Abstract
Background
There are a number of difficulties associated with the study of adaptation. One is a lack of variation in the trait, which is common in adaptations because past selection has removed unfit variants. This lack of variation makes it difficult to determine the relationship between trait variation and fitness. Another difficulty is proving causation in this trait–fitness relationship, because a correlated trait might be the actual adaptation. These difficulties can be ameliorated at least partially by combining studies of natural variation with studies of experimentally manipulated traits and traits whose variance has been augmented by artificial selection.
Scope
We review here a number of our studies on the adaptive value of two aspects of anther position in wild radish (Raphanus raphanistrum, Brassicaceae): anther exsertion, i.e. the degree to which anthers protrude from the mouth of the corolla tube, and anther height dimorphism, i.e. the difference in lengths of the filaments between the two short and four long stamens. We have used both functional analyses, in which the response variable is pollen removal, and measurements of selection, in which the response variable is lifetime male fitness estimated by molecular genetic paternity analyses. In these studies we use both the natural variation in populations as well as manipulated variation, the latter through both stamen removal and artificial selection, to re-create the ancestral trait conditions.
Conclusions
Our work provides convincing evidence that intermediate anther exsertion values are adaptive, and that this is probably an adaptation to a subset of the pollinator fauna, small bees. The picture for anther height dimorphism is much less clear, as the weight of current evidence suggests that current values of this trait might actually be maladaptive; however, if this is true it is difficult to understand how the dimorphism is maintained across the family Brassicaceae.
Key words: Wild radish, Raphanus raphanistrum, adaptation, natural selection, anther position, pollination, pollen removal
INTRODUCTION
The study of floral adaptations for pollination began as early as with Sprengel (1793), and was greatly developed by Darwin (1877a, b). Darwin described the appeal of adaptive traits in the Origin of Species (Darwin, 1859) as ‘ … that perfection of structure and coadaptation which most justly excites our admiration’. Despite the excitement of admiration, proving that a trait is an adaptation can be difficult. The most difficult issue is demonstrating that the trait first originated as an adaptation to a given selective agent. Because this issue can be impossible to overcome, for the remainder of this paper we focus on current utility, i.e. studying whether a trait is an adaptation in present-day populations. Providing convincing evidence for current utility is also difficult for some of the reasons outlined below.
An adaptation can be defined as a trait that helps the organism deal with some challenge in its abiotic or biotic environment (the selective agent), resulting in increased fitness. There are two closely related and complementary approaches to studying adaptation, closely related because they both study present-day populations, and complementary because they have different strengths and weaknesses. The first is directly studying the function of the trait, i.e. what does the trait do, and how does trait variation affect trait function. The advantage of this approach is that it focuses on the direct outcome of the trait interaction with the environment and thus it is easier to make causal inferences; for example, do larger petals result in increased pollinator visitation? The disadvantage of this approach is that the response variable is not fitness, and so it cannot determine whether the trait is really adaptive. This is because the relationship between the functional response variable (pollinator visitation in the cited example) and fitness is often not straightforward. The other approach, with opposite strengths and weaknesses, is to measure selection on the trait by estimating the relationship between variation in the trait and variation in fitness.
Two difficulties with these approaches can be a lack of variation and an inability to prove causality (Grafen, 1988). Low variation is a common problem in the study of adaptation, because if a trait affects fitness, past selection will have eliminated the unfit variants. This results in reduced statistical power to examine the relationship between trait variation and function or fitness, because all members of the population have similar high fitness (Schluter, 1988). The inability to prove causation arises in any observational study because a relationship between the trait and either function or fitness could arise due to a correlated trait that is actually the target of selection.
Both of these problems can be overcome by using experimental manipulation of traits, if that is feasible. If a trait is not easily manipulated, or cannot be manipulated without causing other changes in the organism, artificial selection can be used to expand the range of variation, and then this increased variation can be related to function or fitness (Conner, 2003). This approach cannot prove causality, because other traits that are genetically correlated with the selected trait will also be altered by the selection. However, it can partially decouple correlated traits, because the correlated traits will not evolve as quickly as the selected trait unless the genetic correlation between them equals one, which is extremely rare. If the genetic correlations between the selected trait and other traits are low, the selection will have little effect on the other traits.
Here we review our work bringing all of these approaches (studies of function, estimates of natural selection, experimental manipulation, artificial selection) to bear on whether two aspects of anther position are adaptive in wild radish (Raphanus raphanistrum). Anther exsertion, defined as the difference in the lengths of the long stamen filaments and corolla tube, describes how far the anther protrudes from the opening of the corolla tube (Fig. 1B). The other trait is the difference in length between the two short and four long stamens, which we will refer to as anther height dimorphism (Fig. 1A), although the two stamen morphs are within each flower, not between individuals as in a typical dimorphism. Like most Brassicaceae, wild radish has tetradynamous stamens, and differences in stamen height within flowers are common in other families as well (e.g. Convolvulaceae, Polemoneacae).
Fig. 1.
(A) Side view of a wild radish flower with one sepal and petal removed. Note the anther height dimorphism that is diagnostic of the family Brassicaceae; we define anther height dimorphism as the difference in length between the short and long filaments. (B) A flower from a high exsertion selection line showing the highly positive anther exsertion, defined as filament length minus corolla tube length. (C) A flower from a low exsertion selection line with negative exsertion, i.e. the filaments are shorter than the corolla tube.
There are surprisingly few papers that take any of these approaches to testing whether anther position is adaptive. Most of what has been done has focused on heterostylous species in which there are two or three morphs with complementary stigma and anther heights (reviewed in Barrett, 1992). These studies have shown that heterostyly functions mainly to increase the efficiency of pollen transfer between morphs, but also increases outcrossing rates in the minority of heterostylous species that are self-compatible (Barrett et al., 2000). The L morph, which has the most inserted anthers, also had the lowest male fitness in the tristylous species Eichhornia paniculata (Kohn and Barrett, 1992a). Experimental removal of different anther heights demonstrated that this male fitness effect was due to greater seed siring success of plants with long stamens (which the L morphs lack; Kohn and Barrett, 1992b). Consistent with this finding, functional studies using unmanipulated flowers found that the proportion of pollen removed in single visits to the tristylous species Pontederia cordata was greater from long-level anthers than from the other two anther heights (Wolfe and Barrett, 1989; Harder and Barrett, 1993).
In non-heterostylous species, a functional study using natural variation in the morning glory Ipomoea trichocarpa found that pollen removal in single visits by bumble-bees increased with increasing anther exsertion (Murcia, 1990). In a functional study testing whether multiple anther heights are adaptive, Kudo (2003) experimentally removed pairs of short and long stamen anthers in all possible combinations in rapid-cycling Brassica rapa. Results showed that the presence of short stamen anthers significantly increased the duration of visits by nectar-foraging captive bumble-bees, which in turn led to increased total pollen deposition on stigmas (self and outcross pollen were not distinguishable). Presence or absence of short stamen anthers had no significant effect on pollen removal from long stamen anthers.
Wild radish is a good system to address whether anther position is adaptive, because there is a wealth of ecological, evolutionary and quantitative genetic information about this species (e.g. Stanton et al., 1986; Devlin and Ellstrand, 1990; Mazer and Schick, 1991; Agrawal, 1998; Morgan and Conner, 2001; Snow et al., 2001; Agrawal et al., 2002, 2004; Conner, 2002; Bett and Lydiate, 2003; Conner et al., 2003a; Irwin et al., 2003; Irwin and Strauss, 2005), molecular genetic tools including genetic markers and extensive cDNA sequence information is available (http://radish.plantbiology.msu.edu), and it is a self-incompatible annual, so that lifetime male and female fitness can be estimated (Conner et al., 1996a, b). A total of 14 genera are known to be effective pollinators of wild radish (Sahli and Conner, 2007), with the major pollinators throughout its native and introduced range being small bees, syrphid flies, honey-bees (especially in large plant populations), cabbage butterflies and bumble-bees (Table 1 and Fig. 2).
Table 1.
Visitation frequency (%) of the five major categories of wild radish pollinators in 14 studies from Europe and North America
| Date/Location | SF | SB | HB | BB | Lep | Other |
|---|---|---|---|---|---|---|
| 1975 GB SG | 10·8 | – | 11·7 | 59·0 | 18·5 | – |
| 1975 GB SB | 10·9 | – | 16·9 | 60·9 | 11·3 | – |
| 1975 GB BN | 1·8 | – | 20·8 | 54·4 | 23·0 | – |
| 1975 GB LD | 0·0 | – | 0·0 | 53·8 | 46·2 | – |
| 8/98 Sweden | 58·9 | 8·9 | 21·1 | 10·0 | 1·1 | – |
| 9/99 Sweden | 10·7 | 1·8 | 85·7 | 1·8 | – | – |
| 7/84 CT | – | – | 1·8 | 3·0 | 92·6 | 2·6 |
| 9&10/91 IL | 3·0 | 31·5 | 55·1 | 4·6 | 5·8 | 0 |
| 7&8/92 IL | 40 | 40 | 6 | 0·1 | 5·2 | 8·7 |
| 7&8/93 IL | 38 | 55 | – | – | – | 7 |
| 7&8/95 IL | 19·9 | 67·0 | 0·1 | 0·2 | 2·4 | 10·4 |
| 6/96 IL | 25·8 | 72·0 | 0·2 | 1·2 | 0·8 | – |
| 6–8/01 MI | 48·2 | 40·7 | 1·7 | 1·3 | 8·1 | – |
| 6–8/02 MI | 43·2 | 52·4 | 1·9 | 0·6 | 1·9 | – |
Entries with dashes were either not reported or included in ‘Other’. The three rows in bold are the populations for which selection on anther position was measured using natural variation (see text). The 1991, 1992 and 1996 IL and the Sweden data were from seeds or seedlings planted in the field, the other IL, the MI and CT studies were from potted arrays in the field, and natural populations were observed in Great Britain. SF, small fly; SB, small bee; HB, honey-bee; BB, bumble-bee; Lep, butterfly, mostly the cabbage butterfly. IL, near Champaign, Illinois, USA (Conner et al., 1996; Strauss et al., 2001; K. Lehtilä et al., Södertörn University, Sweden, unpubl. res.); MI, Kellogg Biological Station, Michigan, USA (Sahli and Conner, 2007); CT, Connecticut, USA (Stanton et al., 1989); GB, England and Wales; SG, Singleton; SB, Sketty B; BN, Beenham; LD, Landimore (Kay, 1978); Sweden, K. Lehtila, Södertörn University, Sweden, unpub. res.
Fig. 2.
Four of the five major pollinator groups visiting wild radish. (A) Small bee (mainly sweat bees in the family Halictidae); (B) honey-bee (Apis mellifera); (C) syrphid fly (e.g. Toxomerus); (D) Lepidoptera (mainly cabbage butterflies as shown here, Pieris rapae).
Below we address each of the two traits (anther exsertion and dimorphism) in separate sections. For each, we begin with adaptive hypotheses and evidence bearing on these hypotheses from natural variation, including both functional analyses as well as measurements of natural selection. We also present evidence from experimental manipulation in the case of anther height dimorphism only. For each trait we then turn to measurements of selection on expanded variation produced by artificial selection, including both the overall selection by all pollinators together as well as selection by individual pollinator taxa.
ANTHER EXSERTION
Adaptive hypothesis and evidence from natural variation
The adaptive hypothesis for anther exsertion is that there is stabilizing selection on this trait, i.e. intermediate anther exsertion leads to maximum pollen removal (function) and subsequent seed siring success (male fitness; Conner and Via, 1993). This hypothesis arises from the observation that the visits of all pollinators are similar, i.e. they land on the open part of the corolla (the landing platform) and feed on pollen and/or nectar (Fig. 2; Conner, 1997; our unpubl. res.). Nectar feeding is restricted primarily to the lepidopteran and long-tongued bee visitors – syrphid flies have never been observed feeding on nectar, and small bees rarely do (Conner and Rush, 1996; Rush et al., 1995). Pollen is placed primarily on the pollinator's head and thorax during visitation (our unpubl. res.). Under this hypothesis, anthers that are inserted into the tube would not contact the pollinator's bodies effectively, nor would highly exserted anthers, especially for the smaller pollinators.
This stabilizing selection on exsertion would also select for an increased correlation (i.e. correlational selection) on the component traits, the lengths of the long filament and corolla tube (Phillips and Arnold, 1989; Brodie, 1992). Consistent with this hypothesis, the phenotypic and genetic correlations between filament and corolla tube length are very high, around 0·85, significantly higher than the average correlation among other linear floral dimensions in wild radish (Conner and Via, 1993). This pattern of significantly higher phenotypic correlations between the long filament and corolla tube lengths is consistent across wild radish populations from North America, Europe (including three different native subspecies) and Australia, and is also consistent across years in both the field and the greenhouse (Conner and Sterling, 1995; Conner, 1997; J. K. Conner et al., unpubl. res.).
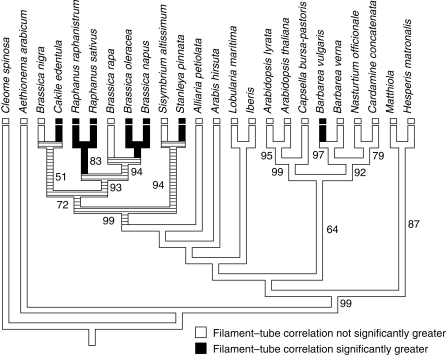
Also consistent with the adaptive hypothesis, the significantly higher correlation between filament and corolla tube compared with the rest of the floral correlations in radish is derived from an ancestral correlation that is not significantly higher than background. A comparative study of 24 species from across the three major clades of Brassicaceae shows that the ancestral condition is no significant difference between the filament–corolla tube correlation and the rest of the floral correlations, and that a significantly higher filament–corolla tube correlation has evolved at least twice (Fig. 3).
Fig. 3.
Phylogenetic tree of 23 species of Brassicaceae plus Cleome spinosa (Cleomeaceae) as the outgroup, based on parsimony analysis of 771 bp of sequence from the chloroplast gene ndhF. Shown is the consensus of three most-parsimonious trees, which differ only in the relationships among Brassica rapa, B. napus and B. oleracea. Bootstrap values above 50% are shown; major topological features are supported by complementary studies (Galloway et al., 1998; Yang et al., 1999; Koch et al., 2001; Johnston et al., 2005; Beilstein et al., 2006). The correlation between filament and corolla tube lengths was mapped onto this phylogeny using parsimony in MacClade. Taxa for which the filament–corolla tube correlation is significantly greater than the average of 12 correlations between six floral dimensions are marked with a black box, whereas white boxes denote species for which the filament–corolla tube correlation is not significantly different than the average correlation (for a description of this test, see Conner and Sterling, 1995). Hatched areas are where the reconstruction of the ancestral state is equivocal.
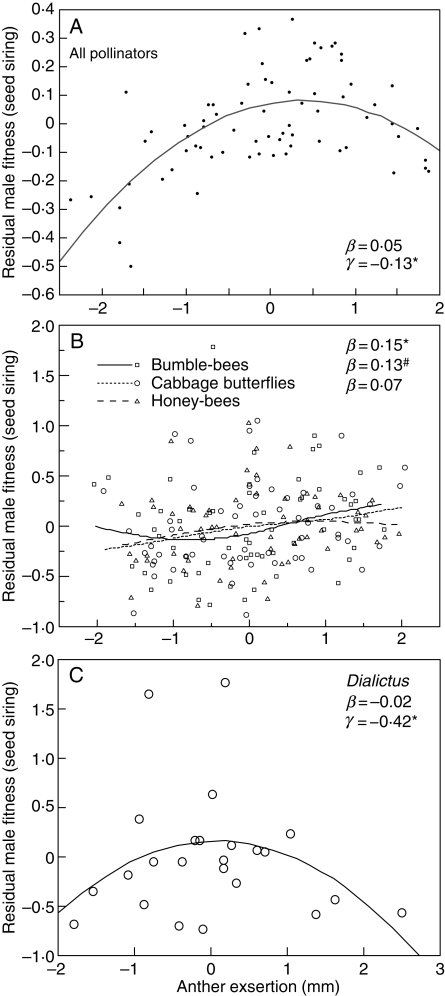
However, when we examine anther exsertion from a functional standpoint, we find support for the adaptive hypothesis for only one of the major pollinator groups. Pollen removal from long stamen anthers during single visits to virgin flowers was measured and related to long stamen anther exsertion by regression (Conner et al., 1995). Pollen removal by syrphid flies was unrelated to anther exsertion, and there was evidence for increased pollen removal by honey-bees and cabbage butterflies with increased anther exsertion (Fig. 4). For one pollinator, small bees, there was evidence for an intermediate maximum in pollen removal, i.e. the highest pollen removal occurred in flowers with slightly exserted anthers, with fewer grains removed with both more inserted and more exserted anthers (Fig. 4).
Fig. 4.
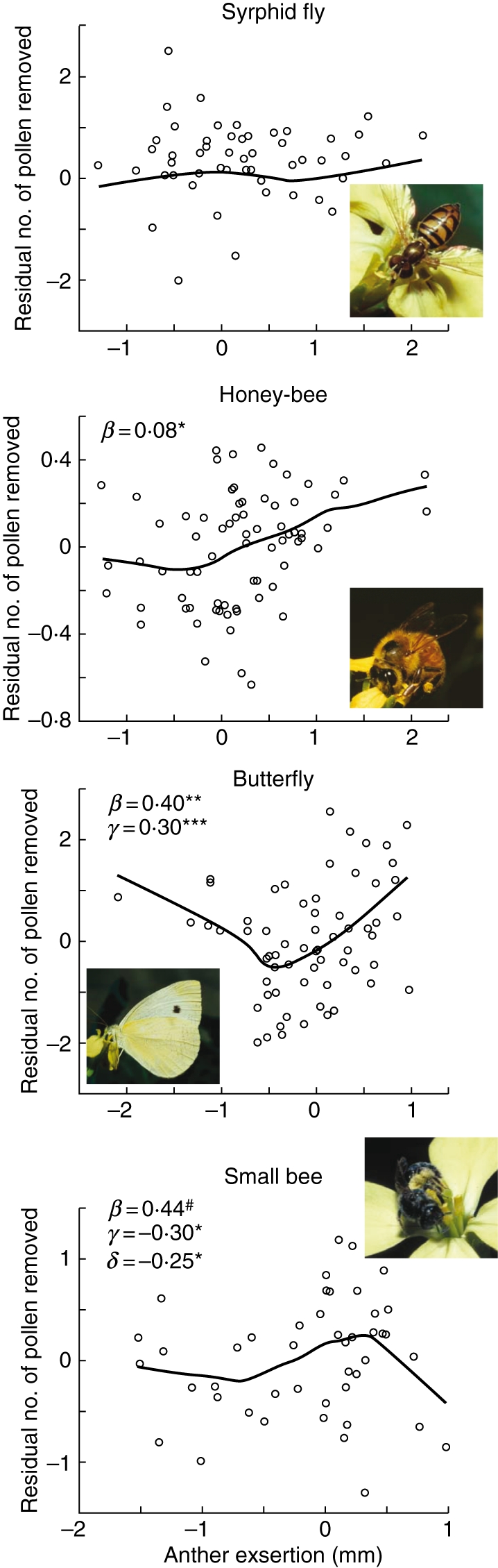
Functional analyses of long stamen anther exsertion. Shown are the relationships between the number of pollen grains removed from long stamen anthers in single visits to virgin flowers by four of the five major groups of wild radish pollinators. Curves were fit with locally weighted least squares (LOWESS; Chambers et al., 1983), with coefficients and significance values from multiple regression analysis that included the number of pollen grains available and the time each visitor spent at the flower; the pollen removal measure is the residual of a regression of these two traits on raw pollen removal data. β is the linear regression coefficient, γ is the quadratic coefficient and δ is the cubic coefficient; only values significant at P < 0·1 are shown. Data from Conner et al. (1995); figure modified from Conner (1997). # P < 0·10, * P < 0·05, ** P < 0·01, *** P < 0·001.
Turning to measurements of selection on anther exsertion (Conner et al., 1996b; Morgan and Conner, 2001), where the response variable is fitness, we again see variable support for the adaptive hypothesis. Lifetime seed siring success (male fitness) was estimated using eight allozyme markers in field arrays over three years (1991–1993). Considering the total selection exerted by all pollinators together, there was strong evidence for stabilizing selection on anther exsertion in the first year, weaker evidence for stabilizing selection in the second, and only directional selection for increased exsertion in the third year (Morgan and Conner, 2001). Therefore, selection seems to be variable, sometimes favouring increased exsertion and sometimes favouring intermediate exsertion; note that this is exactly the same conclusion that can be drawn from the functional analyses based on pollen removal discussed above, if in the first two years the selective agent was predominantly small bees while honey-bees and cabbage butterflies were primarily responsible for selection in the third year. However, this does not match the temporal variation in numerical abundance, as honey-bees were the most common visitor in 1991 but made less than 3% of all visits in 1993, and small bees were always common, but increased in abundance from 1991 through 1993 (Table 1; Conner et al., 1996a). Cabbage butterflies were consistently rare across the three years.
Natural selection on experimentally increased variance
Therefore, the evidence for stabilizing selection is strong in one year only. In addition to the temporal variation in pollination discussed above, a possible explanation for this is that we lack statistical power to detect stabilizing selection due to the low variance (see fig. 4 in Conner, 2003). To overcome this problem, we performed artificial selection for increased anther exsertion in two replicate lines, decreased exsertion in two lines, with two randomly mated control lines. Anther exsertion evolved rapidly in response to this selection, producing phenotypes never seen before in many studies of this population after five generations of selection (Fig. 1B, C). This rapid evolution was in spite of a very high pleiotropic genetic correlation between filament and corolla tube lengths (Conner, 2002). When equal numbers of plants from each of the six lines were combined, the variance in anther exsertion in the resulting synthetic population was almost four times that in the natural population (1·96 vs. 0·28, respectively). Note that this re-creates the ancestral condition in the Brassicaceae, which is a lower correlation between filament and corolla tube lengths compared with radish and most of its close relatives, which in turn leads to greater variation in anther exsertion in the ancestral state.
This synthetic population was then put in the field to measure selection on this expanded variation in anther exsertion. Three arrays of 24 plants each were grown in a pollinator-free greenhouse, and each was exposed to pollinators in the field for a total of 6 d spanning most of the normal flowering season of wild radish. All 72 parents and a random sample of 1060 offspring produced were genotyped at four microsatellite loci; these data and the exsertion measurements from the parents were used to directly estimate selection based on male fitness (Morgan and Conner, 2001). The resulting selection gradient provides further support for the hypothesis of stabilizing selection on anther exsertion, because the linear term is not significant, the quadratic term is significantly negative, and the curve and data clearly show an intermediate optimum (Fig. 5A; J. K. Conner et al., unpubl. res.).
Fig. 5.
Selection gradients based on expanded variation in anther exsertion. Exsertion values are standardized to a mean of zero and standard deviation of one; the y-axis is relative male fitness (seed siring success) after correcting for correlations with other floral traits. (A) Overall selection gradient (all pollinators naturally visiting) showing stabilizing selection. Data from J. K. Conner et al. (unpubl. data). (B) Selection gradients for three individual pollinator taxa from an outdoor flight cage experiment; quadratic terms were not significant. Data from H. F. Sahli and J. K. Conner (unpubl. res.). (C) Selection gradient for the most important wild radish pollinator in Michigan (Sahli and Conner, 2007), the small bee Dialictus (data from H. F. Sahli and J. K. Conner, unpubl. res.). #P < 0·10; *P < 0·05.
As in the previous measurements of selection on anther exsertion, this selection was exerted by all the naturally occurring pollinators together. Indeed, we have little if any data on selection exerted by individual taxa of biotic interactors (e.g. pollinators, herbivores) in any generalist system. We do have data on pollen removal by individual taxa (Fig. 4), but how does this translate into selection through total male fitness? To address this question, two of us (H. F. Sahli and J. K. Conner, unpubl. res.) measured selection on radish anther exsertion that is exerted by each of the seven major genera of wild radish pollinators individually, using single visits to virgin flowers in the field for four genera and outdoor flight cages for the other three. Selection was estimated by regressing male seed siring success estimated using microsatellite markers on the expanded variation in anther exsertion resulting from artificial selection.
The results for honey-bees, bumble-bees and cabbage butterflies show no evidence for stabilizing selection, but rather directional selection for increased exsertion (Fig. 5B). This directional selection is significant for bumble-bees, marginally significant for honey-bees and not significant for cabbage butterflies. There was no significant selection by any of the three main genera of syrphid flies, but there was significant stabilizing selection by the sweat bee Dialictus (Fig. 5C), which was by far the single most important pollinator of wild radish in our field studies (Sahli and Conner, 2007). Note that these selection gradients for individual taxa match the pollen removal data quite well – no significant relationship for syrphids, a positive relationship with anther exsertion for honey-bees and cabbage butterflies, and stabilizing selection by small bees. These relationships are not always statistically significant at P = 0·05 for the selection gradients, but it is difficult to obtain high statistical power for these individual taxon male selection gradients, because male fitness based on molecular markers is estimated with more error than female fitness, and the sample sizes for individual pollinators are less than for fitness measured over all pollinators.
ANTHER HEIGHT DIMORPHISM
Adaptive hypotheses and evidence from natural variation and experimental manipulation
The adaptive hypothesis for anther height dimorphism is less clear. It is possible that it is a key innovation, partly responsible for the success of the Brassicaceae, which currently includes over 3700 species (Beilstein et al., 2008). One hypothesis is that the short stamen anthers serve to restrict pollen removal, which can lead to higher male fitness under conditions of high visitation and high pollen removal (Harder and Thomson, 1989; Conner et al., 2003b). Pollen removal can be very rapid in wild radish – in central Illinois, 84% of pollen produced by virgin flowers was removed after only 1 h of natural pollination in the field (Rush et al., 1995). Therefore, restricting pollen removal may be adaptive in this species. Another hypothesis is that the short stamen anthers manipulate the body position of pollen feeders such that they better contact the long stamen anthers and stigma, or contact them at safe sites on the pollinator's body where pollen is less likely to be groomed off (Harder, 1990). A related hypothesis is that the short stamen anthers are mainly there as a reward for pollen feeders, so that most pollen delivered to stigmas comes from long stamen anthers. Note that none of these hypotheses is mutually exclusive.
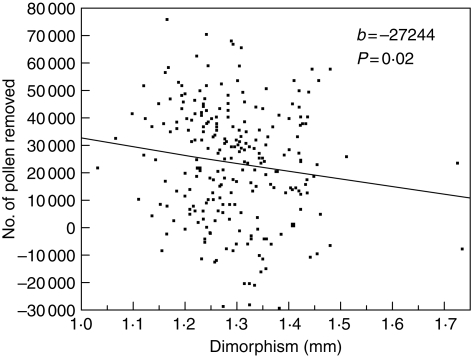
In support of the first hypothesis, and perhaps contradicting the third, is that pollen production per anther is actually greater on the short stamen anthers than on long stamen anthers, but that the percentage removed in single visits by pollen feeders is less (Table 2). This result is very similar to that for the tristylous Pondederia cordata, in which there is a negative relationship between the length of the stamen and per-anther pollen production, but a positive relationship between stamen length and per-visit percentage pollen removal (Wolfe and Barrett, 1989; Harder and Barrett, 1993). The conclusion that having two shorter stamens reduces pollen removal in radish is bolstered by two other results. First, the rates of pollen removal in single visits decline with increased anther height dimorphism, based on a regression using natural variation (Fig. 6). The negative slope of this relationship did not differ significantly among the four major pollinator taxa in this study (small bees, honey-bees, syrphid flies and cabbage butterflies) based on ANCOVA (Conner et al., 2003b).
Table 2.
Mean percentage pollen removed during single visits to virgin flowers by three taxa of pollen feeders (although honey-bees sometimes feed on nectar from wild radish in nature), and the mean number of pollen grains produced per anther on virgin flowers (±1 s.e.m.)
| Long stamen anthers | Short stamen anthers | P | n | |
|---|---|---|---|---|
| Honey-bees | 63 | 38 | <0·0001 | 72 |
| Small bees | 40 | 9 | <0·0001 | 45 |
| Syrphid flies | 29 | 17 | 0·03 | 52 |
| Pollen production | 11 690 (± 510) | 17 356 (± 858) |
P-values are from paired t-tests comparing the percentage removal from long vs. short stamen anthers. The honey-bee experiments were performed in an indoor flight cage, and the other two taxa in the field. Data from Conner et al. (1995).
Fig. 6.
Regression of the number of pollen grains removed in single pollinator visits on anther height dimorphism, where b denotes the unstandardized slope. The regression model also included number of pollen grains on the flower before visitation and pollinator taxon (small bees, syrphid flies, honey-bees and cabbage butterflies); the interaction between pollinator taxon and dimorphism was not significant. Removal was estimated as the difference in pollen counts between adjacent visited and unvisited flowers, so the negative removal values result from experimental error in counting and differences in pollen production between the two flowers. Data from Conner et al. (1995; reanalysed in Conner et al., 2003b).
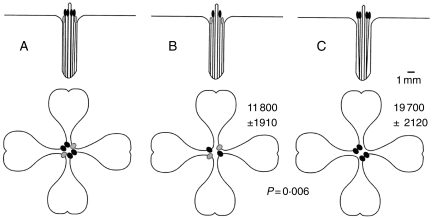
Second, we estimated pollen removal from experimentally manipulated flowers, in which we removed the two short stamen anthers by pinching off the filaments at the base of the anthers (resulting in four anthers of equal height) in one treatment group, and removed two of the long stamen anthers in the other treatment group (dimorphic anther heights, two of each; Fig. 7). The results showed that the monomorphic flowers had higher rates of pollen removal after correcting for differences in pollen production (Fig. 7). However, there was no significant difference in seed set between the two treatments, suggesting that any pollinator body position differences between monomorphic and dimorphic flowers did not affect female fitness (hypothesis 2). This experiment was performed in the field over two years, so the results represent all naturally occurring pollinators together; in these years each of the four major taxa (excluding bumble-bees) were present at greater than 10%.
Fig. 7.
Results of the anther removal experiment. (A) Side and top views of an unmanipulated flower; (B) manipulated dimorphic flower; (C) manipulated monomorphic flower. Least-squares mean number of pollen grains removed in single visits ±1 s.e.m. are shown, with the P-value for the difference between the two manipulated treatments from ANOVA. Modified from Conner et al. (2003b).
However, selection gradient estimates on natural variation suggest that in the case of anther height dimorphism, pollen removal may not translate directly into male fitness. Therefore, the functional analyses above may not be a good guide to how selection is acting on the trait. In the first year, there were significant negative linear and quadratic selection gradients, meaning that there was an intermediate optimum anther height dimorphism just below the current population mean, and fitness dropped off more severely with dimorphism greater than the mean than for dimorphism less than the mean (Fig. 8). In the other two years there were positive linear gradients and negative quadratic gradients, but none was statistically significant.
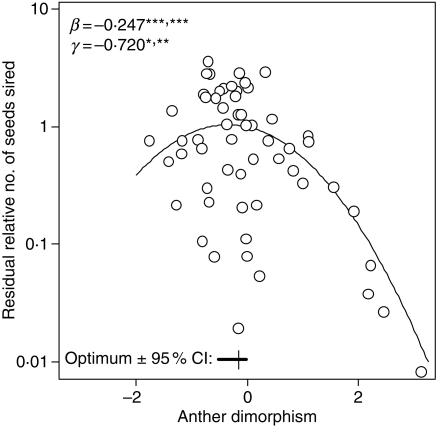
Fig. 8.
Selection gradient for anther height dimorphism using natural variation, showing significant negative linear and quadratic terms. The y-axis is relative male fitness (seed siring success) after correcting for correlations with other floral traits. The optimum ±95% CI is shown. Modified from Morgan and Conner (2001); data from Conner et al. (1996b). Significance values are from both parametric (before slash) and simulation tests. *P < 0.05; **P < 0.01; ***P < 0.005.
Natural selection on experimentally increased variance
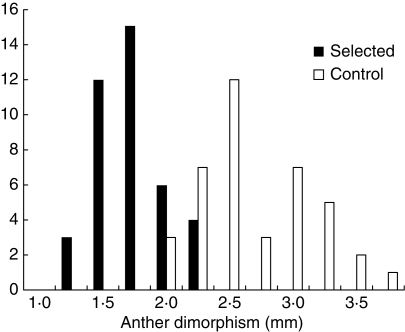
Thus, just as with anther exsertion, the evidence from natural variation on whether anther height dimorphism is adaptive is equivocal. Again, we conducted artificial selection to increase the variance in this trait and to re-create the ancestral condition for this trait. The sister group to the Brassicaceae, the Cleomaceae (Capparaceae), have six or more anthers of equal height (Zomlefer, 1994; Rodman et al., 1998; Hall et al., 2002), so we selected two lines for decreased anther height dimorphism and had two randomly mated control lines. As with anther exsertion, the evolution of dimorphism can only occur through the independent evolution of the two component traits, i.e. the short and long filaments, and this evolution might be expected to be constrained by the extremely strong additive genetic correlation between these traits (rA = 0·91; Conner and Via, 1993), which is also caused by pleiotropy (Conner, 2002). Again, this constraint did not occur, as anther height dimorphism evolved rapidly over five generations of artificial selection; when the control lines are combined with the selected lines, the variance in anther dimorphism is increased by 50%, from 0·24 to 0·36 (Fig. 9).
Fig. 9.
Results of artificial selection on anther height dimorphism. Shown are the distribution of the means of 40 families selected for decreased dimorphism for five generations, and 40 randomly mated control families.
We predicted that there would be positive directional selection on anther height dimorphism in this combined population, because the selected lines had reduced dimorphism relative to the natural population. Therefore, if current levels of dimorphism are adaptive, these reduced dimorphism plants should have lower fitness. However, when we placed these plants in the field and again measured male seed siring success using microsatellite markers, we found exactly the opposite – marginally significant negative directional selection (Fig. 10A). This suggests that natural levels of anther height dimorphism are not adaptive, which is the pattern suggested by the functional analyses of pollen removal reviewed above.
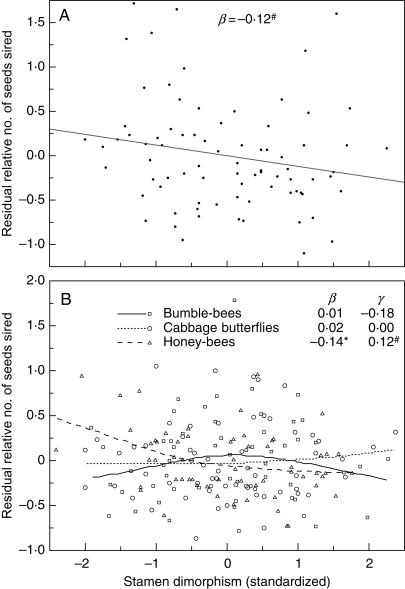
Fig. 10.
Natural selection on expanded variation in anther height dimorphism. Dimorphism values are standardized to a mean of zero and standard deviation of one; the y-axis is relative male fitness (seed siring success) after correcting for correlations with other floral traits. (A) Overall selection gradient (all pollinators naturally visiting). Data from J. K. Conner et al. (unpubl. res.). (B) Selection by three individual pollinator taxa on anther dimorphism; there was no significant selection by small bees and syrphid flies. From H. F. Sahli and J. K. Conner (unpubl. res.). # P < 0·10; * P < 0·05.
In the results for selection through male seed siring success by individual pollinator taxa, we see that the only significant selection was negative directional selection by honey-bees (Fig. 10B). It would be natural to conclude from this that the overall negative selection on expanded variation in dimorphism (Fig. 10A) was due to honey-bees, but they accounted for less than 1% of all visits in the overall selection study, so this seems unlikely. There were non-significant trends for negative directional selection through male fitness by two of the syrphid flies in the individual pollinator study (Toxomerus and Syritta; H. F. Sahli and J. K. Conner, unpubl. res.), and these accounted for about 10% of visits in the overall study. Thus, it is difficult to say with certainty which pollinators were responsible for the negative selection on dimorphism.
CONCLUSIONS
The studies reviewed here, taken together, provide strong evidence that intermediate anther exsertion is adaptive in wild radish, and that this is probably an adaptation to one of the most important subsets of wild radish pollinators, small bees. The correlation between filament and corolla tube lengths is significantly higher than background levels of floral correlation. This high correlation would result from stabilizing selection on exsertion, is stable across populations and environments in wild radish, and is derived from an ancestral lower correlation. Studies using both natural and expanded variation in exsertion show stabilizing selection on exsertion through lifetime male fitness. When both pollen removal and male fitness are estimated based on visits by single pollinator taxa, the pattern of an intermediate optimum occurs only for small bees. There was evidence for increased exsertion being adaptive from functional analyses and/or selection analyses for the larger pollinators, namely honey-bees, bumble-bees and cabbage butterflies. This is consistent with work on other species, in which longer stamens or greater exsertion led to increased pollen removal by bumble-bees (Wolfe and Barrett, 1989; Murcia, 1990; Harder and Barrett, 1993). It also makes sense that larger pollinators effectively contact the more exserted anthers, but small bees make less effective contact the higher the anther is above the landing platform. Current work is examining the molecular genetic basis of anther exsertion and its rapid evolution under artificial selection using mapping of quantitative trait loci and gene expression analyses.
Whether or not dimorphic anther height is an adaptation in wild radish and Brassicaceae in general is much less clear (anther height polymorphisms are clearly adaptive in heterostylous species; see the Introduction). Anther dimorphism might be an adaptation to decrease per-visit pollen removal rates, as it clearly leads to decreased pollen removal, and plants with intermediate dimorphism sired the most seeds (i.e. there was stabilizing selection) in one year (Fig. 8). However, under conditions of lower pollinator visitation, the restriction of pollen removal could well be maladaptive, and the selection gradient based on the expanded variation was negative (but only marginally significant). Even in the year with stabilizing selection there was a strong negative linear gradient as well. However, we know from the artificial selection results that selection for decreased dimorphism results in a rapid response, so if less dimorphism is adaptive, then it is hard to understand why reduced dimorphism has not already evolved. The plants with artificially expanded variation were heavily visited, but interestingly, there was marginally significantly greater visitation to the selected plants, perhaps because the pollen reward was more apparent with less dimorphism. We plan to repeat the anther removal experiment (Fig. 7) and to measure both visitation and seed siring success (instead of pollen removal as was done in the previous experiment) to further test the adaptiveness of anther dimorphism.
ACKNOWLEDGEMENTS
We thank Alan Prather and Anna Monfils for help with the phylogenetic study, Vanessa Koelling for help conducting the artificial selection, Frances Knapczyk for help measuring selection, Martin Morgan for the natural selection analysis in Figs 6 and 11, Kari Lehtilä and Sharon Strauss for sharing unpublished pollinator data, and Christy Stewart, Cindy Mills and many undergraduates for help with all phases of this work. This work was supported by the National Science Foundation (DEB 93 18388, DEB 9903880, DEB 0108354, DBI 0638591 and DDIG DEB-0408055) and by the Cooperative State Research, Education, and Extension Service, US Department of Agriculture (agreement no. 2002*35320-11538).
LITERATURE CITED
- Agrawal AA. Induced responses to herbivory and increased plant performance. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Conner JK, Johnson MTJ, Wallsgrove R. Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution. 2002;56:2206–2213. doi: 10.1111/j.0014-3820.2002.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Conner JK, Stinchcombe JR. Evolution of plant resistance and tolerance to frost damage. Ecology Letters. 2004;7:1199–1208. [Google Scholar]
- Barrett SCH. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany. 2000;85:253–265. [Google Scholar]
- Beilstein MA, Al-Shehbaz IA, Kellogg EA. Brassicaceae phylogeny and trichome evolution. American Journal of Botany. 2006;93:607–619. doi: 10.3732/ajb.93.4.607. [DOI] [PubMed] [Google Scholar]
- Beilstein MA, Al-Shehbaz IA, Mathews S, Kellogg EA. Brassicaceae phylogeny inferred from phytochrome A and ndhF sequence data: tribes and trichomes revisited. American Journal of Botany. 2008;95:1307–1327. doi: 10.3732/ajb.0800065. [DOI] [PubMed] [Google Scholar]
- Bett KE, Lydiate DJ. Genetic analysis and genome mapping in Raphanus. Genome. 2003;46:423–430. doi: 10.1139/g03-026. [DOI] [PubMed] [Google Scholar]
- Brodie ED., III Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution. 1992;46:1284–1298. doi: 10.1111/j.1558-5646.1992.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Chambers JM, Cleveland WS, Kleiner B, Tukey PA. Graphical methods for data analysis. Boston: Duxbury Press; 1983. [Google Scholar]
- Conner JK. Floral evolution in wild radish: the roles of pollinators, natural selection, and genetic correlations among traits. International Journal of Plant Sciences. 1997;158:S108–S120. [Google Scholar]
- Conner JK. Genetic mechanisms of floral trait correlations in a natural population. Nature. 2002;420:407–410. doi: 10.1038/nature01105. [DOI] [PubMed] [Google Scholar]
- Conner JK. Artificial selection: a powerful tool for ecologists. Ecology. 2003;84:1650–1660. [Google Scholar]
- Conner JK, Rush S. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia. 1996;105:509–516. doi: 10.1007/BF00330014. [DOI] [PubMed] [Google Scholar]
- Conner JK, Sterling A. Testing hypotheses of functional relationships: a comparative survey of correlation patterns among floral traits in five insect-pollinated plants. American Journal of Botany. 1995;82:1399–1406. [Google Scholar]
- Conner J, Via S. Patterns of phenotypic and genetic correlations among morphological and life history traits in wild radish, Raphanus raphanistrum. Evolution. 1993;47:704–711. doi: 10.1111/j.1558-5646.1993.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Davis R, Rush S. The effect of wild radish floral morphology on pollination efficiency by four taxa of pollinators. Oecologia. 1995;104:234–245. doi: 10.1007/BF00328588. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rush S, Jennetten P. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). I. Selection through lifetime female fitness. Evolution. 1996a;50:1127–1136. doi: 10.1111/j.1558-5646.1996.tb02353.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rush S, Kercher S, Jennetten P. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). II. Selection through lifetime male and total fitness. Evolution. 1996b;50:1137–1146. doi: 10.1111/j.1558-5646.1996.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Franks R, Stewart C. Expression of additive genetic variances and covariances for wild radish floral traits: comparison between field and greenhouse environments. Evolution. 2003a;57:487–495. doi: 10.1111/j.0014-3820.2003.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rice AM, Stewart C, Morgan MT. Patterns and mechanisms of selection on a family-diagnostic trait: evidence from experimental manipulation and lifetime fitness selection gradients. Evolution. 2003b;57:480–486. doi: 10.1111/j.0014-3820.2003.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. The origin of species by means of natural selection. London: John Murray; 1859. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. Chicago: University of Chicago Press; 1877a. [Google Scholar]
- Darwin C. The various contrivances by which orchids are fertilised by insects. Chicago: The University of Chicago Press; 1877b. [Google Scholar]
- Devlin B, Ellstrand NC. Male and female fertility variation in wild radish, a hermaphrodite. American Naturalist. 1990;136:87–107. [Google Scholar]
- Galloway GL, Malmberg RL, Price RA. Phylogenetic utility of the nuclear gene arginine decarboxylase: an example from Brassicaceae. Molecular Biology and Evolution. 1998;15:1312–1320. doi: 10.1093/oxfordjournals.molbev.a025859. [DOI] [PubMed] [Google Scholar]
- Grafen A. On the uses of data on lifetime reproductive success. In: Clutton-Brock TH, editor. Reproductive success. Chicago: University of Chicago Press; 1988. [Google Scholar]
- Hall JC, Sytsma KJ, Iltis HH. Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. American Journal of Botany. 2002;89:1826–1842. doi: 10.3732/ajb.89.11.1826. [DOI] [PubMed] [Google Scholar]
- Harder LD. Pollen removal by bumble bees and its implications for pollen dispersal. Ecology. 1990;71:1110–1125. [Google Scholar]
- Harder LD, Barrett SCH. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialization. Ecology. 1993;74:1059–1072. [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. Ameriacn Naturalist. 1989;133:323–344. [Google Scholar]
- Irwin RE, Strauss SY. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. American Naturalist. 2005;165:225–237. doi: 10.1086/426714. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Strauss SY, Storz S, Emerson A, Guibert G. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology. 2003;84:1733–1743. [Google Scholar]
- Johnston JS, Pepper AE, Hall AE, et al. Evolution of genome size in Brassicaceae. Annals of Botany. 2005;95:229–235. doi: 10.1093/aob/mci016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay QON. The role of preferential and assortative pollination in the maintenance of flower colour polymorphisms. In: Richards AJ, editor. The pollination of flowers by insects. New York: Academic Press; 1978. [Google Scholar]
- Koch M, Haubold B, Mitchell-Olds T. Molecular systematics of the Brassicaceae: evidence from coding plastidic matK and nuclear Chs sequences. American Journal of Botany. 2001;88:534–544. [PubMed] [Google Scholar]
- Kohn JR, Barrett SCH. Experimental studies on the functional significance of heterostyly. Evolution. 1992a;46:43–55. doi: 10.1111/j.1558-5646.1992.tb01983.x. [DOI] [PubMed] [Google Scholar]
- Kohn JR, Barrett SCH. Floral manipulations reveal the cause of male fitness variation in experimental populations of Eichhornia paniculata (Pontederiaceae) Functional Ecology. 1992b;6:590–595. [Google Scholar]
- Kudo G. Anther arrangement influences pollen deposition and removal in hermaphrodite flowers. Functional Ecology. 2003;17:349–355. [Google Scholar]
- Mazer SJ, Schick CT. Constancy of population parameters for life-history and floral traits in Raphanus sativus L. II. Effects of planting density on phenotype and heritability estimates. Evolution. 1991;45:1888–1907. doi: 10.1111/j.1558-5646.1991.tb02694.x. [DOI] [PubMed] [Google Scholar]
- Morgan MT, Conner JK. Using genetic markers to directly estimate male selection gradients. Evolution. 2001;55:272–281. doi: 10.1111/j.0014-3820.2001.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Murcia C. Effect of floral morphology and temperature on pollen receipt and removal in Ipomoea trichocarpa. Ecology. 1990;71:1098–1109. [Google Scholar]
- Phillips PC, Arnold SJ. Visualizing multivariate selection. Evolution. 1989;43:1209–1222. doi: 10.1111/j.1558-5646.1989.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Rodman J, Soltis P, Soltin D, Sytsma K, Karol K. Parallel evolution of glucosinolate biosysnthesis inferred from congruent nuclear and plastid gene phylogenies. American Journal of Botany. 1998;85:997–1006. [PubMed] [Google Scholar]
- Rush S, Conner JK, Jennetten P. The effects of natural variation in pollinator visitation on rates of pollen removal in wild radish, Raphanus raphanistrum (Brassicaceae) American Journal of Botany. 1995;82:1522–1526. [Google Scholar]
- Sahli HF, Conner JK. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish, Raphanus raphanismum (Brassicaceae) American Journal of Botany. 2007;94:203–209. doi: 10.3732/ajb.94.2.203. [DOI] [PubMed] [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Snow AA, Uthus KL, Culley TM. Fitness of hybrids between weedy and cultivated radish: implications for weed evolution. Ecological Applications. 2001;11:934–943. [Google Scholar]
- Sprengel CK. Das entdeckte Geheimnis der Natur im Bau und in der Befruchtung der Blumen. Berlin: Friedrich Vieweg; 1793. [Google Scholar]
- Stanton ML, Snow AA, Handel SN. Floral evolution: attractiveness to pollinators increases male fitness. Science. 1986;232:1625–1627. doi: 10.1126/science.232.4758.1625. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Snow AA, Handel SN, Bereczky J. The impact of a flower-color polymorphism on mating patterns in experimental populations of wild radish (Raphanus raphanistrum L.) Evolution. 1989;43:335–346. doi: 10.1111/j.1558-5646.1989.tb04231.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Conner JK, Lehtila KP. Effects of foliar herbivory by insects on the fitness of Raphanus raphanistrum: damage can increase male fitness. American Naturalist. 2001;158:496–504. doi: 10.1086/323116. [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Barrett SCH. Patterns of pollen removal and deposition in tristylous Pontederia cordata L. (Pontederiaceae) Biological Journal of the Linnean Society. 1989;36:317–329. [Google Scholar]
- Yang YW, Lai KN, Tai PY, Ma DP, Li WH. Molecular phylogenetic studies of Brassica, Rorippa, Arabidopsis and allied genera based on the internal transcribed spacer region of 18S–25S rDNA. Molecular Phylogenetics and Evolution. 1999;13:455–462. doi: 10.1006/mpev.1999.0648. [DOI] [PubMed] [Google Scholar]
- Zomlefer WR. Guide to flowering plant families. Chapel Hill, NC: University of North Carolina Press; 1994. [Google Scholar]