Abstract
Background and Aims
Coffee seed germination represents an interplay between the embryo and the surrounding endosperm. A sequence of events in both parts of the seed determines whether germination will be successful or not. Following previous studies, the aim here was to further characterize the morphology of endosperm degradation and embryo growth with respect to morphology and cell cycle, and the influence of abscisic acid on these processes.
Methods
Growth of cells in a fixed region of the axis was quantified from light micrographs. Cell cycle events were measured by flow cytometry and by immunocytochemistry, using antibodies against β-tubulin. Aspects of the endosperm were visualized by light and scanning electron microscopy.
Key Results
The embryonic axis cells grew initially by isodiametric expansion. This event coincided with reorientation and increase in abundance of microtubules and with accumulation of β-tubulin. Radicle protrusion was characterized by a shift from isodiametric expansion to elongation of radicle cells and further accumulation of β-tubulin. Early cell division events started prior to radicle protrusion. Abscisic acid decreased the abundance of microtubules and inhibited the growth of the embryo cells, the reorganization of the microtubules, DNA replication in the embryonic axis, the formation of a protuberance and the completion of germination. The endosperm cap cells had smaller and thinner cell walls than the rest of the endosperm. Cells in the endosperm cap displayed compression followed by loss of cell integrity and the appearance of a protuberance prior to radicle protrusion.
Conclusions
Coffee seed germination is the result of isodiametric growth of the embryo followed by elongation, at the expense of integrity of endosperm cap cells. The cell cycle, including cell division, is initiated prior to radicle protrusion. ABA inhibits expansion of the embryo, and hence subsequent events, including germination.
Key words: Abscisic acid, β-tubulin, Coffea arabica, coffee seed, cell morphology, germination, microtubules
INTRODUCTION
The coffee (Coffea arabica) fruit is a drupe containing two seeds. The coffee seed is comprised of an endosperm that envelops an embryo and a peripheral spermoderm or ‘silver skin’ (Krug and Carvalho, 1939; Mendes, 1941). The thickened cell walls of the endosperm are composed mainly of mannans with 2 % of galactose (Wolfrom et al., 1961; Bewley and Black, 1994). The differentiated embryo lies inside an embryo cavity (Rena and Maestri, 1986), has a length of 3–4 mm and consists of a radicle, an axis and two small cotyledons (Mendes, 1941).
Seed germination ‘begins with the water uptake by the seed (imbibition) and ends with the elongation of the embryonic axis, usually the radicle’ (Bewley and Black, 1994). However, the majority of studies on seed germination consider protrusion of the radicle through the covering layers as the termination of the germination phase. For radicle protrusion to occur the expansion force or ‘thrust’ of the embryo must exceed the mechanical restraint of the surrounding layers of tissue, i.e. endosperm and seed coat. In a number of endosperm-retaining species it has been shown that weakening of the endosperm through hydrolytic degradation of the cell walls allows the radicle to overcome endosperm resistance. The weakening of the endosperm cap has been suggested to be a prerequisite for germination in tomato (Groot and Karssen, 1987; Haigh and Barlow, 1987), muskmelon (Welbaum et al., 1995), Datura ferox (de Miguel and Sánchez, 1992), pepper (Watkins et al., 1985) and coffee seed (da Silva et al., 2004). However, there is little information on anatomy and morphology of the coffee seed during germination.
DNA replication is necessary early during the imbibition process for DNA repair (Osborne, 1983). DNA synthesis associated with cell division is a late event during imbibition (Bewley, 1997). Accumulation of β-tubulin, assembly of microtubules, nuclear DNA synthesis and cell division have been shown to occur during imbibition of tomato seeds prior to radicle protrusion (de Castro et al., 1995, 2000). In the coffee seed the embryo grows inside the endosperm, before radicle protrusion by increasing the pressure potential and, presumably, cell wall extensibility of the embryo cells (da Silva et al., 2004), but it is unknown whether this growth is caused by cell elongation, by cell division or both.
Abscisic acid (ABA) is known to inhibit seed germination in many species (Bewley and Black, 1994). During germination ABA may act both in the embryo and in the endosperm (Hilhorst, 1995). In coffee seed, ABA inhibits germination presumably by preventing cell wall loosening in the embryo cells, and it has been shown that endogenous ABA delays germination (da Silva et al., 2004). This has also been observed in Brassica napus (Schopfer and Plachy, 1985). Microtubules play a crucial role in both cell elongation and cell division (Goddard et al., 1994). In the embryo of apple, ABA inhibited the transition of nuclei to the G2 phase of the cell cycle and, consequently, cell division was inhibited (Bouvier-Durand et al., 1989). However, ABA did not inhibit DNA repair in embryos of Avena fatua (Elder and Osborne, 1993). It is not known whether inhibition of coffee seed germination by ABA is targeted at the assembly and organization of microtubules or at other elements of the cell cycle. Therefore, this work aimed to understand the embryo growth process in terms of cell morphology and cell cycle events during coffee seed germination, as well as the effect of ABA.
MATERIALS AND METHODS
Seed source
Coffee seeds from Coffea arabica L. ‘Rubi’ were harvested in Lavras, MG, Brazil. The fruits were mechanically depulped, fermented and the seeds were dried to 12 % moisture content and stored at 10 °C during the course of the experiments.
Germination conditions
Seed coats were removed by hand and the seed surface was sterilized in 1 % sodium hypochlorite for 2 min. Subsequently, seeds were rinsed in water and imbibed on demineralized water or abscisic acid (ABA: Sigma, St. Louis, MO, USA), followed by transfer to water or hydroxyurea (Sigma) solution. The 1 m ABA solution was prepared by dissolving the powder completely in 1 n KOH and dilution in the required amount of water followed by neutralization with 1 n HCl. Four replicates of 25 seeds were placed in 94-mm Petri dishes on filter paper (no. 860, Schleicher & Schuell, Dassel, Germany) in 10 mL of water. During imbibition seeds were kept at 30 ± 1 °C in the dark (Huxley, 1965; Valio, 1976; da Silva et al., 2004). At least three seeds were taken randomly from parallel incubations for microscopy studies. The germination percentage was recorded daily until radicle protrusion no longer occurred.
Light microscopy
The entire imbibed seeds were sectioned using a microtome (Reichert, Austria). Sections of 20–30 µm thickness from the endosperm cap and the rest of the endosperm were first transferred to demineralized water and then fixed in liquid Kaiser's glycerol gelatin (Merck, Germany). Observations were made with a Nikon Optiphot microscope in bright field mode. Digital micrographs were taken with a digital Panasonic Color Video Camera or a Sony CCD Camera DKR 700. Macro images of the coffee fruit, seeds and embryo were taken using a Leica binocular microscope, model Wild MZ8. Embryos were isolated during imbibition on water or on 1000 µm ABA. The embryos were isolated by excision from the endosperm with a razor blade. Embryos were fixed in FAE (formalin 5 %, acetic acid 5 % and ethanol 70 %, v/v) with ascorbic acid (0·1 % w/v); they were vacuum-infiltrated for 1 min and kept overnight in fixative solution. The embryos were then dehydrated in an ethanol series and embedded in Technovit 7100. Longitudinal sections (3 µm thick) were made with a Microm HM-340 microtome and stained with 1 % (w/v) toluidine blue for further observations. The slides were examined under a Nikon Optiphot light microscope with a 10× objective lens. The embryonic axis was divided into ten equal parts along the axis, and width and length of 120 cells per embryo were measured in the 3rd, 7th and 9th parts after 3, 6 and 9 d of imbibition, and after 9 d following radicle protrusion. Three replicate embryos per treatment were used. Measurements were taken from cells that were randomly selected from eight cell layers located at a distance of four cell layers from the epidermis.
Cryo-scanning electron microscopy
For cryo-scanning electron microscopy (cryo-SEM) seeds were longitudinally sectioned with a razor blade and mounted on a cup-shaped holder with tissue-freezing medium. After mounting, the samples were plunge-frozen and stored in liquid nitrogen for subsequent cryo-planing and observations. Cryo-planing, which produces flat surfaces for observations in cryo-SEM, was performed using a cryo-ultramicrotome with a diamond knife (Nijsse et al., 1998). For observations, the specimens were heated up to –90 °C, sputter-coated with platinum and placed in the cryostat of the scanning electron microscope (JEOL 6300 Field emission SEM). Observations were made at –180 °C using a 2·5–5 kV accelerating voltage, and digital images were taken. Alternatively, the seeds were freeze-fractured with a cold scalpel knife, heated up to –90 °C, partially freeze-dried and sputter-coated with 5 nm of Pt.
Flow cytometry
Embryos from water- and ABA-imbibed seeds were isolated and used for flow cytometric measurements. Three replicates containing six embryos each were isolated and chopped with a razor blade in the presence of 1 mL of a nucleus-isolation buffer [10 mm MgSO4, 50 mm KCl, 5 mm HEPES (pH 5·8), 1 mg mL−1 DTT]. After chopping, the suspension with nuclei was passed through a 88-μm nylon mesh and stained with 5 µL of propidium iodide (1·0 mg mL−1 solution in water). After staining, the DNA content of the suspended nuclei was determined with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a 15 mW, 488 nm, air-cooled argon ion laser and cell sorter. At least 10 000 nuclei were analysed in each replication.
Protein extraction and β-tubulin detection
Embryos were isolated from water- and ABA-imbibed seeds during and following germination at intervals of 3 d. Fifteen embryos per sample were isolated and immediately frozen in liquid nitrogen. The embryos were ground with mortar and pestle to a powder and protein was extracted with 150 mL of Laemmli buffer, consisting of 50 mm Tris–HCl, 10 % (v/v) glycerol, 1·5 % DTT and 2 % SDS (de Castro et al., 1995). Protein concentration was determined according to Bradford (1976). Subsequently, 20 mg of total protein was loaded onto a polyacrylamide gel with a running gel composed of 12 % acrylamide, 0·1 % SDS and 375 mm Tris–HCl (pH 8·8) and a stacking gel composed of 5 % acrylamide, 0·1 % SDS and 125 mm Tris–HCl (pH 6·8). Protein samples were separated for 4 h at 30 mA for the running gel and 10 mA for the stacking gel in a Bio-Rad apparatus (BioRad Laboratories, CA). The running buffer used was composed of 196 mm glycine, 0·1 % SDS, 50 mm Tris–HCl (pH 8·3). Two different amounts (10 and 30 ng) of pure bovine brain tubulin were used as references. Proteins were electro-blotted from the gel to a Hybond-P PVDF membrane and optimized for protein transfer (Amersham Pharmacia Biotech) for 70 min at 250 mA at room temperature. For transfer a Mini Trans-Blot electrophoretic transfer cell (BioRad) was used. The transfer buffer consisted of 25 mm Tris, 192 mm glycine and 20 % (v/v) methanol (pH 8·3). After blotting, the membrane was rinsed twice in TBS buffer (0·5 m Tris, pH 7·5, and 1·5 m NaCl). The membrane was blocked in 1 % commercial blocking solution (Boehringer Mannheim, Germany) overnight at 4 °C and probed for 2 h at room temperature with 1 mg mL−1 mouse monoclonal anti-β-tubulin antibody (Boehringer Mannheim). Subsequently, the membrane was washed twice in TBS-T5 (TBS buffer and 5 % Tween 20) followed by a wash in 0·5 % blocking solution. After this step the membrane was incubated with 30 mU mL−1 of a secondary antibody (anti-mouse IgG-POD, fab fragments, Boehringer Mannheim) and diluted in 0·5 % blocking solution for 1 h at room temperature. Thereafter, the membrane was washed twice in TBS-T5 buffer. For detection the immunoblot was incubated in a pre-mixed detection solution (Boehringer Mannheim) for 1–2 min and placed between two plastic sheets inside a cassette. Under safe red light the blot was exposed to a sheet of photographic film (Hyperfilm-ECL, Amersham Pharmacia Biotech) for 15 min and the film was developed according to the manufacturer's instructions.
Immunohistochemical detection of β-tubulin
Embryos from water- and ABA-imbibed seeds were isolated during and following germination. For analysis of the microtubular cytoskeleton the embryos were isolated and plunge-frozen in liquid propane and transferred to freeze-substitution medium containing 0·1 % glutaraldehyde in water-free methanol. The cryo-tubes containing the embryos and freeze-fixation medium were incubated in a freeze-substitution unit (Cryotech-Benelux). After freeze-substitution the freeze-fixation medium was replaced by ethanol, followed by embedding in butylmethylmetacrylate (BMM) and UV polymerization at –20 °C according to Baskin et al. (1992). Longitudinal sections with 3 µm thickness were made and placed on slides. BMM was removed by washing in acetone followed by rinsing the slides in phosphate-buffered saline (PBS), pH 7·3. Sections were blocked in 0·1 m hydroxyl tetra ammonium chloride (HAH) and in 26 mm of bovine serum albumin (BSA). For visualization of the microtubular cytoskeleton (β-tubulin), mouse anti-β-tubulin (Sigma) with a dilution of 1 : 200 (v/v) was applied. The secondary antibody used was goat anti-mouse IgG conjugated with fluorescein-5-isothiocyanate (FITC; Molecular Probes) diluted 1 : 100. The antibodies were diluted in PBS buffer with NaOH (pH 7·3) plus 0·1 % of acetylated BSA (BSAc). Slides without the first antibody were used as a control
Statistical analysis
Statistical analyses were performed by using a general linear model (SPSS 10·0·5), and ANOVA and Student's t-test (Microsoft Excel).
RESULTS
Germination characteristics
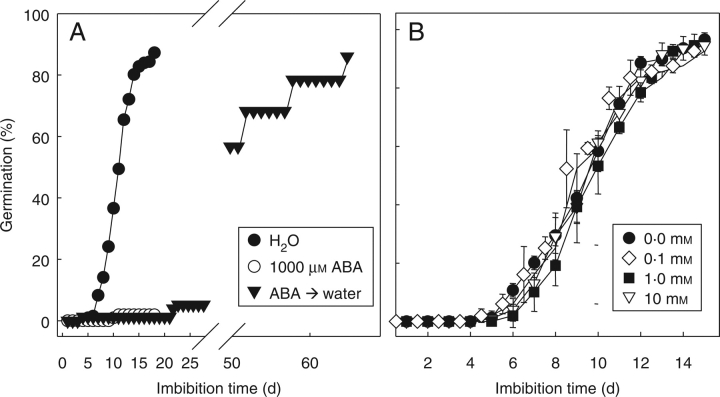
Radicle protrusion first became visible after 5 d of imbibition under optimal conditions (30 °C, in the dark), and after 9 d of imbibition 50 % of the seed population displayed radicle protrusion. After 15 d of imbibition most of the seeds had completed germination (Fig. 1). The formation of a protuberance preceded completion of germination, and became visible after 5 d (Fig. 2 A–D). Imbibition on 1000 mm ABA reduced final germination to 2 % (Fig. 1A) and fully inhibited the formation of the protuberance (data not shown). When seeds were treated with 1000 mm ABA for 18 d and subsequently transferred to water, radicle protrusion took place, albeit slowly (Fig. 1A). Hydroxyurea, a nuclear DNA synthesis inhibitor (Górnik, et al., 1997), did not inhibit germination up to a concentration of 10 mm (Fig. 1B).
Fig. 1.
Germination of coffee seeds (A) in water, 1000 µm ABA, and upon transfer to water after 20 d of imbibition on 1000 µm ABA, and (B) in different concentrations of hydroxyurea. Data points are means of four replicates of 25 seeds each, ± s.d.
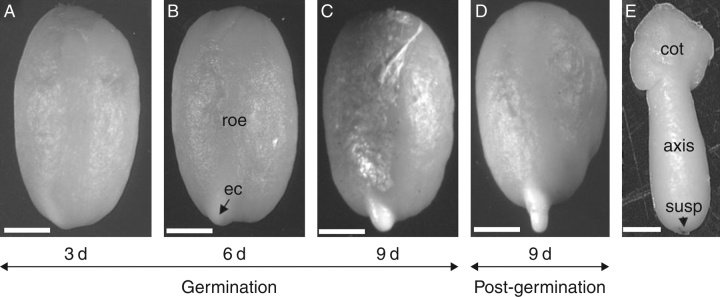
Fig. 2.
Germination and radicle protrusion of the coffee seed. (A) Seed imbibed for 3 d on water. (B) A protuberance is visible from 6 d of imbibition onwards, showing the endosperm cap (ec) and rest of the endosperm (roe). (C) A more prominent protuberance just before radicle protrusion. (D) Radicle protrusion starts after 5 d of imbibition on water, and following germination the radicle grows and the endosperm remains attached to the cotyledons. (E) Imbibed coffee embryo isolated after 6 d showing the cotyledons (cot), the embryonic axis and remnants of the suspensor (susp) on the radicle tip. Sacle bars: (A–D) = 2 mm; (E) = 0·5 mm.
Structure of the endosperm during germination
The embryo is 3–4 mm long and is composed of an axis and two cotyledons (Fig. 2E). The embryonic radicle tip is enclosed by the endosperm cap (Fig. 2B). The rest of the endosperm tissue can be divided into a hard external endosperm and soft internal endosperm (Dedecca, 1957). Endosperm cells have rectangular and polygonal cell types (Fig. 3A, B). The rectangular cells are located adjacent to the embryo and were observed in the region of the internal endosperm, whereas the polygonal cells were located in the external endosperm (Fig. 3A–C). In the endosperm cap region, various compressed cells were evident prior to radicle protrusion after 6 d of imbibition (Fig. 3E). The remnants of the suspensor were observed at the endosperm cap just prior to radicle protrusion (Fig. 3D) and were also observed in the endosperm cap region outside of the seed surface when the protuberance appeared. As germination proceeded, the compressed cells lost their integrity just before radicle protrusion (Fig. 3F). Compressed cells and loss of cell integrity coincided with the appearance of the protuberance, which was observed in the endosperm cap preceding radicle protrusion (Fig. 2B, C). Endosperm cell walls surrounding the embryonic axis, just below the radicle tip, also showed compressed cells, coinciding with the lateral expansion of the embryo inside the endosperm during germination (data not shown). No compressed cells were observed in the endosperm near the cotyledons. Cell walls of the endosperm cap were considerably thinner than those in the rest of the endosperm (Fig. 3G, H). These thick-walled cells in the rest of the endosperm appeared not to be fully hydrated after 6 d, although various degrees of hydration could be observed throughout the rest of the endosperm. After 9 d of imbibition all cells in the rest of the en dosperm appeared fully hydrated (data not shown).
Fig. 3.
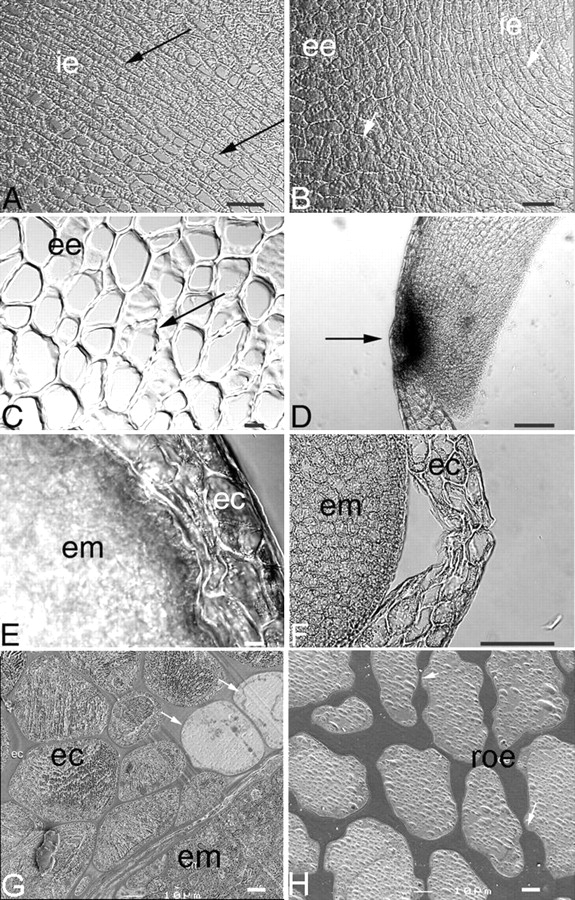
Light micrographs of the endosperm cap and rest of the endosperm of coffee seed during germination. (A) Cells of the rest of the endosperm adjacent to the embryo during imbibition. Note the high degree of uniformity of cell size in this region (arrows); ie, internal endosperm. (B) Cells of the rest of the endosperm during imbibition showing differences in cell morphology in internal (ie) and external (ee) endosperm. The cells of the internal endosperm (arrow) adjacent to the embryo are rectangular in shape and will be the first cells to be consumed following germination. The cells of the external endosperm have a polygonal shape (arrow); these cells will be consumed later. (C) Higher magnification of the external endosperm region of the rest of the endosperm, showing thin-walled areas (arrow). (D) Endosperm cap of a 9-d imbibed seed showing remnants of a suspensor at the radicle tip (dark spot, arrowed). (E) Endosperm cap (ec) region and embryo (em) after 6 d of imbibition, showing compressed cells in the endosperm. (F) Endosperm cap (ec) region and embryo (em) in a 9-d imbibed seed, showing loss of cell integrity just before radicle protrusion. (G) Endosperm cap of a 6-d imbibed seed showing thinner cell walls. Note that some cells are not completely hydrated (arrows) and are surrounded by fully hydrated cells of the endosperm cap (ec) and embryo (em). (H) Cells of the rest of the endosperm (roe) of 6-d imbibed seeds. Note that these cell walls are thicker than the cell walls of the endosperm cap region shown in (G). Scale bars: (C, E, G) = 10 µm; (A, B, D, F, H) = 100 µm.
Dimensions of embryo cells during germination
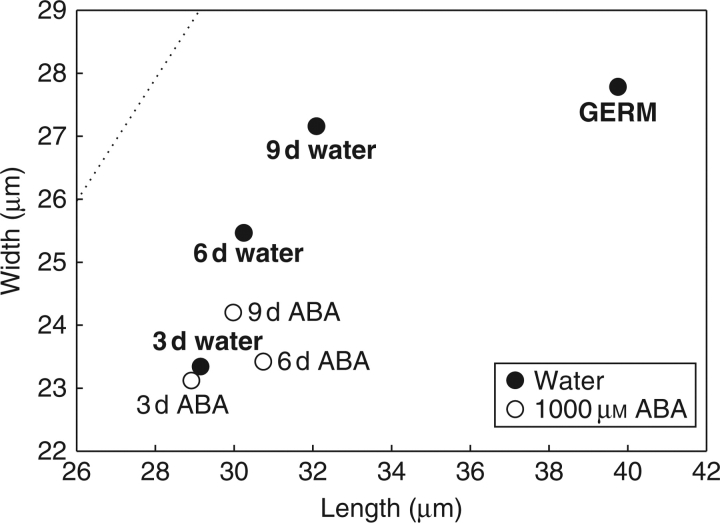
Embryonic axes were used for light microscopy studies since this part of the embryo appears to contribute most to embryo growth during germination (da Silva, et al., 2004). The embryonic axis was divided longitudinally into ten equal parts; microscopic analysis showed that cell growth occurred evenly in these ten regions (data not shown). Therefore, three of the parts in this axis were taken for further analysis, namely parts 3, 7 and 9 from the cortex region. During water-imbibition the increase of cell size followed an isodiametric pattern (Fig. 4). Axis growth at the moment of radicle protrusion seemed to be determined mainly by cell elongation, as cell width increased by only 3 % after 9 d of imbibition until shortly after radicle protrusion, while cell length increased by 24 % (Fig. 4). During imbibition on ABA, a smaller increase in cell width before radicle protrusion was observed compared to imbibition on water (ANOVA: P << 0·001; Fig. 4). However, according to a t-test no differences in cell width upon water- and ABA-imbibition were observed after 3 d, whereas highly significant differences were observed after 6 d (P << 0·001) and 9 d (P << 0·001). ANOVA showed no significant difference in length between water and ABA treatment before radicle protrusion, which was due to the high similarity in length after 3 and 6 d. However, after 9 d a difference in cell length before radicle protrusion was apparent, which was confirmed with a t-test (P < 0·014). Evidently, ABA inhibits cell elongation between 6–9 d of imbibition, but not during the first 6 d. The inhibition of cell elongation by ABA between 6–9 d coincided with the inhibition of the formation of a protuberance.
Fig. 4.
Changes in dimensions of the cells of the embryonic axis upon imbibition on water after 3 d, 6 d and 9 d of imbibition and shortly after radicle protrusion (GERM), and in 1000 mm ABA. The embryonic axis was divided into ten equal parts and the cells in parts 3, 7 and 9 were measured after 3, 6 and 9 d of imbibition, and after 9 d following radicle protrusion. Data represent the mean cell length plotted against mean cell width for 120 cells per point. The dotted line indicates the isodiametric shape.
DNA synthesis and replication
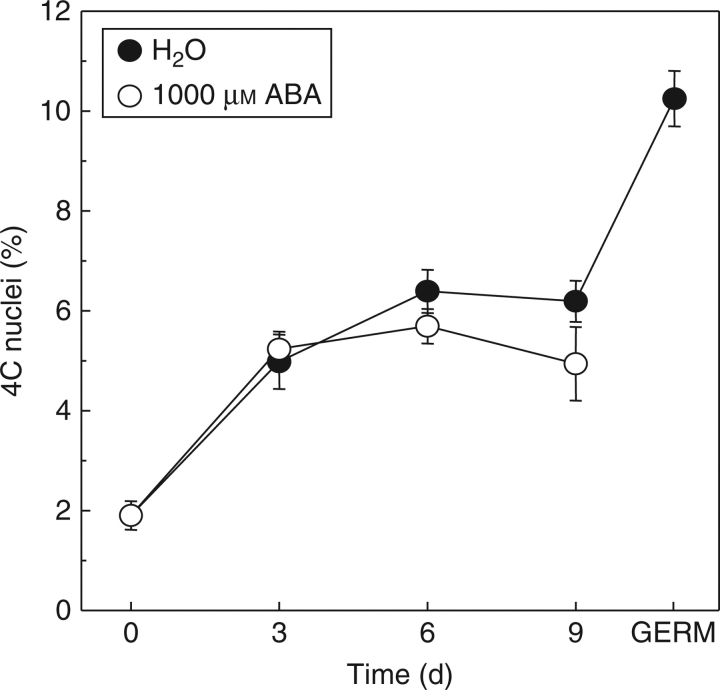
Flow-cytometric analysis of embryos from dry seeds showed a very low peak of 4C nuclei content, which indicates that most of the cells were in the G1 phase of the cell cycle (Fig. 5). The number of 4C nuclei showed a significant increase up to 6 d of imbibition on water (P < 0·001). After 9 d of imbibition on water the number of 4C nuclei was the same as after 6 d. After radicle protrusion, the amount of 4C nuclei increased to almost twice the amount observed after 9 d (Fig. 5). The same trend was found in ABA-imbibed seeds until 6 d. After 9 d of imbibition there was a slightly but significantly lower level of 4C nuclei in ABA-inhibited seeds (P < 0·001). No radicle protrusion was observed when the seeds were imbibed on ABA (Fig. 1A).
Fig. 5.
Frequency of nuclei from embryo cells with 4C DNA content expressed as a percentage of the total number of nuclei (2C + 4C) during imbibition of coffee seed on water or on 1000 mm ABA. Data points are means of three replicates of ten embryos each, ± s.d.
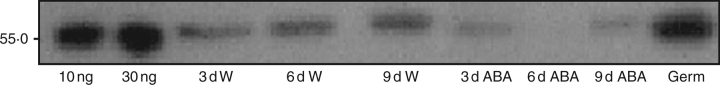
Accumulation of β-tubulin
The relative amount of a 55-kDa β-tubulin in water-imbibed seeds increased from 3 d onwards to radicle protrusion. β-tubulin was also observed in ABA-imbibed seed but at much lower levels (Fig. 6). Upon radicle protrusion in water, β-tubulin abundance increased sharply on the Western blot.
Fig. 6.
Western blot showing a 55-kDa β-tubulin in extracts from coffee embryos during and following germination for water- and ABA-imbibed seeds. Pure bovine brain tubulin (10 and 30 ng) was used as a control.
Configuration of microtubular cytoskeleton
The embryo microtubular cytoskeleton was analysed after 0, 3 and 6 d of imbibition, and after 9 d both prior to and after radicle protrusion. After 0 d the cells exhibited green fluorescent granules and no microtubules were observed (Fig. 7A). After 3 d of imbibition the green fluorescent granules had partially disappeared and microtubules were formed (Fig. 7B). At this stage only a few cells in the axis showed a transversal organization of the microtubules. From 6 d of imbibition onwards, but well before radicle protrusion, more cells showed microtubules that were transversally organized, and pre-prophase bands, mitotic spindles and phragmoplasts were observed, indicating the beginning of cell division (Fig. 7C, D, E). Transversal organization of the microtubules was also observed in the epidermal cells and outer cortex cells shortly before (Fig. 7F) and after radicle protrusion (Fig. 7G). The microtubules also became more abundant from the beginning of imbibition until after radicle protrusion (Fig. 7B, D, F, G). In ABA-imbibed seeds, microtubules were observed initially at low levels (Fig. 7H) and they remained randomly organized after 6 d of imbibition (Fig. 7I). In addition, microtubule numbers greatly reduced or disappeared after 9 d of imbibition on ABA (Fig 7J). When the ABA-imbibed seeds were rinsed in water and the seeds were then imbibed on water for 9 d, microtubules reappeared (Fig. 7K) and radicle protrusion took place (Fig. 1A).
Fig. 7.
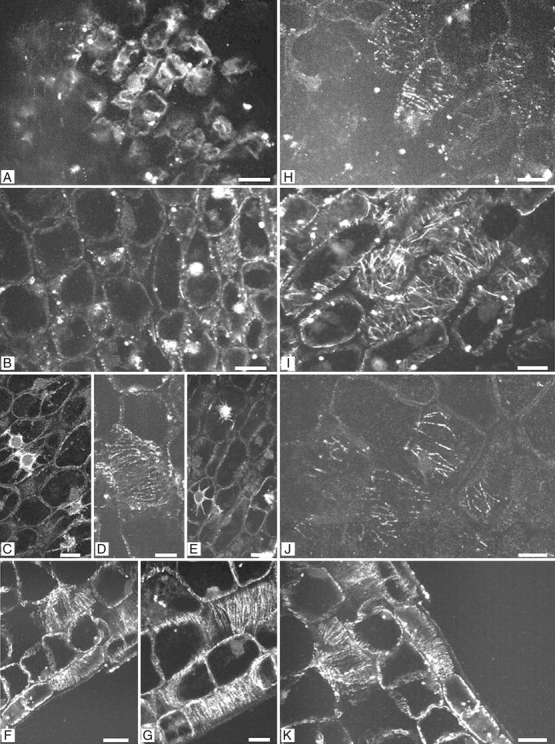
Fluorescence micrographs of longitudinal sections of embryos from water- and ABA-imbibed seed during and following germination. (A) Axis region of dry embryo (0 d of imbibition). Dots represent clusters of preserved β-tubulin. (B) Embryonic axis region from 3-d imbibed seeds on water, showing low microtubule abundance and random microtubular organization. (C–E) Embryonic axis regions from seeds imbibed on water for 6 d, showing transversal microtubular orientation in the outer cortex cells, and mitotic figures before radicle protrusion. (F) Embryonic axis region from 9-d water-imbibed seeds showing transversal microtubular orientation in the epidermal cells. (G) Embryonic axis region after radicle protrusion, showing transversal microtubular orientation and higher abundance of microtubules. (H) Embryonic axis region from 3-d imbibed seeds on ABA showing low abundance of microtubules. (I) Embryonic axis region from 6-d ABA-imbibed seeds, showing random orientation of the microtubules. (J) Embryonic axis region from 9-d imbibed seeds on ABA showing low abundance of microtubules. (K) Embryonic axis region from 9-d ABA-imbibed seeds that were rinsed in demineralised water and transferred to water for 30 d. Note that the microtubules reappeared as in water-imbibed seeds. Scale bars = 2 µm.
DISCUSSION
Coffee seed germination is the result of interplay between embryo and endosperm. The present study addresses several aspects of both these seed tissues during germination.
The endosperm during germination
The coffee seed belongs to the group of seeds that have a relatively high amount of mannans. The main hemicellulose in the cell walls of coffee seeds is an insoluble β-(1 → 4) D-mannan with 2 % of galactose present in the side chains that may serve as a carbohydrate reserve (Wolfrom et al., 1961; Bewley and Black, 1994). The galactose units are also found in arabinogalactans in the coffee seed (Wolfrom and Patin, 1965). Proteins, lipids and minerals are also present in the cytoplasm of the endosperm cells and could be another source of reserves (Dentan, 1985). The difference in cell size and in cell wall morphology between the endosperm cap and rest of the endosperm indicates that the region where the radicle will protrude is predestined in coffee seeds. Just prior to radicle protrusion, the compressed cells and loss of cell integrity observed in the endosperm cap region coincided with the appearance of the protuberance. Thicker cell walls observed in the rest of the endosperm indicate the source of reserves. These cell walls are degraded following germination to provide a source of energy to the growing seedlings.
After 3 d of imbibition the cells appeared turgid, indicating that phase 2 of the germination process had been attained. However, after 6 d of imbibition, there were still cells that were not fully hydrated, surrounded by fully hydrated cells, implying a cellular mechanism controlling water uptake. There were more fully hydrated cells in the endosperm cap than in the rest of the endosperm after 6 d. The thick-walled cells in the rest of the endosperm took longer to fully hydrate. This indicates that either the thickness of the cell walls or a different tissue-specific factor is likely to control the water uptake. The accumulation of solutes, possibly as a result of cell wall hydrolyzing enzymes, could play a role in controlling water uptake during germination (da Silva et al., 2004).
Concomitantly with the morphological changes in the endosperm cap region, porosity of the cell walls was observed after 9 d of imbibition, indicating the absence of cell wall components as a result of an increase in endo-β-mannanase activity and of other cell wall hydrolytic enzymes (Toorop et al., 2000; da Silva et al., 2004). In a previous study, it was shown that weakening of the endosperm cap is a prerequisite for coffee seed germination (da Silva et al., 2004). Weakening of the endosperm cap is mediated by hydrolytic enzymes, of which endo-β-mannanase is rate limiting (Toorop et al., 2000). In coffee seeds, endo-β-mannanase is a target of the inhibitory action of ABA (da Silva et al., 2004.
Light-microscopy observations did not show the presence of an aleurone layer in these endosperm-retaining seeds, which forms the source of hydrolytic enzymes in many monocotyledonous species (Olsen, 2004). In addition, incubation of endosperm slices in tetrazolium (2,3,5-triphenyl-tetrazolium chloride) solutions at 30 °C for 16 h showed a positive reaction (data not shown). This indicates that coffee endosperm cells are alive and are likely to be the source of the hydrolytic enzymes that contribute to the collapse of the endosperm cells.
The embryo during germination
It appears that compressed endosperm cap cells, loss of cell integrity and appearance of the protuberance were the result of embryo growth inside the endosperm prior to radicle protrusion. The fully differentiated coffee embryo is enveloped by the soft endosperm tissue (Krug and Carvalho, 1939; Mendes, 1941), is very small and does not have much storage reserves deposited (Giorgini and Campos, 1992). The embryo is localized close to the convex surface of the seed (Rena et al., 1986) and depends entirely on the endosperm to develop into a seedling (Giorgini and Campos, 1992).
The coffee embryo grows inside the endosperm before radicle protrusion, most likely by increasing the pressure potential and cell wall extensibility (da Silva et al., 2004). Light-microscopic analysis indicated that the embryonic axis grew by isodiametric cell expansion prior to radicle protrusion and by cell elongation following germination. This concurs with the general consensus that radicle protrusion is the direct result of axis-cell elongation. The initial isodiametric growth was followed by a transition of predominantly random orientation of the microtubules to transversal organization. The subsequent longitudinal growth seemed dependent on this predominantly transversal orientation, since both events were inhibited by ABA. In addition, the microtubules became more abundant during and following germination, in both epidermal and cortex cells. The random orientation of the microtubules is apparently a transition state, in preparation of the transverse orientation during embryo elongation. The transition in microtubule orientation and abundance also coincided with the increased accumulation of β-tubulin. This implies that assembly of microtubules, at least partially, depends on de novo synthesis of β-tubulin during imbibition.
ABA may inhibit the coffee embryo growth potential by inhibiting cell wall loosening (da Silva et al., 2004). Schopfer and Plachy (1985) have demonstrated that ABA inhibited cell wall loosening in Brassica napus. In the current study, ABA caused an arrest in elongation after 6 d of imbibition and rigorously restricted the isodiametric swelling of the coffee embryo cells during the germination process. However, ABA did not seem to inhibit seed water uptake (data not shown). Thus, the suppression of embryo growth by ABA was targetted at the phase of predominantly longitudinal growth of the embryo cells during germination. Along with the axis-cell growth in ABA-imbibed seeds, the microtubules were randomly organized and their abundance was low. Ishida and Katsumi (1992) found a similar organization of microtubules in the embryonic hypocotyl cells of growing cucumber seedlings treated with ABA. Therefore, the results found here indicate a relation between cell morphogenesis and microtubule orientation during embryonic axis elongation.
Quantification of DNA levels by flow cytometry indicated that most of the embryonic cells were arrested in the G1 phase of the cell cycle during seed maturation, and that upon imbibition there was an increase in the number of cells in the G2 phase of the cell cycle. Tomato seeds also showed DNA synthesis and an increase in the number of G2 cells during imbibition (Bino et al., 1992; de Castro et al., 2000). The significant increase in 4C nuclei from dry embryos to 6 d of imbibition, both on water and ABA, is probably due to nuclear DNA replication. Nuclear and organellar DNA synthesis found early during imbibition in the coffee embryo probably represents DNA repair, necessary to fix damage caused during maturation drying and storage (Bewley, 1997), whereas DNA duplication at later stages of imbibition represents a preparation for cell division. In ABA-imbibed embryos the amount of 4C nuclei was similar to that of the water control, but after 9 d it was significantly lower. Thus, ABA appears to have an inhibitory effect on DNA duplication as preparation for cell division. There is evidence that ABA inhibits the cell cycle at the G1/S transistion in tobacco BY2 culture cells (Swiatek et al., 2002) or arrests cells in tomato embryos in G1 (Liu et al., 1994). One of the possible targets of ABA in the inhibition of the cell cycle is a cyclin-dependent protein kinase inhibitor (KRP1). This protein interacts with an A-type cyclin dependent kinase (CDKA) and inhibits histone H1 kinase. It has been shown that ABA could induce the expression of the KRP1 gene (Wang et al., 1998). We hypothesize that ABA action is targetted mainly to specific proteins involved in the cell cycle machinery that are associated with cell division but not with repair of DNA synthesis. The DNA repair mechanism seems to be unaffected by the presence of ABA during imbibition. Elder and Osborne (1993) have suggested that continuous DNA synthesis (presumably DNA repair) is important to maintain genome integrity, allowing a continuous but slow maintenance of DNA in dormant or ABA-treated embryos of Avena fatua.
The increase of 4C nuclei in water-imbibed seeds coincided with the accumulation of β-tubulin and with the appearance of mitotic figures in the embryonic axis prior to and after radicle protrusion. In tomato seeds, the presence of 4C nuclei and β-tubulin expression was associated with the presence of mitotic cells in the embryo, as well as with the progression of germination (de Castro et al., 2000). To our knowledge, cell division in the coffee embryo prior to radicle protrusion has never been described before. However, cell division seems no prerequisite for radicle protrusion in coffee since hydroxyurea, which inhibits DNA synthesis (Górnik, et al., 1997), did not inhibit radicle protrusion in the coffee seed. However, it is uncertain to what extent applied hydroxyurea is taken up by the coffee seeds. Cell division is likely to be necessary for further post-germinative embryo growth.
CONCLUSIONS
Embryo growth during and following germination in coffee seeds was found to be the result of cell expansion and elongation, and was accompanied by preparations for cell division, as demonstrated by DNA synthesis, abundance and reorientation of microtubules, and accumulation of β-tubulin. ABA inhibited the accumulation of β-tubulin, cell growth and cell division in the embryo, and transversal organization of microtubules and nuclear DNA replication late during imbibition. Finally, the results show that not only changes in the endosperm tissue but also in embryo growth control germination in coffee seeds.
ACKNOWLEDGEMENTS
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for financial support of the studies of E. A. Amaral da Silva. The seed lab at the Federal University of Lavras (UFLA), Brazil is acknowledged for handling and shipping the seeds to The Netherlands. We are grateful to Paulien van Ekeren at the Laboratory of Plant Cell Biology for measurement of embryo cell dimensions.
LITERATURE CITED
- Baskin TI, Busby CH, Fowke LC, Sammut M, Gubler F. Improvements on immunostaining samples embedded in methacrylate: localization of microtubules and other antigens throughout developing organs in plants of diverse taxa. Planta. 1992;187:405–413. doi: 10.1007/BF00195665. [DOI] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. The Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds. Physiology of development and germination. New York and London: Plenum Press; 1994. [Google Scholar]
- Bino RJ, de Vries JN, Kraak L, van Pijlen JG. Flow cytometric determination of nuclear replication stages in tomato seeds during priming and germination. Annals of Botany. 1992;69:231–236. [Google Scholar]
- Bouvier-Durand M, Real M, Côme D. Changes in nuclear activity upon secondary dormancy induction by abscisic acid in apple embryo. Plant Physiology and Biochemistry. 1989;27:511–518. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- de Castro RD, Zheng X, Bergervoet JHW, Ric de Vos CH, Bino RJ. β-tubulin accumulation and DNA replication in imbibing tomato seeds. Plant Physiology. 1995;109:499–504. doi: 10.1104/pp.109.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro RD, van Lammeren AAM, Groot SPC, Bino RJ, Hilhorst HWM. Cell division and subsequent radicle protrusion in tomato seeds are inhibited by osmotic stress but DNA synthesis and formation of microtubular cytoskeleton are not. Plant Physiology. 2000;122:327–335. doi: 10.1104/pp.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedecca DM. Anatomia e desenvolvimento ontogenético de Coffea arabica L. var. Typica Cramer. Bragantia. 1957;16:315–355. [Google Scholar]
- Dentan E. The microscopic structure of the coffee bean. In: Clifford MN, Wilson KC, editors. Coffee botany, biochemistry and production of beans and beverage. Westport, CT: The Avi Publishing Company; 1985. pp. 284–304. [Google Scholar]
- Elder RH, Osborne ED. Function of DNA synthesis and DNA repair in the survival of embryos during germination and in dormancy. Seed Science Research. 1993;3:43–53. [Google Scholar]
- Giorgini JF, Campos CASP. Changes in the content of soluble sugars and starch synthesis and degradation during germination and seedling growth of Coffea arabica L. Revista Brasileira de Fisiologia Vegetal. 1992;4:11–15. [Google Scholar]
- Goddard RH, Wick SM, Silflow CD. Microtubule components of the plant cell cytoskeleton. Plant Physiology. 1994;104:1–6. doi: 10.1104/pp.104.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górnik K, de Castro RD, Liu Y, Bino RJ, Groot SPC. Inhibition of cell division during cabbage (Brassica oleracea L.) seed germination. Seed Science Research. 1997;7:333–340. [Google Scholar]
- Groot SPC, Karssen CM. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta. 1987;171:525–531. doi: 10.1007/BF00392302. [DOI] [PubMed] [Google Scholar]
- Haigh AM, Barlow EWR. Water relations of tomato seed germination. Australian Journal of Plant Physiology. 1987;14:485–492. [Google Scholar]
- Hilhorst HWM. A critical update on seed dormancy. I. Primary dormancy. Seed Science Research. 1995;5:51–73. [Google Scholar]
- Huxley PA. Coffee germination test recommendations and defective seed types. Proceedings of the International Seed Testing Association. 1965;30:705–715. [Google Scholar]
- Ishida K, Katsumi M. Effects of gibberellin and abscisic acid on the cortical microtubule orientation in hypocotyl cells of light-grown cucumber seedlings. International Journal of Plant Science. 1992;153:155–163. [Google Scholar]
- Krug CA, Carvalho A. Genetical proof of the existence of coffee endosperm. Nature. 1939;144:515. [Google Scholar]
- Liu Y, Bergervoet JHW, Ric de Vos CH, Hilhorst HWM, Kraak HL, Karssen CM, Bino RJ. Nuclear replication activities during imbibition of abscisic acid- and gibberellin-deficient tomato (Lycopersicon esculentum Mill.) seeds. Planta. 1994;194:368–373. [Google Scholar]
- Mendes AJT. Cytological observations in Coffea. VI. Embryo and endosperm development in Coffea arabica L. American Journal of Botany. 1941;28:784–789. [Google Scholar]
- de Miguel L, Sánchez RA. Phytochrome induced germination, endosperm softening and embryo growth potential in Datura ferox seeds: sensitivity to low water potential and time of escape to FR reversal. Journal of Experimental Botany. 1992;43:969–974. [Google Scholar]
- Nijsse J, Erbe E, Brantjes NBM, Schel JHN, Wergin WP. Low-temperature scanning electron microscopic observations on endosperm in imbibed and germinated lettuce seeds. Canadian Journal of Botany. 1998;76:509–516. [Google Scholar]
- Olsen O-A. Nuclear endosperm development in cereals and Arabidopsis thaliana. The Plant Cell. 2004;16:S214–S227. doi: 10.1105/tpc.017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DJ. Biochemical control systems operating in the early hours of germination. Canadian Journal of Botany. 1983;61:3568–3577. [Google Scholar]
- Rena AB, Maestri M. Fisiologia do cafeeiro. In: Rena AB, Malavolta E, Rocha M, Yamada T, editors. Cultura do cafeeiro- fatores que afetam a produtividade. Poços de Caldas. MG, Brazil: Associação Brasileira para a Pesquisa da Potassa e do Fosfato; 1986. pp. 13–85. [Google Scholar]
- Rena AB, Malavolta E, Rocha M, Yamada T, editors. Cultura do cafeeiro-fatores que afetam a produtividade 447. Piracicaba: Potafos; 1986. [Google Scholar]
- Schopfer P, Plachy C. Control of seed germination by abscisic acid. III. Effect on embryo growth potential (minimum turgor pressure) and growth coefficient (cell wall extensibility) in Brassica napus L. Plant Physiology. 1985;77:676–686. doi: 10.1104/pp.77.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva EAA, Toorop PE, van Aelst AC, Hilhorst HWM. ABA regulates embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta. 2004;220:251–261. doi: 10.1007/s00425-004-1344-0. [DOI] [PubMed] [Google Scholar]
- Swiatek A, Lenjou M, Van Bockstaele D, Inze D, Van Onckelen H. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiology. 2002;128:201–211. [PMC free article] [PubMed] [Google Scholar]
- Toorop PE, van Aelst AC, Hilhorst HWM. The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. Journal of Experimental Botany. 2000;51:1371–1379. [PubMed] [Google Scholar]
- Valio IFM. Germination of coffee seeds (Coffea arabica L. cv. Mundo Novo) Journal of Experimental Botany. 1976;27:983–991. [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. The Plant Journal. 1998;15:501–510. doi: 10.1046/j.1365-313x.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Watkins JT, Cantliffe JD, Huber DJ, Nell TA. Gibberellic acid stimulated degradation of endosperm in pepper. Journal of the American Society of Horticulture Science. 1985;110:61–65. [Google Scholar]
- Welbaum GE, Muthui WJ, Wilson JH, Grayson RL, Fell RD. Weakening of muskmelon perisperm envelope tissue during germination. Journal of Experimental Botany. 1995;46:391–400. [Google Scholar]
- Wolfrom ML, Patin DL. Carbohydrates of the coffee bean. IV. An arabinogalactan. Journal of Organic Chemistry. 1965;30:4060–4063. [Google Scholar]
- Wolfrom ML, Laver ML, Patin DL. Carbohydrates of coffee bean. II. Isolation and characterization of a mannan. Journal of Organic Chemistry. 1961;26:4533–4536. [Google Scholar]