. A new species of Leptometopa (Diptera, Milichiidae) from Madagascar pollinating Ceropegia (Asclepiadaceae) Proceedings of the Entomological Society of Washington. 1987;89:242–243. [Google Scholar]
- Sakai S. Aristolochia spp. (Aristolochiaceae) pollinated by flies breeding on decomposing flowers in Panama. American Journal of Botany. 2002;89:527–534. doi: 10.3732/ajb.89.3.527. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in Angiosperms. 1. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Trujillo CG, Sersic AN. Floral biology of Aristolochia argentina (Aristolochiaceae) Flora. 2006;201:374–382. [Google Scholar]
- Vogel S. Die Bestäubung der Kesselfallen-Blüten von Ceropegia. Beiträge zur Biologie der Pflanzen. 1961;36:159–237. [Google Scholar]
- Vogel S. Betrug bei Pflanzen: Die Täuschblumen. Akademie der Wissenschaften und der Literatur, Mainz. Abhandlungen der mathematisch-naturwissenschaftlichen Klasse. 1993;1993(1):5–48. [Google Scholar]
- Vogel S, Martens J. A survey of the function of the lethal kettle traps of Arisaema (Araceae), with records of pollinating fungus gnats from Nepal. Botanical Journal of the Linnean Society. 2000;133:61–100. [Google Scholar]
- Waser NM. Pollination, angiosperm speciation, and the nature of species boundaries. Oikos. 1998;82:198–201. [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Wolda H, Sabrosky CW. Insect visitors to 2 forms of Aristolochia pilosa in Las Cumbres, Panama. Biotropica. 1986;18:295–299. [Google Scholar]
- Wyatt R, Broyles SB. Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics. 1994;25:423–441. [Google Scholar]
- Yamashiro T, Yamashiro A, Yokoyama J, Maki M. Morphological aspects and phylogenetic analyses of pollination systems in the Tylophora-Vincetoxicum complex (Apocynaceae-Asclepiadoideae) in Japan. Biological Journal of the Linnean Society. 2008;93:325–341. [Google Scholar]