Abstract
Background and Aims
The monogeneric Kirkiaceae (Sapindales) were formerly placed as Kirkioideae in Simaroubaceae. However, recent molecular phylogenetic studies indicate that they are not in Simaroubaceae and they appear to be sister to the clade of Anacardiaceae plus Burseraceae. Such affinity was never considered or discussed since the first description of Kirkia. The present study is the first detailed analysis of the floral structure of a representative of Kirkiaceae and the first comparison with other sapindalean families, especially Anacardiaceae and Burseraceae.
Methods
Floral structure of Kirkia wilmsii was studied using transversal and longitudinal microtome section series, scanning electron microscopy and light microscopy.
Key Results
The flowers of Kirkia wilmsii are morphologically bisexual but functionally unisexual. They are polysymmetric, isomerous (tetramerous) and haplostemonous. The ovary is syncarpous and entirely synascidiate. The floral apex forms a hemispherical protrusion on top of the ovary. The styles are free but postgenitally united and apically form a stigmatic head with a compitum. Each carpel is uniovulate (biovulate in a few other species) and ovules are crassinucellar, bitegmic and slightly campylotropous. The micropyle is formed by both integuments and is unusually long. The unusual two radially disposed locules in each carpel in the former genus Pleiokirkia can be explained developmentally by the two offset and tightly contiguous lateral placentae.
Conclusions
Paralleling the molecular results, a suite of floral features supports the position of Kirkiaceae close to the Anacardiaceae–Burseraceae clade, and not in Simaroubaceae.
Key words: Kirkiaceae, floral structure, gynoecium, Sapindales, Anacardiaceae, Burseraceae, monoecy, functional dioecy, heterodichogamy
INTRODUCTION
Kirkia Oliver is a sapindalean genus with six species of small to medium-sized trees in eastern tropical Africa, South Africa and Madagascar (Engler, 1897; Stannard, 1981, 2007). Oliver (1868a, b) first described Kirkia and included it in Simaroubaceae (as Simarubeae). Engler (1896) also placed it in Simaroubaceae and established the monotypic tribe Kirkieae in Simarouboideae, one of the four subfamilies he circumscribed. Three new species were later added to the genus when Engler (1931c) raised Kirkieae to subfamilial level. Based on a similar fruit structure but double the number of carpels, Capuron (1961) described a monotypic genus Pleiokirkia, endemic to Madagascar, and considered it to be close to Kirkia. The close relationship between Kirkia and Pleiokirkia was also supported by fruit anatomy (Fernando and Quinn, 1992). Pleiokirkia was later sunk into Kirkia (Stannard, 2007).
The affinities of Kirkia within Simaroubaceae remained uncertain for a long time despite comparative studies on wood anatomy (Webber, 1936; Heimsch, 1942; Metcalfe and Chalk, 1950), pollen morphology (Erdtman, 1952), gynoecium structure (Ramp, 1988), fruit structure (Fernando and Quinn, 1992) and phytochemistry (Polonsky, 1983; da Silva and Gottlieb, 1987; Simão et al., 1991; Mulholland et al., 2003). Potential relationships of Kirkia with other sapindalean families were never suggested, although Oliver (1868b, p. 27) mentioned that it could be a Burseraceae.
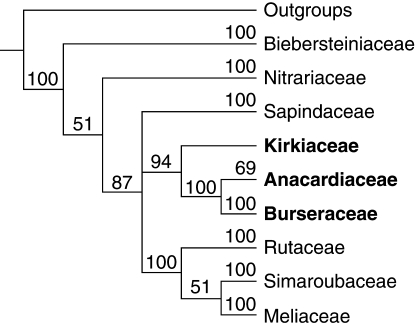
Molecular phylogenetic studies showed that Simaroubaceae are an artificial taxon made up partly of only distantly related components currently placed in Sapindales and Malpighiales (Irvingiaceae), or unplaced in malvids (Picramniaceae) (Fernando et al., 1995; Stevens, 2001 onwards). That Kirkia forms a family, Kirkiaceae, was first suggested by Takhtajan (1966). But its position within Sapindales remained uncertain (Bakker et al., 1998; Fernando et al., 1995; Gadek et al., 1996; Muellner et al., 2007). Depending on taxon sampling, and DNA regions and methods used, Kirkiaceae either appear toward the base of the Sapindales (Gadek et al., 1996; Bakker et al., 1998) or as sister to the Anacardiaceae–Burseraceae clade (Fig. 1; Gadek et al., 1996; Fernando et al., 1995; Muellner et al., 2007).
Fig. 1.
Phylogenetic relationships in Sapindales, based on rbcL sequences (Bayesian posterior probabilities indicated above the branches; simplified from Muellner et al., 2007).
Apart from a short account on the gynoecium (Ramp, 1988), the present analysis of the floral structure is the first in a representative of the family Kirkiaceae. Furthermore, a comparison with the floral structure of the clade Anacardiaceae plus Burseraceae is made possible by the comparative studies on floral morphology plus anatomy and development, with special emphasis of the gynoecium in both families (Bachelier and Endress, 2007; J. B. Bachelier and P. K. Endress, unpubl. res.), and also by the studies on aspects of floral structure in Anacardiaceae by Wannan and Quinn (1991) and Wannan (2006).
MATERIALS AND METHODS
Flowering material of Kirkia wilmsii Engl. fixed in FAA was provided by Mrs D. Fourie (no collection number), National Botanical Garden, Pretoria (South Africa), to E. Ramp in 1987. The material was studied using light microscopy (LM) and scanning electron microscopy (SEM). For LM investigations, the material was embedded in Kulzer's Technovit 7100 (2-hydroxyethyl methacrylate), following a protocol adapted from Igersheim (1993) and Igersheim and Cichocki (1996). Serial microtome sections were made at 5, 7 or 10 µm, using a Microm HM 355 rotary microtome and a standard microtome knife D. The sections were stained with ruthenium red and toluidine blue, and mounted in Histomount (protocol adapted from Weber and Igersheim, 1994). For SEM investigations, specimens were stained with 2 % osmium tetroxide, dehydrated in ethanol and acetone, critical-point dried and sputter coated with gold, and studied at 20 kV with a Hitachi S-4000 scanning electron microscope. The fixed material and permanent slides of serial microtome sections are deposited at the Institute of Systematic Botany, University of Zürich (Z).
RESULTS
Morphology
The flowers are arranged in compound thyrsoids with the cymes dichasial and in higher branching orders monochasial. Although functionally unisexual, the flowers are always morphologically bisexual. They are polysymmetric and isomerous, and mostly tetramerous (Figs 2 and 3). Pentamerous or hexamerous flowers are also found on some low branching orders, and trimerous flowers on high branching orders.
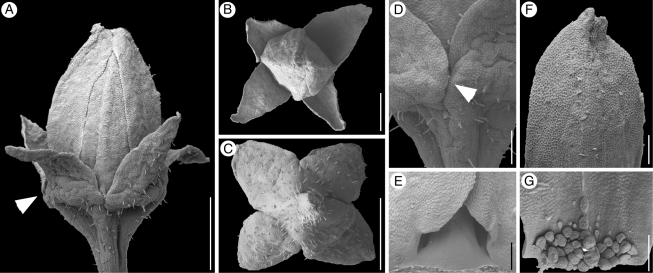
Fig. 2.
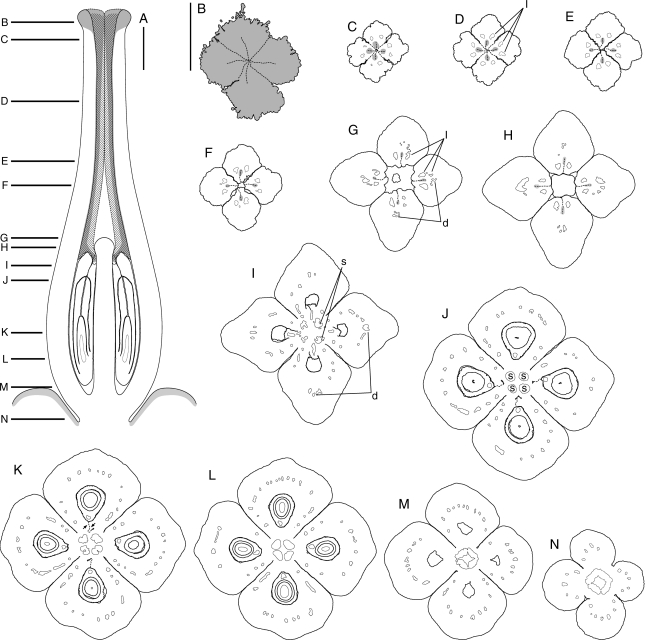
Kirkia wilmsii. Flower buds and parts of flower buds. (A) Bud, lateral view, arrowhead points to close-up in (D). (B) Same bud, from above, with sepals arranged in decussate pairs. (C) Another bud, from below, with sepals arranged in decussate pairs. (D) Bud shown in (A), lateral view, close-up on floral base and overlapping sepal margins (arrowhead). (E) Petal aestivation basally open and imbricate further up. (F) Petal tip, dorsal side. (G) Carpet of secretory hairs on inner side of petal base. Scale bars: A, C = 400 µm; B = 200 µm; D, E, F, G = 100 µm.
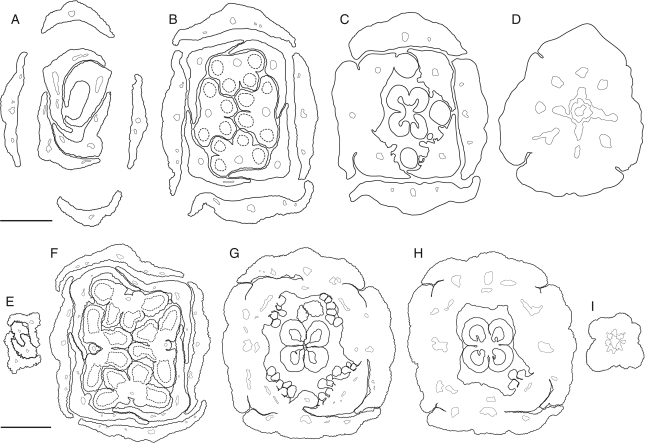
Fig. 3.
Kirkia wilmsii. Transverse microtome section series of two flower buds. Morphological surfaces drawn with thick continuous lines; secondary morphological surfaces drawn with thick dashed lines; vascular bundles drawn with thin continuous lines. (A–D) Male flower bud: (A) open sepal aestivation and imbricate petal tips; (B) contiguous (valvate) sepal aestivation and imbricate petal bases, showing two pairs of antesepalous stamens and introrse anthers with a broad and thick connective; (C) valvate sepal bases and floral cup formed by fusion of the central part of sepal bases, and petal and stamen bases, showing secretory hairs on the inner side of the petal bases and four antepetalous (delayed) sterile carpels; (D) floral base. (E–I) Sterile flower bud: (E) imbricate petal tips, arranged in pairs; (F) sepals and petals arranged in pairs, two pairs of antesepalous sterile stamens, with anthers dorsifixed basally and filament attachment hidden in a pseudopit (for term see Endress and Stumpf, 1991); (G) overlapping free sepal margins and floral cup formed by fusion of the central part of sepal bases, and petal and stamen bases, showing the carpet of secretory hairs on the inner side of the petal bases and four antepetalous carpels; (H) dorsal side of petal bases expanding between the free sepal bases and floral cup surrounding a (sterile) syncarpous and synascidiate ovary with four aborted ovules; (I) pedicel. Scale bars: A–D = 200 µm; E–I = 500 µm.
The flowers are relatively small (<1 cm in diameter). They have long, jointed pedicels and a broad floral base. They are haplostemonous, with the stamens alternipetalous and the carpels antepetalous (Figs 3 and 4A). A short floral cup is formed by congenitally united petal and stamen bases (Fig. 3C, G, H).
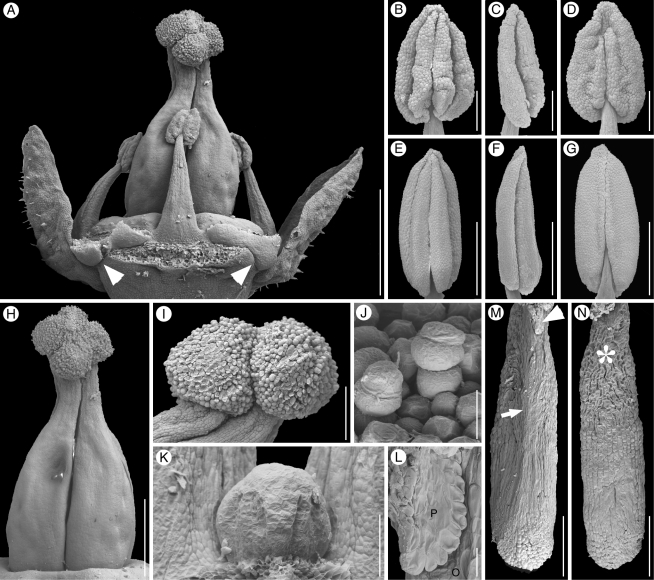
Fig. 4.
Kirkia wilmsii. Reproductive structures. (A) Preanthetic female flower, lateral view, perianth partly removed; arrowheads pointing to protruding petal bases. (B–G) Anthers: (B–D) sterile anther of anthetic female flower; (E–G) fertile anther of male flower bud; (B, E) ventral view; (C, F) lateral view; (D, G) dorsal view, filament attachment hidden between thecae. (H) Preanthetic gynoecium of the flower in (A), lateral view. (I) Same gynoecium, close-up of stigmatic head, lateral view. (J) Stigmatic papillae. (K) Hemispherical protrusion above the ovary, lateral view. (L) Sterile placenta (P) (with collapsed epidermal cells) appressed to the base of the (fertile) ovule (O). (M, N) Fertile ovule: (M) raphal side with arrowhead pointing to sterile placenta and arrow pointing to raphe; (N) antiraphal side with asterisk indicating collapsed enlarged cells of outer integument. Scale bars: A = 500 µm; B, C, D, I, M, N = 90 µm; E, F, G, H = 200 µm; J, L = 10 µm; K = 50 µm.
Sepals are free and triangular (Fig. 2A–C). They are contiguous (valvate) in early stages of development but later the floral base and floral cup enlarge and thus their aestivation becomes open (Fig. 2A–D). The base of the sepals takes part in the floral cup but their extended margins remain free and overlap basally (Figs 2D and 3C, G, H). In tetramerous flowers, the sepals are arranged in pairs with the outer pair in median position (Figs 2A–C and 3). In pentamerous flowers, their aestivation is quincuncial at the base.
Petals are free, linear and acute (Fig. 2A, F). Basally, they expand between the sepal margins with a dorsal bulge (Fig. 4A). In contrast to the sepals, their aestivation is basally open but it is imbricate further up (Fig. 2A, E) and two patterns are observed in tetramerous flowers: (1) one petal inside, one petal outside, and two in between (Fig. 3A, B), or (2) two petals outside and two inside (Fig. 3E, F). The petals protect the inner floral organs in late bud when they become longer than the sepals or even earlier when sepal aestivation changes from valvate to open. Postgenital coherence between the overlapping margins of the petals is formed by interdentation of their papillate surface and striate cuticular ornamentation. At anthesis, the expanded petals are curved slightly inwards and their basal dorsal bulges push the sepal margins away from each other. The arrangement of the sepals in decussate pairs is more conspicuous because the outer pair appears inserted below the inner pair. Calyx and corolla are widely open and androecium and gynoecium are thus exposed (Fig. 4A; see also figures in Immelman, 1984).
Stamens have a broad and thick filament base that narrows and becomes more round further up, and a sagittate and slightly apiculate anther (Fig. 4A). Anthers are dorsally basifixed (Fig. 4A–G). The transition from filament to anther is hidden by the dorsal parts of the thecae, which curve backwards around the constricted tip of the filament and form a pseudopit (Fig. 4D, G; a pit open on one side, here the dorsal side; for term see Endress and Stumpf, 1991). The connective is thick and broad (Fig. 3B, F). Each anther has a shallow dorsal and a deep ventral median (longitudinal) furrow (Fig. 3B, F). The anther is broader on the dorsal than the ventral side, and is thus introrse (Figs 3B, F and 4B, D, E, G). The dehiscence lines extend from the tip of the thecae down to their base and encompass their lower shoulders (Fig. 4B, C, E, F). In our material, the flowers of the low branching orders of the inflorescence had sterile anthers and were thus functionally female (Fig. 4A). In contrast, in flowers terminating axes of higher branching orders, the anthers were more developed than the carpels, and thus were more likely functionally male (Fig. 3A–D). In some flowers, both sexes appeared abortive (Fig. 3E–I). A thick and lobed intrastaminal nectary disc is present but expands only late in development (Figs 4A and 5A).
Fig. 5.
Kirkia wilmsii. Anthetic gynoecium. Morphological surfaces drawn with thick continuous lines; thick dashed lines used in (A) for parts outside the median plane of symmetry, in (B–N) for postgenitally united surfaces; vascular bundles drawn with thin continuous lines; pollen tube transmitting tract dark grey. d, Dorsal vascular bundle; l, lateral vascular bundle; s, synlateral vascular bundle. (A) Schematic median longitudinal section of gynoecium and nectary disc (light grey); postgenitally united surfaces hatched. (B–N) Transverse microtome section series; (B) stigmatic head; (C, D) postgenitally united distal parts of the carpels; (E, F) connivent but free parts of the carpels; (G, H) connivent bases of the free parts of the carpels around the hemispherical protrusion on top of the ovary; (I–M) synascidiate ovary, the two arrows in (K) pointing to the S-shaped line formed by the two lateral placentae (compare with Figs 6F and 7A); (N) gynophore. Scale bars: A, B–N = 500 µm.
The gynoecium is of angiospermy type 4 (Fig. 5; carpels closed entirely by postgenital fusion; for term see Endress and Igersheim, 2000). Superficially, the entire gynoecium gives the impression of being apocarpous because the dorsal part of the carpels is conspicuously bulging (Fig. 4A, H). However, the gynoecium has a syncarpous superior ovary with a short stalk (gynophore) (Fig. 5A, I–N). Above the ovary, the gynoecium is apocarpous (Fig. 5A–H). However, the free parts of the carpels are contiguous, form a conical stylar part (Fig. 5A, E–H), and are distally postgenitally united for half of their length (Fig. 5A–D). They form an oblique and flattened four-lobed receptive plate (‘stigmatic head’), each lobe corresponding to the tip of a carpel (Figs 4A, H, I and 5A, B). The free part of the carpels is plicate and has a ventral median longitudinal slit extending from the stigma down to the ovary (Fig. 5A–H). The (united) stigmas form an external compitum (Fig. 5A, B). The stigmatic surface has unicellular (spherical) and uniseriate multicellular (moniliform) papillae (Figs 4J and 6A) and is covered with secretion. Four pollen tube transmitting tracts differentiate downwards in the inner angle of the ventral slit of the carpels (Fig. 5A–H). Below the short compitum, they extend separately toward the base of the stylar canals and the placentae (Fig. 5A, C–I). The gynoecium is entirely synascidiate in the ovary (Fig. 5A, I–M). A symplicate zone is lacking. Above the ovary, basally between the free parts of the carpels, there is a conspicuous hemispherical protrusion (Figs 4K, 5A, G, H and 6B).
Fig. 6.
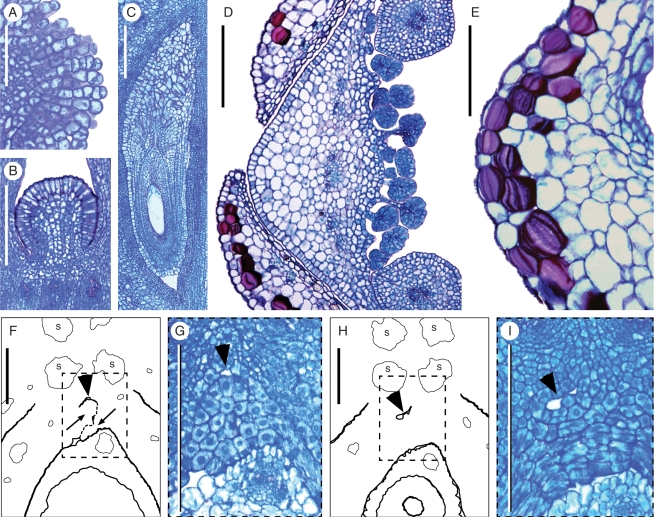
Kirkia wilmsii. (A) Longitudinal section (LS) of uniseriate multicellular papillae of the stigma before anthesis. (B) LS of the hemispherical protrusion in the floral centre above the ovary (compare with Fig. 5A). (C) LS of the slightly campylotropous ovule filling the locule at anthesis, with the expanded large-celled distal parts of the two integuments forming a long micropyle. (D) Transverse section (TS) of base of sepals, petal and stamens before anthesis, showing carpet of secretory hairs (with short multiseriate stalk and large multicellular head) on the inner side of the petal base, and petal base expanding dorsally between the free margins of two sepal bases. (E) LS of sepal base showing the epidermal and sub-epidermal special mucilage cells. (F–I) TS of a preanthetic gynoecium and corresponding enlarged micrographs, showing the inner angle of a fertile locule and centre of the gynoecium (locule dorsal side oriented downwards; compare with Fig. 5K); arrows point to placentae; arrowhead points to the second reduced locule developing on the same radius as the fertile one (compare with Fig. 7A); morphological surfaces drawn with thick continuous lines; postgenitally fused morphological surfaces drawn with dashed lines; vascular bundles drawn with thin continuous lines; ‘s’: synlateral vascular bundle; dash rectangles in (F) and (H) show location of (G) and (I). (F, G) In the upper part of the locule, the endocarp differentiation begins laterally and the ventral inner surface of the carpel is S-shaped (arrowhead). (H, I) Lower down, the endocarp encompasses the inner angle of the locule and the second locule is isolated (arrowhead). Scale bars: A = 30 µm; B, C, D = 200 µm; E, F, G, H, I = 100 µm.
The carpels are uniovulate (Fig. 5I–L). However, they have two axile and almost collateral placentae in the uppermost part of the locule (Figs 5I–K, 6F, G and 7A). The second placenta slightly protrudes in such a way that it resembles a second ovule aborting early in development (Fig. 4L, M). Behind the second placenta, toward the centre of the gynoecium, there is a small gap (Figs 6F–I and 7A). This may correspond to the ‘inner locule’ described in other Kirkia species (Fig. 7B; see Discussion; see also figures in Capuron, 1961).
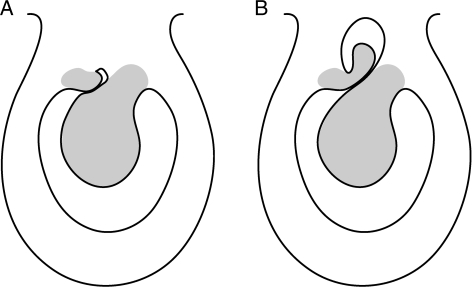
Fig. 7.
Transverse section diagrams of a carpel: (A) Kirkia wilmsii with one locule and a small inner opening; (B) Kirkia leandrii (‘Pleiokirkia’) with two ‘locules’, the inner one corresponding to the small inner opening in Kirkia wilmsii.
The ovule is long and cylindrical (Fig. 4M, N). It is crassinucellar, bitegmic, antitropous (ovule curvature direction opposite to direction of carpel involution; for term see Endress, 1994), and slightly campylotropous with only the very base of the nucellus and embryo sac curved (Fig. 5A). The two integuments surround the nucellus and, although both appear to be of the same thickness, the inner integument comprises three or four cell layers but the outer only two or three cell layers (Figs 5A and 6C). Above the nucellus the integuments are elongate and thickened. At anthesis, the inner integument is about twice as long as the nucellus and the outer even two and a half times (Figs 5A and 6C). Thus, the micropyle is unusually long and comprises two distinct zones. The proximal zone is a straight tubular canal formed by the inner integument, whereas the distal zone is not tubular and is somewhat wavy, and is formed by the second integument (Figs 5A and 6C). The extended part of the integuments above the nucellus comes about by cell enlargement (Fig. 6C). In isolated ovules studied with the SEM, these enlarged cells tend to collapse (Fig. 4 N). The ovule fills the locule and the micropyle is contiguous with the placenta (Fig. 5A).
Anatomy
Sepals have one median and two lateral main vascular bundles, which extend almost through their whole length, and may have one to two smaller, additional lateral bundles in their free parts (Fig. 3A–C, F–H). Toward the sepal base, the smaller lateral bundles merge with one of the two main lateral bundles before extending into the floral base (Fig. 3G, H). Petals have one median main vascular bundle (Fig. 3A–C, E–H) and can have up to three pairs of smaller, lateral bundles at anthesis. Toward the petal bases, all lateral bundles merge together with the main median bundle and a single petal trace extends downwards (Fig. 3C, D, G, H). Stamens have a single bundle, which extends into the upper half of the anthers (Fig. 3B, C, F–H).
In carpels, a pair of lateral vascular bundles differentiates just below the stigmatic head on each side of the ventral slit (Fig. 5C). These laterals extend downwards into the ovary and form synlaterals in the synascidiate zone (Fig. 5D–J). At the upper end of the locule, each synlateral gives off a branch serving an adjacent ovule (Fig. 5I) and ending in the chalaza, whereas lower down, the synlaterals converge toward the centre of the gynoecium and form a ring-shaped central vascular complex (Fig. 5K–N).
In contrast, distinct dorsal bundles are present only far below the zone of postgenital union of the free upper carpel parts (Fig. 5G). Between the dorsal and lateral bundles there are numerous smaller bundles and together they form a reticulate system above the ovary and extend downwards around the locules (Fig. 5G–M). They merge in the gynophore with the ring of the synlaterals.
In the floral base, the petal traces merge with the lateral traces of the sepals whereas the stamen traces merge with the median sepal traces of the same radius. All vascular bundles converge toward the central vasculature of the gynoecium and form a stele with it (Fig. 3D, I).
Histology
Lignified unicellular hairs are sparsely present on the floral base and the dorsal side of sepals, the petal parts, which are not covered by another petal in bud, and the ventral side of the petal base (Figs 2A, C, D, F, G and 4A). Stomata are present on the dorsal side of sepals and petals, on the smooth surface of the nectary disc, and on the carpel tips below the stigmatic head. On the ventral side of the petal bases, there is a carpet of hairs with a multicellular multiseriate stalk and massive head containing dark-staining cells (Figs 2G, 3C, G, H and 6D). Epidermal and subepidermal special mucilage cells (Fig. 6D, E; cells with thickened mucilaginous, layered inner tangential wall; for term see Matthews and Endress, 2006) are present in the sepals and floral base in late buds and anthetic flowers.
DISCUSSION
Sexual system
The presence of functionally unisexual (but morphologically bisexual) flowers appears to be common in Kirkiaceae (this study; Oliver, 1868a; Capuron, 1961; Stannard, 1981; Immelmann, 1984), and is also common in Anacardiaceae, Burseraceae and other Sapindales (J. B. Bachelier and P. K. Endress, unpubl. res.). Functional dioecy by flushes of male and female flowers as described by Immelmann (1984) for Kirkia wilmsii may be morphologically reflected by the presence of female flowers in the lower order branches of the cymes of the thyrsoid inflorescences, and male flowers in the higher order branches, and sequential opening of flowers of successive branching orders (this study). This is a kind of dichogamy and even (imprecise) heterodichogamy if the flowering schemes of different individuals by Immelman (1984) are considered. Heterodichogamy is uncommon in angiosperms (Renner, 2001) but was also recorded among Sapindales, in several species of Acer and in Cupania (Sapindaceae) (Gabriel, 1968; de Jong, 1976; Bawa, 1977; Tatsuhiro, 2000; Sato, 2002; Gleiser and Verdú, 2005; Renner et al., 2007; Kikuchi and Shibata, 2008). The same pattern in the distribution of male and female flowers within an inflorescence as in Kirkia has also been reported in Anacardium (Anacardiaceae) (Copeland, 1962; Moncur and Wait, 1986; Moncur, 1988), in Cedrela, Melia and Toona (Meliaceae) (Styles, 1972; Gouvêa et al., 2008a, b), and in Cupania (Sapindaceae) (Bawa, 1977). In another type of heterodichogamy (in Hernandia, Laurales) it was also found that male and female flowers had a specific distribution pattern in the inflorescence (Endress and Lorence, 2004). Based on inflorescence structure it is to be expected that hitherto unrecognized cases of heterodichogamy may occur among Sapindales.
Floral merism
Flowers in most species of Kirkiaceae are tetramerous and isomerous (Stannard, 1981). Of special interest is the occurrence of species with double the number of carpels, still in one whorl (Capuron, 1961; Stannard, 1981). The tendency of an increase in carpel number is also present in Anacardiaceae (Pleiogynium, up to 13; Wannan and Quinn, 1991) and Burseraceae (Beiselia, up to 12; Forman et al., 1991), and in other families of Sapindales, such as Meliaceae (Turraea, up to 20; Harms, 1940), Rutaceae (Aegle, up to 20; Vasil and Johri, 1964), and also in the unplaced possibly sapindalean fossil Landeenia, approx. 18 (Manchester and Hermsen, 2000). This tendency occurs even more generally in the entire malvids (Endress and Matthews, 2006). Another pattern of interest is the co-occurrence of tetramerous and pentamerous flowers on the same individual, with the pentamerous ones especially on lower-order axes of the inflorescence, as also known from some Rutaceae (Ruta, Eichler, 1878; Skimmia, personal observation). In Kirkia wilmsii trimerous, tetramerous, pentamerous and hexamerous, isomerous, flowers were found on the same individual. Floral isomery is also common in Rutaceae (Engler, 1931b; Gut, 1966; Ramp, 1988) and Simaroubaceae (Engler, 1931c; Ramp, 1988) but less so in Anacardiaceae and Burseraceae (Engler, 1931a; J. B. Bachelier and P. K. Endress, unpubl. res.). Rutaceae also exhibit many genera with tetramerous flowers. In contrast, in Anacardiaceae pentamerous flowers are common, and in Burseraceae trimerous flowers (Lam, 1932; J. B. Bachelier and P. K. Endress, unpubl. res.). However, all numbers, three, four and five, combined with isomery, are present in Anacardiaceae, Burseraceae and Kirkiaceae (and other Sapindales) in different frequencies and distribution. Whereas in Burseraceae isomerous flowers are common, as in Kirkiaceae, in Anacardiaceae they are largely restricted to Spondioideae (S. Pell, Brooklyn Botanical Garden, unpubl. res.).
Floral cup and perianth
The flowers have an expanded base and a shallow floral cup bearing the nectary disc. The cup is formed by the congenitally united bases of petals and stamens, and the median parts of the sepals. The sepal margins remain free and extend downwards as ledges for a short distance. Such free sepal margins on a floral cup or on a floral base without a cup are not restricted to Sapindales but also occur in other rosid groups. Although they are involved in the formation of the floral cup, sepals and petals are free among themselves, thus the perianth is chorisepalous and choripetalous in Kirkiaceae, as in many other Sapindales. According to Merxmüller and Heine (1960), in Kirkia dewinteri, the calyx is united for one-third of its length. If this is correct there would be free and united calyces in the genus.
Sepals are deltoid and have three main vascular traces and thus exhibit the shape and vasculature that is common in many rosids. The petals are acuminate and have a single vascular trace. They become longer than the sepals in older buds and thus have a protective function at this stage in Kirkiaceae, Anacardiaceae and Burseraceae, as also found in various other rosids (e.g. most Celastrales, some Oxalidales, some Crossosomatales and a few Chrysobalanaceae sensu lato; Matthews and Endress, 2002, 2005a, b, 2008). Sepal aestivation of young floral buds is commonly valvate in Kirkiaceae, as in Burseraceae and some Anacardiaceae. However, it becomes open in older buds when the petal bases expand between the sepal bases, as also observed in Bursera (Burseraceae) (J. B. Bachelier and P. K. Endress, unpubl. res.). Petal aestivation is mainly imbricate and sometimes valvate (Oliver, 1868a, b; Stannard, 1981) in Kirkiaceae. Dense groups of secretory hairs with a short multiseriate stalk and large multicellular head at the inner base of the petals in Kirkia (see also Stannard, 1981) are unusual because they were not found in other areas of the flowers. In Beiselia there are secretory hairs with a large multicellular head, especially on the adaxial surface of the petal tips, but they have a uniseriate multicellular stalk as do all secretory hairs in Anacardiaceae and Burseraceae studied (J. B. Bachelier and P. K. Endress, unpubl. res.). These secretory hairs with multiseriate stalk may thus be an apomorphy for Kirkiaceae. Their concentration and restriction to a small area of the flower is suggestive of a specific function. However, since a disc that looks like a normal nectary is present, it is unlikely that the hairs are nectariferous (as is the case in the perianth of some Malvaceae; Vogel, 2000) (see below). It would be of interest to study the hairs in live material.
Androecium
Haplostemony, the presence of only one stamen whorl as consistent in Kirkiaceae is unusual for Burseraceae and occurs in only few genera (Triomma, some species of Canarium, Santiria, Protium and Crepidospermum; Leenhouts, 1956; Daly, 1989; Mitchell and Daly, 1993). In Anacardiaceae, haplostemonous flowers only occur in some Anacardioideae (Mitchell and Daly, 1993). The stamens of haplostemonous flowers are alternipetalous in all three families. Flowers with two stamen whorls in Anacardiaceae and Burseraceae (J. B. Bachelier and P. K. Endress, unpubl. res.) and other Sapindales (Rutaceae, Beille, 1902; Eckert, 1966; Gut, 1966; Simaroubaceae, Nair and Joseph, 1957; Nair and Joshi, 1958; Narayana and Sayeeduddin, 1958; Eckert, 1966) are commonly obdiplostemonous, i.e. the antepetalous stamens have a smaller base than the antesepalous ones, and, in isomerous flowers, the carpels are positioned in the antepetalous radii (contrary to expectation based on regular alternation of whorls) because there is more space available in this position. That the anthetic antepetalous stamens are less developed is commonly obvious also by their shorter filaments, as in Anacardiaceae and Burseraceae (J. B. Bachelier and P. K. Endress, unpubl. res.), and is a common situation also in many other rosids. Haplostemonous flowers with alternipetalous stamens as in Kirkiaceae may be seen as an extreme case in this trend: as complete suppression of the antepetalous stamens. Among other Sapindales, flowers in Meliaceae are mostly obdiplostemonous, rarely diplostemonous (with the carpels alternipetalous), sometimes haplostemonous with the carpels antepetalous, rarely alternipetalous (Harms, 1940); in haplostemonous Rutaceae the stamens are alternipetalous (Beille, 1902; Engler, 1931b), in Simaroubaceae alternipetalous (Brucea, Picrasma) or antepetalous (Picrolemma, Engler, 1931c). Thus the position of stamens in haplostemonous flowers in families of Sapindales other than the clade of Kirkiaceae–Anacardiaceae–Burseraceae appears less fixed.
Stamen shape in Kirkiaceae corresponds to a common type in Sapindales and other rosids with sagittate, slightly dorsifixed, introrse anthers, and with a relatively narrow transition region between filament and anther (Endress and Stumpf, 1991; Matthews and Endress, 2002, 2004, 2005a, b, 2006, 2008; Bachelier and Endress, 2007; J. B. Bachelier and P. K. Endress, unpubl. res.). Especially interesting in Kirkia is the presence of a pseudopit, the enclosure of this transition region between the two dorsal pollen sacs. Among rosids this feature is especially common in Sapindales and was reported for some Anacardiaceae and Burseraceae (Endress and Stumpf, 1991; J. B. Bachelier and P. K. Endress, unpubl. res.).
Nectary disc
A conspicuous intrastaminal nectary disc with nectar pores, often separating the androecium base from the gynoecium base for some distance, as in Kirkia, is also present in Burseraceae and most Anacardiaceae, as well as in Meliaceae, Rutaceae and Simaroubaceae. In Sapindaceae (and Mangifera of Anacardiaceae), the nectary disc is, however, extrastaminal (Ronse De Craene and Haston, 2006).
Gynoecium
The unusual ovary structure in Kirkia can be better understood when fruit differentiation is considered. The dispersal unit is a mericarp which develops from the outward bulging dorsal region of the carpels (including the locule) and detaches from a central part that remains as a column, called the ‘central column’ (Capuron, 1961) or ‘carpophore’ (Engler, 1931c; Stannard, 1981). This carpophore originates by histological differentiation from the central part of the synascidiate ovary. Such mericarps are not present in Anacardiaceae and Burseraceae. In Simaroubaceae carpels are dispersed individually, which was probably one reason why Kirkia was formerly included in Simaroubaceae. However, the morphological basis is different, since in Simaroubaceae the gynoecium is more or less entirely apocarpous (with the styles only postgenitally united) (Nair and Joseph, 1957; Nair and Joshi, 1958; Narayana and Sayeeduddin, 1958; Endress et al., 1983; Ramp, 1988). More or less apocarpous gynoecia (with the styles only postgenitally united) are also present in part of Rutaceae (Gut, 1966; Endress et al., 1983; Ramp, 1988).
Another unusual trait in the ovary is the reported presence of two locules per carpel in radial disposition, each with an ovule, in the former genus Pleiokirkia (Capuron, 1961). This may also be the case in other species of Kirkia (Stannard, 1981). Whereas a compartmentalization of each carpel into two collateral locules is known in various groups of angiosperms, a radial disposition of two locules is highly unusual and morphologically puzzling. However, the analysis of the gynoecium structure in K. wilmsii in the present publication allows a morphological explanation of the two radially disposed locules in the former genus Pleiokirkia. Although in Pleiokirkia there are two ovules, the ovule of the inner locule aborts, and although in Kirkia there is only one ovule, there are two placentae. These placentae are not collateral but somewhat radially displaced.
As in the former Pleiokirkia, only the outer placenta bears a fertile ovule whereas the inner one bears no ovule at all. The two placentae are tightly pressed together as seen in transverse sections, so that the inner surface of the carpels is more or less S-shaped. The fertile locule is on the outer side of the S. As a counterpart, there is a minute gap on the inner side of the S (Figs 6F and G and 7A) which may correspond to the inner locule in the former Pleiokirkia (Fig. 7B). Such transition between the presence of one and two ovules is also present in the Anacardiaceae–Burseraceae clade, with two more or less collateral ovules in most Burseraceae, and one ovule in most Anacardiaceae. Interestingly, there are also rare cases of the reverse situation in the two families: two ovules (the second epitropous) in Dracontomelon (J. B. Bachelier and P. K. Endress, unpubl. res.) and in Spondias (Baillon, 1874), one ovule in Beiselia (J. B. Bachelier and P. K. Endress, unpubl. res.) and in Boswellia (Sunnichan et al., 2005). However, if two ovules are present, the placentae are collateral and not or less radially displaced.
The stigmatic head, a conspicuous feature in Kirkia, consists of the postgenitally united free carpel tips. This construction allows the formation of a compitum, which is absent lower down in the gynoecium because of the apocarpous stylar part and the completely synascidiate ovary. Such an organization of a stigmatic head is also known from Burseraceae but less so from Anacardiaceae (J. B. Bachelier and P. K. Endress, unpubl. res.). A stigmatic head is also present in the more or less completely apocarpous gynoecia of Rutaceae–Rutoideae and Simaroubaceae (Endress et al., 1983), and also occurs in Meliaceae (Gouvêa et al., 2008a, b), in which the gynoecium is usually described as syncarpous (‘carpels united’, Cronquist, 1981; Takhtajan, 1997). However, there are no critical studies on the internal morphological carpel surfaces in Meliaceae, and thus it is uncertain whether the style is really syncarpous or consists only of postgenitally united carpels, although there are some publications with line drawings of transverse sections of styles, which suggest true syncarpy (Narayana, 1958a, b, 1959a; Nair, 1962; Murty and Gupta, 1978a, b; Lal, 1994). The styles are truly syncarpous in Rutaceae–Citroideae (Ramp, 1988). In both Meliaceae and Rutaceae–Citroideae at least the stigmatic head appears to be apocarpous but the carpels are postgenitally united (Ramp, 1988; Gouvêa et al., 2008a, b). The stigma in Kirkia is wet and exhibits unicellular and uniseriate pluricellular (moniliform) papillae, which are also present in Anacardiaceae and Burseraceae (Bachelier and Endress, 2007; J. B. Bachelier and P. K. Endress, unpubl. res.). This differs from the survey in Heslop-Harrison and Shivanna (1977) who reported a non-papillate (smooth) stigma for the only studied genus (Cotinus) of the Anacardiaceae–Burseraceae clade.
A further unifying trait in the gynoecium of Sapindales is the presence of an extensive remnant of the floral apex in the centre of the gynoecium that is not incorporated into the gynoecium architecture. In Kirkia, it forms a dome-shaped or almost spherical protrusion between the carpels where they are free above the synascidiate zone. In groups with entirely apocarpous (only postgenitally united) carpels (e.g. some Rutaceae, Simaroubaceae; Nair and Joshi, 1958; Ramp, 1988), it is present at the base between the free carpels. As the apically postgenitally united carpels are connivent, the protrusion cannot be seen from the outside. This protrusion is commonly hidden in taxa with postgenitally united stigmas/styles (e.g. Beiselia, Burseraceae; Dracontomelon, Anacardiaceae; Rutaceae, Simaroubaceae; J. B. Bachelier and P. K. Endress, unpubl. res.). It is, however, exposed in gynoecia without intercarpellary postgenital union (e.g. Pleiogynum, Spondias p.p., Poupartiopsis, Spondioideae; Mitchell et al., 2006; J. B. Bachelier and P. K. Endress, unpubl. res.). On architectural grounds the dome is especially large in gynoecia with an increased number of carpels (Endress, 2006). Interestingly, a symplicate zone is lacking in Kirkiaceae and in Spondioideae (Anacardiaceae), or very short in Beiselia (Burseraceae). In contrast, in core Burseraceae, a symplicate zone is present and extends from the synascidiate base of the gynoecium to the base of the stigmatic head (J. B. Bachelier and P. K. Endress, unpubl. res.).
Ovules
The crassinucellar, bitegmic ovules in Kirkia are antitropous (epitropous) as are those of Burseraceae (and also Rutaceae, Simaroubaceae and Meliaceae), while those of Anacardiaceae (and also Sapindaceae) are syntropous (apotropous). They are slightly campylotropous (especially involving the basal area of the nucellus), a trait also shared with other Sapindales, especially Burseraceae (Wiger, 1935; Narayana, 1959b, 1960a, b; J. B. Bachelier and P. K. Endress, unpubl. res.), Simaroubaceae (Wiger, 1935; Narayana, 1957), Rutaceae (Mauritzon, 1935; Boesewinkel, 1977, 1984; Souza et al., 2003), and Sapindaceae (e.g. Weckerle and Rutishauser, 2003, 2005). In contrast, Anacardiaceae tend to have anatropous ovules (Bachelier and Endress, 2007; J. B. Bachelier and P. K. Endress, unpubl. res.). The inner integument is thicker than the outer in Kirkia, another tendency shared by many Sapindales and other malvids (Endress and Matthews, 2006).
A peculiarity of the ovules of Kirkia is that they have an exceedingly long micropyle formed by elongation of both integuments. Conspicuous is the cell enlargement of the outer integument accompanying the elongation. These features were not observed in Anacardiaceae and Burseraceae and have not been reported from other Sapindales, and may thus be autapomorphies for Kirkia. The micropylar part of the outer integument is conspicuously wavy. Such wavy micropyles (but in the inner integument, with the outer not involved in micropyle formation) were illustrated for some other Sapindales as well (Burseraceae; Narayana, 1959b, J. B. Bachelier and P. K. Endress, unpubl. res.; Simaroubaceae; Narayana, 1957; Nair and Sukumaran, 1960).
Systematic aspects
Do features of floral structure support the removal of Kirkia from Simaroubaceae and a close relationship with the Anacardiaceae–Burseraceae clade, as is suggested by molecular phylogenetic studies (Muellner et al., 2007)? As seen from the comparative morphological studies on Kirkiaceae (this study) and Anacardiaceae and Burseraceae (J. B. Bachelier and P. K. Endress, unpubl. res.) and from comparison with published work on Sapindales, there is indeed a suite of features that appears to be synapomorphic for Kirkiaceae and Anacardiaceae plus Burseraceae. The pronounced convex remnant of the floral apex on top of the syncarpous and entirely synascidiate ovary, and the almost complete absence of a symplicate zone in the gynoecium, as in Beiselia (Burseraceae) and Spondioideae (Anacardiaceae), appear to be unique for this clade, as they are not known from any other family of Sapindales (not recorded in Simaroubaceae: Engler, 1931c; Nair and Joseph, 1957; Nair and Joshi, 1958; Narayana and Sayeeduddin, 1958; Ramp, 1988; Meliaceae: Garudamma, 1957; Narayana, 1958a; Nair, 1962, 1963; Murty and Gupta, 1978a, b; Lal, 1994; Rutaceae: Gut, 1966; Ramp, 1988; Sapindaceae: Weckerle and Rutishauser, 2003, 2005; Nitrariaceae: Nair and Nathawat, 1958; Ronse De Craene et al., 1996; Biebersteiniaceae: floral structure unstudied). This suite of characters is often associated with an increased number of carpels in a whorl (e.g. more than five in otherwise pentamerous flowers). However, it is also present in Kirkia with only four carpels and some Spondioideae with only three to five carpels. Thus the unique architecture of the gynoecium is not necessarily dependent on an increase in carpel number.
A number of other features of Kirkiaceae occur widely in Sapindales and are thus probably plesiomorphic for the clade of Kirkiaceae and Anacardiaceae plus Burseraceae: anthers with pseudopit, campylotropous ovules, antitropous curvature of ovules, inner integument thicker than outer (Endress and Stumpf, 1991; Endress and Matthews, 2006), and the tendency to form gynoecia with an increased number of carpels (lacking in Simaroubaceae but also present in Rutaceae and Meliaceae). These features are probably synapomorphic at the level of Sapindales or even malvids (see Endress and Matthews, 2006).
CONCLUSIONS
The present comparative study of floral structure is the first in the family Kirkiaceae. It also provides the first structural comparison of Kirkiaceae with the Anacardiaceae–Burseraceae clade within Sapindales. Both the sister relationship of Kirkiaceae and the Anacardiaceae–Burseraceae clade and a more distant relationship with Simaroubaceae, as found in molecular phylogenetic studies, are supported by floral structural features. The unusual two radially disposed locules per carpel in the former genus Pleiokirkia can be explained developmentally by the two offset lateral placentae. The results are a step to a better understanding of the floral evolution in Sapindales.
ACKNOWLEDGEMENTS
We thank Eduard Ramp for kindly providing fixed material of Kirkia wilmsii and microtome sections, which were used in addition to our own section series. We acknowledge the Brunei Forestry Department and Brunei Herbarium for support in the collection of material of Anacardiaceae and Burseraceae. We also thank Joffre Haji Ali Ahmad for organizing the collection trips and Jangarun Eri for his expertise in the field. The Georges-und-Antoine-Claraz-Schenkung is thanked for financial support of the field trip. We thank Mary Endress for reading the manuscript, and two anonymous reviewers for their detailed comments on the manuscript.
LITERATURE CITED
- Bachelier JB, Endress PK. Development of inflorescences, cupules, and flowers in Amphipterygium and comparison with Pistacia (Anacardiaceae) International Journal of Plant Sciences. 2007;168:1237–1253. [Google Scholar]
- Baillon H. Histoire des Plantes V. Paris: Hachette; 1874. [Google Scholar]
- Bakker FR, Vassiliades DD, Morton C, Savolainen V. Phylogenetic relationships of Biebersteinia Stephan (Geraniaceae) inferred from rbcL and atpB sequence comparisons. Botanical Journal of Linnean Society. 1998;127:149–158. [Google Scholar]
- Bawa KS. The reproductive biology of Cupania guatemalensis Radlk. (Sapindaceae) Evolution. 1977;31:52–63. doi: 10.1111/j.1558-5646.1977.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Beille L. Recherches sur le développement floral des Disciflores. Bordeaux: J. Durand; 1902. [Google Scholar]
- Boesewinkel FD. Development of ovule and testa in Rutaceae. I. Ruta, Zanthoxylum, and Skimmia. Acta Botanica Neerlandica. 1977;26:193–211. [Google Scholar]
- Boesewinkel FD. Development of ovule and seed coat in Cneorum tricoccon (Cneoraceae) Acta Botanica Neerlandica. 1984;33:61–70. [Google Scholar]
- Capuron R. Contributions à l'étude de la flore forestière de Madagascar. III. Sur quelques plantes ayant contribué au peuplement de Madagascar. Adansonia n.s. 1961;1:65–92. [Google Scholar]
- Copeland HF. Observations on the reproductive structure of Anacardium occidentale. Phytomorphology. 1962;11:315–325. [Google Scholar]
- Cronquist A. An integrated system of classification of flowering plants. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Daly DC. Studies in Neotropical Burseraceae. II. Generic limits in New World Protieae and Canarieae. Brittonia. 1989;41:17–27. [Google Scholar]
- Eckert G. Entwicklungsgeschichtliche und blütenanatomische Untersuchungen zum Problem der Obdiplostemonie. Botanische Jahrbücher für Systematik. 1966;85:523–604. [Google Scholar]
- Eichler AW. Blüthendiagramme II. Leipzig: Engelmann; 1878. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research. 2006;44:1–61. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences. 2000;161(Suppl.):S211–S223. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Endress PK, Lorence DH. Heterodichogamy of a novel type in Hernandia (Hernandiaceae) and its structural basis. International Journal of Plant Sciences. 2004;165:753–763. [Google Scholar]
- Endress PK, Matthews ML. First steps towards a floral structural characterization of the major rosid subclades. Plant Systematics and Evolution. 2006;260:223–251. [Google Scholar]
- Endress PK, Stumpf S. The diversity of stamen structures in ‘lower’ Rosidae. Botanical Journal of Linnean Society. 1991;107:217–293. [Google Scholar]
- Endress PK, Jenny M, Fallen ME. Convergent elaboration of apocarpous gynoecia in higher advanced angiosperms (Sapindales, Malvales, Gentianales) Nordic Journal of Botany. 1983;3:293–300. [Google Scholar]
- Engler A. Simarubaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien III. Vol. 4. Leipzig: Engelmann; 1896. pp. 202–230. [Google Scholar]
- Engler A. V – Neue Arten aus Transvaal. Notizblatt des Königlichen Botanischen Gartens und Museums Berlin. 1897;2:25–26. [Google Scholar]
- Engler A. Burseraceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. a 19a. Leipzig: Engelmann; 1931. pp. 405–456. [Google Scholar]
- Engler A. Rutaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. b 19a. Leipzig: Engelmann; 1931. pp. 187–359. [Google Scholar]
- Engler A. Simaroubaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. c 19a. Leipzig: Engelmann; 1931. pp. 359–405. [Google Scholar]
- Erdtman G. Pollen morphology and plant taxonomy. Stockholm: Almqvist & Wiksell; 1952. [Google Scholar]
- Fernando ES, Quinn CJ. Pericarp anatomy and systematics of the Simaroubaceae sensu lato. Australian Journal of Botany. 1992;40:263–289. [Google Scholar]
- Fernando ES, Gadek PA, Quinn CJ. Simaroubaceae, an artificial construct: evidence from rbcL sequence variation. American Journal of Botany. 1995;82:92–103. [Google Scholar]
- Forman LL, Brandham PE, Harley MM, Lawrence TJ. Beiselia mexicana (Burseraceae) and its affinities. Kew Bulletin. 1991;44:1–31. [Google Scholar]
- Gabriel WJ. Dichogamy in Acer saccharum. Botanical Gazette. 1968;129:334–338. [Google Scholar]
- Gadek PA, Fernando ES, Quinn CJ, Hoot SB, Terrazas T, Sheahan MC, Chase MW. Sapindales: molecular delimitation and infraordinal groups. American Journal of Botany. 1996;83:802–811. [Google Scholar]
- Garudamma GK. Studies in the Meliaceae. II. Gametogenesis in Melia azadirachta Linn. Journal of the Indian Botanical Society. 1957;36:227–231. [Google Scholar]
- Gleiser G, Verdú M. Repeated evolution of dioecy from androdioecy in Acer. New Phytologist. 2005;165:633–640. doi: 10.1111/j.1469-8137.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- Gouvêa CF, Dornelas MC, Martinelli AP. Characterization of unisexual flower development in the endangered mahogany tree Swietenia macrophylla King. (Meliaceae) Botanical Journal of Linnean Society. 2008;a 156:529–535. [Google Scholar]
- Gouvêa CF, Dornelas MC, Rodriguez APM. Floral development in the tribe Cedreleae (Meliaceae, sub-family Swietenioideae): Cedrela and Toona. Annals of Botany. 2008;b 101:39–48. doi: 10.1093/aob/mcm279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut B. Beiträge zur Morphologie des Gynoeceums und der Blütenachse einiger Rutaceen. Botanische Jahrbücher für Systematik. 1966;85:151–247. [Google Scholar]
- Harms H. Meliaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. 19bI. Leipzig: Engelmann; 1940. pp. 1–172. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. The receptive surface of the angiosperm stigma. Annals of Botany. 1977;41:1233–1258. [Google Scholar]
- Heimsch C. The comparative anatomy of the secondary xylem in the ‘Gruinales’ and ‘Terebinthales'of Wettstein with special reference to taxonomic grouping. Lilloa. 1942;8:83–198. [Google Scholar]
- Igersheim A. The character states of the Caribbean monotypic Strumpfia (Rubiaceae) Nordic Journal of Botany. 1993;13:545–559. [Google Scholar]
- Igersheim A, Cichocki O. A simple method for microtome sectioning of prehistoric charcoal specimens, embedded in 2-hydroxyethyl methacrylate (HEMA) Review of Palaeobotany and Palynology. 1996;92:389–393. [Google Scholar]
- Immelman KL. Flowering in Kirkia wilmsii Engl. Bothalia. 1984;15:151–152. [Google Scholar]
- de Jong PC. Flowering and sex expression in Acer L.: a biosystematic study. Mededelingen van de Landbouwhogeschool te Wageningen. 1976;76:1–201. [Google Scholar]
- Kikuchi S, Shibata M. Development of polymorphic microsatellite markers in Acer mono. Molecular Ecology Notes. 2008;8:339–341. doi: 10.1111/j.1471-8286.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Lal S. A contribution to the floral anatomy of Cedreleae (Meliaceae) Feddes Repertorium. 1994;105:449–455. [Google Scholar]
- Lam HJ. Beiträge zur Morphologie der dreizähligen Burseraceae-Canarieae. I. Aestivation, Meiomerie und Pleiomerie. Annales du Jardin Botanique de Buitenzorg. 1932;42:23–56. [Google Scholar]
- Leenhouts PW. Burseraceae. In: van Steenis CGGJ, editor. Flora Malesiana, I. Vol. 5. Dordrecht: Noordhoff; 1956. pp. 209–296. [Google Scholar]
- Manchester SR, Hermsen EJ. Flowers, fruits, seeds, and pollen of Landeenia gen. nov., an extinct sapindalean genus from the Eocene of Wyoming. American Journal of Botany. 2000;87:1909–1914. [PubMed] [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Cephalotaceae, Brunelliaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae) Botanical Journal of the Linnean Society. 2002;140:321–381. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Datiscaceae, Tetramelaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae) Botanical Journal of the Linnean Society. 2004;145:129–185. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae) Botanical Journal of the Linnean Society. 2005;a 149:129–194. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Crossosomatales (Crossosomatacae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae) Botanical Journal of the Linnean Society. 2005;b 147:1–46. [Google Scholar]
- Matthews ML, Endress PK. Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Systematics and Evolution. 2006;260:199–221. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Chrysobalanaceae s.l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales) Botanical Journal of the Linnean Society. 2008;157:249–309. [Google Scholar]
- Mauritzon J. Über die Embryologie der Familie Rutaceae. Svensk Botanisk Tidskrift. 1935;29:319–347. [Google Scholar]
- Merxmüller H, Heine H. Simaroubaceae. Mitteilungen der Botanischen Staatssammlung München. 1960;3:617–619. [Google Scholar]
- Metcalfe CR, Chalk L. Anatomy of the dicotyledons. Oxford: Clarendon Press; 1950. [Google Scholar]
- Mitchell JD, Daly DC. A revision of the genus Thyrsodium (Anacardiaceae) Brittonia. 1993;45:115–129. [Google Scholar]
- Mitchell JD, Daly DC, Pell SK, Randrianasolo A. Poupartiopsis, gen. nov. and its context in Anacardiaceae classification. Systematic Botany. 2006;31:337–348. [Google Scholar]
- Moncur MW. Floral development of tropical and subtropical fruit and nut species. Melbourne: CSIRO; 1988. [Google Scholar]
- Moncur MW, Wait AJ. Floral ontogeny of the cashew, Anacardium occidentale L. (Anacardiaceae) Scientia Horticulturae. 1986;30:203–211. [Google Scholar]
- Muellner AN, Vassiliades DD, Renner SS. Placing Bierbersteiniaceae, a herbaceous clade of Sapindales, in a temporal and geographic context. Plant Systematics and Evolution. 2007;266:233–252. [Google Scholar]
- Mulholland DA, Cheplogoi P, Crouch NR. Secondary metabolites from Kirkia acuminata and Kirkia wilmsii (Kirkiaceae) Biochemical Systematics and Ecology. 2003;31:793–797. [Google Scholar]
- Murty YS, Gupta S. Morphological studies in Meliaceae. II. A reinvestigation of floral anatomy of members of Swietenieae and Trichilieae. Proceedings of the Indian Academy of Sciences B. 1978;a 87:55–64. [Google Scholar]
- Murty YS, Gupta S. Morphological studies in Meliaceae. III. A reinvestigation of floral anatomy of Azadirachta and Melia. Journal of the Indian Botanical Society. 1978;b 57:195–204. [Google Scholar]
- Nair NC. Studies on Meliaceae. V. Morphology and anatomy of the flower of the tribes Melieae, Trichilieae and Swietenieae. Journal of the Indian Botanical Society. 1962;41:226–242. [Google Scholar]
- Nair NC. Studies on Meliaceae. VI. Morphology and anatomy of the flower of the tribe Cedreleae and discussion on the floral anatomy of the family. Journal of the Indian Botanical Society. 1963;42:177–189. [Google Scholar]
- Nair NC, Joseph TC. Floral morphology and embryology of Samadera indica. Botanical Gazette. 1957;119:104–115. [Google Scholar]
- Nair NC, Joshi RK. Floral morphology of some members of the Simaroubaceae. Botanical Gazette. 1958;120:88–99. [Google Scholar]
- Nair NC, Nathawat KS. Vascular anatomy of the flower of some species of Zygophyllaceae. Journal of the Indian Botanical Society. 1958;10:172–180. [Google Scholar]
- Nair NC, Sukumaran NP. Floral morphology and embryology of Brucea amarissima. Botanical Gazette. 1960;121:175–185. [Google Scholar]
- Narayana LL. Embryology of two Simaroubaceae. Current Science. 1957;26:323–324. [Google Scholar]
- Narayana LL. Floral anatomy and embryology of Cipadessa baccifera Miq. Journal of the Indian Botanical Society. 1958;a 37:147–154. [Google Scholar]
- Narayana LL. Floral anatomy of Meliaceae – I. Journal of the Indian Botanical Society. 1958;b37:365–374. [Google Scholar]
- Narayana LL. Floral anatomy of Meliaceae – II. Journal of the Indian Botanical Society. 1959;a 38:288–295. [Google Scholar]
- Narayana LL. Microsporogenesis and female gametophyte in Boswellia serrata Roxb. Current Science. 1959;b 28:77–78. [Google Scholar]
- Narayana LL. Studies in Burseraceae – I. Journal of the Indian Botanical Society. 1960;a 39:204–209. [Google Scholar]
- Narayana LL. Studies in Burseraceae – II. Journal of the Indian Botanical Society. 1960;b 39:402–409. [Google Scholar]
- Narayana LL, Sayeeduddin M. Floral anatomy of Simaroubaceae – I. Journal of the Indian Botanical Society. 1958;37:517–522. [Google Scholar]
- Oliver D. Flora of tropical Africa. a. London: L. Reeve; 1868. [Google Scholar]
- Oliver D. Kirkia acuminata. Hooker's Icones Plantarum. 1868;b 11:26–27. Ser. 3 plate 1066. [Google Scholar]
- Polonsky J. Chemistry and biological activity of the quassinoids. In: Waterman PG, Grundon MF, editors. Chemistry and chemical taxonomy of the Rutales. London: Academic Press; 1983. pp. 247–266. [Google Scholar]
- Ramp E. Struktur, Funktion und systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. University of Zurich; 1988. Doctoral Dissertation. [Google Scholar]
- Renner SS. How common is heterodichogamy? Trends in Ecology and Evolution. 2001;16:595–597. [Google Scholar]
- Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE. The evolution of dioecy, heterodichogamy, and labile sex expression in Acer. Evolution. 2007;61:2701–2719. doi: 10.1111/j.1558-5646.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Ronse De Craene LP, de Laet J, Smets EF. Morphological studies in Zygophyllaceae. II. The floral development and vascular anatomy of Peganum harmala. American Journal of Botany. 1996;83:201–215. [Google Scholar]
- Ronse De Craene LP, Haston E. The systematic relationships of glucosinolate-producing plants and related families: a cladistic investigation based on morphological and molecular characters. Botanical Journal of the Linnean Society. 2006;151:453–494. [Google Scholar]
- Sato T. Phenology of sex expression and gender variation in a heterodichogamous maple. Acer japonicum. Ecology. 2002;83:1226–1238. [Google Scholar]
- da Silva MF, das GF, Gottlieb OR. Evolution of quassinoids and limonoids in the Rutales. Biochemical Systematics and Ecology. 1987;15:85–103. [Google Scholar]
- Simão SM, Barreiros EL, da Silva MF das GF, Gottlieb OR. Chemogeographical evolution of quassinoids in Simaroubaceae. Phytochemistry. 1991;30:853–865. [Google Scholar]
- Souza LA, Mourão KSM, Moscheta IS, Rosa SM. Morfologia e anatomia da flor de Pilocarpus pennatifolius Lem. (Rutaceae) Revista Brasileira de Botanica. 2003;26:175–184. [Google Scholar]
- Stannard BL. A revision of Kirkia (Simaroubaceae) Kew Bulletin. 1981;35:829–839. [Google Scholar]
- Stannard BL. The inclusion of Pleiokirkia in Kirkia (Kirkiaceae), and corresponding combination. Kew Bulletin. 2007;62:151–152. [Google Scholar]
- Stevens PF. Angiosperm phylogeny website, Version 8, June 2007 (and more or less continuously updated since) 2001. onwards http://www.mobot.org/MOBOT/research/APweb/
- Styles BT. The flower biology of the Meliaceae and its bearing on tree breeding. Silvae Genetica. 1972;21:175–182. [Google Scholar]
- Sunnichan VG, Ram HYM, Shivanna KR. Reproductive biology of Boswellia serrata, the source of salai guggul, an important gum-resin. Botanical Journal of the Linnean Society. 2005;147:73–82. [Google Scholar]
- Takhtajan A. Systema et phylogenia Magnoliophytorum. Moscow: Nauka; 1966. [Google Scholar]
- Takhtajan A. Diversity and classification of flowering plants. New York, NY: Columbia University Press; 1997. [Google Scholar]
- Tatsuhiro A. Dichogamy in fullmoon maple (Acer japonicum Thunb.) Bulletin of the Hokkaido Forest Experiment Station. 2000;37:27–40. [Google Scholar]
- Vasil IK, Johri MM. The style, stigma and pollen tube – I. Phytomorphology. 1964;14:352–369. [Google Scholar]
- Vogel S. The floral nectaries of Malvaceae sensu lato – a conspectus. Kurtziana. 2000;28:155–171. [Google Scholar]
- Wannan BS. Analysis of generic relationships in Anacardiaceae. Blumea. 2006;51:165–195. [Google Scholar]
- Wannan BS, Quinn CJ. Floral structure and evolution in the Anacardiaceae. Botanical Journal of Linnean Society. 1991;107:349–385. [Google Scholar]
- Webber IE. Systematic anatomy of the woods of the Simarubaceae. American Journal of Botany. 1936;23:577–587. [Google Scholar]
- Weber M, Igersheim A. ‘Pollen buds’ in Ophiorrhiza (Rubiaceae) and their role in pollenkitt release. Botanica Acta. 1994;107:257–262. [Google Scholar]
- Weckerle CS, Rutishauser R. Comparative morphology and systematic position of Averrhoidium within Sapindaceae. International Journal of Plant Sciences. 2003;164:775–792. [Google Scholar]
- Weckerle CS, Rutishauser R. Gynoecium, fruit and seed structure of Paullinieae (Sapindaceae) Botanical Journal of the Linnean Society. 2005;147:159–189. [Google Scholar]
- Wiger J. Embryological studies on the families Buxaceae, Meliaceae, Simaroubaceae and Burseraceae. University of Lund; 1935. Doctoral Dissertation. [Google Scholar]