Abstract
Background and Aims
Wind erosion is a severe stress for plants in drylands, but the mechanisms by which plants withstand erosion remain largely unknown. Here, the hypothesis is tested that maintaining rhizome connections helps plants to tolerate erosion.
Methods
Five transects were established across an inland dune in Inner Mongolia, China, and measurements were made of leaf number, biomass per ramet and rhizome depth of Psammochloa villosa in 45 plots. In 40 × 40 cm plots of P. villosa on another dune, the top 15 or 30 cm of sand was removed for 1·5 or 3 months to simulate short- and long-term moderate and severe erosion, respectively, with untreated plots as controls, and the rhizomes at the edges of half of the plots were severed to mimic loss of rhizome connections.
Key Results
Leaf number and biomass per ramet showed quadric relationships with rhizome depth; when rhizomes were exposed to the air, the associated ramets either died or became very weak. Ramet number, leaf number and biomass per plot decreased with increasing erosion severity. Rhizome connections did not affect these traits under control or short-term erosion, but increased them under long-term erosion.
Conclusions
Rhizome connections alleviated the negative effects of erosion on P. villosa, very likely because the erosion-stressed ramets received water and/or photosynthates translocated from those connected ramets that were not subject to erosion. This study provides the first evidence that maintaining rhizome connections helps plants to tolerate erosion in drylands.
Key words: Clonal integration, inland-dune grass, Psammochloa villosa, resource sharing, rhizome severing, wind erosion
INTRODUCTION
Large areas of drylands worldwide have been severely affected by desertification and wind erosion, occurring at various spatial and temporal scales, is a serious problem in these areas (Lopez, 1998; Gomes et al., 2003; Zhao et al., 2006; Reynolds et al., 2007). Erosion can loosen soil constituents and remove small soil particles. During this process, soils become impoverished and productivity is reduced (Lyles, 1975; Dregne, 1983; Kennedy, 1995; Lal, 1998; Dong et al., 2000; Zhao et al., 2006). Wind erosion may also directly affect plant survival and growth by physically damaging below-ground plant structures (i.e. roots and rhizomes; Fryrear et al., 1973) and by exposing them to the air, thereby greatly impairing efficiency of water and nutrient uptake and also photosynthesis (Komlev, 1960; Fryrear et al., 1973). Thus, wind erosion imposes great stress on plants and is a selective force in drylands. To date, however, the mechanisms by which plants withstand wind erosion remain largely untested.
A common pattern in drylands is that many mobile and semi-mobile dunes are colonized by rhizomatous plants that form large, connected clones (Maun, 1984; Evans, 1988; D'Hertefeldt and Jonsdottir, 1999; Dong et al., 1999; Wang et al., 1999; Chen and Dong, 2000; Chen et al., 2001; D'Hertefeldt and Falkengren-Grerup, 2002; Liu et al., 2007). Ramets connected by a large network of below-ground rhizomes are potentially less susceptible to physical damage caused by wind erosion. Moreover, the erosion-stressed ramets may receive carbohydrates, water and/or nutrients from the interconnected ramets growing in more favourable microsites due to clonal integration, and thus their survival, growth and/or reproduction may be greatly improved. This leads us to the hypothesis that rhizome connections may help rhizomatous plants withstand erosion. Although many studies have shown the beneficial effects of rhizome connections on plants (e.g. Hartnett and Bazzaz, 1983; Salzman and Parker, 1985; Evans and Whitney, 1992; Shumway, 1995; D'Hertefeldt and van der Putten, 1998; Dong and Alaten, 1999; Amsberry et al., 2000; Pennings and Callaway, 2000; Yu et al., 2004; Brezina et al., 2006), no study has explicitly tested the roles of rhizome connections in the responses of clonal plants to erosion.
The degree and duration of wind erosion differ greatly in the field (Moreno-Casasola, 1986; Maun, 1998). Moderate erosion (e.g. exposure of a small part of the root and/or rhizome systems) may decrease both survival and growth of ramets, but such negative effects may be greatly alleviated due to clonal integration via rhizome connections. On the other hand, severe erosion (e.g. exposure of most roots and/or rhizomes) will inevitably kill plants because of root desiccation and the resultant loss of capacity for water and nutrient uptake and photosynthesis (Grace, 1977). Under such circumstances, however, the duration of ramet survival may be greatly improved by clonal integration, i.e. connected ramets can survive for a longer time than disconnected ones. Because wind direction may change greatly in the field, the exposed roots and rhizomes may be partially or completely re-buried during the next high wind event. Under such conditions, rhizome connections may contribute to the long-term survival of ramet populations even if severe wind erosion is frequent.
Here, two studies were conducted with the rhizomatous inland-dune grass Psammochloa villosa in the semi-arid region of northern China (Dong, 1999; Dong and Alaten, 1999; Dong et al., 1999). In the first study, P. villosa ramets were excavated in 45 plots of 50 × 50 cm and measurements were made of leaf number, biomass and rhizome depth (i.e. distance to the sand surface of an attached rhizome) along five transects across a semi-mobile dune. The first hypothesis (1) was that leaf number and biomass of a ramet would be correlated with rhizome depth, and that ramets would be weak if the associated rhizomes are exposed. In the second study, simulations were made of relatively long-term (approx. 3 months) and short-term (approx. 1·5 months) moderate and severe wind erosion by removing the top 15 and 30 cm of sand, respectively, in 40 × 40 cm plots of P. villosa, with untreated plots as controls, and the loss of rhizome connections was mimicked by severing the rhizomes along the edge of half of the plots. It was hypothesized (2) that the simulated erosion treatments would greatly reduce growth and survival of P. villosa ramets and that the impact would increase with increasing severity and duration of erosion. It was further hypothesized (3) that rhizome connections would help P. villosa cope with wind erosion, i.e. growth and survival of ramets subject to erosion would be greater in connected than in disconnected plots.
MATERIALS AND METHODS
The species
Psammochloa villosa (Trin.) Bor. is a perennial grass, and occurs in the arid and semi-arid regions of northern and north-western China, as well as in Mongolia Republic (Dong, 1999; Dong et al., 1999). It propagates vegetatively by producing horizontally growing rhizomes and shows a guerrilla-type clonal growth strategy (Dong, 1999; Dong et al., 1999; Yu et al., 2004). Rhizomes of P. villosa root at each node and grow linearly and monopodially (Dong, 1999; Dong et al., 1999; Yu et al., 2004). Usually there is only one bud at each rhizome node, developing into either a ramet or a new rhizome; ramets and rhizome branches are produced alternatively along the main rhizomes (Dong, 1999; Dong et al., 1999). In early spring, ramets of P. villosa start to form leaves that will die in late autumn. Rhizome connections between ramets persist for many years so that large, integrated clones are formed (Dong et al., 1999; Wang et al., 1999; Yu et al., 2004). Psammochloa villosa also reproduces sexually, but abundant seed production occurs only in some wet years and seedlings are rarely found in the field (F.-H. Yu et al., pers. obs.). On mobile and semi-mobile dunes in northern China, P. villosa populations frequently experience various levels of sand burial and erosion imposed by strong wind.
Study site
The studies were conducted near the Ordos Sandland Ecological Research Station (OSES, 39 °29′37·6′′N, 110 °11′ 29·4′′E, 1300 m a.s.l.) of the Chinese Academy of Sciences, located in the eastern Mu Us Sandy Land (37 °30′–39 °20′N, 107 °20′–111 °30′E) and on the semi-arid south-eastern Ordos Plateau in Inner Mongolia, China. The area has a mean annual temperature of 7·5–9·0 °C and mean annual precipitation of 260–450 mm (Zhang, 1994). Historically, this area was highly productive grasslands; today, however, the landscape is seriously desertified, consisting of fixed, semi-mobile and mobile dunes, as well as inter-dune fixed lowland dominated by the semi-shrub Artemisia ordosica (Zhang, 1994). Many mobile and semi-mobile dunes are covered by P. villosa. In this area, erosion by wind occurs frequently during the whole year (Zhang, 1994).
Relationships between ramet growth and rhizome depth
A semi-mobile dune dominated by P. villosa was selected near OSES. The dune occupies an area of about 3000 m2, of which 35 % is covered by P. villosa. On 5 August, 2006, five parallel transects were set up across the dune, and along each transect nine 50 × 50 cm plots were established along an erosion–burial gradient, one in each of the following locations: (1) on the lower, (2) middle and (3) upper part of the windward slope; (4) on the crest of the dune; (5) on the upper, (6) middle and (7) lower part of the leeward slope; (8) in front of the windward slope of the dune where wind erosion is severe (at least part of the roots and rhizomes of P. villosa were exposed); and (9) on a flat area far behind the leeward slope of the dune where neither burial nor erosion is substantial. For each ramet in each plot, rhizome depth was measured, leaf number counted and biomass (without the horizontal rhizome) determined after drying at 70 °C for 48 h. Mean rhizome depth, mean leaf number and mean biomass per ramet was calculated in each plot. Regressions were then used to analyse the relationships between mean rhizome depth and (1) mean biomass and (2) mean leaf number per ramet.
Erosion and rhizome-severing manipulations
Experimental design
On 1 July, 2006, 105 plots (each 40 × 40 cm and containing 6–8 ramets of P. villosa) were set up on the slopes of another semi-mobile dune near OSES. Out of these, 55 plots were randomly selected for rhizome-severing treatments (hereafter referred to as ‘disconnected’ plots) and the remaining 50 plots were used as controls (hereafter referred to as ‘connected’ plots). Along the edges of the disconnected plots the rhizomes of P. villosa were severed by inserting a sharp blade perpendicular to the sand surface to a depth of 50 cm (Yu et al., 2004).
Two days later, the vitality of the P. villosa ramets in each plot was checked and 50 disconnected plots without dead ramets were selected. The 50 disconnected and 50 connected plots were then randomly assigned to five experimental wind erosion treatments: (1) no erosion (control); (2) short-term moderate and (3) short-term severe erosion; (4) long-term moderate and (5) long-term severe erosion. The moderate and severe erosion treatments were simulated by artificially removing the top 15 and 30 cm of sand, respectively; the control plots were left untreated. The top-sand removal was maintained for 1·5 months (46 d) for the short-term erosion treatment and for approx. 3 months (89 d) for the long-term erosion treatment. On 15 August, 2006, all the short-term erosion plots were refilled with sand to the level of the original sand surface. Almost no rhizomes were exposed in the moderate-erosion plots, whereas most (approx. 80 %) were exposed in the severe-erosion plots. During the experiment, each plot was fenced-in with a nylon belt up to 5 cm above the soil surface level in order to minimize sand accumulation. Sand that was occasionally deposited in the erosion plots was removed about once a week. There were ten replicates for each treatment.
Measurements
On 1 July, 2006, ramet number and leaf number were counted in each plot. Between 5 July and 25 September, 2006, ramet number and leaf number were repeatedly counted at intervals of about 1 week. On 26–27 September, 2006, P. villosa ramets in each plot were carefully excavated to a depth of 80 cm and biomass was measured after drying at 70 °C for 48 h.
Data analysis
Two-way ANCOVA was used to investigate the effects of simulated erosion and rhizome severing on biomass per plot, with leaf number and ramet number per plot measured on 1 July as the covariates. Two-way repeated-measures ANCOVA was employed to test the effects of erosion and rhizome severing on ramet and leaf number per plot, with ramet number or leaf number per plot measured on 1 July as the covariate. The P-values for the within-treatment effects were adjusted based on the Huynh–Feldt criteria (von Ende, 2001). When a significant effect of rhizome severing and/or erosion × severing was detected, a contrast between the ‘connected’ and the ‘disconnected’ treatment was conducted for each level of the erosion treatments (Sokal and Rohlf, 1981; SAS Institute Inc., 1999). In order to reduce the heterogeneity of variance, biomass was transformed to the natural log and ramet number to the square root before analyses. SAS v. 8·2 was used for all analyses (SAS Institute Inc., 1999).
RESULTS
Relationships between ramet growth and rhizome depth
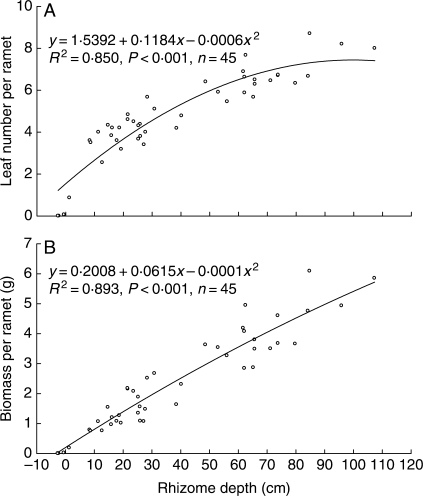
Both quadric and linear models fitted the data very well (R2 > 0·79, P < 0·0001, n = 45 for all regressions). When rhizomes were exposed to the air, the associated ramets died or became very weak (Fig. 1).
Fig. 1.
Quadric relationships between mean rhizome depth per plot of P. villosa and (A) mean leaf number and (B) mean biomass per ramet per plot. Negative values of depth indicate that the rhizomes are exposed to the air.
The regression equations for the relationships between mean leaf number per ramet (y) and mean rhizome depth (x) were y = 1·5392 + 0·1184x – 0·0006x2 (quadric model, R2 = 0·850; Fig. 1A) and y = 2·2967 + 0·0635x (linear model, R2 = 0·797), and those between mean biomass per ramet (y) and mean rhizome depth (x) were y = 0·2008 + 0·0615x – 0·0001x2 (quadric model, R2 = 0·893; Fig. 1B) and y = 0·3208 + 0·0528x (linear model, R2 = 0·891). But quadric models are more realistic because there should be an optimal and a maximum rhizome depth after which performance and survival of P. villosa ramets decreases due to the costs of building long leaf sheaths and/or vertical rhizomes to grow out of the sand.
Effects of erosion and rhizome severing
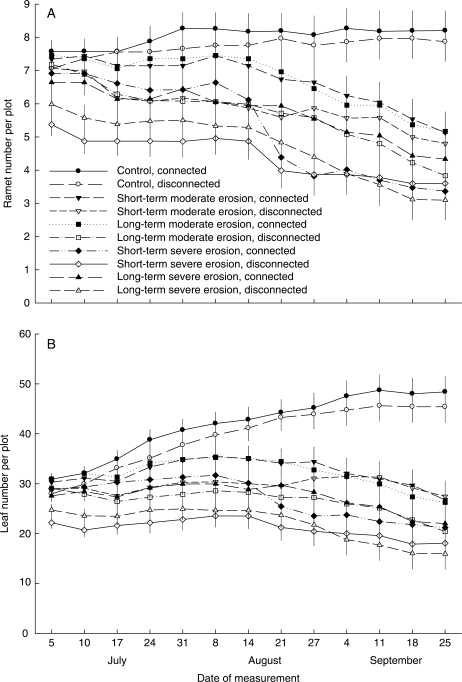
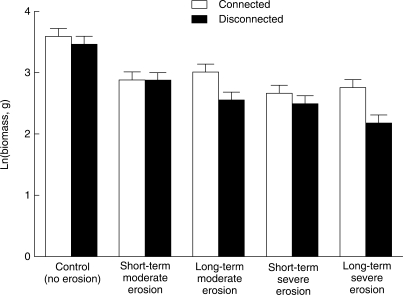
Both erosion and rhizome severing significantly affected ramet number, leaf number and biomass of P. villosa (Table 1). Ramet number, leaf number and biomass decreased greatly with increasing erosion depth (Figs 2A, B and 3). In the absence of erosion (control), severing the rhizome did not affect ramet number (Fig. 2A, ‘connected’ vs. ‘disconnected’; contrast F1,89 = 0·19, P = 0·665), leaf number (Fig. 2B; contrast F1,89 = 0·65, P = 0·424) or biomass of P. villosa (Fig. 3; contrast F1,88 = 0·49, P = 0·485). Likewise, under the short-term moderate or severe erosion treatments, rhizome severing did not affect ramet number, leaf number or biomass (Figs 2 and 3; P > 0·10 for all contrasts). In the plots subject to long-term moderate or severe erosion treatments, however, biomass (Fig. 3; contrast F1,88 = 6·62, P = 0·014 for moderate erosion, and contrast F1,88 = 10·01, P = 0·002 for severe erosion), ramet number (Fig. 2A; contrast F1,89 = 2·95, P = 0·089 for severe erosion) and leaf number (Fig. 2B; contrast F1,89 = 3·35, P = 0·070 for moderate erosion, and contrast F1,89 = 3·25, P = 0·075 for severe erosion) either were or tended to be larger in the connected than in the disconnected plots.
Table 1.
Results of ANCOVA for effects of experimental erosion, rhizome severing and measuring date on ramet number, leaf number and biomass of P. villosa per plot
| Ramet number |
Leaf number |
Biomass |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | F | P | d.f. | F | P | d.f. | F | P |
| Co1 | 1 | 27·32 | <0·001 | – | – | – | 1 | 2·85 | 0·095 |
| Co2 | – | – | – | 1 | 71·59 | <0·001 | 1 | 14·43 | <0·001 |
| Erosion (E) | 4 | 12·15 | <0·001 | 4 | 19·65 | <0·001 | 4 | 20·36 | <0·001 |
| Severing (S) | 1 | 7·59 | 0·007 | 1 | 10·43 | 0·002 | 1 | 10·80 | 0·002 |
| E × S | 4 | 0·25 | 0·908 | 4 | 0·33 | 0·858 | 4 | 1·69 | 0·159 |
| Error | 89 | 89 | 88 | ||||||
| Date (D) | 12 | 1·05 | 0·368 | 12 | 1·51 | 0·214 | |||
| D × Co1 or Co2 | 12 | 0·37 | 0·769 | 12 | 1·48 | 0·224 | |||
| D × E | 48 | 5·33 | <0·001 | 48 | 14·00 | <0·001 | |||
| D × S | 12 | 0·59 | 0·619 | 12 | 0·94 | 0·414 | |||
| D × E × S | 48 | 1·47 | 0·139 | 48 | 0·80 | 0·640 | |||
| Error (D) | 1068 | 1068 | |||||||
F, P and d.f. for ramet number and leaf number are based on three-way repeated measures ANCOVA, and for biomass based on two-way ANCOVA. Covariates 1 (Co1) and 2 (Co2) are ramet number and leaf number per plot measured at the start of the experiment.
Fig. 2.
(A) Ramet number and (B) leaf number of P. villosa per plot under combinations of five erosion and two rhizome-severing treatments. Means ± s.e. were adjusted based on the ANCOVA coefficients.
Fig. 3.
Biomass of P. villosa per plot under combinations of five erosion and two rhizome-severing treatments. Means ± s.e. were adjusted according to the ANCOVA coefficients.
DISCUSSION
In the absence of erosion or burial, the rhizomes of P. villosa are located about 20–40 cm below the sand surface (Dong et al., 1999). In the present correlative study, it was found that some ramets managed to survive even if the attached rhizomes were exposed (up to 4 cm above the sand surface). The manipulative study also showed that some ramets both in the connected and disconnected plots could even survive erosion treatments in which the top 30 cm of sand was removed and most rhizomes in the plots were exposed. These results suggest that P. villosa has a large capacity to survive wind erosion in the field, which may contribute greatly to the persistence of populations in drylands.
On the other hand, the findings strongly support the view that wind erosion imposes great stress on P. villosa, because both leaf number and biomass of the ramets were greatly reduced if the associated rhizomes were exposed to the air, and because the simulated erosion had a significant negative effect on ramet number, leaf number and biomass. The observed negative effects of erosion are very likely due to the fact that without the protection of a sufficiently thick sand layer, roots and rhizomes easily dry out and quickly loose their ability to acquire and transport water, as well as other resources (Grace, 1977).
In the disconnected plots, the number of ramets that survived the long-term moderate and severe erosion treatments decreased by 51 % and 61 %, respectively, in comparison with the disconnected plots not subject to erosion. In the connected plots, by contrast, the corresponding reductions were significantly smaller (37 % in the moderate and 47 % in the severe long-term erosion treatments). In the plots subject to long-term erosion treatments, both biomass and leaf number were greater in the connected than in the disconnected plots. These results support the hypothesis that rhizome connections help P. villosa cope with wind erosion and thus improve the chances for long-term survival. We presume that clonal integration via rhizome connections provided the erosion-exposed ramets with water, nutrients and/or photosynthates translocated from the connected, unstressed ramets, compensating for the reduced efficiency of soil water and/or nutrient absorption by roots and also for the low photosynthesis rate of leaves. These results agree with previous findings that rhizome connections greatly improved the capacity of P. villosa to cope with drought (Dong and Alaten, 1999) and sand burial (Yu et al., 2004) and supported a fast expansion of its rhizome and ramet systems on inland dunes (Dong, 1999; Dong and Alaten, 1999).
In the short-term erosion treatments, however, ramet number, leaf number and biomass of P. villosa were the same in the connected and the disconnected plots. It was therefore apparent that in the study area P. villosa did not depend on rhizome connections (clonal integration) to be able to cope with 1·5 months of erosion. In the case of the 3-month long-term erosion treatment, however, rhizome connections helped P. villosa limit the damage to the same order of magnitude as observed in the short-term erosion treatments. Thus, the adaptive significance of rhizome connections increased with increasing duration and severity of the erosion event.
It is therefore concluded that rhizome connections markedly reduce the negative effects of wind erosion on P. villosa and greatly contribute to its ability to tolerate erosion on inland dunes. Considering that in the field P. villosa can form very large clones (e.g. a whole dune covered by only one clone; Wang et al., 1999) and translocate resources over very long distances (e.g. up to 46 m; Chen et al., 2001), then as well as the beneficial effects of rhizome connections on survival and growth of ramets suffering from wind erosion, sand burial (Yu et al., 2004) and drought (Dong and Alaten, 1999), we propose that these connections are an important adaptive strategy of P. villosa and play a substantial role for its long-term persistence in drylands.
ACKNOWLEDGEMENTS
We thank Guo-Lei Yu, Jun-Qing Yang, Xiao-Jie Li, Lige Bi, Shu-Qin Gao and Da Man for assistance in the field, and Dr Johannes Cornelissen and two anonymous reviewers for valuable comments on an earlier version of the manuscript. The research was supported by the National Key Basic Research Program of China (2007CB106802) and NSFC (30770357, 30500070).
LITERATURE CITED
- Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD. Clonal integration and the expansion of Phragmites australis. Ecological Applications. 2000;10:1110–1118. [Google Scholar]
- Brezina S, Koubek T, Munzbergova Z, Herben T. Ecological benefits of integration of Calamagrostis epigejos ramets under field conditions. Flora. 2006;201:461–467. [Google Scholar]
- Chen Y-F, Dong M. Genet characters of Hedysarum laeve and the characters of its ramet population in different habitats in Mu Us sandy land. Acta Phytoecologica Sinica. 2000;24:40–44. [Google Scholar]
- Chen Y-F, Yu F-H, Zhang C, Dong M. Role of clonal growth of the rhizomatous grass Psammochloa villosa in patch dynamics of Mu Us sandy land. Acta Ecologica Sinica. 2001;21:1745–1750. [Google Scholar]
- D'Hertefeldt T, Falkengren-Grerup U. Extensive physiological integration in Carex arenaria and Carex disticha in relation to potassium and water availability. New Phytologist. 2002;156:469–477. doi: 10.1046/j.1469-8137.2002.00529.x. [DOI] [PubMed] [Google Scholar]
- D'Hertefeldt T, Jonsdottir IS. Extensive physiological integration in intact clonal systems of Carex arenaria. Journal of Ecology. 1999;87:258–264. [Google Scholar]
- D'Hertefeldt T, van der Putten WH. Physiological integration of the clonal plant Carex arenaria and its response to soil-borne pathogens. Oikos. 1998;81:229–237. [Google Scholar]
- Dong M. Effects of severing rhizome on clonal growth in rhizomatous grass species Psammochloa villosa and Leymus secalinus. Acta Botanica Sinica. 1999;41:194–198. [Google Scholar]
- Dong M, Alaten B. Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolian dune, China. Plant Ecology. 1999;141:53–58. [Google Scholar]
- Dong M, Alateng B, Xing X, Wang Q. Genet features and ramet population features in the rhizomatous grass species Psammochloa villosa. Acta Phytoecologica Sinica. 1999;23:302–310. [Google Scholar]
- Dong ZB, Wang XM, Liou LY. Wind erosion in arid and semiarid China: an overview. Journal of Soil and Water Conservation. 2000;55:439–444. [Google Scholar]
- Dregne HE. Soil and soil formation in arid regions. In: Webb RH, Wilshire HG, editors. Environmental effects of off-road vehicles: impacts and management in arid regions. New York: Springer-Verlag; 1983. pp. 15–30. [Google Scholar]
- von Ende CN. Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. Oxford: Oxford University Press; 2001. pp. 134–157. [Google Scholar]
- Evans JP. Nitrogen translocation in a clonal dune perennial Hydrocotyle bonariensis. Oecologia. 1988;77:64–68. doi: 10.1007/BF00380926. [DOI] [PubMed] [Google Scholar]
- Evans JP, Whitney S. Clonal integration across a salt gradient by a nonhalophyte, Hydrocotyle bonariensis (Apiaceae) American Journal of Botany. 1992;79:1344–1347. [Google Scholar]
- Fryrear DW, Stubbendieck J, McCully WG. Grass seedling response to wind and windblown sand. Crop Science. 1973;13:622–625. [Google Scholar]
- Gomes L, Arrue JL, Lopez MV, Sterk G, Richard D, Gracia R, et al. Wind erosion in a semiarid area of Spain: the WELSONS project. Catena. 2003;52:235–256. [Google Scholar]
- Grace J. Plant response to wind. London: Academic Press; 1977. [Google Scholar]
- Hartnett D, Bazzaz FA. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology. 1983;64:779–788. [Google Scholar]
- Kennedy AC. Microbial characteristics of soil quality. Journal of Soil and Water Conservation. 1995;50:243–248. [Google Scholar]
- Komlev AA. Field-protective afforestation and the increase in financial yield of agriculture. Selsk Khoz Povolzhya. 1960;6:43–45. [Google Scholar]
- Lal R. Soil erosion impact on agronomic productivity and environment quality. Critical Reviews in Plant Sciences. 1998;17:319–464. [Google Scholar]
- Liu F-H, Liu J, Yu F-H, Dong M. Water integration patterns in two rhizomatous dune perennials of different clonal fragment size. Flora. 2007;202:106–110. [Google Scholar]
- Lopez MV. Wind erosion in agricultural soil: an example of limited supply of particles available for erosion. Catena. 1998;33:17–28. [Google Scholar]
- Lyles L. Possible effects of wind erosion on soil productivity. Journal of Soil and Water Conservation. 1975;30:279–283. [Google Scholar]
- Maun MA. Colonizing ability of Ammophila breviligulata through vegetative regeneration. Journal of Ecology. 1984;72:565–574. [Google Scholar]
- Maun MA. Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany. 1998;76:713–738. [Google Scholar]
- Moreno-Casasola P. Sand movement as a factor in the distribution of plant communities in a coastal dune system. Vegetatio. 1986;65:67–76. [Google Scholar]
- Pennings SC, Callaway RM. The advantages of clonal integration under different ecological conditions: a community-wide test. Ecology. 2000;81:709–716. [Google Scholar]
- Reynolds JF, Smith DMS, Labin EF, Turner BL, II, Mortimore M, Batterbury SPJ, et al. Global desertification: building a science for dryland development. Science. 2007;316:847–851. doi: 10.1126/science.1131634. [DOI] [PubMed] [Google Scholar]
- Salzman AG, Parker MA. Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia. 1985;65:273–277. doi: 10.1007/BF00379229. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT user's guide, Version 8. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Shumway SW. Physiological integration among clonal ramets during invasion of disturbance patches in a New England salt marsh. Annals of Botany. 1995;76:225–233. [Google Scholar]
- Sokal RR, Rohlf JF. Biometry: the principles and practice of statistics in biological research. New York: W. H: Freeman and Company; 1981. [Google Scholar]
- Wang K, Ge S, Dong M. Allozyme variance and clonal diversity in the rhizomatous grass Psammochloa villosa (Gramineae) Acta Botanica Sinica. 1999;41:537–540. [Google Scholar]
- Yu F-H, Dong M, Krusi B. Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist. 2004;162:697–704. doi: 10.1111/j.1469-8137.2004.01073.x. [DOI] [PubMed] [Google Scholar]
- Zhang X. Ecological background, principles and optimised models for rangeland management of the Maowusu sandland. Acta Phytoecologica Sinica. 1994;18:1–16. [Google Scholar]
- Zhao H, Zhou R, Zhang T, Zhao X. Effects of desertification on soil and crop growth properties in Horqin sandy cropland of Inner Mongolia, north China. Soil & Tillage Research. 2006;87:175–185. [Google Scholar]