Abstract
Background and Aims
Several models predict that the establishment of polyploids within diploid populations is enhanced by non-random mating (i.e. selfing and assortative mating) of cytotypes and by a higher relative fitness of polyploids. This report assesses the role that antheridiogens (i.e. maleness-inducing pheromones) and intercytotype differences in growth rate have on polyploid performance.
Methods
Three buckler-fern species were studied: the allotetraploid Dryopteris corleyi and its diploid parents, D. aemula and D. oreades. In one experiment, gametophytes of these species were cultured under rich growth conditions to compare the timing of gametangia production. The substrata on which these gametophytes had grown were used as antheridiogen sources in a second experiment. The three species were combined as source and target of antheridiogen (i.e. nine species pairs). Timing of antheridia production and gametophyte size were determined after those antheridiogen treatments.
Key Results
Under rich growth conditions the allotetraploid produced archegonia earlier than those of diploid parents. Female gametophytes of the three species produced antheridiogens that inhibited growth and favoured maleness both within and among species. Gametophyte size was similar in the three species but antheridia formed earlier in the allotetraploid.
Conclusions
Unisexuality, promoted by non-specific antheridiogens, enhances random mating both within and among species. The resulting hybridization can favour the reproductive exclusion of the allopolyploid in sites where it is outnumbered by diploids. However, the earlier production of gametangia in the allotetraploid favours assortative mating and may thus counterbalance reproductive exclusion.
Key words: Allopolyploidy, antheridiogen, assortative mating, Dryopteris aemula, Dryopteris corleyi, Dryopteris oreades, gametophytes, gender expression, minority cytotype exclusion
INTRODUCTION
Polyploidy is a major evolutionary force in numerous eukaryotic and prokaryotic lineages (Masterson, 1994; Otto and Whitton, 2000; Tobiason and Seifert, 2006). Polyploid organisms arise in low numbers within diploid parental populations. The minority cytotype exclusion model (Levin, 1975) predicts that, in populations where individuals of different cytotypes mate randomly, newly arisen polyploids (i.e. neopolyploids) will be excluded if they cannot counterbalance their frequency-dependent mating disadvantage, as a consequence of ineffective matings between minority diploid and majority haploid gametes. Several additional evolutionary models have analysed the effects of factors that can enhance polyploid establishment success, such as non-random mating among individuals of different ploidy level, higher relative fitness of polyploids, niche differentiation, recurrent polyploid formation, migration or stochastic events (Felber, 1991; Rodríguez, 1996; Rausch and Morgan, 2005).
Two different types of non-random mating may facilitate the establishment of polyploids: assortative mating based on ploidy-related phenotypic traits (Husband and Sabara, 2004) and selfing (Levin, 1975; Rodríguez, 1996; Rausch and Morgan, 2005). Some recent studies on Chamerion angustifolium show that ploidy-related assortative mating can enhance the performance of autotetraploids (Husband and Sabara, 2004; Kennedy et al., 2006), but more studies are necessary to demonstrate the importance of this factor on a broader range of taxa, including allopolyploids. Polyploids are expected to have increased levels of self-fertilization for several reasons (Barringer, 2007). One of these reasons is that selfing would prevent neopolyploids from hybridizing with diploid individuals, circumventing the fitness disadvantage of being a minority cytotype. This prediction is supported by several empirical studies in angiosperms (Husband and Schemske, 1997; Barringer, 2007; but see Mable, 2004) and homosporous ferns (Masuyama and Watano, 1990).
Ferns are the vascular plant group with the highest proportion of polyploids (Grant, 1981, but see also Vida, 1976; Soltis and Soltis, 1989). Homosporous fern gametophytes are potentially hermaphroditic and may thus self-fertilize, producing completely homozygous sporophytes. However, there is evidence that homosporous ferns actually show environmental sex determination, i.e. the development of female, male or both sex organs largely depends on environmental conditions (Bull, 1981; Korpelainen, 1998). Mainly based on a high cost of female reproduction, Haig and Westoby (1988) predicted female gender expression under favourable growth conditions and male expression under poor growth conditions for homosporous fern gametophytes. In many species, gender is also environmentally determined by antheridiogens, i.e. maleness-inducing pheromones. Antheridiogens are gibberellin-like molecules released by archegoniate (female or hermaphrodite) gametophytes (Yamane, 1998). These compounds inhibit vegetative growth and induce protandry (i.e. antheridium-first gender expression) on asexual gametophytes (Döpp, 1950; Fernández et al., 1999; Tanurdzic and Banks, 2004). Antheridiogens thus promote outcrossing among gametophytes. Antheridiogens induce maleness not only between conspecific gametophytes, but also between gametophytes of different fern species (Näf, 1956; Chiou and Farrar, 1997). In several allopolyploid complexes it has even been found that the gametophytes of polyploid taxa respond to the antheridiogens released by parental species and vice versa (Haufler and Ranker, 1985; Pangua et al., 2003). Thus, antheridiogens may promote hybridization and favour reproductive exclusion.
The competitive interactions between allopolyploid species and their diploid relatives are poorly understood (Levin, 2002). Allopolyploidization (interspecific hybridization either preceded or followed by chromosome doubling) can affect fitness-related traits through two mechanisms. First, chromosome doubling has biophysical effects such as increased nucleus and cell sizes, which can in turn affect whole-plant morphology (Stebbins, 1971). Secondly, hybridization involves important genetic and epigenetic modifications (Wendel, 2000), such as increased heterozygosity, which may increase vigour in some fitness-related traits (e.g. Soltis and Rieseberg, 1986). The homosporous fern genus Dryopteris shows both types of effects as the spores of allotetraploid species are bigger (biophysical effect) and germinate faster (hybrid vigour) than those of diploid parental species (Quintanilla and Escudero, 2006).
In this study two culture experiments were used to elucidate the reproductive and competitive interactions between the gametophytes of the allotetraploid Dryopteris corleyi and its diploid parental species, D. aemula and D. oreades. These interactions may affect mating system and fitness, and therefore influence polyploid establishment. The following hypotheses were tested: (a) gametophytes of D. corleyi and of its two diploid parental species become female under rich growth conditions, as predicted by the Haig and Westoby (1988) model of sex expression; (b) female gametophytes of all three taxa release antheridiogens that reduce the growth rate and induce maleness among neighbouring gametophytes, both within and among species; (c) D. corleyi gametophytes show higher rates of vegetative and reproductive growth than the two diploid parental species.
MATERIALS AND METHODS
Study species
The three following buckler-ferns were studied: the allotetraploid Dryopteris corleyi Fraser-Jenkins (2n = 4x = 164; Fraser-Jenkins and Gibby, 1986) and its diploid parental species, D. aemula (Aiton) O. Kuntze (2n = 2x = 82; Manton, 1950) and D. oreades Fomin (2n = 2x = 82; Manton, 1950). Dryopteris corleyi originated from the hybrid D. × pseudoabbreviata Jermy (D. aemula × D. oreades), as based on morphological and phytochemical traits (Fraser-Jenkins, 1982; Fraser-Jenkins and Widén, 1983) and allozyme markers (A. Jiménez et al., unpubl. res.). It is endemic to a narrow coastal strip of northern Spain, where it inhabits eucalyptus and pine plantations and heathlands from 50 to 650 m a.s.l. (Salvo and Arrabal, 1986). Dryopteris aemula lives in shady riparian forests from sea level to 900 m a.s.l., whereas D. oreades occurs in open, non-calcareous rocky areas and scree slopes from 600 to 2400 m a.s.l. (Salvo and Arrabal, 1986). In some coastal mountains, the three species live in close proximity, especially D. aemula and D. corleyi which form the sterile hybrid D. × arecesiae F.J. Pérez Carro & T.E. Díaz González (Pérez Carro and Díaz González, 1990). Several traits suggest a recent origin of D. corleyi: low genetic divergence from D. aemula at the chloroplast DNA level (Geiger and Ranker, 2005), presence of all allozymes of D. corleyi in its two parental species (A. Jiménez et al., unpubl. res.), high percentage of spore abortion (Quintanilla and Escudero, 2006), narrow distribution range, and occupation of disturbed, potentially recent habitats (Mayor and Fernández, 1988).
Plant material
Spores of the three study species were collected in northern Spain from one population per species: D. corleyi in Asturias province, Pendueles; D. aemula in A Coruña province, Castro River; and D. oreades in León province, Laguna Ferreira (for further information on sampled populations and spore harvesting, see Quintanilla and Escudero, 2006). Spores were stored in glass vials at 5 °C, conditions appropriate for maintaining viability in these species (Quintanilla et al., 2002).
Experiment 1: gender under rich growth conditions
Spores of each species were sown at high density in 5·5-cm-diameter Petri dishes containing sterilized mineral agar (Dyer, 1979) to which Nystatin (100 units mL−1) was added to minimize fungal contamination. Dishes were sealed with Parafilm and placed in a growth chamber with 16 °C light/10 °C dark temperatures and 13-h photoperiod (daylight fluorescent tubes, photon irradiance 30–45 µmol m−2 s−1 within the 400- to 700-nm region). Temperatures and photoperiod were chosen on the basis of late summer and early spring conditions in the studied populations (Quintanilla and Escudero, 2006). Both seasons seem the most favourable for germination: in the former, spores are released, and the latter would represent the end of a hypothetical winter dormancy.
Eight weeks after sowing, 200 presexual gametophytes per species were transplanted to transparent plastic trays completely divided into 25 square cells with 2-cm sides (eight trays per species, 600 gametophytes in total). Into each cell, one gametophyte was transplanted on 3 mL of moist, autoclaved nutrient-rich commercial substratum (Compo Sana Semilleros, COMPO, Barcelona, Spain). Trays were sealed with Parafilm and placed in a growth chamber under the same conditions described above. Trays were periodically randomized to prevent possible effects of environmental heterogeneity within the chamber. For each gametophyte, gender (female, hermaphrodite or male) was determined every 3 weeks using a dissection microscope until week 29. After gender determinations, each gametophyte was watered with three to five droplets of distilled water. Temporal differences among species required to start producing gametangia were analysed with a Kruskal–Wallis test followed by Nemenyi tests (Zar, 1999). Statistical analyses were conducted with SPSS (2003).
Experiment 2: gender and size under antheridiogen effect
A second experiment was performed to study the intra- and interspecific effects of antheridiogen pheromone. The substrata of expt 1, pooled per species, were used as pheromone sources, as all gametophytes of the three species were female at the end of the experiment and had potentially released antheridiogens. To increase the quantity of exudates in the substrata, gametophytes were rinsed with 1 mL distilled water prior to removal. Each of the species-specific substrata was mixed with one-third (volume) of fresh substratum to compensate for nutrient uptake by the removed gametophytes. The resulting substrata were autoclaved and stored at 5 °C.
Dense gametophyte cultures were obtained as in expt 1. The substrata with the exudates of the three species were again distributed into trays divided into 25 square cells (2-cm sides, 3 mL substratum per cell). Eight-week-old presexual gametophytes were transplanted into these trays, one gametophyte per cell, making up the nine possible antheridiogen source-target species combinations (i.e. D. aemula–D. aemula, D. aemula–D. corleyi, etc.). One hundred gametophytes were transplanted per source–target combination (four trays per combination, 900 gametophytes in total). Additionally, 50 asexual gametophytes per species were transplanted into cells with fresh antheridiogen-free substratum. Trays were sealed with Parafilm, placed in the growth chamber under the same conditions as given above, and periodically randomized. For each antheridiogen-target gametophyte, gender was determined every 2 weeks until week 18. Percentages of male gametophytes were calculated on the basis of the four trays (n = 4) per antheridiogen source-target combination. In addition, gametophyte size was measured 12 and 18 weeks after spore sowing. At each date, 25 gametophytes grown without antheridiogens and 25 grown under antheridiogen influence were randomly sampled per species. For this variable, gametophytes of the three antheridiogen-source treatments were pooled per antheridiogen-target species. Images of sampled gametophytes were obtained with a high resolution scanner (94·5 pixel mm−1), and their area was determined with ImageTool 3·0 (http://ddsdx.uthscsa.edu/dig/itdesc.html).
The effects of antheridiogen-source and antheridiogen-target species (both fixed) on arcsine-transformed percentage of male gametophytes were explored with a two-factor ANOVA. A posteriori pairwise comparisons were done with Tukey tests. Differences among target species in time required to initiate antheridia were analysed with a Kruskal–Wallis test followed by Dunn tests for unbalanced data (Zar, 1999). Gametophyte sizes were analysed by ANOVA and Tukey tests with three fixed factors: antheridiogen (present or absent), antheridiogen-target species (D. aemula, D. corleyi and D. oreades) and time (12 and 18 weeks). All statistical analyses were done with SPSS (2003).
RESULTS
Experiment 1
All gametophytes of the three species became female during the 29 weeks of observation (Fig. 1). Time required to initiate archegonia differed significantly among species (H = 184·56, d.f. = 2, P < 0·001, Kruskal–Wallis test). The allotetraploid D. corleyi became female earlier (species means ± standard error, n = 200: 13·7 ± 0·1 week) than the two diploid species, D. aemula (15·9 ± 0·1 week) and D. oreades (15·9 ± 0·2 week). The two diploid species were not significantly different according to Nemenyi tests (P < 0·05).
Fig. 1.
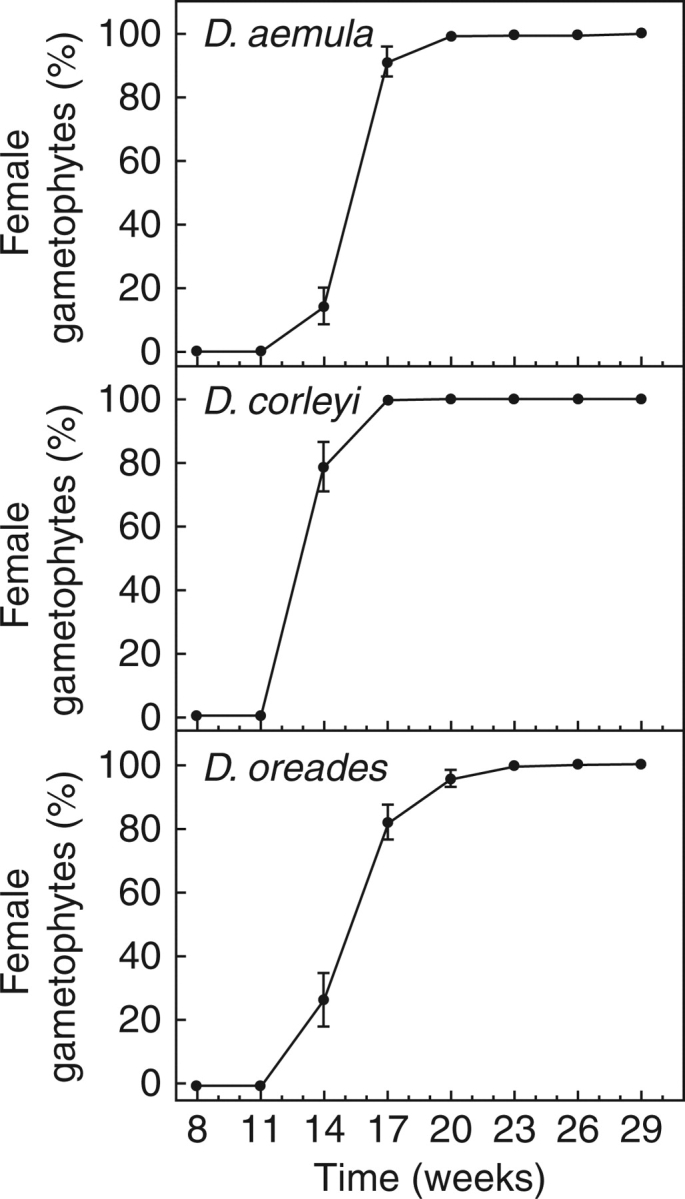
Means (± s.e.) of the percentages of female gametophytes for three Dryopteris species during a 29-week period. Each mean female percentage is for eight trays (n = 8) of 25 gametophytes. No gametophyte became male or hermaphrodite.
Experiment 2
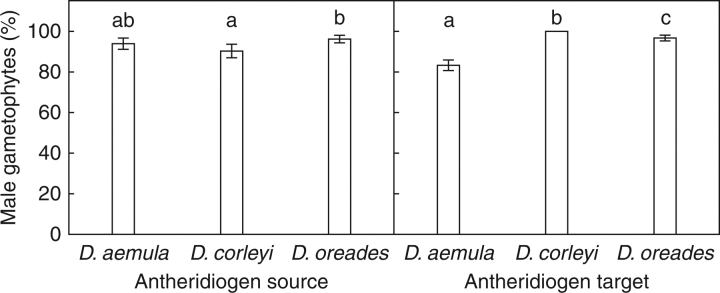
Most gametophytes became male in all the nine antheridiogen source-target species combinations. There were significant differences in the percentages of males among antheridiogen-source species and among antheridiogen-target species (Table 1). The source × target species interaction was not significant. Antheridiogen of D. oreades induced maleness in more gametophytes than D. corleyi antheridiogen, whereas maleness induction of D. aemula antheridiogens was not significantly different from those of D. oreades and D. corleyi (Fig. 2). Percentage of males in antheridiogen-target gametophytes decreased in the order D. corleyi > D. oreades > D. aemula (Fig. 2).
Table 1.
ANOVAs to explore the effects of antheridiogens on arcsine-transformed percentage of male gametophytes and gametophyte size of Dryopteris spp.
| Variable | Effect | d.f. | m.s. | F |
|---|---|---|---|---|
| Male gametophytes (%) | Source species | 2 | 138·220 | 3·800* |
| Target species | 2 | 1699·869 | 46·728*** | |
| Source × target species | 4 | 55·019 | 1·512 n.s. | |
| Error | 27 | 36·378 | ||
| Gametophyte size | Antheridiogen (present or absent) | 1 | 4327·707 | 234·371*** |
| Target species | 2 | 43·199 | 2·340 n.s. | |
| Time | 1 | 5126·119 | 277·609*** | |
| Antheridiogen × target species | 2 | 63·428 | 3·435* | |
| Antheridiogen × time | 1 | 3971·059 | 215·056*** | |
| Target species × time | 2 | 46·756 | 2·532 n.s. | |
| Antheridiogen × time × target species | 2 | 68·493 | 3·709* | |
| Error | 288 | 18·465 |
The analysis of the percentage of male gametophytes included the two fixed factors antheridiogen-source species and antheridiogen-target species (D. aemula, D. oreades and D. corleyi). The analysis of gametophyte size included the three fixed factors antheridiogen absent or present (data from the three source species were pooled), antheridiogen-target species (D. aemula, D. oreades and D. corleyi), and time (12 or 18 weeks).
d.f., Degrees of freedom; m.s., mean square; n.s., non-significant.
*, P < 0·05; **, P < 0·01; ***, P < 0·001.
Fig. 2.
Means (± s.e.) of the percentages of male gametophytes with different antheridiogen-source and antheridiogen-target Dryopteris species. Percentages were calculated on the basis of the four trays (n = 4) per antheridiogen source–target combination. Within each graph, different letters indicate significantly different means (P < 0·05, Tukey tests).
Time required to initiate antheridia differed among species (H = 59·84, d.f. = 2, P < 0·001, Kruskal–Wallis test). Dryopteris corleyi became male significantly earlier (13·2 ± 0·1 weeks, n = 300) than D. oreades (13·7 ± 0·1 weeks, n = 290), which in turn became male earlier than D. aemula (14·6 ± 0·1 weeks, n = 249; P < 0·05, Dunn tests).
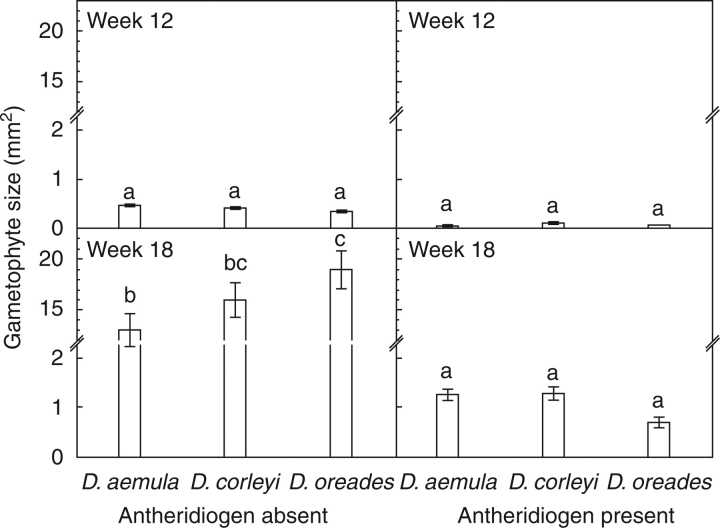
In ANOVA of gametophyte sizes, all factors and interactions were significant except for target species and the target species × time interaction (Table 1). The antheridiogen × time × target species interaction means that differences of size among target species are not independent of the other two factors. At 12 weeks, there were no significant differences in gametophyte size between antheridiogen absent/present or between species (Fig. 3). At 18 weeks, gametophytes grown with antheridiogens were significantly smaller than those grown without antheridiogens. In the antheridiogen-absent treatment, gametophyte size decreased in the order: D. oreades > D. corleyi > D. aemula (Fig. 3).
Fig. 3.
Means (± s.e.) of gametophyte sizes of Dryopteris spp. in the presence or absence of antheridiogens at weeks 12 and 18. Different letters among graphs indicate significantly different means (P < 0·05, Tukey tests).
DISCUSSION
Archegonia-first strategy in rich growth conditions
The hypothesis that archegonia develop before antheridia under rich growth conditions (Haig and Westoby, 1988) was supported by results for all three Dryopteris species studied in the present work. A similar archegonia-first strategy under good growth conditions has been observed in other diploid (e.g. D. ludoviciana, Cousens and Horner, 1970) and allotetraploid (e.g. D. filix-mas, Barker and Willmot, 1985; Korpelainen, 1994) species of Dryopteris as well as in other fern groups (e.g. Phegopteris decursive-pinnata, Masuyama, 1979; Lygodium microphyllum, Lott et al., 2003). Conversely, poor growth conditions (low nutrient availability and low light incidence) induce maleness in the three study species (A. Jiménez et al., unpubl. res.). According to theory, the relationship between plant size (growth rate) and gender expression may be adaptive because of the different costs of male (sperm production) and female (production of egg cells and young sporophyte development) functions. In brief, female function demands a higher energetic investment (Ghiselin, 1969) and a longer time commitment (Paquin and Aarssen, 2004).
Antheridiogen effects within and among species
In agreement with our second hypothesis, it was found that female gametophytes of the three studied species produced antheridiogens which reduced growth rate and gametophyte size and induced maleness in asexual gametophytes within and among species. Maleness induction was more intense with D. oreades as antheridiogen-source species and with D. corleyi as antheridiogen-target species (Fig. 2). However, one should note that maleness induction was very high (>80 %) for all species irrespective of whether they acted as antheridiogen source or target.
Several models predict that polyploids have higher selfing rates than their diploid progenitors (reviewed by Barringer, 2007). The present results do not support this prediction. The gametophytes of the three Dryopteris species studied had an initial unisexual phase, i.e. female under rich growth conditions and male under the effect of antheridiogens. Similarly, Cousens (1975) found high proportions of unisexual gametophytes in eight Dryopteris species, including some allopolyploids. The balance between intragametophytic selfing and intergametophytic mating can be expected to depend on the time periods of the different sexual phases, on whether or not they overlap, and on the length of the period of overlap (Cruden and Lloyd, 1995). Unisexuality, promoted by antheridiogens, favours cross-fertilization in the species studied, or at least the present results suggest that they have similar balances of intra- and intergametophytic mating. In contrast, experimental evidence in homosporous ferns indicates that polyploidy is associated with increased levels of selfing (Masuyama and Watano, 1990; Suter et al., 2000; Pangua et al., 2003). In the genus Dryopteris, Flinn (2006) found higher intragametophytic selfing in the allotetraploid D. carthusiana than in one of its parental species, D. intermedia. However, a number of studies on angiosperms contradict this relationship between polyploidy and inbreeding (Husband and Schemske, 1997; Galloway et al., 2003; Yeung et al., 2005). The most recent review of this topic did support increased selfing in allopolyploids but not in autopolyploids (Husband et al., 2008). The presumed recent origin of D. corleyi (see Materials and Methods: Study species) may explain the proposed deviation from the breeding behaviour expected for allopolyploids. As suggested by Cook and Soltis (1999), if both diploid parental species were outcrossers, then the derived allotetraploid would inherit the traits responsible for cross-fertilization. For self-fertilization to evolve, substantial time would be required for selection to favour it (Cook and Soltis, 1999). Genotype frequencies of D. oreades at allozyme loci show Hardy–Weinberg equilibrium, in accordance with random mating (A. Jiménez et al., unpubl. res.). In D. aemula and D. corleyi, however, determining the mating system could not be accomplished owing to a lack of allozyme variation.
The non-specific antheridiogen system that was found in the three species in the present study could also favour the coexistence among females and males of different species, which might promote random mating among species. Antheridiogens would favour hybridization in multispecies gametophyte communities (Haufler and Ranker, 1985; Schneller et al., 1990) and may thus select against the establishment of minority cytotypes (Levin, 1975).
Intercytotype differences in growth rate
The third hypothesis, that gametophytes of the allotetraploid D. corleyi will have faster vegetative and reproductive growth than those of diploid parental species, was only partially supported by the present results. In expt 2, the three species showed similar vegetative growth rates. The only exception was the antheridiogen treatment at week 18, in which the size of D. corleyi was intermediate between its diploid parental species (Fig. 3). However, in expt 1, archegonia appeared earlier in the allotetraploid than in the diploids. Similarly, in expt 2, the allotetraploid produced antheridia earlier than diploids. It can thus be concluded that reproductive but not vegetative growth rate is faster in D. corleyi. It has been shown that spore germination of some allotetraploid homosporous ferns is faster than that of their diploid parental species (e.g. Dryopteris, Whittier, 1970; Polypodium, Kott and Peterson, 1974). This pattern was also found in the three species studied (Quintanilla and Escudero, 2006). However, it seems that the initial advantage of D. corleyi at germination is not sustained in subsequent vegetative growth of the gametophyte.
A shorter time to sexual maturity of the gametophytes of polyploid species has been observed in other ferns. For example, the allotetraploid Polypodium virginianum attains sexual maturity faster than one of its diploid parental species (Kott and Peterson, 1974), and the autotetraploid Phegopteris decursive-pinnata produces sporophytes earlier than the diploid cytotype (Masuyama, 1979). Early gametangia production found in D. corleyi in the present study may favour ploidy-related assortative mating. Two traits can promote germination of the studied species in the same time and place. First, spore dispersal of the three studied Dryopteris species occurs synchronously in the late summer and thus favours simultaneous germination (Quintanilla and Escudero, 2006). Secondly, microsites suitable for the growth of gametophytes are rare and lead to a spatial clustering of different species (Cousens et al., 1985). However, following germination, the gametophytes of D. corleyi will produce archegonia and release antheridiogens earlier than its two parental species. Furthermore, antheridia formation in response to antheridiogens will also be faster in D. corleyi. This time advantage can promote intraspecific mating in D. corleyi. Given that early sexual maturity reduces hybridization, it may allow D. corleyi gametophytes to circumvent minority cytotype exclusion (Levin, 1975). This assortative fertilization we propose is analogous to the assortative pollination due to flowering asynchrony that has been observed in some autopolyploid flowering plants (Maceira et al., 1993; Husband and Sabara, 2004).
Conclusions
The gametophytes of D. corleyi and its two diploid parental species show two traits with opposite effects for the establishment of polyploids. First, unisexuality, enhanced by non-specific antheridiogens, favours random mating both within and among species. The resulting hybridization may hinder the persistence of D. corleyi in sites where it is outnumbered by diploid parental species. Secondly, earlier gametangia production by D. corleyi promotes intraspecific (assortative) mating and may therefore counterbalance minority cytotype exclusion. Future studies should test if these gametophyte traits observed under culture conditions also occur in nature. Additionally, analysing the distribution and abundance of different cytotypes in polyploid–diploid hybrid zones (Petit et al., 1999) could answer the questions of the importance of hybridization and competition among cytotypes, thus complementing experimental approaches (Baack, 2004).
ACKNOWLEDGEMENTS
We thank R. Holderegger and two anonymous reviewers for their valuable comments on earlier versions of this manuscript, and C. Iriarte for laboratory assistance. This research was supported by the Spanish Ministerio de Educación y Ciencia (project CGL2006-07012).
LITERATURE CITED
- Baack EJ. Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae) American Journal of Botany. 2004;91:1783–1788. doi: 10.3732/ajb.91.11.1783. [DOI] [PubMed] [Google Scholar]
- Barker J, Willmot A. Preliminary studies on the breeding systems of Dryopteris filix-mas (L.) Schott and D. dilatata (Hoffm) A. Gray. Proceedings of the Royal Society of Edinburgh. 1985;86B:455–456. [Google Scholar]
- Barringer BC. Polyploidy and self-fertilization in flowering plants. American Journal of Botany. 2007;94:1527–1533. doi: 10.3732/ajb.94.9.1527. [DOI] [PubMed] [Google Scholar]
- Bull JJ. Evolution of environmental sex determination from genotypic sex determination. Heredity. 1981;47:173–184. [Google Scholar]
- Chiou WL, Farrar DR. Antheridiogen production and response in Polypodiaceae species. American Journal of Botany. 1997;84:633–640. [PubMed] [Google Scholar]
- Cook LM, Soltis PS. Mating systems of diploid and allotetraploid populations of Tragopogon (Asteraceae). I. Natural populations. Heredity. 1999;82:237–244. doi: 10.1038/sj.hdy.6884620. [DOI] [PubMed] [Google Scholar]
- Cousens MI. Gametophyte sex expression in some species of Dryopteris. American Fern Journal. 1975;65:39–42. [Google Scholar]
- Cousens MI, Horner HT. Gametophyte ontogeny and sex expression in Dryopteris ludoviciana. American Fern Journal. 1970;60:13–27. [Google Scholar]
- Cousens MI, Lacey DG, Kelly EM. Life history studies of ferns: a consideration of perspective. Proceedings of the Royal Society of Edinburgh. 1985;86B:371–380. [Google Scholar]
- Cruden RW, Lloyd RM. Embryophytes have equivalent sexual phenotypes and breeding systems: why not a common terminology to describe them? American Journal of Botany. 1995;82:816–825. [Google Scholar]
- Döpp W. Ein die Antheridienbildung bei Farnen fördernde Substanz in den Prothallien von Pteridium aquilinum (L.) Kuhn. Berichte der Deutschen Botanischen Gesellschaft. 1950;63:139–147. [Google Scholar]
- Dyer AF. The culture of fern gametophytes for experimental investigation. In: Dyer AF, editor. The experimental biology of ferns. London: Academic Press; 1979. pp. 253–305. [Google Scholar]
- Felber F. Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. Journal of Evolutionary Biology. 1991;4:195–207. [Google Scholar]
- Fernández H, Bertrand AM, Sierra MI, Sánchez-Tamés R. An apolar GA-like compound responsible for the antheridiogen activity in. Blechnum spicant. Plant Growth Regulation. 1999;28:143–144. [Google Scholar]
- Flinn KM. Reproductive biology of three fern species may contribute to differential colonization success in post-agricultural forests. American Journal of Botany. 2006;93:1289–1294. doi: 10.3732/ajb.93.9.1289. [DOI] [PubMed] [Google Scholar]
- Fraser-Jenkins CR. Dryopteris in Spain, Portugal and Macaronesia. Boletim da Sociedade Broteriana. 1982;55:175–336. [Google Scholar]
- Fraser-Jenkins CR, Gibby M. A new Dryopteris hybrid from Spain. Fern Gazette. 1986;13:113–116. [Google Scholar]
- Fraser-Jenkins CR, Widén CJ. Phloroglucinol derivatives in Dryopteris ardechensis and in D. corleyi (Pteridophyta, Dryopteridaceae) and their putative ancestors. Annales Botanici Fennici. 1983;30:43–51. [Google Scholar]
- Galloway LF, Etterson JR, Hamrick JL. Outcrossing rate and inbreeding depression in the herbaceous autotetraploid. Campanula americana. Heredity. 2003;90:308–315. doi: 10.1038/sj.hdy.6800242. [DOI] [PubMed] [Google Scholar]
- Geiger JMO, Ranker TA. Molecular phylogenetics and historical biogeography of Hawaiian Dryopteris (Dryopteridaceae) Molecular Phylogenetics and Evolution. 2005;34:392–407. doi: 10.1016/j.ympev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Grant V. Plant speciation. 2nd edn. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Ghiselin M. Evolution of hermaphroditism among animals. Quarterly Review of Biology. 1969;44:189–208. doi: 10.1086/406066. [DOI] [PubMed] [Google Scholar]
- Haig D, Westoby M. Sex expression in homosporous ferns: an evolutionary perspective. Evolutionary Trends in Plants. 1988;2:111–119. [Google Scholar]
- Haufler CH, Ranker TA. Differential antheridiogen response and evolutionary mechanisms in Cystopteris. American Journal of Botany. 1985;72:659–665. [Google Scholar]
- Husband BC, Sabara HA. Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolum (Onagraceae) New Phytologist. 2004;161:703–713. doi: 10.1046/j.1469-8137.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. The effect of inbreeding in diploid and tetraploid populations of Epilobium angustifolium (Onagraceae): implications for the genetic basis of inbreeding depression. Evolution. 1997;51:737–746. doi: 10.1111/j.1558-5646.1997.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Ozimec B, Martin SL, Pollock L. Mating consequences of polyploid evolution in flowering plants: current trends and insights from synthetic polyploids. International Journal of Plant Sciences. 2008;169:195–206. [Google Scholar]
- Kennedy BF, Sabara HA, Haydon D, Husband BC. Pollinator-mediated assortative mating in mixed ploidy populations of Chamerion angustifolium (Onagraceae) Oecologia. 2006;150:398–408. doi: 10.1007/s00442-006-0536-7. [DOI] [PubMed] [Google Scholar]
- Korpelainen H. Growth, sex determination and reproduction of Dryopteris filix-mas (L.) Schott gametophytes under varying nutritional conditions. Botanical Journal of the Linnean Society. 1994;114:357–366. [Google Scholar]
- Korpelainen H. Labile sex expression in plants. Biological Reviews. 1998;73:157–180. [Google Scholar]
- Kott LS, Peterson RL. A comparative study of gametophyte development of the diploid and tetraploid races of Polypodium virginianum. Canadian Journal of Botany. 1974;52:91–96. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Lott MS, Volin JC, Pemberton RW, Austin DF. The reproductive biology of the invasive ferns Lygodium microphyllum and L. japonicum (Schizaeaceae): implications for invasive potential. American Journal of Botany. 2003;90:1144–1152. doi: 10.3732/ajb.90.8.1144. [DOI] [PubMed] [Google Scholar]
- Mable BK. Polyploidy and self-compatibility: is there an association? New Phytologist. 2004;162:803–811. doi: 10.1111/j.1469-8137.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- Maceira NO, Jacquard P, Lumaret R. Competition between diploid and derivative autotetraploid Dactylis glomerata L. from Galicia: implications for the establishment of novel polyploid populations. New Phytologist. 1993;124:321–328. doi: 10.1111/j.1469-8137.1993.tb03822.x. [DOI] [PubMed] [Google Scholar]
- Manton I. Problems in cytology and evolution in the Pteridophyta. Cambridge: Cambridge University Press; 1950. [Google Scholar]
- Masterson J. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Masuyama S. Reproductive biology of the fern Phegopteris decursive-pinnata. I. The dissimilar mating systems of diploids and tetraploids. Botanical Magazine Tokyo. 1979;92:275–289. [Google Scholar]
- Masuyama S, Watano Y. Trends for inbreeding in polyploid pteridophytes. Plant Species Biology. 1990;5:13–17. [Google Scholar]
- Mayor M, Fernández M. Comportamiento ecológico de Dryopteris corleyi Fraser-Jenkins. Lazaroa. 1988;10:181–185. [Google Scholar]
- Näf U. The demonstration of a factor concerned with the initiation of antheridia in polypodiaceous ferns. Growth. 1956;20:91–105. [PubMed] [Google Scholar]
- Otto SP, Whitton J. Polyploid incidence and evolution. Annual Review of Genetics. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- Pangua E, Quintanilla LG, Sancho A, Pajarón S. A comparative study of the gametophytic generation in the Polystichum aculeatum group (Pteridophyta) International Journal of Plant Sciences. 2003;164:295–303. [Google Scholar]
- Paquin V, Aarssen LW. Allometric gender allocation in Ambrosia artemisiifolia (Asteraceae) has adaptive plasticity. American Journal of Botany. 2004;91:430–438. doi: 10.3732/ajb.91.3.430. [DOI] [PubMed] [Google Scholar]
- Pérez Carro FC, Díaz González TE. Dryopteris × arecesiae (Aspidiaceae), nuevo híbrido para la pteridoflora cantábrica. Anales del Jardín Botánico de Madrid. 1990;47:239–240. [Google Scholar]
- Petit C, Bretagnolle F, Felber F. Evolutionary consequences of diploid-polyploid hybrid zones in wild species. Trends in Ecology and Evolution. 1999;14:306–311. doi: 10.1016/s0169-5347(99)01608-0. [DOI] [PubMed] [Google Scholar]
- Quintanilla LG, Escudero A. Spore fitness components do not differ between diploid and allotetraploid species of Dryopteris (Dryopteridaceae) Annals of Botany. 2006;98:609–618. doi: 10.1093/aob/mcl137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla LG, Amigo J, Pangua E, Pajarón S. Effect of storage method on spore viability in five globally threatened fern species. Annals of Botany. 2002;90:461–467. doi: 10.1093/aob/mcf224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JH, Morgan MT. The effect of self-fertilization, inbreeding depression, and population size on autopolyploid establishment. Evolution. 2005;59:1867–1875. [PubMed] [Google Scholar]
- Rodríguez D. A model for the establishment of polyploidy in plants. American Naturalist. 1996;147:33–46. [Google Scholar]
- Salvo AE, Arrabal MI. Dryopteris Adanson. In: Castroviejo S, Laínz M, López G, Montserrat P, Muñoz F, Paiva J, Villar L, editors. Flora Iberica. Vol. I. Madrid: CSIC; 1986. pp. 128–143. [Google Scholar]
- Schneller JJ, Haufler CH, Ranker TA. Antheridiogen and natural gametophyte populations. American Fern Journal. 1990;80:143–152. [Google Scholar]
- Soltis DE, Rieseberg LH. Autopolyploidy in Tolmiea menziesii (Saxifragaceae): genetic insights from enzyme electrophoresis. American Journal of Botany. 1986;73:310–318. [Google Scholar]
- Soltis DE, Soltis PS. Polyploidy, breeding systems, and genetic differentiation in homosporous pteridophytes. In: Soltis DE, Soltis PS, editors. Isozymes in plant biology. Portland, OR: Dioscorides Press; 1989. pp. 241–258. [Google Scholar]
- SPSS. SPSS for Windows, version 12·0·1. Chicago, IL: SPSS Inc; 2003. [Google Scholar]
- Stebbins GJ. Chromosomal evolution in higher plants. London: Addison-Wesley; 1971. [Google Scholar]
- Suter M, Schneller JJ, Vogel JC. Investigations into the genetic variation, population structure, and breeding systems of the fern Asplenium trichomanes subsp. quadrivalens. International Journal of Plant Sciences. 2000;161:233–244. doi: 10.1086/314258. [DOI] [PubMed] [Google Scholar]
- Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. The Plant Cell. 2004;16:S61–S71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiason DM, Seifert HS. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS Biology. 2006;4:1069–1078. doi: 10.1371/journal.pbio.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida G. The role of polyploidy in evolution. In: Novák VJA, Pacltová B, editors. Evolutionary biology. Praha: Czechoslovak Academy of Sciences; 1976. pp. 267–294. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Whittier DP. The rate of gametophyte maturation in sexual and apogamous species of ferns. Phytomorphology. 1970;20:30–35. [Google Scholar]
- Yamane H. Fern antheridiogens. International Review of Cytology. 1998;184:1–32. [Google Scholar]
- Yeung K, Miller JS, Savage AE, Husband BC, Igic B, Kohn JR. Association of ploidy and sexual system in Lycium californicum (Solanaceae) Evolution. 2005;59:2048–2055. [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 4th edn. Upper Saddle River, NJ: Prentice-Hall; 1999. [Google Scholar]