Abstract
Background and Aims
Although the ecological and evolutionary consequences of foliar herbivory are well understood, how plants cope with floral damage is less well explored. Here the concept of tolerance, typically studied within the context of plant defence to foliar herbivores and pathogens, is extended to floral damage. Variation in tolerance to floral damage is examined, together with some of the mechanisms involved.
Methods
The study was conducted on Ipomopsis aggregata, which experiences floral damage and nectar removal by nectar-robbing bees. High levels of robbing can reduce seeds sired and produced by up to 50 %, an indirect effect mediated through pollinator avoidance of robbed plants. Using an experimental common garden with groups of I. aggregata, realized tolerance to robbing was measured. Realized tolerance included both genetic and environmental components of tolerance. It was hypothesized that both resource acquisition and storage traits, and traits involved in pollination would mitigate the negative effects of robbers.
Key Results
Groups of I. aggregata varied in their ability to tolerate nectar robbing. Realized tolerance was observed only through a component of male plant reproduction (pollen donation) and not through components of female plant reproduction. Some groups fully compensated for robbing while others under- or overcompensated. Evidence was found only for a pollination-related trait, flower production, associated with realized tolerance. Plants that produced more flowers and that had a higher inducibility of flower production following robbing were more able to compensate through male function.
Conclusions
Variation in realized tolerance to nectar robbing was found in I. aggregata, but only through an estimate of male reproduction, and traits associated with pollination may confer realized tolerance to robbing. By linking concepts and techniques from studies of plant–pollinator and plant–herbivore interactions, this work provides insight into the role of floral traits in pollinator attraction as well as plant defence.
Key words: Compensation, herbivory, indirect effects, Ipomopsis aggregata, male reproductive success, nectar robbing, pollen donation, pollination, resistance, tolerance
INTRODUCTION
Although flowers and their associated rewards are typically studied within the context of pollination, flowers are also besieged by natural enemies (McCall and Irwin, 2006). Consumption is indeed ubiquitous in nature, affecting population densities of the consumer and prey, community structure and ecosystem function (Fussmann et al., 2000; Croll et al., 2005). However, prey are not defenseless against consumer damage, and enemy evasion has driven adaptations and counteradaptations in both parties (Young et al., 2004; Geffeney et al., 2005). In a similar vein, plants that fall prey to foliar herbivores and pathogens are not defenseless to consumer damage, employing at least two defensive strategies: resistance (the ability to decrease the frequency of consumer damage) and tolerance (the ability to maintain fitness after damage, Stowe et al., 2000; Núñez-Farfán et al., 2007). The reproductive structures and rewards of flowering plants are also susceptible to damage from floral predators, especially given their conspicuous and often showy nature, and opportunistic flower feeding was probably involved in the adaptive radiation of flowering plants (Frame, 2003). Given that flowers are often more closely tied to plant fitness than are leaves (Strauss et al., 2004), plants may experience strong selection to defend against floral predators. While the benefits and costs of resistance to floral enemies have received attention (Irwin et al., 2004), the benefits and costs of tolerance to floral enemies remain comparatively unexplored (but see Lehtilä and Syrjänen, 1995; Pilson and Decker, 2002; Ashman et al., 2004; Althoff et al., 2005; Wise et al., 2008). Because flowers and their associated rewards are often ephemeral and produced throughout the growing season, plants may be able to tolerate flower damage and reward removal by changing resource relationships between leaves and flowers, and increasing resource availability per surviving flower (Krupnick and Weis, 1999; McCall, 2007). In addition, tolerance may be a more effective strategy against floral damage than resistance if resistance also results in ecological costs associated with deterring pollinators (Simms, 1992; Strauss, 1997). As a first step in understanding tolerance to floral predators, I asked whether plants varied in tolerance to floral enemies through components of male and female plant reproduction. In addition, I also investigated some of the potential mechanisms involved in variation in tolerance, including mechanisms associated with pollinator attraction and behaviour.
Florivores are a diverse group of organisms that damage buds and mature flowers, steal their resources, and affect plant evolution via natural selection on floral and defensive traits (McCall and Irwin, 2006). For example, nectar robbers are florivores that steal nectar through holes bitten in flowers, often without providing pollination service (Inouye, 1980). Nectar robbers are common parasites on plant–pollinator mutualisms, especially for flowers with concealed nectar in tubes or spurs (Irwin and Maloof, 2002). Nectar robbers can have a diversity of effects on male and female components of plant reproduction, ranging from positive to neutral to negative (Maloof and Inouye, 2000; Irwin et al., 2001), via direct effects (i.e. by damaging plant reproductive structures, Galen, 1983) and indirect effects (i.e. by changing pollinator behaviour, Roubik, 1982). In cases where nectar robbers have strong negative effects on plant reproductive success (Irwin et al., 2001), robbers may constitute a strong selective pressure upon host plants to reduce the negative effect of the interaction. However, the degree to which tolerance mitigates the negative effects of nectar robbers is unknown.
In this study, tolerance to nectar robbing was investigated using the nectar-robbing bumble-bee Bombus occidentalis (Apidae) and its hummingbird-pollinated host plant, Ipomopsis aggregata (Polemoniaceae). On average, nectar robbing reduces male and female components of I. aggregata reproduction by up to 50 %, an effect mediated indirectly through changes in pollinator behaviour (Irwin and Brody, 2000). However, I. aggregata plants and populations vary widely in the degree to which natural enemies, both robbers and herbivores, affect plant fitness (Juenger and Bergelson, 1997; Irwin and Brody, 1999). Given this variation, I predicted that plants may vary in their tolerance to the negative effects of nectar robbing, just as plants vary in their tolerance to herbivory in I. aggregata. Moreover, when resistance is no longer effective at reducing damage, tolerance may constitute the only profitable strategy of defence (Jokela et al., 2000). In this study, an estimate was made of realized tolerance to nectar robbing, which includes both genetic and environmental components of tolerance. This was done using related individuals (hereafter referred to as groups) of I. aggregata with experimentally imposed low and high robbing levels in a field common garden (Rausher, 1992; Simms and Triplett, 1994). Variation in realized tolerance to robbing was identified as a statistical interaction between group and robbing treatment for male and female components of plant reproduction. Although this study does not differentiate between genetic vs. environmental components of tolerance, the work does provide insight into potential patterns of tolerance that can be investigated using further breeding designs (Simms, 2000) in a system in which the interactions among plants, pollinators and nectar robbers are well described (Irwin and Brody, 1998, 2000).
Specifically, two questions were addressed. (1) Do plants vary in realized tolerance to nectar robbing? Variation in realized tolerance was measured through components of male and female function because enemies can differentially affect male and female fitness components, and a full understanding of tolerance requires measurements of both components (Paige et al., 2001; Strauss et al., 2003). Finding that plants varied in realized tolerance, the second question was addressed: (2) what traits are associated with realized tolerance to nectar robbing? As with tolerance to plant–herbivore and plant–pathogen interactions, the ability of plants to tolerate robbing is probably the result of a diverse set of plant traits and life histories (Stowe et al., 2000; Tiffin, 2000). Also, factors intrinsic and extrinsic to the two-way interaction between plants and robbers will probably be important (Rosenthal and Kotanen, 1994), as the consequences of plant–robber interactions can be driven through changes in pollination. Thus, the focus of the study was on traits associated with resource acquisition and storage, and investment in pollination. This study is novel in assessing realized tolerance to nectar robbing and in focusing on estimates of tolerance through male and female components of plant reproduction.
METHODS
Study system
Realized tolerance to nectar robbing was studied from June to August, 2004 in a population of I. aggregata near the Rocky Mountain Biological Laboratory (RMBL), Gothic, Colorado, USA. Throughout western North America, Ipomopsis aggregata is a common herb in montane habitats. The plant is monocarpic, growing as a vegetative rosette for 2–7 years or longer, flowering once and dying (Waser and Price, 1989); thus, lifetime male and female components of reproduction could be measured in a single season. Plants flower for 4–8 weeks, producing numerous red, trumpet-shaped flowers on (typically) a single stalk. The hermaphroditic, protandrous, self-incompatible flowers remain open for 3–5 d, with nectaries at the base of the flower continuously secreting nectar (20–25 % mass/volume, 1–6 µL per 24 h; Pleasants, 1983). Hereafter, for simplicity, male and female components of plant reproduction in this hermaphroditic plant species are referred to as male and female reproduction. Nectar removal does not stimulate nectar production (Pleasants, 1983). Ipomopsis aggregata establishes solely from seeds, and increased seed production translates into increased seedling and offspring recruitment at this site (Price et al., 2008).
The flowers of I. aggregata are pollinated primarily by broad-tailed (Selasphorous platycercus) and rufous (S. rufus) hummingbirds (Price et al., 2005). Pollinator visitation and pollen receipt can limit lifetime seed production in I. aggregata (Campbell and Halama, 1993), and increased pollinator visitation is associated with increased pollen removal and donation (Mitchell and Waser, 1992). A variety of characters, including floral, phenological and nectar characters, influence pollinator visitation rates and subsequent male and female reproduction (e.g. Campbell et al., 1991; Mitchell, 1993; Brody and Mitchell, 1997). Some of these characters also exhibit significant narrow-sense heritability in the field (Campbell, 1996).
Ipomopsis aggregata flowers are nectar robbed by the bumble-bee B. occidentalis. The bees chew holes through the sides of flowers, insert their proboscis and remove the nectar, without pollinating or damaging the reproductive or nectar-producing structures (Irwin and Brody, 1998). Nectar-robbing levels range from 0 to 100 % of flowers robbed at any given time across populations and across individuals within populations, with a mean ± 1 s.e. across 7 years of 66 ± 12 % of flowers robbed at the peak activity of robbing (Irwin and Maloof, 2002). High levels of robbing (≥80 % of available flowers robbed) reduce seeds sired and produced by up to 50 % (Irwin and Brody, 2000), an effect driven indirectly through hummingbird-pollinator avoidance of robbed plants and flowers (Irwin and Brody, 1998).
Do plants vary in realized tolerance to nectar robbing?
To estimate variation in realized tolerance, groups of related plants with experimental low and high robbing levels in a field common garden were used. Eight groups of plants were identified in the field. Within each group, eight individual, single-stalked plants that were growing within 1 m of one another were chosen (n = 64 plants total). Each group of plants was separated from all other groups by at least 20 m. The natural history of this system shows that plants growing closer together in the field are more likely to be genetically related than plants growing farther apart. Ipomopsis aggregata populations around the RMBL exhibit spatial genetic structuring. Plants within populations exhibit isolation by distance; allele frequencies are spatially autocorrelated at distances <4 m and exhibit no autocorrelation at distances from 5 to 35 m (Campbell and Dooley, 1992). Moreover, approx. 84 % of gene flow via pollen is expected within 10 × 10 m (Campbell, 1991), and seeds have no specialized dispersal mechanisms, falling within 1 m of the parent plant (Waser and Price, 1983). It is unlikely that all plants within groups were full- or half-sibs; however, it is likely that plants within groups were more genetically related than plants among groups, and a significant effect of group was found on traits known to exhibit significant narrow-sense heritability in the field (Campbell 1996), including corolla width (F7,56 = 2·22, P = 0·046). This group design provides an estimate of realized tolerance, including genetic, maternal and environmental effects, as well as plant age at the time of flowering, in one estimate.
On 9 June, 2004, the 64 bolting plants were potted individually into peat pots. Plants were watered with root stimulator to reduce transplant shock and were watered and fertilized subsequently on a daily basis for 9 d. The plants were then transplanted into an 8 m square field array on 18 June 2004 by sinking the peat pots into the ground. All plants were assigned to positions at random, and neighbouring plants were 1 m apart. Other I. aggregata were naturally blooming in the area; thus, hummingbirds were actively foraging.
In the field array, one-half of the plants within each group were randomly assigned to a low nectar-robbing treatment (≤10 % of available flowers robbed) and the other half to a high nectar-robbing treatment (≥80 % of available flowers robbed). Robbing levels of <10 % (low) and >80 % (high) represent low and high robbing levels experienced by I. aggregata in the field (Irwin and Brody, 1998). All flowers produced per plant were exposed to the experimental treatments.
Robbing treatments were performed 4 d per week (approx. every other day) throughout the season. Flowers were artificially robbed by cutting a hole, approx. 1 mm in diameter, in the side of the corolla using fine-tipped dissecting scissors. All available nectar was then removed from each flower using a microcapillary tube inserted in the hole. Flowers were not collared to deter nectar robbers from foraging on the control plants because natural robbing levels were low during the study year (see also Irwin and Maloof, 2002). One caveat is that simulated robbing was used to impose damage treatments. Natural and simulated herbivory can elicit different phytochemical responses and effects on plant reproduction (Tiffin and Inouye, 2000). However, for I. aggregata, simulated robbing mimics natural robbing in terms of effects on pollinator behaviour and fruit and seed production (Irwin and Brody, 1998). Moreover, by using simulated robbing levels, resistance to nectar robbing was not confounded with tolerance to robbing.
Male plant reproduction
Pollen donation, a component of male reproduction, was estimated using powdered fluorescent dyes as pollen analogues (Series JST-300, Radiant Color, Richmond, CA, USA). Mean dye donation over multiple flowers provides a reliable estimate of mean pollen donation in I. aggregata (Waser and Price, 1982). Six colours of dye were used. Once per week for 8 weeks, three groups were chosen to act as dye donors; groups and robbing treatments within groups were randomly assigned to one of the six dye colours each week. Dye of the appropriate colour was applied to all of the anthers of flowers in male phase with flat-head toothpicks. Both the number of flowers dyed and the number of flowers open per plant were recorded. Forty-eight hours later, stigmas were collected from the other plants in the array (hereafter referred to as recipient plants) that were not dyed that week. On recipient plants, stigmas were collected from 20 % of the flowers in which the corolla was just falling off. Thus, it was known that the flower was open with a receptive stigma during the time that dye was applied. Collecting stigmas at this stage does not affect probability of fruit set (Waser and Fugate, 1986). The number of dye particles on each stigma was counted under a dissecting microscope (as in Irwin and Brody, 1999). By repeating this procedure for 8 weeks, all groups and treatments within groups acted as dye donors in three trials and as recipient plants in five trials. There were at least 7 d between dye applications, ensuring that any dye from the previous application was no longer in the array on dehiscing flowers or open stigmas.
The mean number of dye particles donated per flower dyed per trial was calculated for each group and robbing treatment. Calculating dye donation on a per-flower dyed basis controlled for differences in the number of flowers dyed among groups and treatments (Campbell, 1989). A previous study has shown that nectar robbing has no effect on the mean or mean-squared distance of dye donation in I. aggregata (Irwin, 2003). A two-way analysis of variance (ANOVA) was performed on dye donation per flower dyed (square-root transformed) with group (random effect), robbing treatment (low vs. high robbing, fixed effect) and their interaction as factors. A significant interaction between group and robbing treatment would suggest that groups varied in their realized tolerance to robbing via dye donation. Groups that undercompensated for robbing were considered those that experienced lower estimates of pollen (dye) donation in the high robbing vs. low robbing treatment. Groups that fully compensated exhibited no difference in pollen (dye) donation between the high and low robbing treatments. Finally, groups that overcompensated for robbing had higher pollen (dye) donation in the high robbing treatment relative to the low robbing treatment.
Female plant reproduction
Four estimates of female reproduction per plant were measured: (1) mean pollen receipt per stigma; (2) proportion of fruit set; (3) mean seeds per fruit; and (4) total seeds. To estimate pollen receipt, on all of the stigmas that were collected to measure dye donation, the pollen was stained in basic fuchsin dye and the number of I. aggregata pollen grains per stigma was counted under a compound microscope. Pollen receipt per plant was calculated as the mean number of pollen grains received per stigma, averaged across the flowering season. To measure fruit and seed production, at the end of the season, all fruits were collected and scored as having set seed or aborted, and all of the seeds produced per plant were counted. Proportion of fruit set was calculated as the number of seed-bearing fruits divided by the total number of flowers per plant, seeds per fruit as the mean number of seeds per seed-bearing fruit, and total seeds per plant as the total number of seeds produced by each plant.
A multivariate analysis of variance (MANOVA) was used to test how robbing treatment, group and their interaction affected pollen receipt, proportion of fruit set (arc-sine square-root transformed), seeds per fruit and total seeds per plant (square-root transformed). A significant interaction between robbing treatment and group would suggest that plants varied in realized tolerance through female function. A significant MANOVA was followed by univariate ANOVAs for each response variable.
What traits are associated with realized tolerance to nectar robbing?
Finding that plants varied in realized tolerance to robbing through an estimate of male reproduction (dye donation, see Results), the question was then asked of what traits were associated with realized tolerance through male function. The focus was on two categories of traits: (1) resource acquisition and storage traits; and (2) traits associated with investment in pollination. For resource acquisition and storage traits, initial root stalk diameter was measured (to the nearest 0·01 mm using digital calipers). Root stalk diameter was measured as the plants started to bolt because I. aggregata is monocarpic and depletes its root tissues as it produces flowers and seeds.
Traits associated with pollination investment were also measured because the effects of nectar robbers are primarily indirect for I. aggregata, mediated through changes in pollination (Irwin and Brody, 1998). Therefore, investment in traits that encourage pollination may also mitigate the negative effects of robbing (Irwin et al., 2008). Corolla length and width, nectar production and sugar concentration, plant height, total number of flowers and bloom duration were measured. Variation in these traits has been shown to influence pollinator visitation or pollination success in this or other systems (see Study system). Corolla length and width were measured to the nearest 0·01 mm using digital calipers on three open flowers at peak bloom. Nectar production was measured on up to three flowers per plant by covering elongated buds with a piece of drinking straw crimped at the end to exclude pollinators and robbers and returning 48 h later to extract the nectar using microcapillary tubes. Nectar sugar concentration was measured using a hand-held refractometer. Measures of floral morphology and nectar are highly repeatable on flowers of the same plant (Campbell et al., 1991; Irwin et al., 2004). Plant height was measured to the nearest 0·1 cm at peak bloom. All of the flowers that plants produced were counted, and bloom duration was measured as the total number of days that plants were in bloom. One caveat about the measured traits is that some exhibit detectable additive genetic variance in I. aggregata (e.g. corolla length and width), some do not (e.g. nectar production and sugar concentration), and, for others, information is not known (Campbell 1996), although the traits may exhibit detectable additive genetic variance in other species. Thus, some of these traits have the potential to respond to selection via tolerance to robbing, while other traits may be important in an ecological context.
To assess which traits were associated with realized tolerance to robbing, multiple regression was used with the mean group value of traits as predictors and group male realized tolerance (square-root transformed) as the response variable (n = 8 groups). Group male realized tolerance was calculated as the ratio in dye donation of high to low robbing; calculating realized tolerance as the difference (Weinig et al., 2003) in dye donation between high and low robbing yielded similar results. For all traits except flower production and bloom duration, the mean trait value of the group was used in the regression. For flower production and bloom duration, the mean ratio (i.e. high/low robbing) in the trait value for each group was calculated. The ratio in flower production and bloom duration for plants with high and low robbing is a measure of the degree of change (i.e. inducibility or plasticity) of these traits following nectar robbing. The reasoning for the different treatment of traits was as follows. In plant tolerance to foliar herbivores, because some plant traits associated with tolerance may change following damage, the change in trait values may be more important in predicting tolerance than simply the mean trait values (Strauss et al., 2003). Unlike foliar damage, however, it seemed unlikely that high levels of robbing would affect resource acquisition (root stalk diameter), floral traits (corolla length and width) or plant height, given that robbing simply involves small holes in flowers and nectar removal. Moreover, I. aggregata does not alter its nectar production with nectar removal or pollination (Pleasants, 1983), so high levels of robbing are unlikely to affect nectar secretion. Although these traits may not change following robbing, variation in their mean values among groups may predict realized tolerance to robbing. However, robbing may affect flower production and bloom duration, especially if robbed plants produce more flowers and bloom over a longer period to compensate for low pollination success (Irwin et al., 2008). Thus, the change in values for these traits between high and low robbing (or the ratio) may be important in predicting realized tolerance. With only eight groups, it was not possible to conduct one multiple regression with all of the predictor traits included. Instead, a stepwise multiple regression was used with variables added to the model at an entry level of P = 0·15 and kept in the model at a stay level of P = 0·10. All variance inflation factors were <2, suggesting no strong collinearity among predictors.
RESULTS
Do plants vary in realized tolerance to nectar robbing?
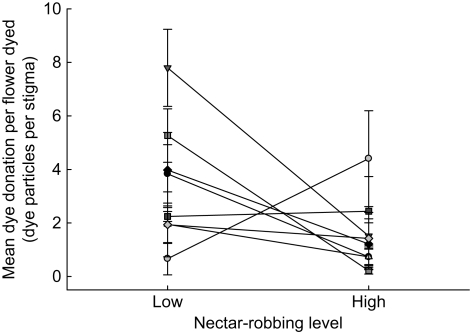
For male reproduction estimated via dye donation, groups varied significantly in their realized tolerance to robbing (ANOVA, robbing × group: F7,32 = 2·95, P = 0·017). It was found that one group fully compensated for robbing, one overcompensated by 7-fold and six undercompensated by 1·3- to 5-fold (Fig. 1). Across all groups, high robbing decreased dye donation by half relative to low robbing (F1,32 = 8·79, P = 0·006), but there was no overall effect of group (F7,32 = 0·82, P = 0·58).
Fig. 1.
Realized tolerance to nectar robbing varied in Ipomopsis aggregata, as indicated through an estimate of male reproduction, mean pollen (dye) donation per flower dyed. Each symbol is a group mean (±s.e.) at either a low or high nectar-robbing level (<10 and >80 % of flowers robbed, respectively). Lines connect groups. Variation in realized tolerance was signified as a statistical interaction between group and robbing treatment for pollen (dye) donation.
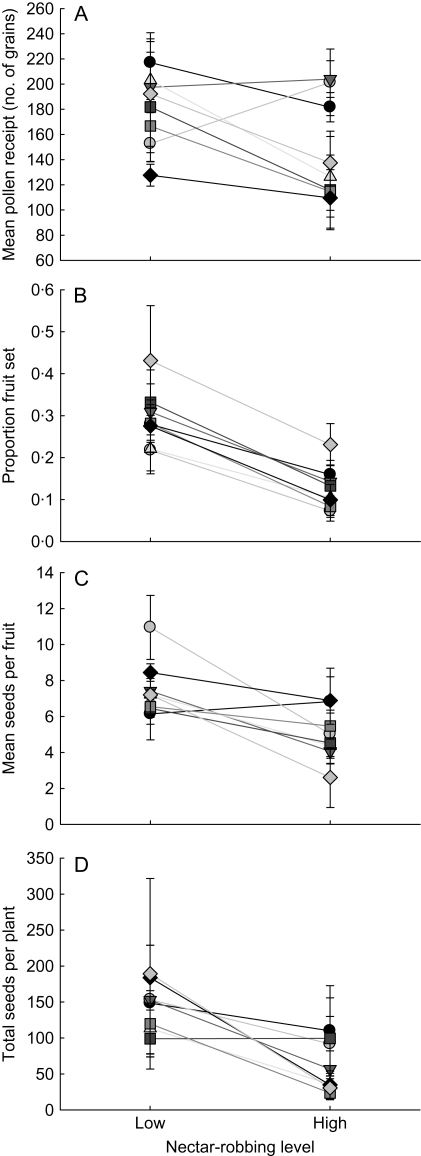
Although groups varied in estimates of female reproduction (MANOVA: λ = 0·41, F28,164 = 1·63, P = 0·03), no evidence was found that groups varied in their realized tolerance to nectar robbing, measured as pollen receipt, percent fruit set, seed set per fruit or total seeds per plant (MANOVA, robbing × group: λ = 0·51, F28,164 = 1·21, P = 0·23; Fig. 2). High robbing did decrease estimates of female reproduction (MANOVA: λ = 1·09, F4,45 = 12·28, P < 0·0001). Plants with low levels of nectar robbing received 17 % more pollen (F1,48 = 5·68, P = 0·02), had 56 % higher fruit set (F1,48 = 34·88, P < 0·0001), produced 34 % more seeds per fruit (F1,48 = 20·22, P < 0·0001) and produced 58 % more total seeds (F1,48 = 17·90, P = 0·0001) compared with plants with high robbing. Taken together, these results suggest that I. aggregata do vary in their realized tolerance to robbing, but only through an estimate of male reproduction.
Fig. 2.
Realized tolerance to nectar robbing did not vary in Ipomopsis aggregata, as indicated through through female reproduction, estimated as (A) pollen receipt, (B) proportion of fruit set, (C) seed set per fruit and (D) total seeds per plant. Each symbol is a group mean (±s.e.) at either a low or high nectar-robbing level (<10 and >80 % of flowers robbed, respectively). There was no statistically significant interaction between group and robbing treatment for components of female reproduction.
What traits are associated with realized tolerance to nectar robbing?
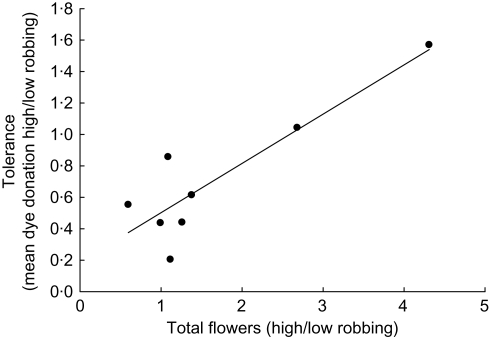
Only one trait, plasticity in flower production, was significantly related to variation in realized tolerance to nectar robbing through male function (Fig. 3). The ratio of mean group high robbing relative to mean group low robbing in flower production was significantly positively related to realized tolerance (β = 0·56, F1,6 = 36·39, P = 0·0009), explaining 86 % of the variation in realized tolerance through dye donation per flower dyed. In other words, plants that had higher inducibility of flower production following high robbing had higher realized tolerance to robbing. There was also a positive relationship between tolerance and mean group flower production for high robbing (univariate regression: r2 = 0·83, β = 0·01, F1,6 = 28·81, P = 0·002). The fit of the relationships did not improve using a non-linear model. These results suggest that higher flower production benefits plants with high robbing and that a higher degree of inducibility in flower production may benefit plants following robbing, with groups producing more flowers after high levels of robbing being more able to compensate. No other resource acquisition and storage or pollination-related traits entered into the stepwise regression.
Fig. 3.
Positive relationship between the ratio in mean group flower production for high and low robbing and mean group realized tolerance measured via pollen (dye) donation per flower dyed. Each point represents a group mean (n = 8 groups with eight plants per group).
DISCUSSION
Plants commonly exhibit variation in tolerance to insect–herbivore, mammalian–herbivore and pathogen damage (e.g. Simms and Triplett, 1994; Fornoni and Núñez-Farfán, 2000; Stinchcombe and Rausher, 2002; Weinig et al., 2003). Here I show variation in realized tolerance to another type of plant antagonist, nectar robbers, finding groups that fully, over- and undercompensated for robbing. In a similar vein, wild radish (Raphanus raphanistrum) families exhibit full, over- and undercompensation to foliar damage (Agrawal et al., 1999). It is unknown whether plants can compensate more fully for nectar robbing than for leaf or pathogen damage. Factorial studies assessing genetic variation in tolerance to robbers, herbivores and pathogens are needed to make such comparisons. One relevant hypothesis is that it may be easier for plants to compensate for flower damage and nectar removal than leaf damage (McCall and Irwin, 2006). Flowers and nectar are generally considered resource sinks, whereas leaves are important for photosynthate production. Thus, nectar robbing may alter resource source–sink relationships within a plant to compensate for robbing. For example, plants may be able to increase resource availability to unrobbed flowers or shunt resources to increase flower production to compensate for poor pollination success in robbed flowers (Irwin et al., 2008). In contrast, herbivore damage to leaves may change resource acquisition and decrease overall plant resource status, making it more difficult for plants to compensate for herbivory than for robbing. Nonetheless, the degree of compensation to robbing vs. herbivory may depend on levels of damage, the costs of producing more flowers and/or nectar, whether plants are annuals vs. perennials and how much plants can shunt resources from sources to sinks (McCall and Irwin, 2006).
Two caveats are important in the interpretation of the results. First, I did not use full- or half-siblings to estimate whether there was a genetic basis to tolerance to robbing. The natural history of I. aggregata suggests that plants growing closely together are more likely to be genetically related than plants growing further apart (see Methods); thus, it is likely that I estimated variation in realized tolerance to robbing using groups of related plants. However, realized tolerance may also be partially attributable to maternal, age, and environmental factors. To provide estimates of variation in tolerance that are comparable with those from studies of plant–herbivore and plant–pathogen interactions and to understand the potential evolution of tolerance to robbing, studies are needed that use plants from controlled breeding designs (e.g. Mauricio et al., 1997). Thus, in this study, I sacrificed the ability to identify solely genetic effects associated with tolerance for the benefit of working in a system where the interactions among plants, pollinators and nectar robbers are well studied (Irwin and Brody, 1998, 2000). Moreover, although realized tolerance in this study may be driven by factors other than genotype, such as plant age or access to resources, that the environment may also affect plant tolerance to robbing has important ecological implications within the context of variation in plant fitness (Núñez-Farfán et al., 2007).
The second caveat is that I used only eight groups to examine variation in tolerance and mechanisms of tolerance. With eight groups, I found group variation in tolerance through male reproduction but not female reproduction. Studies that examine variation in tolerance using breeding designs typically use more plant families to estimate tolerance (12 inbred lines, Fineblum and Rausher, 1995; 17 full- or half-sib families, Agrawal et al., 1999; 24 full-sib families, Tiffin, 2002). That I could detect variation in realized tolerance through male but not female function using only eight groups may suggest that male function is more sensitive to variation in tolerance than female function, just as male function is often more sensitive to changes in species interactions than female function (Young and Stanton, 1990). In addition, with only eight groups, the relationship between flower production (and inducibility in flower production) and male tolerance was strongly affected by two groups. Increasing the number of groups using a breeding design would provide further insight into the observed pattern.
Under the experimental conditions of this study, I found variation only in the degree to which tolerance mitigated the negative effects of robbing through an estimate of male reproduction, dye donation per flower dyed, but not through estimates of female reproduction. In I. aggregata, estimates of dye donation (per flower dyed) are correlated with realized seeds sired per plant (Irwin and Brody, 1999, 2000). Few studies of plant–herbivore or plant–pathogen interactions have measured tolerance through male and female function (Strauss and Agrawal, 1999). Of the studies that have measured tolerance through both fitness components, there are cases in which plants vary in tolerance through both male and female components (Agrawal et al., 1999), in which tolerance is more variable through female compared with male components (Wise et al., 2008) and in which plants compensate more fully through female than male function (Ashman et al., 2004). It is important to note that many of the studies that have measured tolerance through male and female function have done so in the greenhouse (i.e. measured allocation to traits or components of male function, such as pollen production or flower number) and have not incorporated how changes in pollinator behaviour may differentially affect tolerance through male vs. female function. Thus, studies may be underestimating tolerance through male function given that male function is often more sensitive to changes in pollinator visitation than female function (Young and Stanton, 1990), and investment in allocation to male function and pollinator response to those traits may be non-additive.
Identifying the mechanisms (or traits) associated with plant tolerance to consumer damage is important for understanding the ecological and evolutionary dynamics of both plants and consumers as well as the genetic, allocation, and ecological constraints that may limit the expression and evolution of tolerance (Tiffin, 2000). I found evidence only for flower production and inducibility in flower production to confer tolerance to robbing through male function. Because the effects of nectar robbers are mediated through pollinators for I. aggregata, investment in traits that encourage pollination are important in mitigating the negative effects of robbing. Hummingbirds often preferentially visit plants with more flowers (Brody and Mitchell, 1997) and, for I. aggregata, the benefits of multiple flower visitation can outweigh any potential costs associated with within-plant self-pollen transfer (Irwin, 2003). Here, increased flower production probably results in increased per-plant and per-flower visitation by hummingbirds and subsequent per-flower dye donation. Because high robbing generally reduces seed production through reduced pollination (Irwin and Brody, 1998), plants may shunt some resources from provisioning seeds to producing more flowers (also see Stanton et al., 1987). However, it is important to note that increased flower production may still benefit total seed set in plants with high robbing by providing more opportunities for pollinators to confer fitness benefits to compensate for poor pollination in robbed flowers (Irwin et al., 2008). Higher flower production is weakly correlated with male reproduction in I. aggregata (Campbell, 1998) and more strongly correlated with male reproduction in other plant species (Conner et al., 1996; Strauss et al., 2001). Moreover, higher flower production has also been associated with tolerance to herbivory (Paige and Whitham, 1987; Lennartsson et al., 1998), and increased pollination can mitigate the negative effects of herbivory (Juenger and Bergelson, 1997).
Evolutionary response to selection can only occur when there is heritable variation in traits that affect fitness. Although I found evidence to suggest group variation in tolerance associated with increased flower production, flower number of I. aggregata in a decade-long field study at the RMBL showed no significant heritability and was strongly influenced by maternal effects (Campbell, 1997), and can also be affected by environmental microsite and resource acquisition (Campbell and Halama, 1993). The degree to which inducibility in flower production shows detectable additive genetic variation is unknown. Even within a species for the same trait, the magnitude of heritability is context dependent (Mazer and Schick, 1991), and the heritability of flower number is variable in other species. For example, in R. raphanistrum, studies have found significant broad- and narrow-sense heritability of flower production for some plant families, but not others (Mazer, 1987; Agrawal et al., 1999). One alternative interpretation of the results presented here is that the positive relationship between mean group ratio in flower production and tolerance was simply driven by the environmental microsite that groups grew in as rosettes. Nonetheless, traits that vary due to environmental conditions may be important in mitigating the negative effects of robbers via changes in pollination.
The ability of plants to resist floral damage has garnered recent attention (Irwin et al., 2004); however, the ability of plants to tolerate floral damage has remained comparatively unexplored (but see Ashman et al., 2004). Here I show that the concept of tolerance can be successfully applied to understand plant variation in response to nectar robbing. Under the experimental conditions of this study, I found that I. aggregata exhibited variation in realized tolerance to nectar robbing through an estimate of male function, but not female function, and that one trait associated with realized tolerance was flower production. How common these results are for other plant–robber systems remains to be tested. Exciting challenges remain in the study of plant tolerance to nectar robbing and floral damage more generally. Of particular interest are studies that use breeding designs to estimate tolerance to robbing on known genotypes through male and female function and that ascribe mechanisms to variation in tolerance. Moreover, given that plants are often damaged simultaneously by multiple enemies, including herbivores, florivores, nectar robbers and seed predators (Morris et al., 2007), the degree to which tolerance to one type of enemy provides cross-tolerance or impedes tolerance to others will yield additional ecological and evolutionary insights (Núñez-Farfán et al., 2007). Finally, plants can both resist and tolerate foliar damage (Fineblum and Rausher, 1995); the degree to which there is a trade-off between resistance and tolerance for robbing remains unexplored.
ACKNOWLEDGEMENTS
I thank E. Bruneau, M. Erhart, K. Ritter and B. Wilke for help in the field and lab, the RMBL for providing access to field sites, and S. Armbruster, A. Brody, J. Bronstein, D. Campbell, S. Elliott, A. Siepielski and S. Strauss for comments on the manuscript. Research was funded by the National Science Foundation (DEB-0089643) and Dartmouth College.
LITERATURE CITED
- Agrawal AA, Strauss SY, Stout MJ. Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution. 1999;53:1093–1104. doi: 10.1111/j.1558-5646.1999.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Althoff D, Segraves KA, Pellmyr O. Community context of an obligate mutualism: pollinator and florivore effects on Yucca filamentosa. Ecology. 2005;86:905–913. [Google Scholar]
- Ashman TL, Cole DH, Bradburn M. Sex-differential resistance and tolerance to herbivory in a gynodioecious wild strawberry. Ecology. 2004;85:2550–2559. [Google Scholar]
- Brody AK, Mitchell RJ. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed predation in the hummingbird-pollinated plant Ipomopsis aggregata. Oecologia. 1997;110:86–93. doi: 10.1007/s004420050136. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Measurements of selection in a hermaphroditic plant: variation in male and female pollination success. Evolution. 1989;43:318–334. doi: 10.1111/j.1558-5646.1989.tb04230.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Comparing pollen dispersal and gene flow in a natural population. Evolution. 1991;45:1965–1968. doi: 10.1111/j.1558-5646.1991.tb02702.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Evolution of floral traits in a hermaphroditic plant: field measurements of heritabilities and genetic correlations. Evolution. 1996;50:1442–1453. doi: 10.1111/j.1558-5646.1996.tb03918.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Genetic and environmental variation in life-history traits of a monocarpic perennial: a decade-long field experiment. Evolution. 1997;51:373–382. doi: 10.1111/j.1558-5646.1997.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Variation in lifetime male fitness in Ipomopsis aggregata: tests of sex allocation theory. American Naturalist. 1998;152:338–353. doi: 10.1086/286173. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Dooley JL. The spatial scale of genetic differentiation in a hummingbird-pollinated plant: comparison with models of isolation by distance. American Naturalist. 1992;139:735–748. [Google Scholar]
- Campbell DR, Halama KJ. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology. 1993;74:1043–1051. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. Components of phenotypic selection: pollen export and flower corolla width in Ipomopsis aggregata. Evolution. 1991;45:1458–1467. doi: 10.1111/j.1558-5646.1991.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rush S, Kercher S, Jennetten P. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). II. Selection through lifetime male and total fitness. Evolution. 1996;50:1137–1146. doi: 10.1111/j.1558-5646.1996.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- Fineblum WL, Rausher MD. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature. 1995;377:517–520. [Google Scholar]
- Fornoni J, Núñez-Farfán J. Evolutionary ecology of Datura stramonium: genetic variation and costs for tolerance to defoliation. Evolution. 2000;54:789–797. [PubMed] [Google Scholar]
- Frame D. Generalist flowers, biodiversity and florivory: implications for angiosperm origins. Taxon. 2003;54:681–685. [Google Scholar]
- Fussmann GF, Ellner SP, Shertzer KW, Hairston NGJ. Crossing the Hopf Bifurcation in a live predator–prey system. Science. 2000;290:1358–1360. doi: 10.1126/science.290.5495.1358. [DOI] [PubMed] [Google Scholar]
- Galen C. The effects of nectar thieving ants on seedset in floral scent morphs of Polemonium viscosum. Oikos. 1983;41:245–249. [Google Scholar]
- Geffeney SL, Fujimoto E, Brodie ED, Brodie ED, Ruben PC. Evolutionary diversification of TTX-resistant sodium channels in a predator–prey interaction. Nature. 2005;434:759–763. doi: 10.1038/nature03444. [DOI] [PubMed] [Google Scholar]
- Inouye DW. The terminology of floral larceny. Ecology. 1980;61:1251–1253. [Google Scholar]
- Irwin RE. The impact of nectar robbers on estimates of pollen flow: conceptual predictions and empirical outcomes. Ecology. 2003;84:485–495. [Google Scholar]
- Irwin RE, Brody AK. Nectar robbing in Ipomopsis aggregata: effects on pollinator behavior and plant fitness. Oecologia. 1998;116:519–527. doi: 10.1007/s004420050617. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK. Nectar-robbing bumble bees reduce the fitness of Ipomopsis aggregata (Polemoniaceae) Ecology. 1999;80:1703–1712. [Google Scholar]
- Irwin RE, Brody AK. Consequences of nectar robbing for realized male function in a hummingbird-pollinated plant. Ecology. 2000;81:2637–2643. [Google Scholar]
- Irwin RE, Maloof JE. Variation in nectar robbing over time, space, and species. Oecologia. 2002;133:525–533. doi: 10.1007/s00442-002-1060-z. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK, Waser NM. The impact of floral larceny on individuals, populations, and communities. Oecologia. 2001;129:161–168. doi: 10.1007/s004420100739. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Adler LS, Brody AK. The dual role of floral traits: pollinator attraction and plant defense. Ecology. 2004;85:1503–1511. [Google Scholar]
- Irwin RE, Galen C, Rabenold JJ, Kaczorowski R, McCutcheon ML. Mechanisms of tolerance to floral larceny in two animal-pollinated wildflowers, Polemonium viscosum and Ipomopsis aggregata. Ecology. 2008;89:3093–3104. doi: 10.1890/08-0081.1. [DOI] [PubMed] [Google Scholar]
- Jokela J, Schmid-Hempel P, Rigby MC. Dr. Pangloss restrained by the Red Queen – steps towards a unified defence theory. Oikos. 2000;89:267–274. [Google Scholar]
- Juenger T, Bergelson J. Pollen and resource limitation of compensation to herbivory in scarlet gilia, Ipomopsis aggregata. Ecology. 1997;78:1684–1695. [Google Scholar]
- Krupnick GA, Weis AE. The effect of floral herbivory on male and female reproductive success in Isomeris arborea. Ecology (Washington DC) 1999;80:135–149. [Google Scholar]
- Lehtilä K, Syrjänen K. Compensatory responses of two Melampyrum species after damage. Functional Ecology. 1995;9:511–517. [Google Scholar]
- Lennartsson T, Nilsson P, Tuomi J. Induction of overcompensation in the field gentian, Gentianella campestris. Ecology. 1998;79:1061–1072. [Google Scholar]
- Maloof JE, Inouye DW. Are nectar robbers cheaters or mutualists? Ecology. 2000;81:2651–2661. [Google Scholar]
- Mauricio R, Rausher MD, Burdick DS. Variation in the defense strategies of plants: are resistance and tolerance mutually exclusive? Ecology. 1997;78:1301–1311. [Google Scholar]
- Mazer SJ. The quantitative genetics of life history and fitness components in Raphanus raphanistrum L. (Brassicaceae): ecological and evolutionary consequences of seed-weight variation. American Naturalist. 1987;130:891–914. [Google Scholar]
- Mazer SJ, Schick CT. Constancy of population parameters for life-history and floral traits in Raphanus sativus L. II. Effects of planting density on phenotype and heritability estimates. Evolution. 1991;45:1888–1907. doi: 10.1111/j.1558-5646.1991.tb02694.x. [DOI] [PubMed] [Google Scholar]
- McCall AC. Leaf damage and gender but not flower damage affect female fitness in Nemophila menziesii (Hydrophyllaceae) American Journal of Botany. 2007;94:445–450. doi: 10.3732/ajb.94.3.445. [DOI] [PubMed] [Google Scholar]
- McCall AC, Irwin RE. Florivory: the intersection of pollination and herbivory. Ecology Letters. 2006;9:1351–1365. doi: 10.1111/j.1461-0248.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. Adaptive significance of Ipomopsis aggregata nectar production – observation and experiment in the field. Evolution. 1993;47:25–35. doi: 10.1111/j.1558-5646.1993.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Waser NM. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology. 1992;73:633–638. [Google Scholar]
- Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, et al. Direct and indirect interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology. 2007;88:1021–1029. doi: 10.1890/06-0442. [DOI] [PubMed] [Google Scholar]
- Núñez-Farfán J, Fornoni J, Valverde PL. The evolution of resistance and tolerance to herbivores. Annual Review of Ecology, Evolution and Systematics. 2007;38:541–566. [Google Scholar]
- Paige KN, Whitham TG. Overcompensation in response to herbivory: the advantage of being eaten. American Naturalist. 1987;129:407–416. [Google Scholar]
- Paige KN, Williams B, Hickox T. Overcompensation through the paternal component of fitness in Ipomopsis arizonica. Oecologia. 2001;128:72–76. doi: 10.1007/s004420100647. [DOI] [PubMed] [Google Scholar]
- Pilson D, Decker KL. Compensation for herbivory in wild sunflower: response to simulated damage by the head-clipping weevil. Ecology. 2002;83:3097–3107. [Google Scholar]
- Pleasants JM. Nectar production in Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1983;70:1468–1475. [Google Scholar]
- Price MV, Waser NM, Irwin RE, Campbell DR, Brody AK. Temporal and spatial variation in pollination of a montane herb: a seven year study. Ecology. 2005;86:2106–2116. [Google Scholar]
- Price MV, Campbell DR, Waser NM, Brody AK. Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology. 2008;89:1596–1604. doi: 10.1890/07-0614.1. [DOI] [PubMed] [Google Scholar]
- Rausher MD. Natural selection and the evolution of plant–insect interactions. In: Roitberg BD, Isman MB, editors. Insect chemical ecology: an evolutionary approach. New York: Chapman and Hall; 1992. pp. 20–88. [Google Scholar]
- Rosenthal JP, Kotanen PM. Terrestrial plant tolerance to herbivory. Trends in Ecology and Evolution. 1994;9:145–148. doi: 10.1016/0169-5347(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Roubik DW. The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology. 1982;63:354–360. [Google Scholar]
- Simms EL. Costs of plant resistance to herbivores. In: Fritz RS, Simms EL, editors. Plant resistance to herbivores and pathogens. Ecology, evolution, and genetics. Chicago: University of Chicago Press; 1992. pp. 392–425. [Google Scholar]
- Simms EL. Defining tolerance as a norm of reaction. Evolutionary Ecology. 2000;14:563–570. [Google Scholar]
- Simms EL, Triplett J. Costs and benefits of plant responses to disease: resistance and tolerance. Evolution. 1994;48:1973–1985. doi: 10.1111/j.1558-5646.1994.tb02227.x. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Bereczky JK, Hasbrouck HD. Pollination thoroughness and maternal yield regulation in wild radish Raphanus raphanistrum (Brassicaceae) Oecologia. 1987;74:68–76. doi: 10.1007/BF00377347. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JR, Rausher MD. The evolution of tolerance to deer herbivory: modifications caused by the abundance of insect herbivores. Proceedings of the Royal Society B, Biological Sciences. 2002;269:1241–1246. doi: 10.1098/rspb.2002.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annual Review of Ecology and Systematics. 2000;31:565–595. [Google Scholar]
- Strauss SY. Floral characters link herbivores, pollinators, and plant fitness. Ecology. 1997;78:1640–1645. [Google Scholar]
- Strauss SY, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology and Evolution. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Conner JK, Lehtilä KP. Effects of foliar herbivory by insects on the fitness of Raphanus raphanistrum: damage can increase male fitness. American Naturalist. 2001;158:496–504. doi: 10.1086/323116. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Watson W, Allen MT. Predictors of male and female tolerance to insect herbivory in Raphanus raphanistrum. Ecology. 2003;84:2074–2082. [Google Scholar]
- Strauss SY, Irwin RE, Lambrix V. Optimal defense theory and flower petal colour predict variation in the secondary chemistry of wild radish. Journal of Ecology. 2004;92:132–141. [Google Scholar]
- Tiffin P. Mechanisms of tolerance to herbivore damage: what do we know? Evolutionary Ecology. 2000;14:523–536. [Google Scholar]
- Tiffin P. Competition and time of damage affect the pattern of selection acting on plant defense against herbivores. Ecology. 2002;83:1981–1990. [Google Scholar]
- Tiffin P, Inouye BD. Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution. 2000;54:1024–1029. doi: 10.1111/j.0014-3820.2000.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Waser NM, Fugate ML. Pollen precedence and stigma closure: a mechanism of competition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia. 1986;70:573–577. doi: 10.1007/BF00379906. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. A comparison of pollen and fluorescent dye carry-over by natural pollinators of Ipomopsis aggregata (Polemoniaceae) Ecology. 1982;63:1168–1172. [Google Scholar]
- Waser NM, Price MV. Optimal and actual outcrossing, and the nature of plant–pollinator interaction. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 341–359. [Google Scholar]
- Waser NM, Price MV. Optimal outcrossing in Ipomopsis aggregata: seed set and offspring fitness. Evolution. 1989;43:1097–1109. doi: 10.1111/j.1558-5646.1989.tb02554.x. [DOI] [PubMed] [Google Scholar]
- Weinig C, Stinchcombe JR, Schmitt J. Evolutionary genetics of resistance and tolerance to natural herbivory in Arabidopsis thaliana. Evolution. 2003;57:1270–1280. doi: 10.1111/j.0014-3820.2003.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Wise MJ, Cummins JJ, de Young C. Compensation for floral herbivory in Solanum carolinense: identifying mechanisms of tolerance. Evolutionary Ecology. 2008;22:19–37. [Google Scholar]
- Young HJ, Stanton ML. Influences of floral variation on pollen removal and seed production in wild radish. Ecology. 1990;71:536–547. [Google Scholar]
- Young KV, Brodie ED, Brodie ED. How the horned lizard got its horns. Science. 2004;304:65. doi: 10.1126/science.1094790. [DOI] [PubMed] [Google Scholar]