Abstract
Background and Aims
Consistent abiotic factors can affect directional selection; cyclones are abiotic phenomena with near-discrete geographic limits. The current study investigates selective pressure of cyclones on plants at the species level, testing for possible natural selection.
Methods
New World Arecaceae (palms) are used as a model system, as plants with monopodial, unbranched arborescent form are most directly affected by the selective pressure of wind load. Living specimens of known provenance grown at a common site were affected by the same cyclone. Data on percentage mortality were compiled and analysed in biogeographic and phylogenetic contexts.
Key Results
Palms of cyclone-prone provenance exhibited a much lower (one order of magnitude) range in cyclone tolerance, and significantly lower (P < 0·001) mean percentage mortality than collections from cyclone-free areas. Palms of cyclone-free provenance had much greater variation in tolerance, and significantly greater mean percentage mortality. A test for serial independence recovered no significant phylogenetic autocorrelation of percentage mortality.
Conclusions
Variation in cyclone tolerance in New World Arecaceae correlates with biogeography, and is not confounded with phylogeny. These results suggest natural selection of cyclone tolerance in cyclone-prone areas.
Key words: Abiotic selection, Arecaceae, biogeography, cyclone, hurricane, phylogenetic independence
INTRODUCTION
The role of abiotic natural selection has been considered since Darwin's era. Darwin thought abiotic environmental factors to be generally too random to provide directionality to selection (Niklas, 1992). Studies of selection can advance a leading role for biotic factors (Allmon and Ross, 1990), or abiotic factors (Totland, 2001). Over long time scales, consistent abiotic factors can affect directional selection on plants (Caruso et al., 2003).
Consideration of plant evolution in an abiotic context of high winds is increasingly relevant. Tropical cyclones have increased in frequency and intensity since 1970, and the trend is expected to continue (Emmanuel, 2005). High winds have a demonstrated influence on vegetation (Coutts and Grace, 1994). Relationships among high winds and canopy dynamics, seedling recruitment and stand demographics have been demonstrated throughout tropical, subtropical and temperate regions (Gresham et al., 1991; Zimmermann et al., 1994; Asner and Goldstein, 1997; Hirsh and Marler, 1997; Quine and Bell, 1998). Cyclones can directly influence vegetation, forest structure, canopy height, community composition, structural adaptations and evolutionary patterns (Webb, 1958; Hopkins, 1990; Clarke and Kerrigan, 2000; Grove et al., 2000; Horvitz and Koop, 2001; de Gouvenain and Silander 2003).
Palms (Arecaceae) offer an apt model system for investigating evolution of tolerance to mechanical stress via wind. Many palm species have rigidly determined unbranched monopodial stem architecture, derived entirely from primary growth (Tomlinson, 2006). Damage from wind load has a potentially more direct selective effect on individual plants with unbranched monopodial architecture than on branched plants. Mature palms also have an easily quantified, limited number of leaves, facilitating estimations of surface area, and enabling accurate estimations of potential wind load (Sterken, 2007). In terms of general biomechanical study of palms, a robust canon exists (Tomlinson, 1962; Rich, 1987; Isnard et al., 2005; among others). Palms are described as mechanically efficient (Tomlinson, 2006) and well-adapted to wind stress (Lippincott, 1995; Fetcher et al., 2000), and palms are sometimes observed as prominent surviving plant groups following high wind events (Loope et al., 1994; Vandermeer, 1994; Ostertag et al., 2005).
Intrinsic biotic factors may have a primary influence in cyclone tolerance in some Arecaceae (J. L. Dowe, unpubl. res.). Studies of primary axis anatomy, lignification and stress suggest that the palm trunk is adapted to distribute high load stress efficiently through flexibility (Tomlinson, 1990; Huang et al., 2002; Kuo-Huang et al., 2004). Palm stem and leaf mechanical properties have both been considered in the context of adaptive value for high wind environments (Rich, 1986; Tomlinson, 1990; Niklas, 1999). Knowledge about broad patterns of high wind tolerance in Arecaceae can inform and guide further studies in these areas.
Large-scale unplanned disturbances such as cyclones illuminate natural systems in ways that controlled experimentation may not (Holt, 2006; Schoener and Spiller, 2006; cf. Cooper-Ellis et al., 1999). Live plant collections affected by an unplanned natural disturbance offer potentially broader insights (Klein, 1992; Fisher et al., 1996; Dosmann, 2006). Taxonomically and biogeographically, broad plant collections may offer insight into natural selection via pressure from catastrophic wind events in the context of biogeography and phylogeny. The current study investigates the effects of a cyclone on a taxonomically and geographically diverse collection of palms growing in a single location.
MATERIALS AND METHODS
Plants studied
Arecaceae specimens at Montgomery Botanical Center (Coral Gables, FL, USA; 25°39'N, 80°16'W) were used in the current study. Specimens were obtained and cultivated through ongoing ex situ conservation collection development operations. The study group was circumscribed to include only Arecaceae specimens: (a) from the New World; (b) of mature age; (c) with adequate numbers (>10 individuals per taxon) represented; and (d) with solitary, self-supporting, arborescent axes (excluding other palm habits such as clumping palms with multiple axes and lianas). Taxa studied and respective sample sizes are detailed in Table 1.
Table 1.
Collections, provenance and mortality data for the current study
| Taxon | Latitude | Longitude | n Sampled | n Killed | Voucher specimen |
|---|---|---|---|---|---|
| Acrocomia aculeata Lodd. ex Mart. | –1·58 | –48·75 | 43 | 7 | Noblick 5019, FTG |
| Attalea phalerata Mart. ex Spreng. | –1·58 | –48·75 | 27 | 0 | Noblick 5018, FTG |
| Attalea pindobassu Bondar | –11·78 | –40·74 | 13 | 0 | Noblick 4600, FTG |
| Butia capitata Becc. | –14·76 | –46·35 | 12 | 0 | Noblick 5090, FTG |
| Butia eriospatha Becc. | –26·58 | –53·83 | 11 | 1 | Noblick 4878, FTG |
| Coccothrinax argentata (Jacq.) L. H. Bailey | 24·67 | –81·37 | 13 | 0 | Noblick 5076, FTG |
| Coccothrinax barbadensis Becc. | 16·28 | –61·45 | 106 | 2 | Hahn 7651, NY |
| Coccothrinax scoparia Becc. | 18·12 | –71·62 | 34 | 0 | Hahn 7751, NY |
| Coccothrinax spissa L. H. Bailey | 18·33 | –70·37 | 27 | 0 | Hahn 7710, NY |
| Gaussia attenuata Becc. | 18·45 | –66·94 | 18 | 0 | Proctor s.n., NY |
| Pseudophoenix vinifera (Mart.) Becc. | 18·48 | –70·71 | 37 | 0 | Noblick 5459, FTG |
| Roystonea oleracea O. F. Cook | 16·32 | –61·78 | 41 | 3 | Hahn 7646, NY |
| Roystonea regia O. F. Cook | 26·02 | –81·4 | 146 | 0 | Noblick 5250, FTG |
| Sabal minor (Jacq.) Persoon | 27·82 | –81·8 | 20 | 0 | Noblick 5452, FTG |
| Sabal palmetto Hort. | 30·48 | –81·5 | 204 | 2 | Noblick 5444, FTG |
| Syagrus amara Mart. | 16·2 | –61·65 | 13 | 0 | Hahn 7649, NY |
| Syagrus botryophora Mart. | –15·85 | –38·9 | 29 | 17 | Noblick 5002, FTG |
| Syagrus cearensis Noblick | –7·38 | –34·96 | 42 | 1 | Noblick 5132, FTG |
| Syagrus × costae Glassman | –8·88 | –36·5 | 13 | 1 | Noblick 4980, FTG |
| Syagrus oleracea (Mart.) Becc. | –14·51 | –46·36 | 11 | 3 | Noblick 5084, FTG |
| Syagrus orinocensis (Spruce) Burret | 5·67 | –67·67 | 13 | 3 | Noblick 4946, FTG |
| Syagrus picrophylla Barb.Rodr. | –16·92 | –41·48 | 17 | 0 | Noblick 5156, FTG |
| Syagrus romanzoffiana (Cham.) Glassman | –25·65 | –54·47 | 61 | 15 | Noblick 5112, FTG |
| Syagrus vermicularis Noblick | –5·03 | –47·02 | 16 | 1 | Noblick 4974, FTG |
| Thrinax radiata Mart. | 24·7 | –81·03 | 124 | 5 | Noblick 5081, FTG |
Wind event
The study plants were investigated for the effects of Hurricane Wilma, a cyclonic storm which affected the collections on 24 October 2005. Wind speeds of up to 95–105 knots (175·9–194·5 k.p.h.) were recorded as the cyclone crossed the study site (Pasch et al., 2006).
Data collection
Data related to each plant's condition and mortality were compiled during the period 25 October to 10 November 2005. Data on numbers of plants killed by Hurricane Wilma were extracted from these records, as well as from collections census data from before the wind event (Table 1).
Analysis
Percentage mortality resulting from the wind event was calculated for each taxon using the compiled data. Resulting percentage mortality for each taxon was investigated in two contexts: (1) biogeographic patterns; and (2) phylogenetic dependence.
Biogeographic analysis
The geographic limits of Atlantic cyclone activity were determined via a review of meteorological data from 1851 to 2006 (NOAA, 2007; described by Jarvinen et al., 1984). These data were used to circumscribe cyclone-influenced and cyclone-free geographic regions. Provenance data for the taxa studied were mapped and compared with the known geographic limits of Atlantic cyclones. Percentage mortality by taxon was plotted against latitude of provenance. Via comparison of the geographic limits of cyclone activity and provenance data, taxa were divided into cyclone-prone and cyclone-free groups. Average percentage mortality for taxa from cyclone-prone and cyclone-free taxa was calculated. Tests for significance were performed on the two subgroups via the binomial test (Hollander and Wolfe, 1999), using the overall average proportion of mortality for the whole dataset (ignoring provenance) as the null hypothesis for group comparison.
Phylogenetic independence analysis
In the absence of a complete cohesive molecular phylogeny for all Arecaceae, a diagram of putative relationships was constructed using available information. Relationships among the taxa studied were compiled via a review of available work (Nauman and Sanders, 1991; Gunn, 2004; Asmussen et al., 2006; A. W. Meerow, USDA-ARS, USA, unpubl. res.; L. R. Noblick, unpubl. res.). To evaluate potential autocorrelation of percentage mortality via synapomorphic derivation of cyclone tolerance, the percentage mortality for each taxon was mapped onto this estimation of relationships, and a randomization test version of von Neumann's test (von Neumann et al., 1941) for serial independence (Abouheif, 1999) was performed on these data given the a priori phylogeny. The test for serial independence compared the distribution of mortality data on the a priori putative phylogeny against 1000 random re-distributions of mortality data on the phylogeny, as implemented by Phylogenetic Independence v2·0 (Reeve and Abouheif, 2003). This test for serial independence was repeated ten times to evaluate the consistency of the results obtained.
RESULTS
Taxa and percentage mortality
The average sample size per taxon for the taxa studied was 44, and ranged from 11 to 204. The percentage mortality for each taxon is presented in Figs 1 and 2. Values for percentage mortality per taxon ranged from 0·00 % to 58·62 %, with average percentage mortality per taxon of 7·58 %, and overall percentage mortality for the entire study of 5·59 %. The mortality rates do not correlate with the sample size per taxon (Table 1).
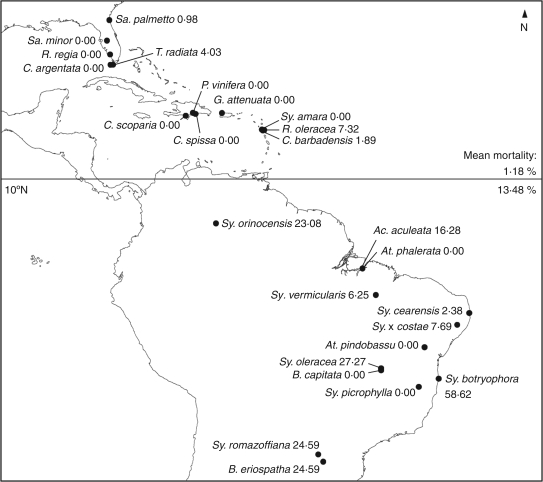
Fig. 1.
Provenance of specimens studied relative to the occurrence of cyclones. Numbers after each taxon are the percentage mortality of the taxon following Hurricane Wilma in 2005 (calculated from Table 1). Taxa from south of 10°N latitude (see Table 2) had mean observed percentage mortality over ten times greater than those northward (P < 0·001).
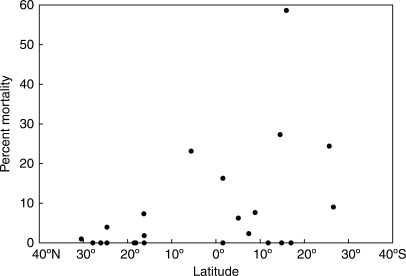
Fig. 2.
Latitude of provenance versus percentage mortality of taxa studied. Specimens native to cyclone-prone latitudes (north of 10°N) have significantly lower (P < 0·001) observed percentage mortalities following Hurricane Wilma.
Circumscription of cyclone geography
Table 2 presents an overview of Atlantic cyclone data by decade from 1851 to 2006. A total of 1363 Atlantic cyclones are recorded for the period. All cyclone tracks occurred at least partially north of 10°N, 97·87 % of Atlantic cyclone activity occurred entirely north of 10°N latitude, and all Atlantic cyclone activity over this period occurred north of 7°N latitude, with only one exception. For the purposes of this study, the effective cyclone-prone region of the geographic area of interest was determined to have a south boundary of 10°N latitude (Fig. 1).
Table 2.
Frequency and location of Atlantic cyclone activity, 1851–2006
| Period | No. of cyclones | No. of cyclones active south of 10°N* | Southern maximum |
|---|---|---|---|
| 1851–1860 | 60 | 0 | 12·0°N |
| 1861–1870 | 76 | 1 | 9·5°N |
| 1871–1880 | 75 | 1 | 8·5°N |
| 1881–1890 | 82 | 1 | 9·9°N |
| 1891–1900 | 84 | 2 | 8·7°N |
| 1901–1910 | 79 | 3 | 7·7°N |
| 1911–1920 | 54 | 0 | 10·3°N |
| 1921–1930 | 56 | 1 | 9·5°N |
| 1931–1940 | 104 | 1 | 8·8°N |
| 1941–1950 | 98 | 0 | 10·2°N |
| 1951–1960 | 98 | 0 | 10·1°N |
| 1961–1970 | 98 | 3 | 8·0°N |
| 1971–1980 | 96 | 0 | 10·0°N |
| 1981–1990 | 96 | 6 | 7·2°N |
| 1991–2000 | 111 | 6 | 8·3°N |
| 2001–2006 | 96 | 4 | 8·9°N |
Data adapted from NOAA (2007).
* In all but one case, cyclones active south of 10°N also tracked north of that line. The only recorded cyclone occurring entirely south of 10°N, in the south Atlantic, occurred in 2004 (Pezza and Simmonds, 2005).
Biogeography
Figure 1 presents provenance of the taxa included in the current study. Based on the determination of cyclone-prone geographic areas, the taxa were divided into those from cyclone-prone regions north of 10°N latitude, and taxa with provenance south of 10°N latitude which fall into the region determined as cyclone-free. Specimens from the cyclone-prone region are all of Antillean or peninsular Floridian provenance, and specimens from the cyclone-free region are exclusively of South American provenance, and each of these two groups occurs over a wide geographic area.
Percentage mortality data differed significantly based on latitude (Fig. 2). Much higher variation of percentage mortality occurred in the taxa from south of 10°N. The range of mortality values per taxon was 0·00–7·32 % in the cyclone-prone area, and 0·00–58·62 % in the cyclone-free area. The mean percentage mortality per taxon for the cyclone-free area was 13·48 %, and 1·18 % for the cyclone-prone area. Mean percentage mortality of each group differed significantly from that of the overall data set according to the results of the binomial test (P < 0·001 in both cases).
Phylogenetic independence
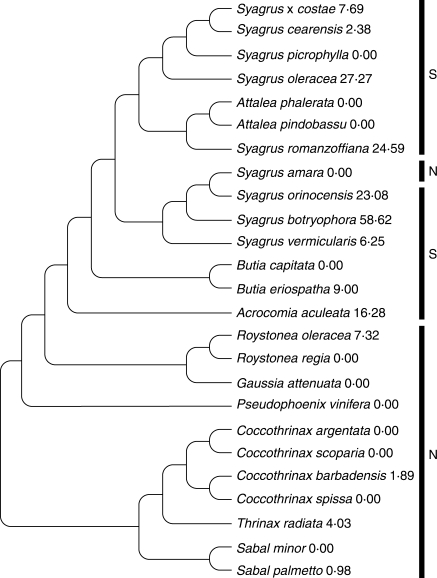
Hypothesized relationships among the taxa studied are detailed in Fig. 3. The putative phylogeny broadly aligns with the biogeography of the collections studied, with a monophyletic clade of taxa from the cyclone-free area (‘S,’ Fig. 3.) circumscribing Acrocomia, Attalea, Butia and Syagrus (with the notable exception of S. amara).
Fig. 3.
Putative relationships among the taxa studied, for testing autocorrelation. Diagram adapted from known phylogenetic information (Nauman and Sanders, 1991; Gunn, 2004; Asmussen et al., 2006; A. W. Meerow, USDA-ARS, USA, unpubl. res.; L. R. Noblick, unpubl. res.). Numbers after taxa are percentage mortality data. Bars to the right indicate the biogeographic group: N = north of 10°N latitude; S = south of 10°N latitude.
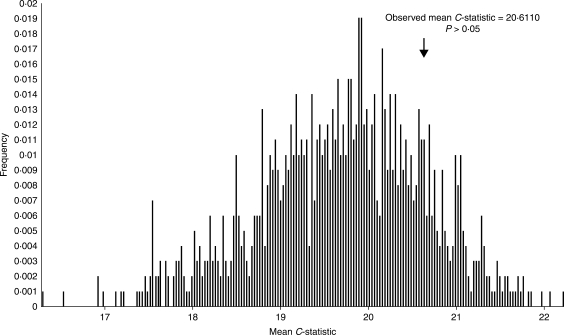
Viewing the percentage mortality data as mapped onto the putative phylogeny suggests differential distribution of cyclone tolerance. The randomization test for serial independence of the percentage mortality data, given the relationships in Fig. 3, found no evidence of significant phylogenetic autocorrelation (Table 3 and Fig. 4). The average C-statistic for the ten runs was 20·5747, and the average P-value was 0·1688. This result rejects the hypothesis of synapomorphy as an influence on cyclone tolerance.
Table 3.
Test for serial independence
| Replication | Observed mean C-statistic | P-value |
|---|---|---|
| 1 | 20·54 | 0·189 |
| 2 | 20·59 | 0·167 |
| 3 | 20·58 | 0·164 |
| 4 | 20·57 | 0·184 |
| 5 | 20·57 | 0·202 |
| 6 | 20·57 | 0·155 |
| 7 | 20·61 | 0·159 |
| 8 | 20·57 | 0·139 |
| 9 | 20·59 | 0·156 |
| 10 | 20·57 | 0·173 |
| Average | 20·5747 | 0·1688 |
Fig. 4.
Phylogenetic independence of cyclone mortality: representative randomization test for serial independence of cyclone mortality data, given the hypothesized phylogeny (Fig. 3). The observed mean C-statistic for the given series (20·6110) does not fall far to the right of the distribution of mean C-statistics for these 1000 randomized replicates (P = 0·1590). Cyclone tolerance is not confounded with phylogeny; lack of evidence of phylogenetic autocorrelation for cyclone tolerance leaves open the possibility of selection for higher cyclone tolerance dependent on geographic distribution.
DISCUSSION
Abiotic condition
The observed geographic limits of Atlantic cyclone activity (Table 2 and Fig. 1) create essentially discrete conditions which approximate a formal experiment with a test group and control group, albeit on very large geographic and temporal scales.
From 1851 to 2006, an average of nearly eight cyclones per year has occurred north of 10°N latitude, and a small percentage of these have tracked south of this parallel. Only one cyclone, Cyclone Catarina (2004), has occurred entirely south of 10°N latitude, between 25°S and 31°S latitude (Pezza and Simmonds, 2005). More accurate cyclone positional records correspond with the advent of aerial monitoring in 1944 (Neumann et al., 1999): for the period 1944–2006, 667 cyclones were recorded, 97·15 % of these occurred entirely north of 10°N latitude, and the southern maximum did not extend beyond 7°N latitude, with the exception of Cyclone Catarina.
Recent work has measured Caribbean cyclone activity in the 19th and 20th centuries (Miller et al., 2006; Nyberg et al., 2007), written records exist of Caribbean cyclone activity in the 16th century (Ludlum, 1963), and the sedimentary record shows evidence of cyclones in the Caribbean for at least 5000 years (Donnelly and Woodruff, 2007). Assuming that wind shear forces in the Atlantic have been present in more or less the same form during the Holocene, the regular presence of cyclones in the Caribbean and their absence in South America are conditions that likely existed on a time scale of at least that length. This creates two very different long-term adaptive regimes, one with frequent and regular cyclones as a possible selective pressure, and one without. Relative to the long-lived perennial habit of the plants studied, the frequency and history of the differential abiotic condition is temporally consistent, and therefore expected to affect directional selection (Levins, 1968; Reboud and Bell, 1997).
Model system
As noted in the Introduction, palm collections provide a suitable model system for investigating potential selective pressure from high winds. The intersection of the differential abiotic condition (frequent regular high winds over many generations) with a particularly vulnerable habit (unbranched monopodial arborescence) presents an opportunity to investigate empirical patterns of natural selection in response to this abiotic selective pressure.
A potential challenge in the current study may be the degree to which potentially confounding variables affect the analysis. For the current study, the specimens were not planted in exacting identical conditions in specific anticipation of a cyclone. Rather, curatorial practice made the collections useful for research unanticipated at accession (Dosmann, 2006). Edaphic, topographical and other factors associated with the siting of individual plants may introduce systematic variation into the model system, potentially obscuring effects of the environmental factor of interest. Previous studies demonstrate that topography and protectedness of specific sites can influence cyclone damage (Bellingham, 1991; Brokhaw and Grear, 1991; Frangi and Lugo, 1991), but the relationship to mortality is not consistent (Putz and Shanz, 1991). The effect of variation in site conditions, even between widely separated and topographically variable sites, may be less influential than intrinsic biotic factors with regard to cyclone tolerance in palms (J. L. Dowe, unpubl. res.). Total elevation difference at the study site is <6 m, and the specimens are more or less uniformly exposed. Potential effects of microsite variation throughout the collections are mitigated by the sample sizes and breadth of locations of individual specimens throughout the landsite.
Testing the hypothesis
Insights gleaned from unplanned disasters (Klein, 1992; Holt, 2006; Schoener and Spiller, 2006) can be more rigorous if hypotheses are carefully stated, model systems are defined, and records are available. The central question of this study addresses whether cyclones exert a selective effect on plants. The null hypothesis is that there is no difference in cyclone tolerance between palms of cyclone-prone and cyclone-free provenance. The null hypothesis is rejected with a high degree of statistical confidence (P < 0·001; Figs 1 and 2). Therefore, it is possible that the frequent and regular occurrence of cyclones has exerted a selective pressure towards increased wind tolerance in Caribbean Arecaceae.
The effective boundary of Atlantic cyclone geography (10°N latitude) bisects the natural distributions of two of the taxa included in this study, Acrocomia aculeata and Roystonea oleracea. Acrocomia aculeata is widespread through the Caribbean basin, from Cuba and Mexico southward throughout Paraguay and into southern Brazil (Henderson et al., 1995); Roystonea oleracea is found throughout the Lesser Antilles, to Trinidad, westward through Venezuela, and into Colombia (Henderson et al., 1995). Estimating the centre of origin for these two taxa is beyond the scope of the current study, but it is worth noting that the centre of diversity for Roystonea is the Caribbean basin, while the other Acrocomia species (A. hassleri) occurs in Paraguay and southern Brazil (Henderson et al., 1995). Reanalysis of the percentage mortality data excluding these two taxa may offer a more discrete estimation of the differences between the two geographic groups: with A. aculeata and R. oleracea excluded, the overall mean percentage mortality changes from 7·58 % to 7·21 %, the mean percentage mortality per taxon for the cyclone-prone group falls by half, from 1·18 % to 0·63 %, while the mean percentage mortality per taxon of the cyclone-free group changes slightly, from 13·48 % to 13·25 %. The differences between the groups remain significant (P < 0·001). Whether R. oleracea is included or excluded, the range of observed percentage mortality (either 0·00–7·32 % or 0·00–4·03 %) for the Caribbean group remains below the average mean percentage mortality for the entire study (either 7·58 % or 7·21%). Geographically dependent differences in mortality between the groups are well supported.
Phylogenetic considerations
Having rejected the null hypothesis that the geographic groups display no difference in cyclone tolerance, the potential confounding effect of shared ancestry must be considered, as biogeography and phylogeny appear correlated (Fig. 3). The results of the randomization test for serial independence (Table 3 and Fig. 4) show no significant evidence that the observed percentage mortality data is phylogenetically autocorrelated (i.e. the result of shared ancestry). This suggests that the observed variation by biogeography is not confounded by phylogenetic factors.
As above, reanalysis of the data with exclusion of Acrocomia aculeata and Roystonea oleracea could be advisable, as exclusion of these two species divides the study organisms into very discrete groups based on the 10°N latitude line. Excluding these two taxa and re-running the test for serial independence, the null hypothesis (that the percentage mortality data are not phylogentically autocorrelated) is still not rejected (P = 0·320).
Wind as a selective pressure
Based on the significant differences in cyclone tolerance based on geography observed in the model system (Figs 1 and 2), along with the lack of autocorrelation in the hypothesized phylogeny (Table 3 and Fig. 4), empirical evidence from the current study suggests that frequent and regular high wind events exert a selective pressure towards increased tolerance. Ultimate cause of mortality in all cases was damage from high winds, but proximate causes showed some variation. Major causes of death were stem failure just below the primary meristem (crown), stem failure in mid-stem, and uprooting and subsequent desiccation. Anecdotal observations of variation in leaf shedding, petiole and stem flexibility, and root depth may suggest specific adaptive mechanisms for cyclone tolerance. Thorough analysis of morphological, anatomical or edaphic characters associated with cyclone tolerance is outside the scope of the current study, but these types of observations can provide a starting point for further investigation.
The Caribbean palm taxa included in this study possess a wide range of stem, leaf and crown morphologies but, as a group, these taxa have little variation in and low observed percentage mortality, i.e. greater cyclone tolerance. Pseudophoenix and Coccothrinax provide two examples of widely different morphology, showing pinnate versus palmate leaves, crownshaft versus no crownshaft habit, and very different stem heights, stem diameters and stem allometry, yet these genera are basically identical with regard to cyclone tolerance. In the South American taxa studied, much greater variation in cyclone tolerance is observed.
Coccothrinax and Syagrus are two genera with diverse stem allometry. Among Coccothrinax, very little variation in percentage mortality is observed, whereas in Syagrus, percentage mortality data appear to correlate with stem height to diameter ratio (M. P. Griffith, unpubl. res.).
Broader applications
The Arecaceae are pantropical and very widely distributed in the subtropics (Uhl and Dransfield, 1987). The current study utilizes New World Arecaceae of a certain habit, but the hypotheses tested here can be more broadly examined using collections from other areas. One report (Jones, 2006) suggests that cyclone tolerance may vary in African palms (Hyphaene, Borassus).
In the context of observed increasing frequency and intensity of cyclones (Emmanuel, 2005), a model system to investigate long-term natural selection in vegetation will be of use. The current study presents evidence that long-lived perennial vegetation can adapt to the selective pressure of frequent high winds at the species level, and provides a starting point for more specific investigation of allometric adaptation.
Elucidating the mechanism or mechanisms of cyclone tolerance in palms will be of interest. Further examination for morphological or anatomical adaptations will be of use. Results here can inform studies of specific adaptive mechanisms of cyclone tolerance, and provide a framework for other investigations into adaptation to wind stress.
ACKNOWLEDGEMENTS
We thank Lee Anderson, Charles Bauduy, Laurie Danielson, Vickie Murphy, Sandra Rigotti-Santos, Randy Russ, Arantza Strader, Laura Vasquez and Ericka Witcher, for help with collections care and data collection; William Hahn and George Proctor for providing material from field research; and Jack Fisher for discussion. This work was funded by generous support of the Kelly Foundation.
LITERATURE CITED
- Abouheif E. A method for testing the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research. 1999;1:895–899. [Google Scholar]
- Allmon WD, Ross RM, editors. Causes of evolution: a paleontological perspective. Chicago, IL: University of Chicago Press; 1990. [Google Scholar]
- Asmussen CB, Dransfield J, Deickmann V, Barfod AS, Pintaud J-C, Baker WJ. A new subfamily classification of the palm family (Arecaceae): evidence from plastid DNA phylogeny. Botanical Journal of the Linnaean Society. 2006;151:15–38. [Google Scholar]
- Asner GP, Goldstein G. Correlating stem biomechanical properties of Hawaiian canopy trees with hurricane wind damage. Biotropica. 1997;29:145–150. [Google Scholar]
- Bellingham PJ. Landforms influence patterns of hurricane damage: evidence from Jamaican montane forests. Biotropica. 1991;23:427–439. [Google Scholar]
- Brokhaw NVL, Grear JS. Forest structure before and after Hurricane Hugo at three elevations in the Luquillo Mountains, Puerto Rica. Biotropica. 1991;23:386–392. [Google Scholar]
- Caruso CM, Peterson SB, Ridley CE. Natural selection on floral traits of Lobelia (Lobeliaceae): spatial and temporal variation. American Journal of Botany. 2003;90:1333–1340. doi: 10.3732/ajb.90.9.1333. [DOI] [PubMed] [Google Scholar]
- Clarke PJ, Kerrigan RA. Do forest gaps influence the population structure and species composition of mangrove stands in northern Australia. Biotropica. 2000;32:642–652. [Google Scholar]
- Cooper-Ellis S, Foster DR, Carlton G, Lezberg A. Forest response to catastrophic wind disturbance: results from an experimental hurricane. Ecology. 1999;80:2683–2696. [Google Scholar]
- Coutts MP, Grace J, editors. Wind and trees. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Donnelly JP, Woodruff JD. Intense hurricane activity over the past 5,000 years controlled by El Niño and the West African monsoon. Nature. 2007;447:465–468. doi: 10.1038/nature05834. [DOI] [PubMed] [Google Scholar]
- Dosmann MS. Research in the garden: averting the collections crisis. Botanical Review. 2006;72:207–234. [Google Scholar]
- Emmanuel KA. Increasing destructiveness of cyclones over the past 30 years. Nature. 2005;436:686–688. doi: 10.1038/nature03906. [DOI] [PubMed] [Google Scholar]
- Fetcher N, Cordero RA, Voltzow J. Lack of ecotypic differentiation: plant responses to elevation, population origin, and wind in the Luquillo Mountains, Puerto Rico. Biotropica. 2000;32:225–234. [Google Scholar]
- Fisher JB, Burch JN, Noblick LR. Stem structure of the Cuban Belly Palm (Gastrococcus crispa) Principes. 1996;40:125–128. [Google Scholar]
- Frangi JL, Lugo AE. Hurricane damage to a flood plain forest in the Luquillo Mountains of Puerto Rico. Biotropica. 1991;23:324–335. [Google Scholar]
- de Gouvenain RC, Silander JA., Jr Do tropical storm regimes influence the structure of tropical lowland rain forests. Biotropica. 2003;35:166–180. [Google Scholar]
- Gresham CA, Williams TM, Lipscomb DJ. Hurricane Hugo wind damage to southeastern U.S. coastal forest tree species. Biotropica. 1991;23:420–426. [Google Scholar]
- Grove SJ, Turton SM, Siegenthaler DT. Mosaics of canopy openness induced by tropical cyclones in lowland rain forest with contrasting management histories in northeastern Australia. Journal of Tropical Ecology. 2000;16:883–894. [Google Scholar]
- Gunn BF. The phylogeny of the Cocoeae (Arecaceae) with emphasis on Cocos nucifera. Annals of the Missouri Botanical Garden. 2004;91:505–522. [Google Scholar]
- Hirsh H, Marler T. Damage and recovery of Cycas micronesica after Typhoon Paka. Biotropica. 1997;34:598–602. [Google Scholar]
- Henderson A, Galeano G, Bernal R. Field guide to the palms of the Americas. Princeton, NJ: Princeton University Press; 1995. [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd edn. New York, NY: Wiley-Interscience; 1999. [Google Scholar]
- Holt RD. Making a virtue out of a necessity: hurricanes and the resilience of community organization. Proceedings of the National Academy of Sciences of the USA; 2006. pp. 2005–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins MS. Disturbance – the forest transformer. In: Webb LJ, Kikkawa J, editors. Australian tropical rainforests: science – values – meaning. East Melbourne: CSIRO; 1990. pp. 40–52. [Google Scholar]
- Horvitz CC, Koop A. Removal of non-native vines and post-hurricane recruitment in tropical hardwood forests of Florida. Biotropica. 2001;33:268–281. [Google Scholar]
- Huang YS, Chen SS, Lin TP, Chen YS. Growth strain in coconut palm trees. Tree Physiology. 2002;22:261–266. doi: 10.1093/treephys/22.4.261. [DOI] [PubMed] [Google Scholar]
- Isnard S, Speck T, Rowe NP. Biomechanics and development of the climbing habit in two species of the South American palm genus Desmoncus (Arecaceae) American Journal of Botany. 2005;92:1444–1456. doi: 10.3732/ajb.92.9.1444. [DOI] [PubMed] [Google Scholar]
- Jarvinen BR, Neumann CJ, Davis MAS. A tropical cyclone data tape for the North Atlantic Basin, 1886–1983: contents, limitations, and uses. NOAA Technical Memorandum NWS NHC 22. 1984 [Google Scholar]
- Jones C. After the hurricanes of 2005. Palms. 2006;50:57–59. [Google Scholar]
- Klein WMcK. Fairchild Tropical Garden hit by Hurricane Andrew. Principes. 1992;36:225–227. [Google Scholar]
- Kuo-Huang LL, Huang YS, Chen SS, Huang YR. Growth stresses and related anatomical characteristics in coconut palm trees. IAWA Journal. 2004;25:297–310. [Google Scholar]
- Levins R. Evolution in changing environments. Princeton, NJ: Princeton University Press; 1968. [Google Scholar]
- Lippincott C. Reintroduction of Pseudophoenix sargentii in the Florida Keys. Principes. 1995;39:5–13. [Google Scholar]
- Loope L, Duever M, Herndon A, Snyder J, Jansen D. Hurricane impact on uplands and freshwater swamp forest. Bioscience. 1994;44:238–246. [Google Scholar]
- Ludlum D. Early American Hurricanes, 1492–1870. Boston, MA: American Meteorological Society; 1963. [Google Scholar]
- Miller DL, Mora CI, Grissino-Mayer HD, Mock CJ, Uhle ME, Sharp Z. Tree-ring isotope records of tropical cyclone activity. Proceedings of the National Academy of Sciences of the USA; 2006. pp. 14294–14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman CE, Sanders RW. Preliminary classificatory studies in Coccothrinax (Palmae: Coryphoideae) Selbyana. 1991;12:91–101. [Google Scholar]
- Neumann CJ, Jarvinen BR, McAdie CJ, Hammer GR. Tropical cyclones of the North Atlantic Ocean 1871–1998. Historical Climatology Series 6. 1999 [Google Scholar]
- von Neumann JR, Kent H, Bellinson HR, Hart BI. The mean square successive difference. Annals of Mathematical Statistics. 1941;12:153–162. [Google Scholar]
- Niklas KJ. Plant biomechanics: an engineering approach to plant form and function. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Niklas KJ. A mechanical perspective on foliage leaf form and function. New Phytologist. 1999;143:19–31. [Google Scholar]
- NOAA. Re-analysis project. Atlantic Oceanographic and Meteorological Laboratory, Hurricane Research Division; 2007. [Google Scholar]
- Nyberg J, Malmgren BA, Winter A, Jury MR, Kilbourne KH, Quinn TM. Low Atlantic hurricane activity in the 1970s and 1980s compared to the past 270 years. Nature. 2007;447:698–702. doi: 10.1038/nature05895. [DOI] [PubMed] [Google Scholar]
- Ostertag R, Silver WL, Lugo AE. Factors affecting mortality and resistance to damage following hurricanes in a rehabilitated subtropical moist forest. Biotropica. 2005;37:16–24. [Google Scholar]
- Pasch RJ, Blake ES, Cobb HD, III, Roberts DP. Tropical cyclone report: Hurricane Wilma, 15–25 October 2005. Miami: National Oceanic and Atmospheric Administration; 2006. [Google Scholar]
- Pezza AB, Simmonds I. The first South Atlantic hurricane: unprecedented blocking, low shear and climate change. Geophysical Research Letters. 2005;32:L15712. [Google Scholar]
- Putz FE, Shanz RR. Hurricane damage to old-growth forest in Congaree Swamp National Monument, South Carolina, U.S.A. Canadian Journal of Forest Research. 1991;21:1765–1770. [Google Scholar]
- Quine CP, Bell PD. Monitoring of windthrow occurrence and progression in spruce forests in Britain. Forestry. 1998;71:87–97. [Google Scholar]
- Reboud X, Bell G. Experimental evolution in Chlamydomonas. III. Evolution of specialist and generalist types in environments that vary in time and space. Heredity. 1997;78:507–514. [Google Scholar]
- Reeve J, Abouheif E. Phylogenetic independence. Department of Biology, McGill University; 2003. Version 2·0. [Google Scholar]
- Rich PM. Mechanical architecture of arborescent rain forest palms. Principes. 1986;30:117–131. [Google Scholar]
- Rich PM. Mechanical structure of the stem of arborescent palms. Botanical Gazette. 1987;148:42–50. [Google Scholar]
- Schoener TW, Spiller DA. Nonsynchronous recovery of community characteristics in island spiders after a catastrophic hurricane. Proceedings of the National Academy of Sciences of the USA; 2006. pp. 2220–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterken P. The elastic stability of palms. Brussels: Peter Sterken; 2007. [Google Scholar]
- Tomlinson PB. The leaf base in palms – its morphology and mechanical biology. Journal of the Arnold Arboretum. 1962;43:23–45. [Google Scholar]
- Tomlinson PB. The structural biology of palms. Oxford: Oxford University Press; 1990. [Google Scholar]
- Tomlinson PB. The uniqueness of palms. Botanical Journal of the Linnaean Society. 2006;151:5–14. [Google Scholar]
- Totland O. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology. 2001;82:2233–2244. [Google Scholar]
- Uhl NW, Dransfield J. Genera Palmarum. Lawrence, KS: Allen Press; 1987. [Google Scholar]
- Vandermeer J. Effects of Hurricane Joan on the palms of the Caribbean coast rainforest of Nicaragua. Principes. 1994;38:182–189. [Google Scholar]
- Webb LJ. Cyclones as an ecological factor in tropical lowland rainforest, north Queensland. Australian Journal of Botany. 1958;6:220–228. [Google Scholar]
- Zimmermann JK, Everham EM, III, Waide RB, Lodge DJ, Taylor CM, Brokaw NK. Responses of tree species to hurricane winds in subtropical wet forest in Puerto Rico: implications for tropical tree life histories. Journal of Ecology. 1994;82:911–922. [Google Scholar]