Abstract
Background and Aims
French wheat grains may be of little value on world markets because they have low and highly variable grain protein concentrations (GPC). This nitrogen-yield to yield ratio depends on crop nitrogen (N) fertilization as well as on crop capacity to use N, which is known to vary with climate and disease severity. Here an examination is made of the respective roles that N remobilization and post-anthesis N uptake play in N yield variations; in particular, when wheat crops (Triticum aestivum) are affected by leaf rust (Puccinia triticina) and Septoria tritici blotch (teleomorph Mycosphaerella graminicola).
Methods
Data from a 4-year field experiment was used to analyse N yield variations in wheat crops grown either with a third or no late N fertilization. Natural aerial epidemics ensured a range of disease severity, and fungicide ensured disease-free control plots. The data set of Gooding et al. (2005, Journal of Agricultural Science 143: 503–518) was incorporated in order to enlarge the range of conditions.
Key Results
Post-anthesis N uptake accounted for a third of N yield whilst N remobilization accounted for two-thirds in all crops whether affected by diseases or not. However, variations in N yield were highly correlated with post-anthesis N uptake, more than with N remobilization, in diseased and also healthy crops. Furthermore, N remobilization did not significantly correlate with N yield in healthy crops. These findings matched data from studies using various wheat genotypes under various management and climatic conditions. Leaf area duration (LAD) accurately predicted N remobilization whether or not crops were diseased; in diseased crops, LAD also accurately predicted N uptake.
Conclusions
Under the experimental conditions, N yield variations were closely associated with post-anthesis N uptake in diseased but also in healthy crops. Understanding the respective roles of N uptake and N remobilization in the case of diseased and healthy crops holds the promise of better modelling of variations in N yield, and thus in GPC.
Key words: Triticum aestivum, Puccinia triticina, leaf rust, Mycosphaerella graminicola, Septoria tritici blotch, N uptake, N remobilization, N yield, Leaf area duration
INTRODUCTION
On international markets, grain protein concentration (GPC) is a key criterion of trade. Farmers must meet strict thresholds in order to sell their harvest profitably, or even to sell it at all (Dimmock and Gooding, 2002b). Being directly derived from the ratio of nitrogen (N) to dry mass (DM) in grain at harvest, GPC largely depends on N yield – the source–sink determination of which is far less understood than that of DM yield, even in healthy crops (Martre et al., 2003) let alone those affected by disease. N yield is derived from two pools of nitrogen: (1) N stored before anthesis and remobilized to the grain during grain filling; and (2) N taken up during grain filling. N remobilization from the vegetative parts accounts for the major part of final N in grains (Simpson et al., 1983), but Barbottin et al. (2005) have found N remobilization is not enough to explain variations in N yield; under their conditions, it also greatly depended on both N uptake and disease pressure during the grain-filling period. Thus, breeding wheat genotypes with a high potential for late N uptake is one way that is being studied with the aim of increasing GPC and N yield (Brancourt-Humel et al., 2003, 2005; Laperche, 2005). Using both genotype variability and physiological markers, Kichey et al. (2007) have recently shown that post-anthesis N uptake and GPC were highly correlated with nitrate reductase activity, which suggests the important role late N uptake may play in increasing GPC. An agronomic way to increase N yield and GPC – one that has been widely used in France over the past 10 years – is to apply a late fertilization around the time of heading (Hébrard, 1999). However, to our knowledge, the respective contributions of N remobilization and late N uptake to N yield variation in healthy crops has not been thoroughly investigated, either from a genetic or from an agronomic point of view.
Although efficient in many cases, late N fertilization has raised several questions about the susceptibility and/or the response of genotypes to diseases. First of all, does late N fertilization play a role in favouring diseases? It is well known that late N application commonly increases leaf N concentration, with contrasting effects on disease severity. Von Tiedemann (1996), Leitch and Jenkins (1995) and Solomon et al. (2003) observed that high leaf N concentration often increased epidemics in both biotrophic and necrotrophic fungi. In contrast, other authors (Talbot et al., 1997; Hoffland et al., 1999; Snoeijers et al., 2000) have observed that susceptibility to necrotrophic fungi was sometimes increased in the case of low N availability because it leads to weaker plants that are unable to defend themselves. Indeed, whether or not leaf N concentration plays a direct role in the severity of fungal epidemics at the field scale remains controversial. This discrepancy in findings may arise from the fact that, at the field level, early N fertilization modifies both leaf N concentration and crop structure (through tillering), such that their respective roles in disease severity are difficult to differentiate (Savary et al., 1995).
In turn, what effect do diseases have on N yield? Whereas extensive work has been carried out on foliar disease effects in terms of DM yield losses (Bastiaans, 1993; Shtienberg, 1990; Robert et al., 2004), foliar disease effects on N yield and GPC have received little attention. In a review summarizing data sets from the literature focusing on wheat leaf diseases, Dimmock and Gooding (2002b) proposed some general trends. In most cases, but not always, leaf rust (Puccinia tritici) would reduce GPC whereas STB (Septoria tritici blotch; Mycosphaerella graminicola) would either not affect or increase it. The authors also suggested that perhaps some of the discrepancy between experiments could be due to varying effects of wheat foliar diseases on both the capture and utilization of either carbon or nitrogen pools, i.e. either photosynthesis and carbohydrate reserves or N uptake and remobilization. No attempt was made to delve any deeper into the matter. Thus, the respective impact of disease severity on either N remobilization or post-anthesis N uptake that leads to N yield variations requires further investigation (Savary et al., 2006).
Based on these insights, we hypothesize that the lack of evidence of a direct link between disease and variations in GPC could arise from the fact that diseases may affect DM and N fluxes differently and thereby either increase, decrease, or stabilize GPC, mainly depending on environmental conditions. Advances have been made in the comprehension of losses in DM growth and yield. Following Robert et al. (2004) and Dimmock and Gooding (2002a), losses could arise from the decline in the green leaf area index (GLAI; m2 of green leaf per m2 of soil) and thus in leaf area duration (LAD). Foliar diseases have often been shown to decrease N remobilization to grain through N retention in the diseased plant parts (Verreet and Hoffmann, 1990; Bastiaans, 1993; Kremer and Hoffmann, 1993; Garry et al., 1996). In cereals, foliar diseases have been shown to reduce N uptake in the case of early epidemics occurring before flowering; however, late foliar diseases are rarely reported to affect N uptake (Verreet and Hoffmann, 1987; Bastiaans, 1993; Kremer and Hoffmann, 1993). Experiments carried out by Gooding et al. (2005) have suggested that late foliar diseases in wheat crops decrease N uptake, but the study did not examine the relative contribution of N uptake to N yield. Under French crop management practices, post-anthesis N uptake usually represents a small proportion of total N uptake (Girard, 1997; Barbottin et al., 2005); therefore, late foliar disease effects on post-anthesis N uptake has generally been thought to be negligible. However, as regards late nitrogen fertilization, this assumption may no longer hold because, in the case of late N fertilization under French conditions, post-anthesis N uptake rate can reach the same levels as those observed in the UK (1 kg N ha−1 d−1; Gooding et al., 2005).
The aim of the current study was to examine the respective roles that N remobilization and post-anthesis N uptake play in N yield variations due to late foliar diseases and, in particular, in the case of wheat crops benefiting or not from late N fertilization. Crops of the wheat variety ‘Soissons’ were grown either with or without a late N fertilization over 4 years of field experiments in Grignon, France. We relied on natural aerial epidemics of leaf rust and STB to create a range of disease severity. Each year a full fungicide protection program also ensured disease-free control plots. The data obtained by Gooding et al. (2005) in the UK were integrated into the study in order to enlarge the range of genotypic and environmental conditions, as well as to evaluate the robustness of our findings. In this paper we (1) assess the effects of leaf rust and STB epidemics on N remobilization and post-anthesis N uptake; (2) relate plant N fluxes to nitrogen yield in the grain; and (3) account for decreases in N fluxes by using LAD as a simple plant indicator. The insights provided by this approach in terms of the respective roles of N uptake and N remobilization in the response of N yield to late fertilization and diseases are discussed.
MATERIALS AND METHODS
Experimental design
Four field experiments were carried out at Grignon, 50 km west of Paris, France (INRA-EGC Station; 48°51′N, 1°57′E) from 1997 to 2004. The trials were part of a triennial crop succession typical of this region, e.g. maize–wheat–barley, a set-up that favours nitrogen management mainly through fertilization – the previous maize crop minimizing nitrogen supply from soil. The trials were carried out on a deep silt loam (>1 m deep), a typical Eutrochrept soil according to soil taxonomy. P and K were applied at sufficiently high levels to prevent them from being limiting factors. For the different years, all crops were sown at the rate of 250 plants m−2 between 20 and 22 October, close to the optimal sowing date at the experimental site. Plots (42 m2), containing nine 30-m long rows, were spaced 0·5 m apart. We relied on weather variations to create a range of epidemics, while full crop protection ensured disease-free control plots during wheat development in spring. The winter wheat ‘Soissons’ was chosen, firstly, because of its high susceptibility to both leaf rust and STB, and secondly, because of its potential for both high yield and GPC. The range of conditions was effectively enlarged by integrating the data set of Gooding et al. (2005), which contains full combinations of three genotypes over three experimental years under different patterns of N fertilization and with either full or no crop fungicide protection.
Treatments
Different experimental treatments were carried out in order to analyse interacting effects of climate as well as late N and fungicide applications on N fluxes in the wheat crop. Thus, a treatment is characterized by a combination of three factors as presented in Table 1, where (1) is the experimental year (4 years: 1997, 1999, 2001, 2004), (2) is late nitrogen fertilization (yes or no), and (3) is fungicide application (yes or no). The local climate at the site is similar to a typical semi-oceanic temperate one. Minimum and maximum air temperature (measured 2 m above the soil) as well as rainfall and global incident radiation were recorded and stored hourly at a local weather station less than 500 m from the experimental plots. Potential evapo-transpiration was calculated daily according to the Penman–Monteith equation (Monteith, 1965). Table 2 summarizes the average climatic conditions during the grain-filling period for each year. In order to avoid water stress during stem elongation and grain filling, additional water was supplied to crops when needed through a soil-irrigation system at ground level, which was triggered by tensiometers.
Table 1.
Summary of the different treatments used in the present study, and their abbreviated codes (as referred to in the text and in the figures). The initial letter indicates the year, the first number indicates high (1) or low (0) fertilization, and the second number indicates either fungicide-controlled (1) or diseased crops (0)
| Low N |
High N |
|||
|---|---|---|---|---|
| Year | No fungicide | Plus fungicide | No fungicide | Plus fungicide |
| 1996–1997 | A00 | A01 | A10 | A11 |
| 1998–1999 | B00 | B01 | B10 | B11 |
| 2000–2001 | C00 | C01 | C10 | C11 |
| 2003–2004 | D00 | D01 | D10 | D11 |
Table 2.
Crop and climate variables characterizing each experimental year during the grain-filling period
| 1997 (0–1)* | 1999 (0) | 1999 (1) | 2001 (0–1) | 2004 (0–1) | |
|---|---|---|---|---|---|
| Anthesis date | 25 May | 21 May | 26 May | 28 May | 27 May |
| Grain filling duration (d) | 48 | 46 | 45 | 44 | 46 |
| Cumulated global radiation (MJ) | 935 | 1002 | 993 | 975 | 933 |
| Mean daily temperature (°C; ± s.d.) | 16·0 (2·3) | 16·6 (2·6) | 17·0 (2·5) | 17·5 (3·3) | 16·7 (2·6) |
| Cumulated rainfall (mm) | 122 | 56·5 | 56·5 | 119·6 | 61·4 |
| Potential evapo-transpiration (mm) | 186 | 197·5 | 197·5 | 198·6 | 194·4 |
* High (1) and low (0) nitrogen fertilizations were applied each year as follows: Treatment 1 consisted each year in a total 24 g m−2 N supply in 3 applications: 6 g m−2 at GS 25; 10 g m−2 at GS 30; 8 g m−2 at GS 55, ** Treatment 0 consisted in the same two first N applications as treatment 1, and a third application at GS 55 of only 3 g m−2 (1996–1997), 0 g m−2 (1998–1999), 3 g m−2 (2000–2001), 0 g m−2 (2003–2004).
In order to limit soil-borne diseases, a fungicide (cyprodinyl; 60 mg m−2) was applied to every plot early on, at the beginning of stem elongation (GS 30). Fungicide-treated control plots were maintained as follows: epoxiconazole (Opus®, 12·5 mg m−2) was sprayed in mid-April, during early stem elongation (GS 31–32), and in May, when flag leaves were fully expanded (GS 39); cyproconazole (Alto®, 8 mg m−2) was sprayed in mid-June at mid-grain filling (GS 75). There were no differences in plant and spike density between healthy and diseased crops (data not shown).
The ‘high fertilization’ treatment reflected the climatic potential for yield usually associated with maximum disease development (Savary et al., 2006) in the given years. In this treatment, 24 g m−2 of nitrogen was supplied in three applications: 6 g m−2 in mid-March (GS 25); 10 g m−2 at the beginning of April (double-ridge or beginning of stem elongation, GS 30), and 8 g m−2 close to heading (GS 55). In the ‘low fertilization’ treatment, the last application was either omitted or limited to 3 g m−2. In both cases, plots were managed in order to achieve an identical potential yield of 9 t ha−1 with varying GPC.
As a result of year-to-year variation, as well as varying fertilization and fungicide applications, a range of epidemics representative of wheat crop conditions in northern France was obtained. All of this combined to provide a full cross-factor experimental set-up designed to analyse individual effects of year as well as fertilization and fungicide applications, plus interactions of these three factors. All experiments were laid out as a randomized block design with three field replicates per treatment. As these three field replicates never showed significant differences (P > 0·10; see below), they were regarded as statistical repetitions.
Plant sampling and assessments
Crop dry mass (DM) and green leaf area index (GLAI) per leaf layer were measured weekly from heading (mid-May) or anthesis (end of May) to physiological maturity (around 15 July) by collecting two plant samples (0·18 m2 each) from each plot. Thermal-time (base zero; °Cd) was calculated to in order to standardize the development from anthesis to maturity across years. This time period was chosen based on the fact that even though STB develops early (between tillering and heading), its long latency period allows three to four new leaves to fully develop before symptoms appear on an infected leaves. Thus, crop losses rarely occur before heading or even anthesis. In our experiments, no difference in crop growth between healthy and diseased crops was found at anthesis (data not shown). On each sampling date, main stems were separated from tillers. A sub-sample of 15 main stems was put aside, selected on the basis of an individual fresh weight being as close as possible to the average of the main stem fresh weight (± 5 %). The upper five leaf blades, stems plus leaf sheaths, as well as ears, were separated. The DM and N concentrations (Dumas, 1831) of each of the upper four leaves, dead lower leaves, stems plus sheaths, chaff, and grain were measured. To simplify the analysis, the DM and N content of stems plus sheaths and chaff were pooled because these organs showed the same behaviour; these are termed ‘stems plus chaff’. At harvest, grains were counted, and the weight of one thousand grains (WTG) and the nitrogen content of one thousand grains (NTG) were determined.
Apart from the main stem sub-sample described above, both main stems and tillers were separately oven-dried at 80 °C for 48 h and then weighed separately for spikes and vegetative parts. The DM ratio of each main stem sub-sample to the whole 0·36 m2 sample was calculated, and its average over time was used to extrapolate sub-sample data at the per m2 level. Comparisons between main stem and tiller samples indicated disease affected both of them identically (data not shown).
N uptake and net remobilization from vegetative organs were calculated as described in Ruske et al. (2003a):
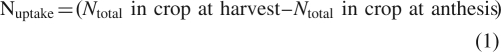 |
1 |
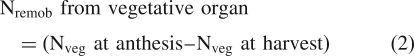 |
2 |
Assessment of disease severity, total and green leaf area
The severity of the two main diseases, leaf rust and STB, was estimated visually from heading to maturity. The standard Peterson's scale (Peterson et al., 1948) was used to assess leaf rust severity between 0 and 100 %. STB severity was directly recorded as the percentage of overall leaf area necrosis, including both sporulating and non-sporulating necrotic areas (Robert et al., 2004), minus the mean natural apical necrosis measured on the healthy crops. Leaf area index (LAI; leaf area m2 per soil m2) and green leaf area index (GLAI) were measured weekly on the sub-sample of 15 main stems per block and per treatment, selected as described above. The upper five leaf blades, when still green (the 5th leaf down from the top was dry at anthesis or very soon after), were colour-scanned separately. Total, green, apical-necrotic and infected areas per leaf layer were then measured by image analysis using the Optimas software (Media Cybernetics, Silver Spring, MD). In 1997, the scanned images of the 4th leaf blade down from the top were unusable, and the GLAI was slightly under-estimated that year. Total and per-leaf-layer GLAI were fitted to logistic curves and an integration from 0 to 1000 °Cd gave an accurate estimation of leaf area duration (LAD; °Cd m2 m−2) for the crop and the individual leaf layers.
In order to account for the differences in disease dynamics, the areas under the disease progressive curves (AUDPCs) for leaf rust and STB (including STB and induced-necrosis) were calculated for the varying leaves of the diseased crops from anthesis to maturity, according to Robert et al. (2004). For the calculation, we used the classical equation AUDPC(X) = Σj[(Xj + Xj+1) × (TTj+1 – TTj)/2] where X is either sporulating leaf rust severity or STB severity minus the apical senescence of healthy crops, and TTj is the thermal time (base zero) accumulated from anthesis till day j (°Cd). Following Dimmock and Gooding (2002a) and Ruske et al. (2003a), the LAD of the varying leaves was used to characterize the leaf rust and STB epidemics of each year.
Data analysis
Multiple analyses of variance were conducted using Statgraphics Plus (Manugistics, Inc., Maryland, USA) to examine the effects of treatments on disease severity and yield components, as well as N amount and concentration per organ. Individual effects of year, nitrogen and fungicide applications were analysed as well as the first level interactions, i.e. year × fertilization, year × fungicide, and fertilization × fungicide. Statistically significant differences were then determined using a Student Neuman–Keuls test. The overall error rate was α = 0·05. Means are given in the text followed by standard deviation (s.d.).
The contribution of N uptake and remobilization to N yield in grains was analysed by simple linear regression. Slopes and intercepts of regression lines between healthy and diseased crops were also compared using the specific Stagraphics Plus procedure. For the parameters of the linear regressions, s.d. and number of observations (n) are given in the text.
RESULTS
Effects of treatments on diseases and crops
Effects of treatments on disease severity
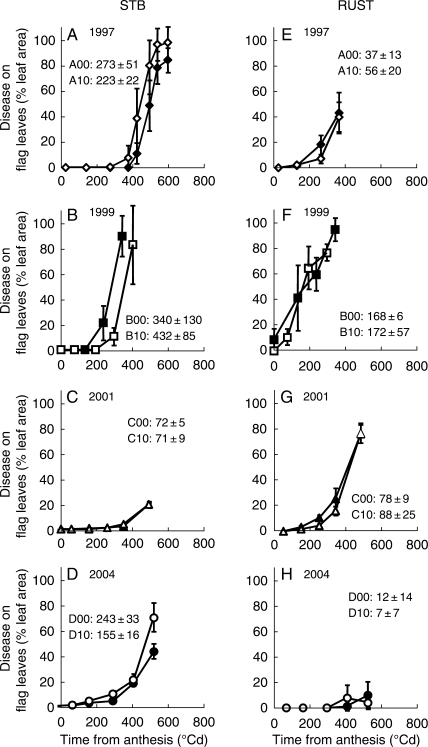
Figure 1 illustrates the epidemics of leaf STB and rust that occurred on wheat flag leaves in the study years. Epidemics differed in timing, with either early (1999), mid- (1997, 2001) or late-season (2004) outbreaks. Epidemics also differed as regards the combination of leaf rust and STB, with severities of either high leaf rust–high STB (1999), low leaf rust–low STB (2004), high leaf rust–low STB (2001) or low leaf rust–high STB (1997). During the grain-filling period the area under the disease progress curve (AUDPC) of leaf rust and STB (% disease °Cd) of leaves at the different leaf levels was used to analyse the overall effects of fertilization and year on the disease epidemics. Analysis of variance confirmed that ‘year’ induced highly significant variations of both leaf rust and STB AUDPC (P < 10−4; d.f. = 23). Late fertilization had no influence on either leaf rust or STB AUDPC (P > 0·05). There was no evidence of interactions between year and fertilization on leaf rust or STB AUDPC (P > 0·05).
Fig. 1.
(A–D) Septoria tritici blotch (STB) and (E–H) leaf rust epidemics on flag leaves for the years 1997 (A, E), 1999 (B, F), 2001 (C, G) and 2004 (D, H) for low (open symbols) and high (closed symbols) levels of late nitrogen fertilization. Each point is the mean of three replicates of main shoots and bars indicate s.d. Areas under the disease progressive curves (AUDPCs) are indicated for the individual treatments: see Table 1 for treatment codes.
As the two diseases were not present in fully crossed combinations among the years, we did not use these data sets to study the individual effects of leaf rust or STB on N concentration or fluxes in diseased crops. The combined effects of diseases were further analysed by examining the LAD variations they induced.
Effects of treatments on DM and N yield components
The effects of the different treatments on grain number and on DM and N yield components measured at harvest are presented in Table 3. The number of grains m−2 varied considerably and significantly with year in healthy crops (P < 10−3; d.f. = 23; data not shown); 1997 being significantly higher (treatment A11: 29 600 grains m−2) than the other three years [from 24 800 (C11) to 25 600 (G11) grains m−2]. No overall effect of fertilization or fungicide on grain number was observed; however, significant interactions between these factors and year were found (Table 3). Thus, a significant decrease in number of grains (P < 10−3) was only observed in diseased crops in 1999, which represented a 15 % loss in the case of this early and severe epidemic. Similarly, in 1997, late fertilization had a significant effect (P < 0·05), representing a 10 % gain in grain number. The dry weight of one thousand grains (WTG) ranged from 30 g to 41·6 g and from 22·9 g to 37·6 g in healthy and diseased crops, respectively, and showed a significant variation with year (P < 10−4; d.f. = 47) and with fungicide application (P < 10−4), but not with late fertilization (P > 0·05). Full fungicide protection significantly increased WTG by 8 g on average (range of increase 3–15 g). The N content of one thousand grains (NTG) showed variations with treatments similar to WTG, both in trends and in quantity, except that late fertilization did have a very significant effect on NTG (P < 10−4). In healthy crops, NTG ranged from 0·49 g in 1997 (A01: low N) to 0·86 g in 2004 (D11: high N). Fungicide application significantly increased NTG by 0·18 g on average, although there was a wide range (0·08–0·32 g). Lastly, late fertilization increased NTG by 0·07 g, within a much narrower range (0·05–0·09 g). A significant interaction between year and fungicide was found as expected for both WTG and NTG, mainly due to the large range of disease severity among years. In contrast, interactions between year and fertilization or between fertilization and fungicide were not detected (P > 0·05).
Table 3.
Effects of treatments on the components of grain yield and nitrogen yield: grain number per m2 (GN), weight of thousand grains (WTG), nitrogen of thousand grains (NTG), grain protein concentration (GPC)
| Treatment† | Gain number (m−2) | Weight of 1000 grains (g) | Nitrogen content of 1000 grains (g) | Grain protein concentration (%DM) |
|---|---|---|---|---|
| A00 | 25 402 | 26·4 | 0·47 | 10·2 |
| A01 | 26 674 | 30·0 | 0·49 | 9·5 |
| A10 | 27 555 | 24·7 | 0·49 | 11·4 |
| A11 | 29 641 | 31·5 | 0·62 | 11·3 |
| B00 | 21 841 | 23·8 | 0·41 | 9·9 |
| B01 | 25 336 | 38·4 | 0·68 | 10·2 |
| B10 | 22 335 | 22·9 | 0·41 | 10·4 |
| B11 | 25 441 | 38·6 | 0·78 | 11·6 |
| C00 | 28 060 | 32·4 | 0·55 | 9·8 |
| C01 | 24 848 | 39·6 | 0·76 | 11 |
| C10 | 25 557 | 34·8 | 0·65 | 10·7 |
| C11 | 25 112 | 41·6 | 0·85 | 11·7 |
| D00 | 25 869 | 37·3 | 0·67 | 10·4 |
| D01 | 25 636 | 39·4 | 0·79 | 11·5 |
| D10 | 25 930 | 37·6 | 0·76 | 11·7 |
| D11 | 25 308 | 41·2 | 0·86 | 11·9 |
| ANOVA | ||||
| Year (Y) | *** | *** | *** | *** |
| Nitrogen (N) | ns | ns | *** | *** |
| Fungicide (F) | ns | *** | *** | *** |
| Y × N | * | ns | ns | ns |
| Y × F | *** | *** | *** | *** |
| N × F | ns | ns | ns | ns |
† See Table 1 for treatment codes.
Multiple factor ANOVA was carried out fully combining effects of year, nitrogen and fungicide; first-order interactions between factors were examined. *, 0·01 < P < 0·05; **, 0·001 < P < 0·01; ***, P < 0·001; ns, non-significant effect.
Grain protein concentration (GPC) significantly varied with year in healthy crops (P < 10−3; d.f. = 23): from 9·5 %DM in 1997 (A01) to 11·9 %DM in 2004 (D11). It increased with fungicide application (P < 10−4; d.f. = 47) and late fertilization (P < 10−4; d.f. = 47), by 0·5 %DM and 1·0 %DM on average, respectively. An interaction between year and fungicide application was, however, observed: disease significantly decreased GPC (P < 0·05; d.f. = 11) in 1999, 2001 and 2004, but no significant effect was found in 1997 (P > 0·05; d.f. = 11). In contrast, interactions between year and fertilization or between fertilization and fungicide were not detected (P > 0·05).
Evolution of crop N pools in healthy and diseased crops
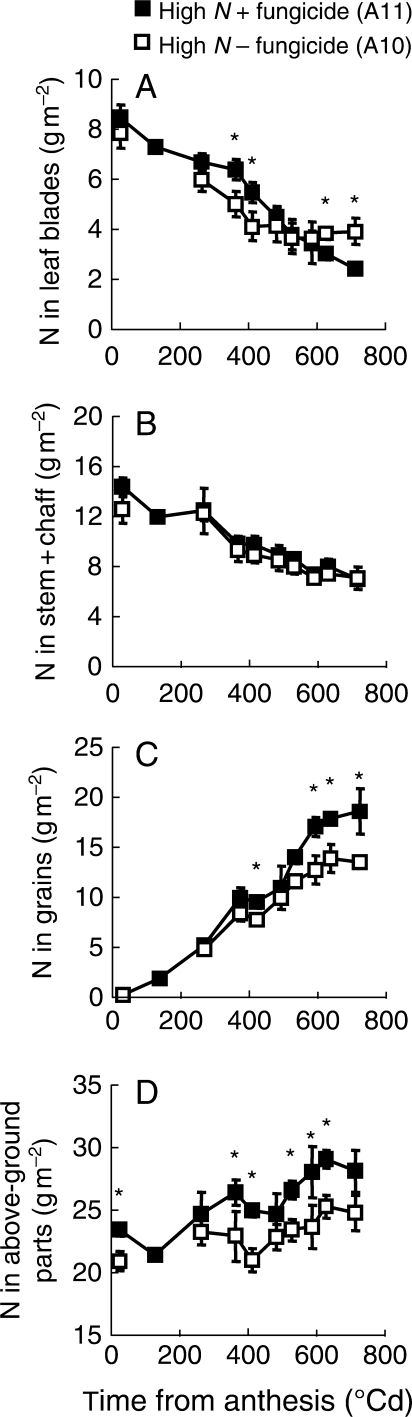
The changes in crop N pools during grain filling in healthy and diseased crops under high fertilization in 1997 serves as an example of how late leaf diseases affected some but not all N fluxes in the wheat crop (Fig. 2). The N pool of healthy leaves (Fig. 2A), starting at 8·5 ± 0·5 g m−2, decreased throughout the grain-filling period, the rate of decrease slowing down after 500 °Cd but not stabilizing until 700 °Cd, to reach 2·4 ± 0·2 g m−2 at the end of the grain-filling period. The N pool of diseased leaves was significantly different from the one of healthy leaves. It displayed a two-step evolution: when diseases increased (Fig. 1A, E), N flow out of diseased leaves first accelerated (from 150 °Cd until 400 °Cd after anthesis in treatment A0) and then suddenly stopped. At maturity, the N pool of diseased leaves reached 3·9 ± 0·5 g m−2, a significantly higher value than that of healthy ones (P < 10−4; d.f. = 47). N remobilization from healthy stems plus chaff (Fig.2B) was within the same magnitude as that from the leaves, despite the fact the N pool was bigger in stems plus chaff than in leaves throughout grain filling. Starting at 14·5 ± 0·8 g m−2, the N pool in stems plus chaff first remained steady for 100–200 °Cd and then declined until about 600 °Cd, stabilizing progressively thereafter and declining to 7·1 ± 0·4 g m−2 in treatment A1. In contrast to other plant parts, N fluxes out of stems plus chaff were apparently not affected by the diseases (Fig. 2B). N yield in the grain (Fig. 2C) increased until 600 °Cd post-anthesis and remained steady thereafter at 18·1 ± 0·4 g m−2 in treatment A1. N yield in the grain of diseased crops (Fig. 2C) was lower than that of healthy crops quite early on, but the difference only became significant later (from 525 °Cd to maturity in treatment A0). N of total above-ground plant parts (Fig. 2D) increased slightly during grain filling in the healthy crops, from 23·6 ± 0·5 g m−2 to 28·3 ± 1·7 g m−2 in treatment A1. In diseased crops (Fig. 2D), N always stopped accumulating in above-ground plant parts earlier than in healthy crops. Despite the fact that significant differences were not always detected, generally speaking, diseases decreased N accumulation in aerial parts.
Fig. 2.
Variation of nitrogen in (A) leaf blades, (B) stems plus chaff, (C) grain and (D) above-ground parts from anthesis to maturity in treatments A11 and A10. Data were extrapolated from sampling three replicates of average main shoots (see Methods). A point-by-point ANOVA was carried out for each curve and asterisks indicate a significant difference at the 5 % level (Newmann–Keuls test).
Changes in N content were similar for all organs for a given year, and these were used to calculate N balance between anthesis and maturity (Table 4). An analysis of variance was first carried out for healthy crops (d.f. = 23; data not shown) in order to explore the effects of year and late fertilization on the different N pools. In healthy crops, across all years N yield ranged from 13–21 g m−2, depending on both year (P < 10−3) and fertilization (P < 10−3). N remobilization by healthy leaves ranged from 5·5–6·5 g m−2 without any effect of year or fertilization (P > 0·05), while N remobilization by stems plus chaff ranged from 5·1–7·4 g m−2 with significant effects of year (P < 0·01), fertilization (P < 0·05) and interaction between these factors (P < 0·05), as fertilization significantly increased N remobilization only in 1997 and 1999. High variation in N uptake was noted: from 2·3 to 10·3 g m−2, with both year (P < 10−4) and fertilization effects (P < 0·01), but without any interaction between factors. The classification of the years was the same for both N yield and N uptake: 1997 ≤ 1999 ≤ 2001 ≤ 2004. However, the classification of years was different for N remobilization of stems plus chaff, and of vegetative parts: 2001 ≤ 2004 = 1997 ≤ 1999.
Table 4.
Effects of treatments on N balance (ΔN; g m−2) between anthesis and maturity for the main plant parts: grains (g), leaves (l), stems plus chaff (s), vegetative parts (v) and aerial parts (a).
| Treatment† | ΔNg | ΔNl | ΔNs | ΔNv | ΔNa |
|---|---|---|---|---|---|
| A00 | 12·13 | −3·79 | −4·64 | −8·43 | 3·71 |
| A01 | 12·98 | −5·48 | −5·21 | −10·70 | 2·29 |
| A10 | 13·22 | −3·95 | −5·51 | −9·46 | 3·76 |
| A11 | 18·16 | −6·09 | −7·43 | −13·52 | 4·64 |
| B00 | 8·61 | −2·87 | −5·34 | −8·21 | 0·40 |
| B01 | 16·77 | −5·95 | −6·42 | −12·37 | 4·40 |
| B10 | 9·29 | −3·10 | −5·54 | −8·64 | 0·64 |
| B11 | 19·15 | −6·39 | −7·34 | −13·73 | 5·42 |
| C00 | 15·74 | −4·85 | −6·29 | −11·14 | 4·59 |
| C01 | 19·17 | −5·67 | −5·34 | −11·01 | 8·16 |
| C10 | 17·04 | −3·90 | −6·47 | −10·37 | 6·67 |
| C11 | 20·97 | −5·63 | −5·06 | −10·69 | 10·28 |
| D00 | 17·61 | −5·03 | −5·73 | −10·76 | 6·85 |
| D01 | 19·00 | −6·47 | −6·18 | −12·66 | 6·34 |
| D10 | 19·86 | −5·75 | −6·83 | −12·58 | 7·28 |
| D11 | 20·82 | −5·82 | −5·98 | −11·80 | 9·02 |
| ANOVA | |||||
| Year (Y) | *** | *** | ns | * | *** |
| Nitrogen (N) | *** | ns | ** | * | ** |
| Fungicide (F) | *** | *** | ns | *** | *** |
| Y × N | ns | ns | ns | ns | ns |
| Y × F | *** | *** | *** | *** | *** |
| N × F | ns | ns | ns | ns | ns |
† See Table 1 for treatment codes.
Multiple factor ANOVA was carried out fully combining effects of year, nitrogen and fungicide; first-order interactions between factors were examined. *, 0·01 < P < 0·05; **, 0·001 < P < 0·01; ***, P < 0·001; ns, non-significant effect.
An analysis of variance was carried out to compare N balance per organ between anthesis and maturity in healthy and diseased crops. Considering all years, late leaf diseases strongly affected N yield (P < 10−4; d.f. = 47), resulting in losses of 0·8–9·9 g m−2, while N remobilization from leaves decreased by 0·1–3·3 g m−2 (P < 10−4). Variations in N uptake due to diseases (P < 10−4) ranged from a decrease of 4·8 g m−2 to an increase of 1·4 g m−2 (not significantly different from zero). However, diseases did not lead to a significant effect on N remobilization of stems plus chaff (P > 0·05; Table 4). A systematic interaction between year and fungicide application was revealed (P < 0·01), reflecting differences in disease severity between years. Interactions between fertilization and fungicide applications were not found to be significant (P > 0·05), and the interaction between fertilization and year was not significant (P > 0·05) when both healthy and diseased crops were considered.
Considered all years over time, diseased leaves always displayed a loss of N before healthy leaves. The time for maximum N loss in diseased leaves varied between 300 °Cd and 420 °Cd depending on year, but not on late fertilization. Analysis of variance at the time of maximum N loss indicated that leaf N in diseased crops was 0·7 g m−2 less than in healthy crops (P < 10−3). However, at the same time, N yield in diseased crops was 0·4 g m−2 less than in healthy crops (P < 0·05). When early loss of N occurred in diseased leaves, there was no accompanying temporary increase in N grain filling.
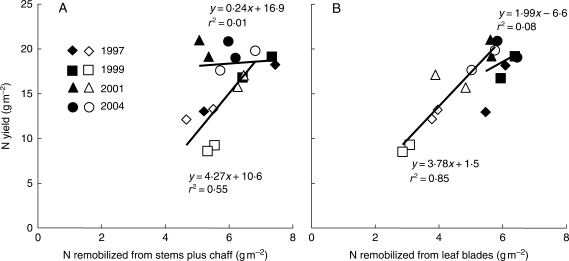
Relating N yield in the grain to plant N fluxes
N yield in relation to N pools from remobilization and post-anthesis N uptake are shown for all years in Fig. 3. In healthy crops, no matter which plant organ was considered, no significant relationship appeared between N remobilization and N yield (r2 < 0·08; P > 0·05). In diseased crops, N remobilized by stems plus chaff (Fig. 3A) was generally related to N yield (r2 = 0·55; P < 0·05; d.f. = 6), with a quite clear year effect due to very early and very strong 1999 epidemics: a loss in N remobilized from stems plus chaff accompanied the loss in N yield. In contrast, for all years, N remobilization from leaves (Fig. 3B) was highly correlated to N yield in diseased crops (r2 = 0·85; P < 10−3). The regression line between N yield and N remobilization from diseased leaves showed an intercept not significantly different from 0 (P > 0·05) and a slope of 3·8 ± 1·6 (n = 8), which was significantly higher than 1 (P < 0·01).
Fig. 3.
Correlation with N yield of N remobilized from stems plus chaff (A) or leaf blades (B) during grain filling. Each point is the mean of three replicates of main shoots extrapolated to the crop level. Years are as indicated; filled symbols represent fungicide-treated crops and open symbols represent diseased crops. Duplicate symbols represent the data for the two late nitrogen fertilization treatments that were applied for each year/disease–control combination.
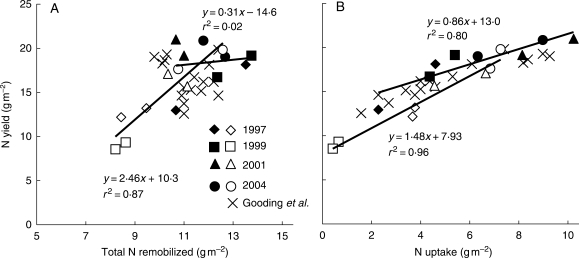
Total N remobilization (leaves, stems and chaff) from diseased crops (Fig. 4A) was highly correlated with N yield (r2 = 0·87; P < 10−3). The regression line with N yield showed a slope of 2·5 ± 0·9 (n = 8), which was significantly higher than 1 (P < 0·01), and an intercept significantly lower than 0 (–10·2 ± 9·3; n = 8; P < 0·05), thus suggesting that N yield would have been zero had the remobilization been below 4·2 g m−2. The correlation between N yield and post-anthesis N uptake (Fig. 4B) was highly significant in healthy (r2 = 0·80; P < 0·01; d.f. = 6) and diseased (r2 = 0·96; P < 10−4) crops; in all cases, slopes were significantly different from 0 (P < 10−3). In both cases, the intercepts were significantly higher than 0 (P < 10−4), thus suggesting that N yield would have remained positive if no post-anthesis N uptake had occurred, something which actually did happen in 1999 for diseased crops. The intercept was significantly higher in healthy crops, with values of 12·9 ± 3·1 g m−2 compared with 7·9 ± 1·6 g m−2 for diseased crops (n = 8). Furthermore, slopes significantly differed between the healthy and diseased data sets (P < 0·01). In diseased crops, the slope was 1·5 ± 0·3 (n = 8), significantly higher than 1 (P < 0·01); however, in healthy crops it was 0·9 ± 0·4 (n = 8) and thus not significantly different from 1 (P > 0·05).
Fig. 4.
Correlation with N yield of total N remobilization (A) and N uptake (B) during grain filling. Years are as indicated; filled symbols represent fungicide-treated crops and open symbols represent diseased crops. Crosses represent data recalculated from Gooding et al. (2005).
Figure 4A is recalculated from the findings of Gooding et al. (2005). For both diseased and healthy crops, their data indicated no significant correlation between N yield and N remobilization (r2 = 0·05; d.f. = 14), even when diseased and healthy crops data sets were separated (r2 = 0·38; P > 0·05; d.f. = 6). The findings involving healthy crops in the present experiment matched the findings of Gooding et al. (2005) for both diseased and healthy crops. In contrast, in both cases, the relation between N uptake and N yield was highly significant (r2 = 0·89; d.f. = 14) and revealed no differences, either in the slopes (r2 = 0·82; P = 0·81; d.f. = 14) or in the intercepts (12·2 g m−2; P = 0·45; d.f. = 14). The results involving healthy crops in the present experiment therefore matched the results of Gooding et al. (2005), but we observed a specific relationship between N yield and either N uptake or remobilization in the case of diseased crops while Gooding et al. (2005) did not.
Relating N fluxes to LAD
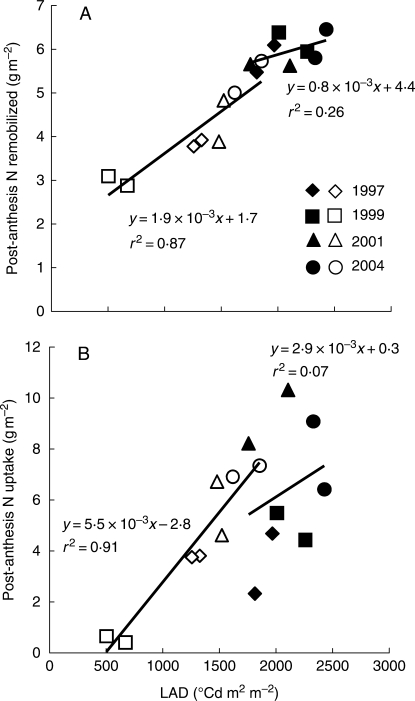
Because leaf area duration (LAD) has been identified as a good indicator for crop losses in terms of dry matter (DM), its role was investigated as an indicator for individual N fluxes that feed the grains. LAD was related to final N remobilization from leaves (Fig. 5A) and to post-anthesis N uptake (Fig. 5B) in healthy and diseased crops. In healthy crops, the LAD range varied only narrowly from 1800 to 2400 °Cd m2 m−2, whereas it was clearly reduced and much more variable in diseased crops, from 500 to 1900 °Cd m2 m−2. The LAD of healthy crops was never significantly related to either N remobilization from leaves (r2 = 0·27; P > 0·05; d.f. = 6) or post-anthesis N uptake (r2 = 0·15; P > 0·05). In contrast, the LAD of diseased crops was significantly related to both N remobilization from leaves (r2 = 0·83; P < 0·01) and post-anthesis N uptake (r2 = 0·91; P < 0·01). The regression line between N remobilization from leaves and LAD of diseased crops showed a positive intercept value significantly different from zero (P < 10−3), thus suggesting even if LAD had been reduced to zero, as much as 1·7 ± 1·2 g m−2 (n = 8) would nevertheless have been remobilized from the leaves. Otherwise, the regression line between post-anthesis N uptake and LAD of diseased crops showed a significantly (P < 0·01) negative intercept of –2·8 ± 2·4 g m−2 (n = 8): N uptake became zero when LAD was equal to or below 504 °Cd m2 m−2. The slopes of these two regression lines were significantly different (P < 10−3): the relationship between LAD and N uptake revealed a slope about three times greater than the one found between LAD and N remobilization from leaves (5·5 ± 1·7 vs. 1·9 ± 0·7 mg °Cd−1 m−2; n = 8).
Fig. 5.
Correlations of total leaf area duration (LAD) from anthesis to maturity (as an indicator of leaf disease intensity) with N fluxes in the crops: (A) post-anthesis nitrogen remobilization from leaf blades and (B) post-anthesis nitrogen uptake by the crop. Years are as indicated; filled symbols represent fungicide-treated crops and open symbols represent diseased crops.
Interestingly, the regression lines for LAD and N remobilization by leaves in both healthy and diseased crops were not statistically different (P > 0·05): in fact, the regression line for healthy crops could be considered as a continuation of the one for diseased crops. This did not hold for N uptake: when the relationship between LAD and N uptake in healthy crops was compared to that in diseased crops, in most cases, N uptake in the former case was lower than in the latter one.
DISCUSSION
In this study, a range of wheat-leaf rust and blotch epidemics, varying in severity and timing due to different yearly effects of climate, was used to assess their global effect on N remobilization and post-anthesis N uptake in comparison to wheat crops in which diseases were controlled. Whatever the amount of late N fertilization, when crossed with a range of diseases (mainly leaf Septoria tritici blotch, STB) a decrease in grain protein concentration (GPC) was observed most of the time, which somewhat contradicts previous trends (Dimmock and Gooding, 2002b; Gooding et al., 2005) and suggests that late N fertilization applications may well shift that trend. More generally speaking, variations between N uptake and N yield remained highly correlated whether or not the crop suffered from late foliar diseases. The dynamics in GLAI that diseases induced, accounted for by measured LAD, was a good indicator of the decrease in both leaf N remobilization and post-anthesis N uptake. LAD might thus be a valuable predictor of N yield in diseased crops, despite the fact it is not in the case in healthy crops.
Effects of treatments on disease severity and crop functioning
Year and fungicide application had a significant effect on disease severity; however, late N fertilization did not affect disease severity. Furthermore, there was no interaction between the effects of late N fertilization and either year or fungicide application. Moreover, the results show that whether or not there was a late N fertilization, the dynamics of disease severity were the same; thus, in this case, late N fertilization did not increase crop susceptibility to leaf rust and STB.
As is generally the case, late N fertilization in this experiment had no effect on crop structure, while analyses of variance indicated that in both healthy and diseased crops late N fertilization significantly increased N content in the leaves at anthesis. Late N fertilization only slightly modified N remobilization, whilst it significantly increased post-anthesis N uptake. Late N fertilization also increased LAD, N yield and GPC; however, in no case was a significant interaction between the effects of N fertilization and fungicide application observed.
Contribution to N yield of N remobilization from vegetative parts in healthy and diseased crops
In healthy crops, N remobilization from the vegetative parts was on average 12·1 ± 1·5 g m−2, thus contributing 67 ± 11 % to N yield and falling within the usual range as reported in the literature (Cox et al., 1985; Barbottin et al., 2005). Few variations in this ratio were observed with differing years; the experimental design was set up to highlight the effects of late N fertilization and therefore did not allow us to specify variations in crop N at anthesis.
Leaf rust and STB induced a decrease in total N remobilization that ranged from 0 to 35 % of N yield in the experiments, which is much wider than the 0–12 % range found by Gooding et al. (2005). Except in the case of the very early and very strong 1999 epidemics, N remobilization from stems plus chaff was not affected by late leaf diseases, which agrees with the findings of Gooding et al. (2005) for wheat crops affected by the same two diseases. This was also the case in pea crops affected by Ascochyta blight (Garry et al., 1996). Conversely, Bastiaans (1993) found a lower N remobilization from stems in rice crops affected by leaf blast; but leaf blast was also shown to obstruct leaf vessels, suggesting a fungal expansion within the tissues that could reach the stem. To our knowledge, this has never been demonstrated in the case of either wheat-leaf rust or STB. On the other hand, N remobilization ceased early on in diseased leaves, leading to N retention in these tissues that was not compensated for by an increased remobilization from the stems plus chaff. It has previously been hypothesized that, for healthy crops grown under experimental conditions or using models, once N remobilization is initiated it might be regulated by current N availability in varyious plant parts, and not by the sink demand of grains. Feller (1979) and MacKown and van Sanford (1988) have shown that N remobilization and associated proteolytic activities in leaves were only slightly affected by spike excision, suggesting sink strength affected C more than N fluxes. Jamieson and Semenov (2000) and Martre et al. (2003) have also shown that N grain filling was more accurately modelled when calculated from N availability in vegetative plant parts rather than from grain N demand. In light of these other findings, the fact that in our case diseased crops behaved like healthy ones leads us to consider that N remobilization in diseased plants was not driven by sink demand but rather by N availability in the still-living plant parts.
Being the largest N pool for grain filling, N remobilization has often been regarded as the main process responsible for variations in N yield. Crops affected by late foliar diseases are therefore supposed to follow a similar pattern: many studies involving different pathosystems have revealed N retention in diseased organs, the extent of which depends on both the disease severity and timing (Verreet and Hoffmann, 1987; Bastiaans, 1993; Kremer and Hoffmann, 1993; Leitch and Jenkins, 1995; Garry et al., 1996; Barbottin et al., 2005). Using a large range of genotype × environment combinations, Barbottin et al. (2005) have shown that N remobilization efficiency, i.e. the proportion of vegetative N at anthesis remobilized to grains, was decreased by both high post-anthesis N uptake and high disease severity during grain filling in susceptible genotypes. In healthy crops, the results from the current experimental field set-up followed this general trend: there were moderate variations in N remobilization without any correlation to N yield irrespective of the particular vegetative parts of the plant considered. But in diseased crops, a highly significant correlation was found between N yield and N remobilization from the shoot; this explained as much as 85 % of N yield variations. Nevertheless, its slope was significantly greater than 1, suggesting that another source of N was used to fill the grains, a source which was affected by disease in a way similar to N remobilization. A significant and positive correlation (r2 = 0·70; P < 0·01; d.f. = 6) was found between total N remobilization and N uptake in diseased but not in healthy crops (r2 = 0·10; P >0·1; d.f. = 6).
Is post-anthesis N uptake closely associated with variations in N yield?
The second N pool that supplies grains, post-anthesis N uptake from soil, was 6·3 ± 2·8 g m−2 in the healthy crops (roughly half the value for N remobilization) but it had a much higher variability. Thus, coefficients of variation (CV) reaching 45 and 12 % were found in healthy crops for N uptake and N remobilization, respectively; this variability was increased in diseased crops, with CV values reaching 66 and 17 %, respectively. Unlike N remobilization, N uptake varied significantly not only with disease but also with year and N fertilization in both healthy and diseased crops. Furthermore, the variability of N yield was strongly related to that of N uptake in both diseased and healthy crops. In healthy crops, the slope was close to 1, highlighting that under our fertilizing regime most variations in N yield were closely linked to post-anthesis N uptake. In diseased crops, the correlation was even stronger but the slope was higher than 1, reflecting the above-mentioned impact of disease on N remobilization. In both cases, intercepts were significantly different from zero, and close to the average total N remobilization for healthy crops. Interestingly, working with data sets from Gooding et al. (2005) produced similar results: in their study, disease affected N uptake more than N remobilization in three wheat cultivars over 3 years under quite different environmental and genotypic conditions. Furthermore, still using their data sets, we found a similar strong correlation between N uptake and N yield, which leads us to consider that our findings might apply under more general conditions. The most important difference is that, in their case, the slope from N yield to N uptake was close to 1 in healthy as well as diseased crops, because no disease effect on N remobilization was found. This discrepancy needs further investigation.
Does LAD accurately predict both N uptake and remobilization in healthy and diseased plants?
In the literature, LAD has often been shown to correlate strongly with disease severity (Sadras et al., 2000; Ruske et al., 2003b) and it has been proposed as a useful means to account for the effects of multiple foliar attacks on yield (Gaunt, 1995; Dimmock and Gooding, 2002a; Robert et al., 2004; Bancal et al., 2007). Both yield and N yield have been shown to increase linearly with FLAD (LAD of the flag leaf), but the correlation between FLAD and GPC was found to vary with either genotype or environment, or disease severity (Ruske et al., 2003a); thus its use as a predictor of GPC cannot be applied to other sites and genotypes. Under our conditions, LAD correlated slightly better with both N yield and GPC than FLAD did. However, the results also suggest that neither FLAD nor LAD are good direct indicators of GPC as the decrease in LAD affects post-anthesis N uptake more than N remobilization. This may explain how LAD relates to N yield and GPC differently, depending on environment or genotype.
Under our experimental conditions, distinguishing between the two N fluxes reveals the possibility of extending the correlation relating LAD with N remobilization from diseased to healthy crops with a high degree of significance and without any significant bias. This result suggests that LAD may be a means to accurately predict N remobilization from leaves, diseased or not, at least in the case of the variety ‘Soissons’ (RMSE = 0·5 g m−2). Its extension to other genotypes needs further investigation, as suggested by the results of Dimmock and Gooding (2002b) and Ruske et al. (2002b).
Perhaps surprisingly, LAD in the current study was also closely related to N uptake in diseased crops but not in healthy crops, suggesting post-anthesis N uptake in healthy crops was limited by other factors, e.g. N soil availability. In the case of diseased crops, the tight relationship between LAD and N uptake suggests that LAD is the main limiting factor. Because N uptake requires energy, it it is possible that its correlation with LAD could indicate that absorbed PAR (PARa) drove N uptake. We therefore calculated PARa in the crops according to Bancal et al. (2007), and checked to assess whether or not it correlated with N uptake. However, the value of r2 obtained was only 0·85, clearly less than r2 = 0·91 obtained in the case of N uptake and LAD. This suggests that in the calculation of PARa variables or parameters other than LAD not yet taken into account may vary with disease. The relationship between N uptake and LAD showed a negative intercept, which may be an indication of the necessity for a minimum LAD for nitrate absorption. On the other hand, N remobilization by leaves looked as though it still continued after leaves died; this tended to match the systematic increase in GPC at the end of grain filling (Gooding et al., 2005). Once again, this points to the fact that LAD, in terms of post-anthesis N uptake, operates differently from LAD in terms of N remobilization.
CONCLUSIONS
This study has shown that under the experimental conditions applied, late foliar diseases decreased N yield through variations in N uptake rather than N remobilization during grain filling. Even though post-anthesis N uptake only accounted for a third of N yield when compared with N remobilization, N yield variations were closely associated with N uptake variations in the case of diseased and also healthy crops. N remobilization by leaves and vegetative parts contributed less to N yield variations, and only in the case of diseased crops. Given that our findings match those based on other conditions (Gooding et al., 2005), we suggest it is possible to relate N yield to post-anthesis N uptake more generally. We also suggest LAD is a possible indicator of the effect of leaf rust and STB in combination on both N remobilization and post-anthesis N uptake. Differentiating between the role of N uptake and N remobilization holds the promise of better modelling of variations in N yield, and thus GPC.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge J. Troizier and his team for technical help, J. Jean-Jacques, B. Le Fouillen and F. Lafouge for their work in processing the plant samples and in their analysis, and P. Belluomo for developing the adapted programs for image analysis. We are very grateful to J.-F. Castell, O. Bethenod and D. Mirallès for fruitful scientific discussions. We also thank S. Tanis-Plant for discussions and thorough editorial advice with regard to the use of English. This study was funded by the Institut National de la Recherche Agronomique (INRA).
LITERATURE CITED
- Bancal M-O, Robert C, Ney B. Accounting for wheat crop growth and yield losses by accelerated green leaf layer losses due to late leaf rust and blotch epidemics. Annals of Botany. 2007;100:777–789. doi: 10.1093/aob/mcm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbottin A, Lecompte C, Bouchard C, Jeuffroy MH. Nitrogen remobilization during grain filling in wheat: genotypic and environmental effects. Crop Science. 2005;45:1141–1150. [Google Scholar]
- Bastiaans L. Effects of leaf blast on growth and production of a rice crop. 1. Determining the mechanism of yield reduction. Netherland Journal of Plant Pathology. 1993;99:323–334. [Google Scholar]
- Brancourt-Hulmel M, Doussinault G, Lecomte C, Bérard P, LeBuanec B, Trottet M. Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Science. 2003;43:37–45. [Google Scholar]
- Brancourt-Hulmel M, Heumez E, Pluchard P, Beghin D, Depatureaux C, Giraud A, Le Gouis J. Indirect versus direct selection of winter wheat for low input or high input levels. Crop Science. 2005;45:1427–1431. [Google Scholar]
- Cox MC, Qualset CO, Rains DW. Genetic variation for nitrogen assimilation and translocation in wheat. II. Nitrogen assimilation in relation to grain yield and protein. Crop Science. 1985;25:435–440. [Google Scholar]
- Dimmock JPRE, Gooding MJ. The effect of fungicides on the rate and duration of grain filling in relation to the maintenance of flag leaf green area. Journal of Agricultural Science. 2002;a 138:1–16. [Google Scholar]
- Dimmock JPRE, Gooding MJ. The influence of foliar diseases, and their control by fungicide, on protein concentration in wheat grain: a review. Journal of Agricultural Science. 2002;b 138:349–366. [Google Scholar]
- Dumas JBA. Procédés de l'analyse organique. Annales de Chimie et de Physique. 1831;247:198–213. [Google Scholar]
- Feller U. Effect of changed source/sink relations on proteolytic activities and on nitrogen mobilization in field-grown wheat (Triticum aestivum L.) Plant and Cell Physiology. 1979;20:1577–1583. [Google Scholar]
- Garry G, Tivoli B, Jeuffroy MH, Citharel J. Effects of Ascochyta blight by Mycosphaerella pinodes on the translocation of carbohydrates and nitrogenous compounds from the leaf and hull to the seed of dried-pea. Plant Pathology. 1996;45:769–777. [Google Scholar]
- Gaunt RE. The relationship between plant disease severity and yield. Annual Review of Plant Physiology and Molecular Biology. 1995;33:119–144. doi: 10.1146/annurev.py.33.090195.001003. [DOI] [PubMed] [Google Scholar]
- Girard ML. France: Institut National Agronomique Paris-Grignon; 1997. Modélisation de l'accumulation de biomasse et d'azote dans les grains de blé tender d'hiver (Triticum aestivum L.); simulation de leur teneur en protéines à la récolte. PhD Thesis. [Google Scholar]
- Gooding MJ, Gregory PJ, Ford KE, Pepler S. Fungicide and cultivar affect post-anthesis patterns of nitrogen uptake, remobilization and utilization efficiency in wheat. Journal of Agricultural Science. 2005;143:503–518. [Google Scholar]
- Hébrard JP. Azote et qualité des blés: fractionner pour plus de protéines. Perspectives Agricoles. 1999;244:84. [Google Scholar]
- Hoffland E, van Beusichem ML, Jeger MJ. Nitrogen availability and susceptibility of tomato leaves to Botrytis cinerea. Plant Soil. 1999;210:263–272. [Google Scholar]
- Jamieson PD, Semenov MA. Modelling nitrogen uptake and redistribution in wheat. Field Crops Research. 2000;68:21–29. [Google Scholar]
- Kichey T, Hirel B, Heumez E, Dubois F, Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilization to the grain correlates with agronomic and nitrogen physiological markers. Field Crop Research. 2007;102:22–32. [Google Scholar]
- Kremer M, Hoffmann GM. Effekte von Blattinfektionen durch Drechlera tritici-repentis auf den Kohlenhydrat- und Stickstoffhaushalt von Weizenpflanzen. Journal of Plant Diseases and Protection. 1993;100:259–277. [Google Scholar]
- Laperche A. Etude du déterminisme génétique de la tolerance à une carence en azote chez le blé tender d'hiver. France: Université de Technologie de Compiègne; 2005. PhD Thesis. [Google Scholar]
- Leitch MH, Jenkins PD. Influence of nitrogen on the development of Septoria epidemics in winter wheat. Journal of Agricultural Science. 1995;124:361–368. [Google Scholar]
- MacKown CT, van Sanford DA. Nitrogen allocation with altered sink demand in wheat. Crop Science. 1988;28:133–136. [Google Scholar]
- Martre P, Porter JR, Jamieson PD, Triboï E. Modeling grain nitrogen accumulation and protein composition to understand sink/source regulations of nitrogen remobilization for wheat. Plant Physiology. 2003;133 doi: 10.1104/pp.103.030585. 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith JL. Evaporation and environment. Symposium of the Society for Experimental Biology. 1965;19:205–234. [PubMed] [Google Scholar]
- Peterson RF, Campbell AB, Hannah AE. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Canadian Journal of Research. 1948;26:496–500. [Google Scholar]
- Robert C, Bancal M-O, Nicolas P, Lannou C, Ney B. Analysis and modelling effects of leaf rust and Septoria tritici blotch on wheat growth. Journal of Experimental Botany. 2004;55:1079–1094. doi: 10.1093/jxb/erh108. [DOI] [PubMed] [Google Scholar]
- Ruske RE, Gooding MJ, Jones SA. The effects of triazole and strobilurin fungicide on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. Journal of Agricultural Science. 2003;a 140:395–407. [Google Scholar]
- Ruske RE, Gooding MJ, Jones SA. The effects of adding picoxystrobine, azoxystrobine and nitrogen to a triazole programme on disease control, flag leaf senescence, yield and grain quality of winter wheat. Crop Protection. 2003;b 22:975–987. [Google Scholar]
- Sadras VO, Quiroz F, Echarte L, Escande A, Pereyra VR. Effect of Verticillium dalhiae on photosynthesis, leaf expansion and senescence of field-grown sunflower. Annals of Botany. 2000;86:1007–1015. [Google Scholar]
- Savary S, Castilla NP, Elazegui FA, Mc Laren CG, Ynalvez MA, Teng PS. Direct and indirect effects of nitrogen supply and disease source structure on rice sheath blight spread. Phytopathology. 1995;85:959–965. [Google Scholar]
- Savary S, Teng PS, Willocquet L, Nutter FW. Quantification and modelling crop losses: a review of purposes. Annual Review of Phytopathology. 2006;44:89–112. doi: 10.1146/annurev.phyto.44.070505.143342. [DOI] [PubMed] [Google Scholar]
- Shtienberg D, Bergeron SN, Nicholson AG, Fry WE, Ewing EE. Development and evaluation of a general model for yield loss assessment in potatoes. Phytopathology. 1990;80:466–472. [Google Scholar]
- Simpson RH, Lambers H, Dalling MJ. Nitrogen redistribution during grain growth in wheat (Triticum aestivum L.) Plant Physiology. 1983;71:7–14. doi: 10.1104/pp.71.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeijers SS, Perez-Garcia A, Joosten MHAJ, De Wit JGM. The effect of nitrogen on disease development and gene expression in bacterial and fungal plant pathogens. European Journal of Plant Pathology. 2000;106:493–506. [Google Scholar]
- Solomon PS, Tan KC, Oliver RP. The nutrient supply of pathogenic fungi; a fertile field for study. Molecular Plant Pathology. 2003;4:203–210. doi: 10.1046/j.1364-3703.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- Talbot NJ, McCafferty HPK, Ma M, Moore K, Hamer JE. Nitrogen starvation of rice blast fungus Magnaporthe grisea may act as an environmental cue for disease symptom expression. Physiological and Molecular Plant Pathology. 1997;50:179–195. [Google Scholar]
- von Tiedemann A. Single and combined effects of nitrogen fertilization and ozone on fungal leaf diseases on wheat. Journal of Plant Diseases and Protection. 1996;103:409–419. [Google Scholar]
- Verreet JA, Hoffmann GM. Effects of infection by Septoria nodorum at different development stages of wheat on the level of production. Plant Diseases and Protection. 1987;94:283–300. [Google Scholar]
- Verreet JA, Hoffmann GM. Effect of leaf and ear infection by Septoria nodorum at different growth stages of wheat on plant N content and amino acid composition. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 1990;97:1–12. [Google Scholar]