Abstract
Background and Aims
Reproductive assurance, the ability to produce seeds when pollinators or mates are scarce, is thought to be the major advantage of selfing in flowering plants. However, few studies have performed a direct cost–benefit analysis of the selective advantage of selfing, particularly given a long-term perspective among populations or across several flowering seasons within population. This study examined the fertility consequences of autonomous selfing in Roscoea schneideriana (Zingiberaceae), a small perennial Himalayan ginger typically found in habitats at around 3000 m a.s.l.
Methods
The floral biology of R. schneideriana was studied in natural populations; the capacity for autonomous selfing was estimated using pollinator exclusion experiments; the timing of selfing was quantified by anther removal at different times during flowering; whether autonomous selfing increases seed production was tested by emasculating flowers; and the magnitude of inbreeding depression was estimated by comparing relative performance of progeny from self- and cross-pollinations. Pollinator observations were also conducted in the natural populations.
Key Results
The hooked stigmas of most flowers curl towards the anther and can contact pollen grains at an early stage of anthesis. Flowers with potential pollinators excluded set of as many seeds per fruit as hand-selfed and opened flowers. Autonomous selfing mostly occurs within 2 d of anthesis and can increase seed production by an average of 84 % in four populations during the flowering seasons of 2005–2007. Visits by effective pollinators were extremely rare. The cumulative inbreeding depression of R. schneideriana was 0·226.
Conclusions
Autonomous selfing in R. schneideriana is achieved by stigmas curling towards the anthers early in flowering. It is suggested that under the poor pollination conditions, autonomous selfing has been selected for in this alpine ginger because it provides substantial reproductive assurance with very low costs.
Key words: Zingiberaceae, Roscoea, autonomous self-pollination, reproductive assurance, inbreeding depression, pollinator failure, Himalayan species
INTRODUCTION
Selfing is common among angiosperms with approx. 20 % of species using self-fertilization as their predominant mating strategy (Barrett, 2002). Indeed, the transition from outcrossing to selfing is one of the most frequent evolutionary trends in plants (Stebbins, 1974). Selection for reproductive assurance, where selfing ensures seed production when lack of pollinators or inefficient pollen transfer limits reproductive success, is the most widely accepted hypothesis for the evolution of selfing. Selfing can also be favoured because of gene transmission advantages (reviewed in Lloyd, 1992; Holsinger, 2000; Barrett, 2002; Eckert and Herlihy, 2004). Although selfing has many benefits, such as gene transmission advantage and reproductive assurance, it is also associated with diverse costs, including inbreeding depression, gamete discounting and seed discounting (Lloyd, 1992; Eckert and Herlihy, 2004; Goodwillie et al., 2005; Eckert et al., 2006). Thus a complete understanding of why selfing evolves requires consideration of both its benefits and its costs. Although this transition has been well documented in genetic aspects, i.e. transmission advantage versus inbreeding depression (e.g. Lande and Schemske, 1985; Charlesworth and Charlesworth, 1987), less is known about its ecological fitness consequences and its ecological context (Lloyd, 1992; Kalisz and Vogler, 2003; Kalisz et al., 2004).
The ecological benefits and costs of selfing depend on how and when selfing occurs (Lloyd, 1979, 1992), and its ecological context, e.g. the availability of pollinators (Kalisz et al., 2004). Selfing can occur within flowers (autogamy) or among flowers on the same plant (geitonogamy); autogamy can be facilitated by pollinators or occur autonomously in the absence of pollinators. Autonomous selfing can occur before, during, or after opportunities for outcrossing (prior, competing and delayed selfing, respectively) (Lloyd, 1992). In general, geitonogamy and facilitated selfing, which both occur when pollinators transfer pollen, provide little or no reproductive assurance and are not adaptive (Lloyd, 1992). In contrast, all modes of autonomous selfing provide reproductive assurance. When pollen is not limited, delayed selfing incurs no pollen or seed discounting, while prior and competing selfing can cause costs of gamete discounting. Theoretically, strong inbreeding depression can disfavour selfing (Lloyd, 1992).
Since Darwin (1876), reproductive assurance has been proposed as a principal benefit of selfing in flowering plants when seed production is limited by pollen transfer. This can be tested by removing the anthers before they dehisce, and then comparing seed production of emasculated flowers with that of intact flowers. If selfing provides reproductive assurance, emasculated flowers should set fewer seeds than intact flowers (Schoen and Lloyd, 1992). Despite the widely accepted importance of reproductive assurance, there are few experiments to directly test whether the capacity for selfing increases seed set in natural populations (Eckert and Schaefer, 1998; Eckert and Herlihy, 2004; Goodwillie et al., 2005; Eckert et al., 2006). Even fewer studies estimated the benefit of selfing among populations or across years within populations (Eckert and Herlihy, 2004; Eckert et al., 2006). In addition, costs incurred by selfing should be considered when the benefit of reproductive assurance is evaluated. For example, seed production by autonomous selfing may contribute limited fitness benefit because of strong inbreeding depression (Herlihy and Eckert, 2002). However, few empirical studies have directly quantified this selective advantage of autonomous selfing within a cost–benefit framework and in natural ecological conditions (but see Herlihy and Eckert, 2002; Kalisz et al., 2004; Brunet and Sweet, 2006; Vaughton et al., 2008).
The Zingiberaceae is a large family of animal-pollinated tropical monocotyledons (Endress, 1994). While the family is largely restricted to the tropical lowlands, the Himalayan endemic genus Roscoea, is found at high elevations (1200–4880 m a.s.l.) from Kashmir in the west to south-west China (Cowley, 1982; Ngamriabsakul et al., 2000). Roscoea species have striking orchid-like flowers with a long floral tube, apparently adapted for pollination by specialized long-tongued insects (Fenster et al., 2004). In previous investigations of Roscoea schneideriana (Fig. 1A), it was observed that it had much higher fruit set compared with its sympatric species in natural populations. It was presumed that this alpine ginger has the ability for autonomous selfing. In this study, three specific questions are addressed: (1) Is R. schneideriana capable of autonomous self-pollination? If so, then (2) how and when does self-fertilization occur in R. schneideriana? And (3) why has autonomous selfing been selected for in this alpine ginger?
Fig. 1.
(A) The plants (about 60 mm in height) and flower of Roscoea schneideriana (Zingiberaceae) in a natural population; (B) variations of sigma morphology of R. schneideriana: from left to right, before anthesis, then 12, 24 and 48 h after anthesis. Petals were removed to reveal the anthers and styles.
MATERIALS AND METHODS
Study sites and species
The study was conducted during four flowering seasons from 2004 to 2007. Most of the field work was carried out in the population of Roscoea schneideriana at Ganhaizi (GH population), Lijiang, Yunnan province, SW China (27°05′N, 100°16′E; 3120 m a.s.l.). Flower emasculation experiments were also conducted in three other populations located near Lijiang City: Wenbifeng (WB population) (26°48′N, 100°11′E; 2760 m a.s.l.), Xiangshan (XS population) (26°53′N, 100°14′E; 2500 m a.s.l.) and Lijiang Alpine Botanical Garden (LB population) (27°00′N, 100°1′E; 2830 m a.s.l.). Lijiang region, the core area of Hengduan Mountains, is considered the area of highest diversity of Roscoea species (Cowley, 1982; Ngamriabsakul et al., 2000; Wu and Larsen, 2000). The annual precipitation of Lijiang City (2393 m a.s.l.) is 934·9 mm, and the peak rainy season is from July to August (1951–1981, Meteorological department of Yunnan province, unpubl.data). There is more precipitation at higher elevation of this mountains region with an increasing rate of 103 mm per 100 m (Su and Pu, 1996).
Roscoea schneideriana (Loesener) Cowley (Zingiberaceae) is a small perennial herb that inhabits shady habitats of mixed forest, or open stony slopes and ledges of mountain cliffs, commonly around 3000 m a.s.l. (Cowley, 1982; Wu and Larsen, 2000). Plants are 9–45 cm tall, with annual pseudostem from erect, reduced rhizome; roots are fascicled, tuberous and fusiform. The flowering season of this species is usually from July to August. The inflorescence has a short peduncle. Its slender-tubed flowers are purple or white, comprising an erect hooded dorsal petal, a large labellum which has two erect petal-like staminodes near its base, and two narrow lateral petals; the dorsal petal and two erect leaf-like staminodes form a floral chamber, where anther and style stand (Fig. 1A).
Floral biology
Phenological observations were made in the GH population in the flowering season of 2004. Sixty plants were randomly selected, and the number of inflorescences, flowers per inflorescence, flower longevity, and stigma movement recorded. Flowering phenology of the GH population was also quantified at 1-week or 10-d intervals in 2007. Thirty flower buds were randomly chosen, and the anthers and ovaries fixed in FAA solution separately for pollen and ovule counting. A haemocytometer was used to estimate pollen production per flower following the methods of Dafni (1992). The number of ovules in each ovary was also counted under the dissecting microscope. For each flower, the pollen : ovule (P/O) ratio was calculated as the number of pollen grains divided by the number of ovules. If possible, means (± 1 s.e.) are given (all data in this paper are presented as means ± 1 s.e., unless otherwise noted).
Capacity for autonomous self-fertilization
Pollinator exclusion combined with hand-self pollination was used to quantify the capacity for autonomous self-fertilization. In the flowering season of 2004, >100 individuals were randomly selected from the GH population, and visitors excluded with nylon mesh bags. Plants were evenly and randomly assigned among three different pollination treatments: (1) flowers were emasculated at the onset of anther dehiscence, hand self-pollinated and bagged; (2) flowers were left intact and unpollinated; (3) flowers were left unpollinated and their anthers were removed before they dehisced. All treated flowers were prevented from receiving visitors with nylon mesh bags for the duration of flowering. Fruits were collected 30 d after pollination, and the full seeds in each fruit were counted. Flowers that did not make a fruit were considered as producing zero seeds. Treatments 1 and 2 were repeated in the flowering season of 2005. To assess the capacity for autonomous selfing, a two-way ANOVA was used to compare seed production in flowers receiving treatments 1 and 2 in two flowering seasons, with treatment and year as fixed factors. An index of autofertility was also estimated as: mean seed production of treatment 2/mean seed production of outcross flowers (Schoen and Lloyd, 1992); these data on seed production of outcrossed flowers were obtained from the inbreeding data set (see below). Treatment 3 was included to confirm that autonomous seed set did not occur through either apomixis or accidental pollination by insects that entered the bags.
Timing of autonomous selfing
The proportion of autonomously selfed seed was quantified at different times in the life of flowers in the GH population in 2006. More than 150 plants with buds were selected and prevented from receiving visitors with nylon mesh bags for the duration of flowering. Plants were evenly and randomly assigned among five anther removal treatments (one flower per plant): (1) anthers were removed just prior to the anthesis; (2) anthers were removed 12 h after anthesis; (3) anthers were removed 24 h after anthesis; (4) anthers were removed 48 h after anthesis; (5) anthers were left intact. Seeds per flower were counted after 30 d. To test the relationship between timing of anther removal and mean seed production, polynomial regression analysis was used. Then a one-way ANOVA was used to compare the reproductive output at different anthesis stages.
Flower visitors
Visitors of R. schneideriana were observed continuously from 0930 to 1630 for 3 d in the GH population during the flowering season of 2004 and 2005, and for 2 d in the XS population during the flowering season of 2007. All the observations were conducted on fine sunny days. All types of flower visitors were photo-recorded and preserved in the insect collections of Xishuangbanna Tropical Botanical Garden.
Experiment to quantify reproductive assurance
The extent autonomous selfing increased seed production was determined by removing the capacity for autogamy by emasculating flowers before anther dehiscence. Experimental emasculations were conducted in one population (GH) in 2004, two populations (GH and WB) in 2005, and three populations (GH, XS and LB) in 2006. In each population 60–100 plants with buds were randomly selected. Plants were evenly and randomly assigned for one of the following pollination treatments: (1) anthers of flowers were removed before anthesis; (2) flowers were left intact. It is not possible to remove the whole anther of a flower because like most gingers, the soft styles penetrate through the anther. Fruits were collected after about 30 d and seeds per fruit were counted. Seed production was compared using a two-way ANOVA with population/year and treatment as fixed factors. The increase in seed production was calculated via autogamy for each population as the difference between the mean seed production of intact flowers and that of emasculated flowers (Lloyd, 1992; Schoen and Lloyd, 1992). Reproductive assurance was estimated as: 1 – (mean seed production of emasculated flowers/mean seed production of intact flowers) (Eckert et al., 2006).
Inbreeding depression
To examine inbreeding depression, 60 plants with buds were randomly selected in the GH population used to assess self-compatibility and the performances of selfed and crossed seeds compared. Visitors were excluded from flowers with nylon mesh bags for the duration of flowering. Flowers on these plants received one of two hand-pollination treatments: (1) flowers of 30 plants received pollen from the same flower (selfed); (2) flowers were emasculated and then hand pollinated with pollen from other plants at least 3 m away (outcrossed). Fruits were collected after 30 d and the seeds counted. These seed count data were used to calculate an index of autofertility (see above). To determine seed mass, seeds were mixed from the same treatment and 180 selfed seeds and 180 crossed seeds were randomly selected from each treatment; seeds of each treatment were evenly and randomly divided into six groups. Seeds were dried in a roaster set at 130°C for 24 h, and then weighed. To measure germination rate, 45 selfed seeds and 45 crossed seeds were randomly selected from both self- and cross-pollination treatments, and the seeds placed in a moist germination cabinet set at 25°C with a 12-h light : 12-h dark photoperiod. The number of germinated seeds was recorded over the next 2 weeks. For seed set and seed mass, a t-test was used to compare the mean values of selfed and outcrossed progenies. For fruit set, seed germination, and seedling survival, 2 × 2 contingency tables were used to compare values of selfed and outcrossed progenies. Inbreeding depression was estimated at five stages: fruit set, seed set, seed mass, seed germination and seedling survival. Inbreeding depression (δ) was calculated for each stage as δ = 1 – (ws/wo), where ws and wo are the fitness of selfed and outcrossed progeny, respectively. Cumulative inbreeding depression was calculated by multiplying fitness values for each cross-type across all life stages and then applying the formula above.
RESULTS
Floral biology
Phenological investigations of Roscoea schneideriana in the GH population indicated that flowering of this species occurs mainly from July to mid-August, which is the peak period of the rainy season in this area. R. schneideriana produces one inflorescence per plant with one to four flowers per inflorescence (Table 1); Inflorescences generally flower for 4–15 d and produce one flower after another at intervals of 2–6 d (3·4 d ± 0·72, n = 11). Flowers usually begin anthesis in the early morning and last for 4 d (Table 1). The anthers usually dehisce and stigmas produce stigmatic fluid at the beginning of anthesis. The distance between anthers and hooked stigmas is about 2 mm at that time. The funnel-like stigma progressively elongates and curls towards the erect anther during flowering (Fig. 1B). Of the flowers surveyed, stigmas of 66·7 % flowers (30 flowers, n = 54) could touch the dehisced anther about 24 h after anthesis and kept curling; 80 % of them (36 flowers) could be self-pollinated in this way within 2 d of flowering.
Table 1.
Floral characteristics of Roscoea schneideriana based on sample from GH population
| No.flowers/inflorescence | Longevity of flower (d) | Pollen grains | Ovules | P/O ratio | |
|---|---|---|---|---|---|
| Mean ± s.e. | 1·6 ± 0·1 | 4·0 ± 0·5 | 5791 ± 17 | 31 ± 1 | 195 ± 16 |
| Range | 1–4 | 3·5–5 | 1333–9967 | 15–45 | 45–344 |
| n | 60 | 60 | 30 | 30 | 30 |
Flowers do not produce nectar and no odour was detected. The number of pollen grains produced per flower was 5791 ± 417; the number of ovules was 31 ± 1. Hence, the P/O ratio of R. schneideriana flowers was 195 ± 16 (Table 1).
Capacity for autonomous self-pollination
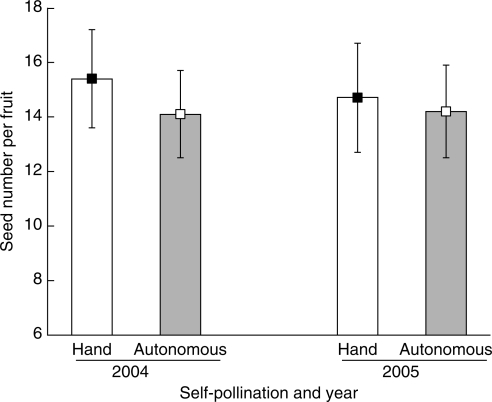
Bagged emasculated flowers did not set seeds, suggesting that autonomous seed set did not occur through either apomixis or accidental pollination by insects that entered the bags. Bagged intact flowers set as many seeds as flowers self-pollinated by hand both in 2004 (autonomous selfed: 14·1 ± 1·5; hand-selfed: 15·4 ± 2·0; t = 0·545, d.f. = 59, P = 0·59) and 2005 (autonomous selfed: 13·6 ± 1·7; hand-selfed: 13·9 ± 2·0; t = 0·114, d.f. = 83, P = 0·91), indicating a well-developed capacity for autonomous selfing (Fig. 2). In a two-way ANOVA, year, treatment, and year × treatment interaction were not significant, and treatments affected seed production similarly in both years (Table 2). Relative autofertility measures were 1·0 and 0·96, respectively, in the two flowering seasons studied.
Fig. 2.
Capacity for autonomous selfing of Roscoea schneideriana. Bagged flowers (autonomous selfing) and hand-selfed flowers did not significantly differ in seed set in two flowering seasons (P < 0·05). Analysis of these data is presented in Table 2.
Table 2.
Two-way ANOVA examining the effect of year and pollination treatment on seed production per flower in Roscoea schneideriana
| Source | SS | d.f. | MS | F | P |
|---|---|---|---|---|---|
| Year | 2·73 | 1 | 2·73 | 0·024 | 0·88 |
| Treatment | 28·25 | 1 | 28·25 | 0·244 | 0·62 |
| Year × treatment | 5·79 | 1 | 5·79 | 0·050 | 0·82 |
| Error | 15950·22 | 138 | 115·58 |
Timing of autonomous selfing
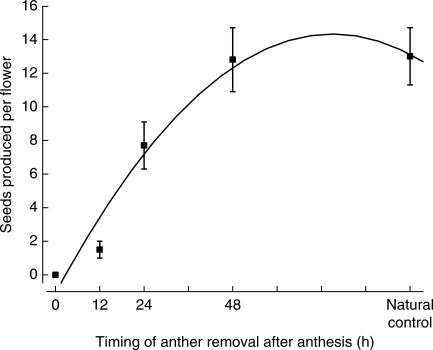
The timing of anther removal and mean seeds per flower show a significant quadratic line relationship (r2 = 0·96, F2,2 = 26·3, P = 0·03). One-way ANOVA analysis also shows a significant effect on the number of seeds produced per flower (one-way ANOVA, F4,154 = 20·01, P < 0·0001; Fig. 3). When anthers were removed before flower opening, flowers did not set fruits and seeds, indicating that autonomous selfing did not occur through prior selfing. When anthers were removed about 12 h and 24 h after anthesis, the numbers of seeds produced per flower were 11·3 % and 60·0 % that of natural control flowers, respectively. Two-day flowers, however, produced as many seeds as natural control flowers (2-d flowers: 12·8 ± 1·9; control flowers: 13·0 ± 1·7; t = 0·081, d.f. = 65, P = 0·94). Because almost all seeds in each fruit were produced during the early life of 4-d flowers, autonomous selfing in R. schneideriana most likely occurred during the period of ‘competing selfing’ (Lloyd, 1992).
Fig. 3.
The relationship between the timing of anther removal and self-seed production in the GH population of R. schneideriana.
Flower visitors
Few insects were observed visiting flowers of R. schneideriana in natural populations. A small Curculionid beetle Rhadinomerus sp. (∼3 mm in length) was the most common visitor to R. schneideriana with 39 visits recorded during the 56-h observation of 40–50 flowers. These beetles most commonly landed on the labellum or outside of the dorsal petal, before entering the floral chamber. The beetles spent a long time in the chamber eating pollen from the lower part of the anthers. They are not effective pollinators because they occasionally transfer pollen within the same flower when eating the pollen. A visit by a small bee, Anthophora sp., was also observed in the XS population, and occasional visits by ants, but they did not collect pollen or contact the sexual organs.
Reproductive assurance
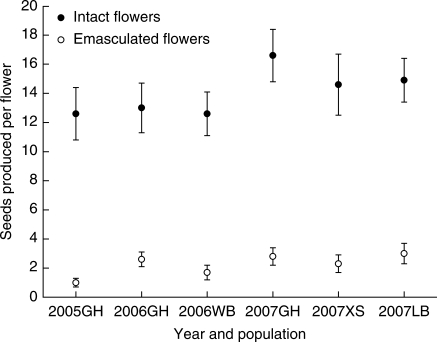
Emasculations were carried out in the GH population during three flowering seasons and in three other populations (WB, LB and XS) during one flowering season. Experimentally preventing autonomous self-fertilization reduced seed production in all the populations of R. schneideriana studied (Fig. 4), and the variation in seed set among populations or within populations was very minor compared with the effect of autogamy (Table 3). Reproductive assurance measures (RA) were 0·92, 0·80 and 0·83, respectively, in the GH population for the flowering seasons of 2005, 2006 and 2007. For the WB, LB and XS populations in the flowering seasons studied, the measures of RA were 0·87, 0·80 and 0·84, respectively. The mean RA was 0·84.
Fig. 4.
The effect of eliminating autogamy by emasculation on mean seed production in natural populations of Roscoea schneideriana. Emasculated flowers had pollen removed to eliminate autogamy. Both intact and emasculated flowers experienced natural pollinator visitation. Analysis of these data is presented in Table 3.
Table 3.
Two-way ANOVA on the effect of population/year and treatment on mean seed production per flower of Roscoea schneideriana
| Source | SS | d.f. | MS | F | P |
|---|---|---|---|---|---|
| Population/year | 463·02 | 5 | 92·60 | 1·25 | 0·28 |
| Treatment | 16233·31 | 1 | 16233·31 | 219·62 | 0·00 |
| Population/year × treatment | 134·34 | 5 | 26·87 | 0·36 | 0·87 |
| Error | 34518·57 | 467 | 73·92 |
Inbreeding depression
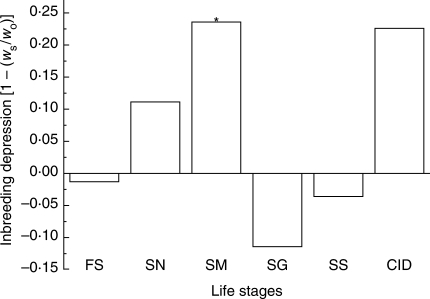
Mean values of five traits of self versus outcross progenies were compared, and the results indicated that only seed mass had a significant difference (self: 95·5 ± 1·4; outcross: 73·0 ± 3·3; t = –6·23, d.f.= 11, P <0·01); fruit set (self: 86·5 %; outcross: 85·4 %; χ2 = 0·02, d.f. = 1, P = 0·89), seeds per fruit (self: 16·0 ± 1·9; outcross: 18·0 ± 1·9; t = –0·7, d.f. = 66, P = 0·49), seed germination (self: 86·7 %; outcross: 77·8 %; χ2 = 1·23, d.f. = 1, P = 0·27), and seedling survival (self: 94·9 %; outcross: 91·6 %; χ2 = 0·38, d.f. = 1, P = 0·85) were not significantly different. Inbreeding depression of fruit set, seed set, seed mass, seed germination and seedling survival were –0·013, 0·111, 0·236, –0·114 and –0·036, respectively; and the cumulative inbreeding depression of R. schneideriana was 0·226 (Fig. 5), lower than 0·5, the threshold at which inbreeding depression balances the genetic transmission advantage of selfing (Lande and Schemske, 1985).
Fig. 5.
Inbreeding depression of Roscoea schneideriana. For inbreeding depression, ws and wo are performance of selfed and outcrossed progeny, respectively. FS, Fruit set; SN, seed number per fruit; SM, seed mass; SG, seed germination; SS, seedling survival; CID, cumulative inbreeding depression. * Significant difference between fitness of selfed and outcrossed offspring (P < 0·05).
DISCUSSION
Roscoea schneideriana has a well-developed capacity for autonomous selfing by curling its stigma towards its dehisced anthers during the early stage of flowering. The estimated timing of autonomous selfing fell potentially within the period of ‘competing selfing’ (Lloyd, 1992) for the GH population. Pollinator observations and seed production for emasculated flowers indicated that pollinator service was unreliable in the natural populations studied. Emasculations conducted over three flowering seasons in six populations showed that autonomous selfing provided substantial reproductive assurance in this alpine ginger. Because of pollination failure and the lack of strong inbreeding depression, the gamete and seed discounting costs incurred by autonomous selfing were low. These results support the hypothesis that autonomous selfing by R. schneideriana has been selected because it provides reproductive assurance with low costs.
The mechanism and mode of autonomous selfing
The present phenological observations suggest that flowers of R. schneideriana do not appear to be dichogamous because anthers dehisced and stigmas produced stigmatic fluid at the beginning of anthesis. No fruits and seeds were set when anthers were removed just before flower opening, and few seeds were set when anther removal was performed on flowers 12 h after anthesis (Fig. 3). Thus, spatial separation of stigmas and anthers appears to be the mechanism preventing autonomous selfing and promoting pollinator-mediated outcrossing in the early floral life-span. However, in R. schneideriana, during anthesis stigmas gradually elongate and curl towards the dehisced anthers and in the second day of flowering the stigmas generally reach the pollen sac and contact pollen grains (Figs 1B and 3). The anther removal experiments also demonstrated that curling stigmas result in autonomous selfing. It is clear that autonomous selfing in R. schneideriana is achieved by hooked stigmas curling towards dehisced anthers. Similar mechanisms for autonomous selfing have been observed in several other angiosperm species. For example, delayed selfing in Hibiscus laevis is achieved by stigmas progressive curling towards anthers (Klips and Snow, 1997). In blue-eyed Mary, Collinisia verna, stigmas elongate towards the dehisced anther during late floral development (Kalisz et al., 1999). Conversely, in incompletely protogynous Aquilegia canadensis anthers curve downward toward the stigmas (Eckert and Schaefer, 1998).
Three modes of autonomous selfing (prior, competing and delayed) can be distinguished based on when each occurs relative to the opportunity of outcrossing (Lloyd, 1992). As discussed above, there is a period when potential outcrossing can occur before selfing in R. schneideriana, suggesting autonomous selfing could be considered as delayed selfing. However, selfing also occurs early in flowering so can be considered competing selfing rather than delayed selfing (Kalisz and Vogler, 2003). Although previous studies indicated that the predominant mode of autonomous selfing in plants is delayed selfing (Fenster and Martén-Rodríguez, 2007), in reality, selfing does not always fit neatly into ‘prior’, ‘competing’ or ‘delayed’ categories. It seems that R. schneideriana experiences a brief period during which outcrossing alone can occur, but then experiences competing selfing and potential outcrossing or facilitated selfing.
Selective value of autonomous selfing
Few visitors were observed in the natural populations of R. schneideriana, suggesting that geitonogamy and facilitated selfing, which both depend on pollen vectors, have little opportunity to occur. The present results indicated that geitonogamy has little chance of occuring in R. schneideriana even when visitors are abundant. This is because (a) R. schneideriana has small fascicled, tuberous, fusiform roots so is not strongly branching (clonal) like other tropical gingers, and (b) its floral display is very small with just a few flowers opening one by one at intervals of about 4 d. Thus the reproductive output of this species is mostly the result of autonomous selfing.
Bagged flowers of R. schneideriana, which could set seeds only by autonomous selfing, have the same seed production as opened flowers, corroborating the well-developed capacity for autonomous selfing (Fig. 2). These results suggested that autonomous selfing could ensure seed production when pollinators are scarce or absent. By comparing emasculated and intact flowers under natural pollination conditions, it was found that outcross pollen was limited and autonomous selfing strongly boosted seed production (Fig. 4). The mean reproductive assurance of the populations studied was 0·84, which was greater than for most other taxa for which reproductive assurance has been quantified in previous studies (Eckert et al., 2006).
Inbreeding depression is a major selective cost that prevents the evolution of selfing (Charlesworth and Charlesworth, 1987). Theoretically, if 0·5 < δ< 1, selfing will lose the benefit of transmission advantage; if δ = 1, then the reproductive assurance is negated (Lloyd, 1992; Vaughton et al., 2008). Inbreeding depression throughout the entire life cycle is expected to be higher, especially under natural field conditions. Although inbreeding depression was measured only up to seedling survival stage in relatively benign growth chamber conditions, the inbreeding depression (0·226, Fig. 5) in R. schneideriana should not be high enough to counteract selfing benefits. The early arrival of selfing pollen may usurp ovules that could be outcrossed, producing lower-quality inbred seeds, and reducing the number of outcrossed and higher-fitness seeds. Because visitors to flowers were chronically scarce, there was little or no gamete discounting cost for autonomous selfing in R. schneideriana (Lloyd, 1992).
Pollen limitation and autonomous selfing
Roscoea species have showy orchid-like flowers with a long, slender corolla tube, suggesting specialized pollination by long-tongued insects, but the present results clearly show that R. schneideriana has a well-developed capacity for autonomous self-fertilization (Fig. 2). Fenster and Martén-Rodríguez (2007) suggested that the ability for autonomous self-pollination can evolve independently of pollination specialization. When pollen limitation reduces plant reproductive success, selection could favour reproductive strategies that make plants less dependent on pollinators to reduce pollen limitation (Totland and Sottocornola, 2001; Ashman et al., 2004). Several studies documented that even in plants with showy floral structure and display, autonomous selfing can be selected for under poor pollination conditions (e.g. Wang et al., 2004; Zhang et al., 2005). Using floral emasculation, the present results show that an inadequate quantity of outcross pollen strongly limits reproductive output in all the populations studied (Fig. 4). Thus, reproductive assurance is likely to drive the evolution of autonomous modes of selfing in R. schneideriana, such that this species is not dependent on insects for reproductive success.
It seems that pollinator failure is the reason for pollen limitation in R. schneideriana. Here, two possible causes which may account for the unreliable pollinator service are assumed. First, pollinator failure in the natural populations of R. schneideriana in this area may be caused by too much rain during the flowering season. Based on 30 years (1951–1981) of meteorological data, the flowering seasons of R. schneideriana were found to be exactly coincident with the peak of the rainy season. Rainy weather may be the cause of the chronic pollinator limitation in these populations because insects are highly dependent on their environment. The other explanation for pollinator failure is that the principal pollinator of the genus Roscoea may be lost in the Chinese Himalayas. According to floral morphology, flowers of most Roscoea species require long-tongued, nectar-foraging pollinators for successful pollination. Fletcher and Son (1931) and Dierl (1968) described how a long-proboscid fly Corizoneura longirostris visited flowers of Roscoea species for nectar in the Nepal Himalayas. However, in the present study this or similar insect visitors were not found on R. schneideriana and several other Roscoea species over many years of observations (Z. Q. Zhang, unpubl. data). Breakdown of mutualism can occur if pollinators are lost in highly specialized pollination systems (Bond, 1994). One recent study also demonstrated that long-proboscid pollinators were absent from long-corolla-tubed Pedicularis flowers in Chinese Himalaya (Huang and Fenster, 2007). Collapse of a pollination mutualism may have caused R. schneideriana and its related species in the Chinese Himalayas to have become dependent on autonomous selfing or generalist visitors to achieve successful pollination. It is speculated that the nectarless flowers may have released this species from the selective constraint associated with long-proboscid pollinators, and, more importantly, the low cost and substantial benefit of reproductive assurance was responsible for the selection of autonomous selfing in R. schneideriana in the harsh environment of the Chinese Himalayas.
ACKNOWLEDGMENTS
We sincerely thank W.-J. Xie for field assistance; C. R. Herlihy, C. H. Cannon and two anonymous reviewers for their valuable suggestions; X.-S. Chen, S. Sun and G.-Y. Hao for comments on earlier versions of the manuscript; D.-R. Yang for identifying insects; and Y.-J. Luo for helping with the statistics. We give special thanks to Professor S. Hiscock for his constructive suggestions and very detailed help in revising our use of the English language. This research was supported by National Basic Research Program of China (973 Program) 2007CB411603, and the Fund for Top One Hundred Young Scientists of Chinese Academy of Sciences.
LITERATURE CITED
- Ashman T-L, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell D, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Bond WJ. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 1994;344:83–90. [Google Scholar]
- Brunet J, Sweet HR. The maintenance of selfing in a population of the Rocky Mountain columbine. International Journal of Plant Sciences. 2006;167:213–219. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Cowley EJ. A revision of Roscoea (Zingiberaceae) Kew Bulletin. 1982;36:747–777. [Google Scholar]
- Dafni A. Pollination ecology: a practical approach. Oxford: Oxford University Press; 1992. [Google Scholar]
- Darwin CR. The effects of cross and self-fertilization in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Dierl W. Zur Nahrungsaufnahme von Corizoneura longirostris (Hardwicke) (Diptera: Tabanidae) Khumbu Himal. 1968;3:76–81. [Google Scholar]
- Eckert CG, Herlihy CR. Using a cost-benefit approach to understand the evolution of self-fertilization in plants: the perplexing case of Aquilegia canadensis (Ranunculaceae) Plant Species Biology. 2004;19:159–173. [Google Scholar]
- Eckert CG, Schaefer A. Does self-pollination provide reproductive assurance in wild columbine, Aquilegia canadensis (Ranunculaceae)? American Journal of Botany. 1998;85:919–924. [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder L, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Thomson JD, Dudash MR. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Fenster CB, Martén-Rodríguez S. Reproductive assurance and the evolution of pollination specialization. International Journal of Plant Sciences. 2007;168:215–228. [Google Scholar]
- Fletcher TB, Son SK. A veterinary entomology for India, Part XIV. Indian Journal of Veterinary Science and Animal Husbandry. 1931;1:192–199. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating system in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics. 2005;36:47–79. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. Reproductive systems and evolution in vascular plants. Proceedings of the National Academy of Sciences of the USA. 2000;97:7037–7042. doi: 10.1073/pnas.97.13.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-Q, Fenster CB. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. International Journal of Plant Science. 2007;168:325–332. [Google Scholar]
- Kalisz S, Vogler DW. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology. 2003;84:2928–2942. [Google Scholar]
- Kalisz S, Vogler D, Fails B, Finer M, Shepard E, Herman T, et al. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae) American Journal of Botany. 1999;86:1239–1247. [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Habkey KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Klips RA, Snow AA. Delayed autonomous self-pollination in Hibiscus laevis (Malvaceae) American Journal of Botany. 1997;84:48–53. [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plant. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist. 1979;113:67–79. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Science. 1992;153:370–380. [Google Scholar]
- Ngamriabsakul C, Newman MF, Cronk CB. Phylogeny and disjunction in Roscoea (Zingiberaceae) Edinburgh Journal of Botany. 2000;57:39–61. [Google Scholar]
- Schoen DJ, Lloyd DG. Self-fertilization and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press; 1974. [Google Scholar]
- Su Z, Pu JC. The developing conditions, numbers, and form of glaciers in Hengduan mountain range. In: Li JJ, editor. Glaciers in Hengduan mountain range. Beijing: Science Press; 1996. [Google Scholar]
- Totland O, Sottocornola M. Pollen limitation of reproductive success in two sympatric alpine willows (Salicaceae) with contrasting pollination strategies. American Journal of Botany. 2001;88:1011–1015. [PubMed] [Google Scholar]
- Vaughton G, Ramsey M, Simpson I. Does selfing provide reproductive assurance in the perennial herb Bulbine vegans (Asphodelaceae)? Oikos. 2008;117:390–398. [Google Scholar]
- Wang Y-Q, Zhang D-X, Renner SS, Chen Z-Y. A new self-pollination mechanism. Nature. 2004;431:39–40. doi: 10.1038/431039b. [DOI] [PubMed] [Google Scholar]
- Wu TL, Larsen K. Zingiberaceae. In: Wu ZY, Peter HR, editors. Flora of China. Beijing: Science Press; 2000. pp. 322–377. [Google Scholar]
- Zhang L, Barrett SCH, Gao J-Y, Chen J, Cole WW, Liu Y, et al. Predicting mating patterns from pollination syndromes: the case of ‘sapromyiophily’ in Tacca chantrieri (Taccaceae) American Journal of Botany. 2005;92:517–524. doi: 10.3732/ajb.92.3.517. [DOI] [PubMed] [Google Scholar]