Abstract
Background and Aims
Experimental evidence in the literature suggests that O2•− produced in the elongation zone of roots and leaves by plasma membrane NADPH oxidase activity is required for growth. This study explores whether growth changes along the root tip induced by hyperosmotic treatments in Zea mays are associated with the distribution of apoplastic O2•−.
Methods
Stress treatments were imposed using 150 mm NaCl or 300 mm sorbitol. Root elongation rates and the spatial distribution of growth rates in the root tip were measured. Apoplastic O2•− was determined using nitro blue tetrazolium, and H2O2 was determined using 2′, 7′-dichlorofluorescin.
Key Results
In non-stressed plants, the distribution of accelerating growth and highest O2•− levels coincided along the root tip. Salt and osmotic stress of the same intensity had similar inhibitory effects on root elongation, but O2•− levels increased in sorbitol-treated roots and decreased in NaCl-treated roots.
Conclusions
The lack of association between apoplastic O2•− levels and root growth inhibition under hyper-osmotic stress leads us to hypothesize that under those conditions the role of apoplastic O2•− may be to participate in signalling processes, that convey information on the nature of the substrate that the growing root is exploring.
Key words: Root tip growth, Zea mays, salt stress, reactive oxygen species, ROS
INTRODUCTION
Production of reactive oxygen species (ROS) appears to be a general characteristic of expanding plant cells and organs (Foreman et al., 2003; Peleg-Grossman et al., 2007; Potocky et al., 2007). A requirement for ROS for elongation of tip-growing cells has been demonstrated for root hairs using NADPH oxidase mutants (Foreman et al., 2003; Carol and Dolan, 2006); infection threads in symbiotic relationships (Peleg-Grossman et al., 2007) and pollen tubes (Potocky et al., 2007) also require ROS for active growth. Expanding organs such as embryonic axes (Puntarulo et al., 1988), growing roots (Jon et al., 2001), and germinating seeds (Schopfer et al., 2001) can produce and extrude ROS. In maize, pharmacological treatments that reduce O2•− levels indicate that growth of leaves (Rodríguez et al., 2002) and roots (Liszkay et al., 2004) depends on active apoplastic ROS production. ROS production occurs in almost all cell compartments (Mittler et al., 2004), including the apoplast, where it has been repeatedly associated with NADPH oxidase activity as a major source. Similar experiments have shown that elongation of Arabidopsis roots depends on the generation of OH• by cell wall peroxidases and this reaction, in turn, depends on the apoplastic supply of H2O2 resulting from the dismutation of O2•−, which is produced by an NADPH-oxidase-like enzyme in the plasma membrane (Dunand et al., 2007). Ten NADPH-oxidase catalytic subunit genes exist in the Arabidopsis genome (Torres et al., 1998; Foreman et al., 2003) and atrbohF null mutants of Arabidopsis have reduced root elongation as compared to wild types (Kwak et al., 2003), providing functional evidence for the need of NADPH-oxidase-generated ROS for root elongation.
On the other hand, increased oxidative damage is often the result of stress conditions and, specifically in roots, it has been reported for drought (Porcel and Ruiz-Lozano, 2004; Sofo et al., 2004), salinity (Katsuhara et al., 2005) and heavy metals (Schutzendubel and Polle, 2005; Singh et al., 2007). Stimulated NADPH-oxidase-mediated ROS production occurs under both hyper-osmotic (Kawano et al., 2001; Beffagna et al., 2005) and hypo-osmotic stress conditions (Cazalé et al., 1998; Rouet et al., 2006), and it is known to be the result of complex regulatory mechanisms including changes in cytosolic free Ca2+ concentration (Foreman et al., 2003), pH (Beffagna et al., 2005) and phospholipids with signal functions. The plant NADPH-oxidase complex can be activated by phosphatidylinositol 3-phosphate (PI3P), the product of phosphatidylinositol 3-kinase (PI3K; Joo et al., 2005). Under salt stress, enhanced PI3K activity has been found to lead to an endocytosis-dependent intracellular activation of NADPH oxidase (Leshem et al., 2007). In Arabidopsis, phospholipase Da1 (PLDa1) and phosphatidic acid (PA) have also been implicated in increasing NADPH oxidase activity and ROS production (Sang et al., 2001).
The association between changes in ROS production and growth reductions under salt and osmotic stress has not been thoroughly explored. Decreased apoplastic O2•− production, resulting from ion-specific effects on plasma membrane NADPH oxidase (Rodríguez et al., 2007), was observed in the growing region of leaf blades from salt-treated maize plants (Rodríguez et al., 2004), where it was shown to contribute to the reduction in leaf elongation. The current study explores whether growth changes along the root tip and the distribution of apoplastic O2•− are associated in maize (Zea mays) roots subject to growth inhibition by hyper-osmotic stress treatments. A detailed analysis is presented of salt and osmotic stress effects on the spatial distribution of O2•− production along the root tip, and its correlation with the pattern of distribution of relative growth rate. The results indicate that root elongation reductions by NaCl or sorbitol occur under low and high apoplastic O2•− production, respectively.
MATERIAL AND METHODS
Root elongation measurements
Maize (Zea mays L.) ‘Prozea 30’, (Produsem, Pergamino, Argentina) was used for this study. Seeds were imbibed on moist filter paper, and after 3 d germinated seeds were transferred to plastic pouches (SCS®) containing half-strength Hoagland solution (Hoagland and Arnon, 1950). Two small styrofoam cubes inserted half way down the pouch prevented the plastic surface from sticking to the moist paper lining and helped air from the upper portion (where seeds separated the plastic and the lining) reach the roots. Pouches were kept at 21 °C under a 16-h photoperiod of 65 µE m−2 s−1.
For the short-term treatments, the root medium was changed to one containing 150 mm NaCl or 300 mm sorbitol, and root elongation rates were measured for 3 h immediately before the change and in the following 3-h treatment period. To assess the effects of stress release, the salt or sorbitol treatments were initiated when most emerged roots in the pouch were at least 2 cm long. In this case, NaCl or sorbitol were added on two consecutive days to final concentrations 150 mm (NaCl) or 300 mm (sorbitol). Two days after the final concentration was reached, the root medium was changed to half the concentration of salt or sorbitol (controls were changed to a medium of the same composition). Elongation rates were assessed by measuring the displacement over a 3- or 4-h interval using marks indicating the position of the root apices on the pouch surface. There were at least 20 seeds per experiment, and experiments were repeated up to four times. Only roots selected for uniformity of elongation rate (within +15 % of the mean per pouch) were included in the final analysis.
In order to estimate the distribution of growth rates along the root tip, a flap was opened in the pouch and marks were made on the root surface with an ultra-fine ball pen (‘Uni-Ball’, Faber Castell, Germany), at approx. 1-mm intervals for 12 mm from the root apex, as described by Sharp et al. (1988). Photographs were taken under a stereoscopic microscope with a NIKON DS camera (DS Camera Control Unit DS-L1, DS Camera Head DS-5M and DS Cooled Camera Head DS-5Mc) immediately after marking (time 0) and 3 h later. Spatial growth patterns were obtained by computing distances between marks using image-processing software (Optimas 6·1, Optimas Corporation, Bothell, WA). Segmental growth was quantified as a relative growth rate (Hunt, 1978)
where Li and Lf denote the initial and final segment length, respectively, and Δt is the measurement period. For statistical analysis, data were grouped into 0·5-mm intervals and subject to ANOVA followed by Tukey's test, using InfoStat (InfoStat/Profesional ver. 2007p, Grupo InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina).
Histochemical analyses
O2•− detection
Presence of O2•− in root tips was determined with nitro blue tetrazolium (NBT), which reacts with O2•− to produce a blue formazan precipitate. Excised root tips (approx. 1 cm long) were placed in an Eppendorf tube containing 0·01 % NBT solution of the medium in which the roots had been growing and incubated in darkness for 15 min. Note that NBT was always dissolved in the appropriate root medium, as hyper- or hypo-osmotic shocks alter O2•− levels in the tissue. Staining the root tips directly on glass slides was found to produce uneven staining and was therefore avoided; shorter and longer incubation periods were also tested. Stained roots were mounted on glass slides, in the same medium but devoid of NBT, and colour images were obtained under a stereoscopic microscope, as described above. Control staining reactions included 10 mm MnCl2, a highly effective O2•− dismutating catalyst agent (Hernández et al., 2001); this was used rather than superoxide dismutase because it readily penetrates cell walls.
The distribution of stain intensity was measured as luminance (Lu, scale 0–255 from darkest to lightest) with the image processing software, and transformed to optical density, OD = log(Lu)−1.
H2O2 detection
Roots were treated with 10 µm 2′, 7′-dichlorofluorescin (DCFH-DA) for 5 min. Epifluorescence was observed with an Axiophot microscope (Zeiss, Germany) with excitation filter BP 450–490 and emission filter LP 520. Images were taken with the camera described above, using identical exposure settings for each root zone (tip or root-hair zone).
RESULTS
Elongation rates and apoplastic O2•− distribution in root tips under hyper-osmotic stress
Maize roots elongated at a rate of approx. 1 mm h−1 in Hoagland solution, and in plants treated for 3 h with 150 mm NaCl or 300 mm sorbitol elongation rates were 0·51 and 0·56 mm h−1, respectively. Thus, these treatments decreased elongation rates by approx. 50 % and there were no statistical difference between them; however, both differed from the rate in control plants (P < 0·05). Reductions in elongation rates were even higher in roots of plants subjected to longer (4-d) stress treatments.
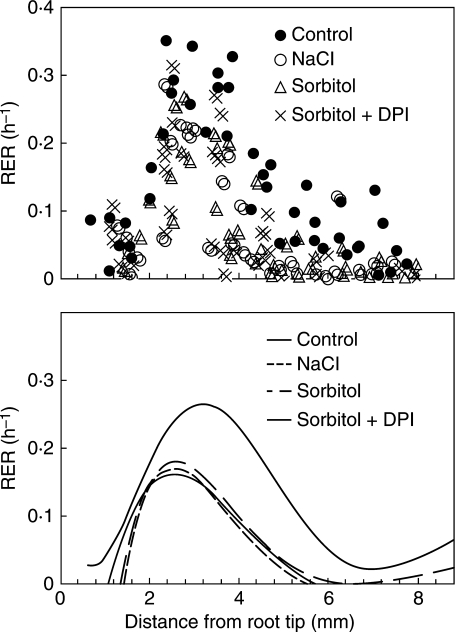
The distribution of elongation rates within root segments is shown in Fig. 1. In non-stressed roots, elongation rates increased up to approx. 3 mm from the root tip and then decreased. In the growth-acceleration zone, elongation rates were similar in controls and in roots stressed for 3 h, but peaked closer to the tip in the latter. Elongation rates in the decelerating zone were lower in both stress treatments than in control roots (P < 0·05), and the elongation zone was approx. 1 mm shorter in stressed roots compared with control roots. The distribution of growth rates in this analysis, which were determined over 3-h intervals, may be shifted towards the tip, as would be expected when intervals between marking and measurement depart from being instantaneous (Erickson and Silk, 1980). Nevertheless, the data show that stress treatments significantly affected elongation rates in the growth-decelerating zone, but not closer to the tip as previously reported by Sharp et al. (1988) and Fan and Neumann (2004) for roots under water stress.
Fig. 1.
Effect of iso-osmotic NaCl or sorbitol treatments on segmental relative elongation rates (RER) in maize roots. Plants were treated for 3 h with either 150 mm NaCl, 300 mm sorbitol or 300 mm sorbitol + 50 µm DPI. (A) RER for each segment plotted against the initial position of the mark on the root closest to the apex. (B) Profiles of RER obtained by fitting sixth-order polynomials to the data points. Data from six control roots and from seven samples for each stress treatment.
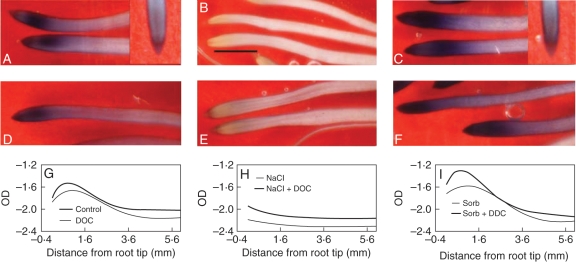
The level of O2•− was high up to 1 mm from the root tip and then decreased (Fig. 2). Staining intensity increased in sorbitol-treated roots and was decreased by NaCl treatments (Fig. 2A–C). Beyond the initial 2 mm, the stress treatments produced either a relatively high or reduced apoplastic presence of ROS. This is clearly seen when densitometry measurements of formazan distribution along the roots are compared (Fig. 2G–I; to correctly interpret these figures it must be kept in mind that the OD units are presented on a logarithmic scale). These results indicate that alterations of the root growth profile by osmotic and salt stresses of similar magnitude were significant beyond 3 mm from the root tip. Growth changes, however, were not associated with changes in apoplastic O2•− levels, not only because the treatments had different effects on O2•− levels, but also because those effects became evident closer to the apex, where growth was essentially not altered. DPI reduced O2•− levels in sorbitol-treated roots (Fig. 2C, inset); however, it had no effect on the growth of such roots (Fig. 1).
Fig. 2.
Effect of a 3-h NaCl (150 mm) or sorbitol (300 mm) treatment on O2•− levels in root tips. (A–F) NBT staining in those roots; (A, D, G) non-stressed controls; (B, E, H) NaCl treatment; (C, F, I) sorbitol treatment. (D–F) Roots treated with 1 mm DDC to inhibit SOD activity. The inset image in (A) is a control with 10 mm MnCl2, and the inset in (C) is a root treated with sorbitol + 50 µm DPI. Scale bar in (B) = 2 mm. (G–I) Densitometry measurements of stain intensity; curves are fitted with sixth-order polynomials on average measurements, n = 5.
SOD activity and H2O2 in stressed roots
Superoxide dismutase (SOD) activity and increased internal ROS generation were assessed as possible causes for differences in O2•− levels between salt- and sorbitol-treated roots. In order to determine stress-treatment effects on SOD, roots were treated with Na-diethyldithiocarbamate (DDC), an inhibitor of SOD activity (Ogawa et al., 1996); results are included in Fig. 2. DDC slightly increased stain intensity, indicating SOD activity in all cases (in Fig. 2, compare each panel in the second row, which included DDC, with its control above; corresponding densitometry measurements are below). However, in NaCl-treated roots NBT staining still remained distinctly lighter than in non-stressed roots when DDC was added (Fig. 2D vs. E), suggesting that changes in SOD activity were not responsible for the observed low apoplastic levels of O2•−.
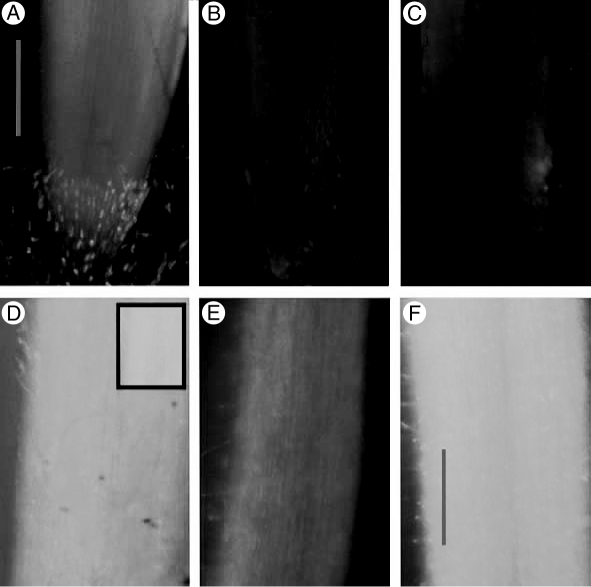
Internalization of NADPH oxidase activity could also have reduced apoplastic O2•− levels in salt-treated roots, while increasing internal ROS generation (Leshem et al., 2007). However, fluorescence microscopy of DCFH-DA-treated samples did not show increased ROS levels in the growing region of root tips for either stress treatment (Fig. 3A–C). Only further from the tip, in the root-hair region, did sorbitol-treated roots show increased fluorescence (Fig. 3D–F). These results indicate that the differential effect of these stresses on O2•− levels in the roots did not seem to be related to SOD activity, and apparently also not to increased generation of internal H2O2 in the growth zone.
Fig. 3.
Intracellular ROS detection by DCFH-DA fluorescence microscopy. (A, D) control; (B, E) 150 mm NaCl for 3 h; (C, F) 300 mm sorbitol treatment for 3 h. (A–C) Root tips, exposure time 1 s; (D–F) root hair region, exposure time 0·5 s; inset in (D) indicates fluorescence intensity in the same roots when exposure was 1 s. Sacle bars = 1 mm.
Growth stimulation and O2•− production in roots after stress relief
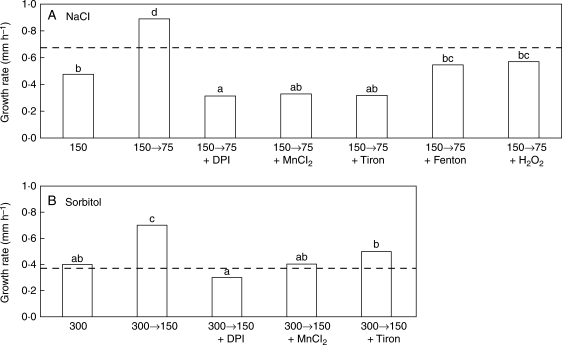
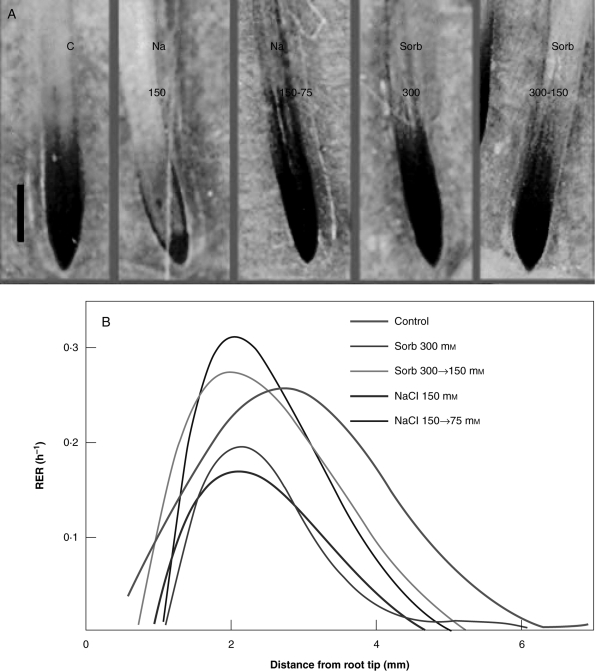
Stressed roots can be stimulated to grow by increasing the water potential in the medium. This was used to determine whether different apoplastic oxidative conditions generated by salt or osmotic stress may influence root elongation when it is stimulated by relief from stress. Plant roots were treated for 2 d with either 150 mm NaCl or 300 mm sorbitol and root elongation was then stimulated by decreasing the concentration of the growth medium. Elongation rates were measured immediately before and 4 h after the change to the root medium (Fig. 4). Root elongation rates nearly doubled when stressed roots were transferred to a medium with higher water potential (Fig. 4A: NaCl, 150 vs. 150→75; Fig. 4B: sorbitol, 300 vs. 300→150). Growth stimulation clearly depended on water uptake and was decreased by the aquaporin inhibitor HgCl2 (data not shown). The addition of DPI and of the O2•− scavengers MnCl2 and Tiron reduced growth in these experiments, indicating that O2•− production is required for growth stimulation under these conditions. However, the addition of oxidative reagents such as H2O2 or a Fenton reaction mixture to the NaCl step-down experiments also inhibited root elongation (these oxidative treatments were not applied to sorbitol-treated roots as ROS levels were already high in them). The Tiron concentration used in these experiments (assuming it did not ionize) would have added 50 mm to the 75 mm NaCl and the 150 mm sorbitol medium. In the NaCl treatment, it would have reduced the water potential gradient that it was intended to produce, but in the case of sorbitol the gradient would have still existed as the medium would be at 67 % of its original osmolarity.
Fig. 4.
Root elongation rates in stress-relief experiments. Plants were grown in Hoagland solution for 4 d and gradually treated with either 150 mm NaCl or 300 mm sorbitol. Average elongation rates (4 h) were measured on day 6, before and after transfer to a hypo-osmotic medium. The dashed lines across the figures indicate the mean elongation rate before transfer, the bars represent elongation rates after transfer; results are means of 34–119 roots. (A) Roots kept in 150 mm NaCl, or transferred to medium containing 75 mm NaCl (150 → 75) with the following additions as indicated: 50 µm DPI, 10 mm MnCl2, 50 mm Tiron, a Fenton mixture (0·2 mm ascorbate + 0·2 mm CuCl2 + 0·2 mm H2O2 ) or 100 µm H2O2. (B) Roots kept in 300 mm sorbitol, or changed to medium containing 150 mm sorbitol (300 → 150) with the following additions as indicated: 50 µm DPI, 10 mm MnCl2, 50 mm Tiron. Different letters indicate significant differences among elongation rates after transfer within each stress treatment (P < 0·05).
Stimulated root elongation in the osmotic step-down experiments was due to increases in growth rates in the region beyond 1 mm from the tip, but these were statistically higher only between 2·5 and 3 mm from the tip (Fig. 5B). Increased O2•− was detected in the growth region and beyond after reducing the NaCl concentration in the growth medium (Fig 5A). In sorbitol-treated roots, NBT staining was also higher after transfer but it was not significantly different from the initial state, which was already high in these roots. Thus, independent of the initial oxidative status of the apoplast, the stimulation of root elongation growth by stress relief was accompanied by O2•− production.
Fig. 5.
(A) NBT staining and (B) segmental elongation rates in stress-relief experiments. Plants were grown in Hoagland solution for 4 d and gradually treated with either 150 mm NaCl or 300 mm sorbitol; elongation rates were measured on day 6, before and after transfer to a hypo-osmotic medium (from 150 to 75 mm NaCl, or from 300 to 150 mm sorbitol); C indicates roots from non-stressed plants. Scale bar = 1 mm. (B) Segmental elongation rates measured immediately before and during the period following stress relief, both for 3 h. RER for each segment is plotted against the initial position of the mark on the root closest to the apex. Data are from 4–8 roots from each treatment and were fitted with sixth- or seventh-order polynomials.
DISCUSSION
O2•− levels and root growth under stress
Cell elongation depends on the hydrostatic pressure, derived from water uptake in response to a water-potential gradient generated by solute accumulation, and the yielding properties of cell walls. OH•, derived from apoplastic metabolism of O2•− (Schopfer et al., 2001), is involved in wall-polymer cleavage (Fry, 1998, 2004) and the control of ion- (Demidchik and Maathuis, 2007) and water-channel (Henzler et al., 2004) activity. These functions, in part, provide the basis for the observation that O2•− is required for the elongation of plant cells, and consequently participates in root elongation regulation. Evidence from various sources points to a role of ROS in root growth (Gapper and Dolan, 2006): atrbohF null mutants of Arabidopsis had reduced root elongation as compared to wild types (Kwak et al., 2003); in maize roots, inhibitor studies have indicated that that OH• formation resulting from apoplastic O2•− produced by NADPH oxidase activity is essential for normal root growth (Liszkay et al., 2004). In the current study, in non-stressed plants the distribution of accelerating growth and highest O2•− levels coincided along the root tip, supporting the association between growth and ROS. Root elongation stimulation after NaCl stress relief also supports this idea, as increased O2•− levels and stimulated growth coincided. However, salt and osmotic stress of the same intensity, which had similar inhibitory effects on root elongation, were not associated with consistent changes in apoplastic O2•− levels. The alterations of the root growth profile by NaCl and sorbitol hyper-osmotic stress were observed beyond 3 mm from the tip, as has been reported for water-stressed roots by Sharp et al. (1988) and Fan and Neumann (2004). O2•− levels, however, increased in sorbitol-treated roots and were decreased by NaCl treatments. Furthermore, these effects were also observed closer to the tip, in the region where growth was essentially not altered by stress, highlighting that changes in other factors dominated the growth response to stress conditions.
In the case of water stress, it has been suggested that spatial differences in proton pumping into expanding cell walls (Fan and Neumann, 2004) and cell wall metabolism (Wu et al., 2001; Zhu et al., 2007) contribute to the growth response of roots to this stress. Hardening of cell walls (Neumann et al., 1994), but not reduced proton-pumping activity (Zidan et al., 1990), have been associated with reduced maize root elongation under salinity. Growth stimulation after stress reduction implies that the cells involved had not yet experienced permanent changes in wall stiffening. Wu et al. (1996) have shown higher abundance of expansin and susceptibility to expansin action in the apical 5-mm of roots grown at low compared with high water potential, in agreement with the suggestion that there is increased yielding ability of the cell walls in this region previously made by Spollen and Sharp (1991). In leaves of salt-treated Chloris gayana plants increased wall tightening and peroxidase activity were found in the growth-decelerating zone (Ortega et al., 2006). Peroxidase activity has been detected beyond the zone of accelerated growth in maize roots (L. Ortega, IFFIVE-INTA, Argentina, pers. comm.) and it may be associated with the observed growth reductions.
While external supply of apoplastic ROS has been shown to stimulate growth in leaf segments of salt-treated plants (Rodríguez et al., 2004), the same treatment was ineffective in roots, and prevented recovery after stress relief. Oxidative damage was not detected in salt-treated roots in these experiments, indicating that this level of salinity was not sufficient to produce oxidative stress; however, supplementary ROS may have produced it, and a differential sensitivity of root and leaf tissues may explain a different response to ROS. Increased oxidative damage has been documented in roots under several stress conditions (Porcel and Ruiz-Lozano, 2004; Sofo et al., 2004; Schutzendubel and Polle, 2005; Singh et al., 2007), including salinity (Shalata et al., 2001; Katsuhara et al., 2005), and antioxidants have been implicated in stimulated growth under stress (Córdoba-Pedregosa et al., 2005), highlighting that in root tips ROS balance must be very tightly controlled.
Differential effects of NaCl and sorbitol stress treatments on apoplastic O2•− levels
O2•− abundance is the result of the balance of production and dismutation activity, each one subject to complex regulation (Mittler et al., 2004). The dual role of ROS in growth stimulation and inhibition by oxidative damage is now well established, and the balance between stimulatory and inhibitory activities depends on the type of ROS and its concentration (Knight, 2007). NADPH oxidases, pH-dependent cell wall peroxidases, germin-like oxalate oxidases and amine oxidases have been proposed to generate ROS at the apoplast. ROS-scavenging mechanisms include enzymes such as superoxide dismutase, ascorbate peroxidase, catalase, glutathione peroxidase and peroxiredoxin, which together with the antioxidants ascorbic acid, glutathione and Mn provide alternative and convergent ways to control ROS concentration. In addition, membranes are protected by α-tocopherol, which is kept in its reduced state by the pool of reduced ascorbic acid. Elements of these mechanisms are located in almost all plant organs (Blokhina et al., 2003) and cell compartments, including the apoplast (Pignocchi and Foyer, 2003), and their concerted action is responsible for the fine tuning of ROS-specific activities. Both increased (Arbona et al., 2003; Pang et al., 2005; Sekmen et al., 2007) and reduced O2•− dismutating activity under salinity has been reported (Hernández et al., 2001; Aktas et al., 2005). Drought and salinity had different effects on SOD activity when assayed in lupin leaves (Yu and Rengel, 1999). If decreased O2•− levels under salinity had resulted from increased SOD activity, DDC inhibition would be expected to increase NBT staining in the NaCl treatment, but not in sorbitol. Alternatively, if salt and sorbitol had exerted differential effects on O2•− production (and not on SOD activity), DDC would not be expected to differentially increase NBT staining in either of the stress treatments, and the presence of H2O2, a product of O2•− dismutation would have been expected to be higher in sorbitol than in NaCl. Our results are consistent with the second alternative. The inhibition of SOD activity increased staining in both treatments, suggesting that SOD activity was not differentially affected by the stress treatments we imposed. Increased SOD protein was detected in apoplastic fluid from the growth-accelerating zone of water-stressed maize roots (Zhu et al., 2007), and the results from both sorbitol-treated and NaCl-treated roots also suggest increased SOD activity in that region. The metabolic trasformation of O2•− by SOD would have resulted in increased levels of OH• and H2O2. Verifying the level of those ROS along the root would also indicate whether alterations in growth profile by stress that do not produce oxidative damage are related to other ROS (Knight, 2007) and, in this context, whether the action of sorbitol as an effective OH• scavenger (Smirnoff and Cumbes, 1989) could also be related to reduced growth of roots under that treatment. This analysis would also shed light on the perplexing finding that growth was maintained in NaCl-stressed roots whilst apoplastic levels of O2•− were drastically reduced.
Salt, but not osmotic stress, increases internalization of NADPH oxidase activity in Arabidopsis roots (Leshem et al., 2007). Internalization would result in lowering apoplastic O2•− while increasing internal ROS generation, as reported by Leshem et al. (2007). However, in our work increased DCFH-DA fluorescence in stressed roots was observed beyond the elongation zone, and only in sorbitol-treated roots. The differences between our results and those of Leshem et al. (2007) could perhaps be due to the different species studied and the duration of the treatments (3 h in our case). Direct effects of NaCl on plasma membrane NADPH oxidase activity, as suggested by Rodríguez et al. (2007), could provide an alternative explanation for those results.
Root growth rates appear to be specifically limited by the inner tissues of the stele, rather than by the epidermis (Pritchard, 1994; Fan et al., 2006). However, in the growing part of maize root tips, O2•− formation is greatest in the epidermis and vascular tissue (Liszkay et al., 2004). In this context, the lack of association between growth inhibition by salt and osmotic stress and epidermal apoplastic O2•− levels may be interpreted as indicating that ROS produced in the apoplast of the epidermis of the expanding zone could participate in an early ‘alert’ system, conveying information on the substrate quality that the new growing root is exploring, a role that has been suggested for ROS in Arabidopsis root hairs under ion-deficiency conditions (Shin et al., 2005). Cells closer to the tip, whose growth is apparently unaffected by stress, will then progress onto regions beyond it, where both osmotic and NaCl stress result in inhibited growth. The signals originating from the region closer to the tip may convey specific information under various environmental conditions, and have an influence on the adaptation of the plant to them. Clues to the differential components of this system, for the case of salinity, may reside in the genes commonly regulated by both salt and sorbitol + DPI; such analysis is under currently being undertaken.
ACKNOWLEDGMENTS
ET, HRL, ES, DB and LV are fellows of CONICET (Consejo de Investigaciones Científicas y Técnicas de la República Argentina). Funding is gratefully acknowledged from Agencia Nacional de Promoción Científica y Técnica (PICT 00270/2 to ET); Instituto Nacional de Tecnología Agropecuaria (AEEV1513 to ET) and Fundación Antorchas.
LITERATURE CITED
- Aktas H, Karni L, Chang DC, Turhan E, Bar-Tal A, Aloni B. The suppression of salinity-associated oxygen radicals production, in pepper (Capsicum annuum) fruit, by manganese, zinc and calcium in relation to its sensitivity to blossom-end rot. Physiologia Plantarum. 2005;123:67–74. [Google Scholar]
- Arbona V, Flors V, Jacas J, García-Agustín P, Gómez-Cadenas A. Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant and Cell Physiology. 2003;44:388–394. doi: 10.1093/pcp/pcg059. [DOI] [PubMed] [Google Scholar]
- Beffagna N, Buffoli B, Busi C. Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant and Cell Physiology. 2005;46:1326–1339. doi: 10.1093/pcp/pci142. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. Journal of Experimental Botany. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Cazalé A-C, Rouet-Mayer M-A, Barbier-Brygoo H, Mathieu Y, Laurière C. Oxidative burst and hypoosmotic stress in tobacco cell suspensions. Plant Physiology. 1998;116:659–669. doi: 10.1104/pp.116.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba-Pedregosa MC, Villalba JM, Córdoba F, González-Reyes JA. Changes in intracellular and apoplastic peroxidase activity, ascorbate redox status, and root elongation induced by enhanced ascorbate content in Allium cepa L. Journal of Experimental Botany. 2005;56:685–694. doi: 10.1093/jxb/eri051. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist. 2007;175:387–404. doi: 10.1111/j.1469-8137.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist. 2007;174:332–341. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Erickson R, Silk W. The kinematics of plant growth. Scientific American. 1980;242:134–151. [Google Scholar]
- Fan L, Neumann PM. The spatially variable inhibition by water deficit of maize root growth correlates with altered profiles of proton flux and cell wall pH. Plant Physiology. 2004;135:2291–2300. doi: 10.1104/pp.104.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiology. 2006;140:603–612. doi: 10.1104/pp.105.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate induced hydroxyl radicals. Biochemical Journal. 1998;332:505–515. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiology. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler T, Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell and Environment. 2004;27:1184–1195. [Google Scholar]
- Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F. Antioxidant systems and O2•−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiology. 2001;127:817–831. doi: 10.1104/pp.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular. 1950;347:1–32. [Google Scholar]
- Hunt R. Plant growth analysis. London: Edward Arnold; 1978. [Google Scholar]
- Jon JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiology. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Letters. 2005;579:1243–1248. doi: 10.1016/j.febslet.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Otsuka T, Ezaki B. Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Science. 2005;169:369–373. [Google Scholar]
- Kawano T, Kawano N, Muto S, Lapeyrie F. Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence. Plant Cell and Environment. 2001;24:1235–1241. [Google Scholar]
- Knight MR. New ideas on root hair growth appear from the flanks. Proceedings of the National Academy of Sciences of the USA. 2007;104:20649–20650. doi: 10.1073/pnas.0710632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mor IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. The EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. The Plant Journal. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2•−, H2O2, and OH•) by maize roots and their role in wall loosening and elongation growth. Plant Physiology. 2004;136:3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–496. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Neumann PM, Azaizeh H, Leon D. Hardening of root cell walls: a growth inhibitory response to salinity stress. Plant Cell and Environment. 1994;17:303–309. [Google Scholar]
- Ogawa K, Kanematsu S, Asada K. Intra- and extra cellular localization of ‘cytosolic’ CuZn-superoxide dismutase in spinach leaf and hypocotyl. Plant and Cell Physiology. 1996;37:790–799. [Google Scholar]
- Ortega L, Fry SC, Taleisnik E. Why are Chloris gayana leaves shorter in salt-affected plants? Analyses in the elongation zone. Journal of Experimental Botany. 2006;57:3945–3952. doi: 10.1093/jxb/erl168. [DOI] [PubMed] [Google Scholar]
- Pang C-H, Zhang S-J, Gong Z-Z, Wang B-S. NaCl treatment markedly enhances H2O2-scavenging system in leaves of halophyte Suaeda salsa. Physiologia Plantarum. 2005;125:490–499. [Google Scholar]
- Peleg-Grossman S, Volpin H, Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. Journal of Experimental Botany. 2007;58:1637–1649. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Current Opinion in Plant Biology. 2003;6:379–389. doi: 10.1016/s1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Pritchard J. The control of cell expansion in roots. New Phytologist. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Puntarulo S, Sanchez R, Boveris A. Hydrogen peroxide metabolism in soybean axes at the onset of germination. Plant Physiology. 1988;86:626–630. doi: 10.1104/pp.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, Grunberg K, Taleisnik E. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiology. 2002;129:1627–1632. doi: 10.1104/pp.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, Ortega L, Córdoba A, Taleisnik E. Decreased reactive oxygen species concentration in the elongation zone contributes to the reduction in maize leaf growth under salinity. Journal of Experimental Botany. 2004;53:1383–1390. doi: 10.1093/jxb/erh148. [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Lascano H, Bustos L, Taleisnik E. Salinity-induced reductions in NADPH oxidase activity in the maize leaf blade elongation zone. Journal of Plant Physiology. 2007;164:223–230. doi: 10.1016/j.jplph.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Rouet M-A, Mathieu Y, Barbier-Brygoo H, Lauriere C. Characterization of active oxygen-producing proteins in response to hypo-osmolarity in tobacco and Arabidopsis cell suspensions: identification of a cell wall peroxidase. Journal of Experimental Botany. 2006;57:1323–1332. doi: 10.1093/jxb/erj107. [DOI] [PubMed] [Google Scholar]
- Sang Y, Cui D, Wang X. Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiology. 2001;126:1449–1458. doi: 10.1104/pp.126.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiology. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzendubel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany. 2005;53:1351–1365. [PubMed] [Google Scholar]
- Sekmen AH, Turkan I, Takio S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiologia Plantarum. 2007;131:399–411. doi: 10.1111/j.1399-3054.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- Shalata A, Mittova V, Volokita M, Guy M, Tal M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiologia Plantarum. 2001;112:487–494. doi: 10.1034/j.1399-3054.2001.1120405.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiology. 1988;87:50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant and Cell Physiology. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Singh H, Batish D, Kohli R, Arora K. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regulation. 2007;53:65–73. [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Sofo A, Dichio B, Xiloyannis C, Masia A. Lipoxygenase activity and proline accumulation in leaves and roots of olive trees in response to drought stress. Physiologia Plantarum. 2004;121:58–65. doi: 10.1111/j.0031-9317.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- Spollen W, Sharp R. Spatial distribution of turgor and root growth at low water potentials. Plant Physiology. 1991;96:438–443. doi: 10.1104/pp.96.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91ph°x) The Plant Journal. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ. Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiology. 1996;111:765–772. doi: 10.1104/pp.111.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Thorne ET, Sharp RE, Cosgrove DJ. Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiology. 2001;126:1471–1479. doi: 10.1104/pp.126.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Rengel Z. Drought and salinity differentially influence activities of superoxide dismutases in narrow-leafed lupins. Plant Science. 1999;142:1–11. [Google Scholar]
- Zhu J, Alvarez S, Marsh EL, LeNoble ME, Cho I-J, Sivaguru M, et al. Cell wall proteome in the maize primary root elongation zone. II. Region-specific changes in water soluble and lightly ionically bound proteins under water deficit. Plant Physiology. 2007;145:1533–1548. doi: 10.1104/pp.107.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidan I, Azaizeh H, Neumann PM. Does salinity reduce growth in maize root epidermal-cells by inhibiting their capacity for cell-wall acidification. Plant Physiology. 1990;93:7–11. doi: 10.1104/pp.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]