Abstract
Background and Aims
Previous studies have shown that silica in grass leaves defends them against small herbivores, which avoid high-silica grasses and digest them less efficiently. This study tested the idea that silica can reduce digestibility by preventing the mechanical breakdown of chlorenchyma cells.
Methods
Both the percentage of total chlorophyll liberated from high- and low-silica grass leaves by mechanical grinding and the chlorophyll content of locust faeces were measured.
Key Results
High-silica grasses released less chlorophyll after grinding and retained more after passing through the gut of locusts, showing that silica levels correlated with increased mechanical protection.
Conclusions
These results suggest that silica may defend grasses at least in part by reducing mechanical breakdown of the leaf, and that mechanical protection of resources in chlorenchyma cells is a novel and potentially important mechanism by which silica protects grasses.
Key words: Grass, silica, locust, digestibility, defence, Lolium perenne, Festuca ovina
INTRODUCTION
Silica can constitute 2–6 % of the dry weight of the leaves of grasses, many times higher than is typical in dicotyedonous plants (Russel, 1961). It is actively taken up as silicic acid from the soil (Ma, 2006), and the majority is deposited as hydrated amorphous silica within the lumen of epidermal cells, forming bodies known as phytoliths, whose shapes are characteristic of individual grass taxa (Parry and Smithson, 1964; Kaufmann et al., 1985).
It has been suggested that phytoliths act as a defence against both vertebrate and invertebrate herbivores by increasing the abrasiveness of grass leaves; they wear down the teeth of herbivores and hence deter feeding. The fossil record supports this, since the evolution of high crowned teeth among the Ungulata, continuously growing teeth among the Rodentia and Lagamorpha, and enlarged mandibles in the Lepidoptera and Orthoptera, have all been linked to a diet of grass (Isely, 1944; Simpson, 1951; Stebbins, 1981; Jernvall and Fortelius, 2002). Furthermore a diet of silica-rich grass apparently causes microwear scratches on teeth (Baker et al., 1959; Walker et al., 1978).
Recent experimental work has also supported this hypothesis. Grasses grown with additional silica were significantly more abrasive than those grown without (Massey et al., 2006), while in interspecific comparisons, abrasiveness of grass leaves was proportional to silica content (Massey et al., 2007).
Palatability studies also showed that high silica deterred feeding in three small herbivores with chewing mouthparts: field voles, locusts and army worms (Massey and Hartley, 2006; Massey et al., 2006, 2007), all herbivores preferentially eating low-silica grasses when given a choice. When these herbivores were forced to eat high-silica grasses, they also grew more slowly than when fed on low-silica grass (Massey and Hartley 2006; Massey et al., 2006). However, this was not because they ate less high-silica grass, but because they absorbed a smaller proportion of its total carbohydrates (Massey et al., 2006) and nitrogen (Massey and Hartley, 2006). This suggests that silica defends grasses by reducing its digestibility, not just palatability. How? One possibility is that the silica acts chemically, preventing digestion or absorption. Another, is that herbivores might reduce the amount they chew when eating high-silica grass, to avoid excessive abrasion. This would reduce mechanical breakdown of the cells. A third alternative is that silica particles directly protect the protein- and starch-filled chlorenchyma cells in the leaf from being broken down by chewing. Previous work on orthopterans has shown that, because they lack enzymes that can break down the cell wall, they need to chew their food to break open or disrupt the walls of the chlorenchyma cells (Hochuli, 1996; Clissold et al., 2004). This is necessary to extract starch and proteins; starch is largely stored in chloroplasts, where Rubisco, by far the most common leaf protein, and other important photosynthetic proteins are also located (Raven et al., 1999). Since the vast majority of silica particles have dimensions between 10 µm and 20 µm (Sanson et al., 2007), they are of similar size to grass chlorenchyma cells (Raven et al., 1999) and could keep the cusps of the mouthparts apart, so preventing them crushing the chlorenchyma cells. Conversely, milling theory (Lowrison, 1974) suggests that the silica particles would be too small to crush the chlorenchyma cells, because the cells could readily slip out from between them.
To test this mechanical protection hypothesis, two sets of experiments were carried out. First, high- and low-silica leaves were briefly ground mechanically and then the integrity of their chlorenchyma cells compared. Integrity was determined by comparing the amount of chlorophyll that could be readily extracted from the ground leaves with the total amount of chlorophyll that they contained. How much chlorophyll remained in the faeces was then compared after high- and low-silica grasses had been eaten by and passed through the digestive tract of locusts. This would determine how successful the grasses were at preventing the contents of their chlorenchyma cells from being digested and hence, by implication, their cell walls from being disrupted by the locusts' mouthparts.
MATERIALS AND METHODS
Study species
The grasses chosen for study were Lolium perenne L. and Festuca ovina L., and the herbivore was the locust Schistocerca gregaria Forskal, all of which had been successfully used in previous studies (Massey et al., 2006). The grasses were chosen because they had contrasting palatability to locusts (Massey et al., 2006), with Lolium being more palatable, yet silica acted as a defence in both species; high-silica plants were eaten significantly less by locusts than low-silica plants, while locusts fed exclusively on high-silica grasses performed less well and digested the grasses less efficiently than those fed on low-silica grasses (Massey et al., 2006).
Plant growth conditions
Lolium perenne and Festuca ovina (Emorsgate seeds) were grown under greenhouse conditions (15–25 °C, light : dark 16 : 8). Seeds were sown at a rate of approx. 0·5 cm−2 in sward trays (20 × 30 × 5 cm, split into 24 5 × 5 cm compartments) in washed perlite, an inert growth medium. Grasses were grown from September 2006 until February 2007 for grinding experiments and until March 2007 for locust feeding experiments. Grasses were watered twice weekly with 100 mL of Hoagland's solution, with or without 150 mg L−1 of soluble silica in the form of NaSiO3·9H20. All plants also received tap water ad libitum.
Foliar silica content
Foliar silica content (n = 10 per silica treatment from different tray compartments) was determined in March 2007 by fusing oven-dried leaf samples (approx. 0·2 g) in sodium hydroxide followed by analysis using the colorimetric silicomolybdate technique (Allen, 1989; Massey et al., 2006).
Grinding experiments
To simulate locust chewing, grass leaves were mechanically ground in as far as possible a repeatable way using a pestle and mortar. While this may not exactly simulate locust mandibles there is sufficient precedent (Caswell and Reed, 1976) to use this technique to estimate differential mechanical extraction. Leaf tissue, 0·25 g from each of the high- and low-silica trays of grass of each species (n = 10), was placed into a mortar together with 10 mL of 100 % ethanol (Fisher Scientific). The tissue was ground by a single researcher (J. W. Hunt) turning a pestle 50 times in the mortar over the grass and applying as far as possible a constant crushing force.
Chlorophyll release occurs when chlorenchyma cell walls are ruptured, so the percentage of the total chlorophyll released represents a readily quantifiable measure of the mechanical breakdown of these cells. Extracted chlorophyll was filtered and analysed spectrophotometrically within 5 min of grinding (Jespersen and Chistoffersen, 1987) to determine the chlorophyll content. The grass tissue left in the filter was then returned to the mortar along with 10 mL of ethanol and the amount of chlorophyll remaining was determined by thoroughly grinding the tissue, leaving for it for 10 min for full chlorophyll extraction, and repeating the spectrophotometric analysis. Control samples held in ethanol for 5 min without grinding showed no discernable leakage of chlorophyll, showing that the action of ethanol alone was unable to cause release of chlorophyll over this short time period.
Locust feeding experiments
To investigate the relative amount of intact chlorenchyma after locusts had eaten grass, the chlorophyll content of their faeces was determined. Fourth instar locusts Schistocerca gregaria, obtained from Blades Biological, were maintained on Poa pratensis grass. This ensured that locusts had no previous experience of the experimental grasses. For the tests, locusts were individually caged (n = 10) in 1-L sandwich boxes, and starved for 24 h, much longer than the clearance time of locusts, so all food eaten before, would have passed through their guts. Any faeces were removed and each locust was assigned to a diet treatment (Festuca or Lolium; low or high silica). Locusts were given surplus fresh grass leaves (approx. 0·5 g) daily. After 3 d, locust faeces were collected from each cage and 0·1-g samples were analysed for chlorophyll content. Faeces were ground, together with 10 mL ethanol until all of the chlorophyll had been extracted and chlorophyll content was again determined using the spectrophotometer.
RESULTS
Silica content of grasses
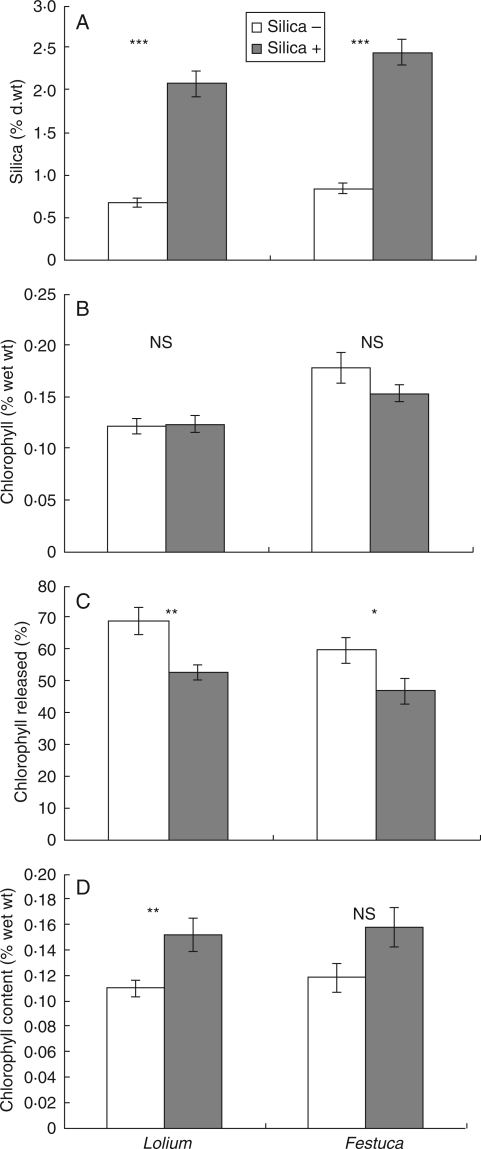
Plants given added silica had levels that were approximately three times as great as those without added silica (Fig. 1A), results which were highly significant (Lolium t18 = 8·72, P < 0·001. Festuca t18 = 9·72, P < 0·001), despite the possible confounding effect of silica in the added tap water.
Fig. 1.
The effects of added silica on the silica and chlorophyll content of leaves, and on their resistance to grinding and digestion. (A) Mean silica content ( % dry weight) of the leaves of high- and low-silica grasses. (B) Mean chlorophyll content ( % wet weight) of high- and low-silica grass leaves. (C) Mean percentages of total chlorophyll released by high- and low-silica grass leaves after a short period of grinding. (D) Mean chlorophyll content ( % wet weight) of locust faeces from high- or low-silica grasses. In all figures, mean ± s.e. are shown, together with the significance of differences between high- and low-silica plants.
Grinding experiments
There were no significant differences in total leaf chlorophyll levels between silica treatments for either grass species (Fig. 1B). In contrast, the percentage of the total chlorophyll released after initial grinding was significantly lower in grasses subjected to high-silica treatment than low-silica treatment (Fig. 1C). The percentage released was around 16 % lower in the high-silica plants in Lolium (t18 = 3·25, P = 0·004) and 13 % lower in Festuca (t18 = 2·19, P = 0·042).
Locust feeding experiments
The chlorophyll content of the faeces from locusts fed high- and low-silica grasses is shown in Fig. 1D. The chlorophyll content of faeces from locusts fed high-silica grass was 38 % higher for Lolium and 33 % for Festuca. However, though the difference for Lolium was significant (t18 = 2·95, P = 0·009) it was not quite significant for Festuca (t15 = 2·03, P = 0·060) due to high variability.
DISCUSSION
The data described here is to some extent preliminary. Further study could have examined the loss of starch and protein in crushed leaves directly, mimicked tooth action better, and looked at more species. Nevertheless, the results of the two series of tests suggest that silica can affect the mechanical disruption of the walls of chlorenchyma cells in these grasses; proportionately less chlorophyll is released from high-silica grasses by mechanical grinding, while more chlorophyll is left in the faeces of locusts after they have eaten, chewed and digested grass. More chlorenchyma cells must therefore have remained intact in the high-silica plants. Since chlorenchyma cells contain high levels of starch and proteins, especially Rubisco, the mechanical protection of these cells by silica could well be at least partially responsible for the reduced digestibility of high-silica grasses (Massey et al., 2006).
So how could high silica protect chlorenchyma cells? It is unlikely that it reinforces their cell walls because earlier studies have failed to show significant levels of silica in chlorenchyma cells. Silica is also extremely brittle, so it would be poor at preventing cell wall disruption. It is more likely that the solid phytoliths keep apart the pestle and mortar and the cusps of the molar regions of the locusts' jaws, preventing crushing of the chlorenchyma cells. Certainly in these species the two sets of cells are of near identical size. In Festuca both the silica bodies (pers. obs.) and mesophyll cells have a diameter of 10 µm (Metcalfe, 1960), while in Lolium the longer silica bodies are 10–15 µm in width, comparable to the diameter of the mesophyll cells (Metcalfe, 1960). The suggested mechanical protection function of silica bodies sheds light on their non-spherical shapes. Many, such as those of Lolium, are essentially cuboidal while others have concave regions within them; those of Festuca are heart-shaped, while other species have saddle- or dumbbell-shaped phytoliths. Such bodies are far more likely to jam between mouthparts than spherical ones, and may also be more abrasive and provide more friction between the mouthparts.
Of course though silica can directly protect cells, this does not preclude other postulated mechanisms of defence. The abrasiveness of silica bodies might still have a role, despite Sanson's recent finding (Sanson et al., 2007) that phytoliths are not as hard as enamel. They might still be hard enough to abrade insect mouthparts, so that locusts are either unable to process food so efficiently or reduce chewing to prevent abrasion. The silica could also damage the gut lining or act chemically to disrupt digestion. Further experiments are clearly needed to determine the relative importance of the different mechanisms.
Though phytoliths reduce the digestibility of grasses to small herbivores such as locusts which digest only the cell contents, they may not have the same effect for large vertebrate herbivores such as ruminants. In the fermenting rumen, cell walls are broken down by symbiotic bacteria (Alexander, 1993), so large herbivores could digest high-silica grasses whether chewing has mechanically disrupted their cell walls or not (Van Soest and Jones, 1968; Smith et al., 1971). However, reduced mechanical breakdown could slow down the rate of digestion, so ruminants might have to chew high-silica grass for longer and reduce their consumption rate. In support of this, recent work (F. P. Massey, A. R. Ennos and S. E. Hartley, unpubl. obs.) has shown that high silica reduces the bite rate of sheep. Clearly more research is needed to fully understand the functional morphology of phytoliths. However, it is concluded that mechanical protection of resources in chlorenchyma cells is a novel and potentially important mechanism by which silica protects grasses.
LITERATURE CITED
- Alexander RMcN. The relative merits of foregut and hindgut fermentation. Journal of Zoology. 1993;231:391–401. [Google Scholar]
- Allen SE. Chemical analysis of ecological materials. 2nd edn. London: Blackwell Press; 1989. [Google Scholar]
- Baker G, Jones LHP, Wardrop ID. Causes of wear in sheeps' teeth. Nature. 1959;184:1583–1584. doi: 10.1038/1841583b0. [DOI] [PubMed] [Google Scholar]
- Caswell H, Reed FC. Plant–herbivore interactions: the indigestibility of C4 bundle sheath cells by grasshoppers. Oecologia. 1976;26:151–156. doi: 10.1007/BF00582893. [DOI] [PubMed] [Google Scholar]
- Clissold FJ, Sanson GD, Read J. Indigestibility of plant cell wall by the Australian plague locust. Chorticoidcetes terminifera. Entomologia Experimentalis et Applicata. 2004;112:159–168. [Google Scholar]
- Hochuli DF. The ecology of plant/insect interactions: implications of digestive strategy for feeding by phytophagous insects. Oikos. 1996;75:133–141. [Google Scholar]
- Isely FB. Correlation between mandibular morphology and food specificity in grasshoppers. Annals of the Entomological Society of America. 1944;37:47–67. [Google Scholar]
- Jernvall J, Fortelius M. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature. 2002;417:538–540. doi: 10.1038/417538a. [DOI] [PubMed] [Google Scholar]
- Jespersen A-M, Christoffersen K. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Archiv fûr Hydrobiologie. 1987;109:445–454. [Google Scholar]
- Kaufman PB, Dayanandan P, Franklin CI, Takeoka Y. Structure and function of silica bodies in the epidermal system of grass shoots. Annals of Botany. 1985;55:487–507. [Google Scholar]
- Lowrison GC. Crushing and grinding: the size reduction of solid materials. London: Butterworth; 1974. [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, et al. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Massey FP, Hartley SE. Experimental demonstration of the antiherbivore effects of silica in grasses: impacts on foliage digestibility and vole growth rates. Proceedings of the Royal Society of London, B. 2006;273:2299–2304. doi: 10.1098/rspb.2006.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and phloem feeders. Journal of Animal Ecology. 2006;75:595–603. doi: 10.1111/j.1365-2656.2006.01082.x. [DOI] [PubMed] [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. Grasses and the resource availability hypothesis: the importance of silica-based defences. Journal of Ecology. 2007;95:414–424. [Google Scholar]
- Metcalfe CR. Anatomy of the monocotyledons. Oxford: Clarendon Press; 1960. [Google Scholar]
- Parry DW, Smithson F. Types of opaline silica depositions in leaves of British grasses. Annals of Botany. 1964;28:169–185. [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE. Biology of plants. 6th edn. New York, NY: W. H. Freeman; 1999. [Google Scholar]
- Russel EW. Soil conditions and plant growth. New York, NY: Longmans Green; 1961. [Google Scholar]
- Sanson GD, Kerr SA, Gross KA. Do silica phytoliths really wear mammalian teeth? Journal of Archaeological Science. 2007;34:526–531. [Google Scholar]
- Simpson GG. Horses: the story of the horse family in the modern world and through sixty million years of history. New York, NY: Natural History Library; 1951. [Google Scholar]
- Smith GS, Nelson AB, Boggino EJA. Digestibility of forages in vitro as affected by content of “silica”. Journal of Animal Science. 1971;33:466–471. doi: 10.2527/jas1971.332466x. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Coevolution of grasses and herbivores. Annals of the Missouri Botanical Gardens. 1981;68:75–86. [Google Scholar]
- Van Soest PJ, Jones LHP. Effect of silica in forages upon digestibility. Journal of Dairy Science. 1968;51:1644–1648. [Google Scholar]
- Walker A, Hoeck H, Perez L. Microwear of mammalian teeth as an indicator of diet. Science. 1978;201:908–910. doi: 10.1126/science.684415. [DOI] [PubMed] [Google Scholar]