Abstract
Background and Aims
Anther appendages play diverse roles in anther dehiscence and pollen dispersal. This study aims to explore the pollen-dispensing mechanism triggered by special anther appendages in Incarvillea arguta.
Methods
Field studies were conducted to record floral characteristics, pollinator visitations, and flower–pollinator interactions. Measurements of flowers and pollinators were analysed statistically. Pollen counts following a series of floral manipulations were used to evaluate pollen dispensing efficiency and function of the anther appendages.
Key Results
Field observations determined that two species of Bombus (bumble-bees) were the primary pollinators of I. arguta with a mean visiting frequency of 1·42 visitations per flower h−1. The results display a diminishing pollen dispensing pattern; the proportion of remaining pollen removed by pollinators decreased from 27 % to 10 % and 7 % in subsequent visits. Anther appendages act as a trigger mechanism to dispense pollen. The arrangement of the anthers and appendages function to control pollen load and timing. Mechanical stimulation experiments revealed that one set of appendages is only triggered by stimulation in the direction moving into the flower, while the other set is only triggered by stimulation in the opposite direction (exiting the flower).
Conclusions
The anther appendage is a pollen-dispensing trigger mechanism. The configuration of the stamens and duel trigger system has evolved to allocate pollen in allotments to enhance male function.
Key words: Incarvillea arguta, anther appendage, pollination biology, Bignoniaceae, stamen morphology, pollen dispensing
INTRODUCTION
The individuals of hermaphroditic plants commonly have both a ‘male’ and a ‘female’ sexual function. If reproductive resources are limited, resource allocation to one sexual function could diminish the alternate sexual function (Charnov, 1979; Charlesworth and Charlesworth, 1981; Lloyd, 1984). Evolutionary methods for enhancement of male sexual success have been documented in many studies (Willson and Price, 1977; Charnov, 1982; Lloyd and Yates, 1982; Sutherland and Delph, 1984; Bell, 1985; Stanton et al., 1986). Ultimately, male sexual fitness depends on the proportion of pollen that reaches receptive stigmas (Thomson and Barrett, 1981; Lloyd and Yates, 1982). Male fitness may be reduced if all pollen is removed in one pollination event (Harder and Wilson, 1994). Therefore, in the face of diminishing returns, a plant with limited resources for male function could have a selective advantage in maximizing pollen dispersal by restricting pollen removal during each pollinator visit (Harder and Thomson, 1989). As a result, selection could favour a lengthened male phase (the duration of pollen presentation) to allow more successful pollinator visits per flower in order to increase male success (Lloyd and Yates, 1982).
In general, there are two mechanisms used by plants to restrict the pollen load available to pollinators: packaging (staggering the maturation of anthers, flowers or inflorescences) and dispensing (restricting the pollen amount available during a single visit) (Lloyd and Yates, 1982; Brantjes, 1983; Harder and Thomson, 1989). Effective dispensing and/or packaging strategies allow multiple floral visitors to participate in pollen dissemination. The pattern of pollen allocation (Harder and Thomson, 1989; Harder and Wilson, 1994; Harder, 1990; Galen and Stanton, 1989; Young and Stanton, 1990) predicts that the proportion of pollen remaining in the anther after each successful floral visit should decline during a sequence of visits. However, the loss in fertilization success due to time-dependent factors (e.g. pollen precedence and declining viability) favours a pattern of continuously increasing the proportion of pollen removed during successive visits. Hence, Harder and Wilson (1994) proposed optimal dispensing schedules in order to balance the benefits of restricted removal with the advantages of increased removal to avoid time-dependent losses in fertilization. Their hypothesis suggests that dynamic dispensing (theoretical advantages of pollen allocation that adjusts to the frequency of pollinator visits) should be taken into account in relative experimental and theoretical studies.
Anther appendages or spurs have been proposed to provide an accessory function in anther dehiscence and pollen dispersal (Buchmann, 1983; Hermann and Palser, 2000). The anthers of several members of the Orobanchaceae have elongated spurs and their function has been hypothesized as acting as levers to tilt the anthers in order to deposit a pollen load upon the floral visitor (Kuijt, 1969). Endress (1994) has also hypothesized that the function of anther appendages in Globba (Zingiberaceae) are as a lever, increasing the likelihood of the anther making full contact with a pollinator, and Armstrong (1992) reported a similar mechanism in Torenia (Scrophulariaceae), where the flange-like anther structures shed pollen forcibly via a lever action. In addition, the concave, spade-like awns on the anthers of some species of Viola and Hybanthus (Violaceae; Beattie, 1971, 1974; Augspurger, 1980) are filled with pollen grains that will be dumped on nectar-seeking insects. However, in each of these cases there is little direct evidence linking these appendages to pollinator behaviour in controlling anther dehiscence and pollen load.
Incarvillea arguta (Bignoniaceae) is a small shrub occurring throughout the western Himalayas in Nepal and the Chinese provinces of Yunnan and Sichuan. The plants have large, pink, insect-pollinated flowers. The flowers are hermaphroditic and self-compatible. A unique type of anther morphology in the genus has been described based on studies of the horticultural species, Incarvillea delavayi (Cutting, 1921). Cutting (1921) hypothesized that an insect passing through the floral tube of Incarvillea would make repeated contact with appendages on the anther lobes; contact with each appendage would result in a separate pollen-shedding event. He also speculated that the unequal surface of the pollen sac serves a double function; first, to prevent the pollen from being shed all at once, and secondly to allow more pollen to be shed directly on the insect. Since his description was based on the observations of cultivated, garden plants, he suggested that the exact details of pollination can only be made in the field where the natural pollinators occur. We chose I. arguta as our study subject because it shares this unique anther morphology and pollination strategy and it is common in high elevation habitats (1300–2700 m) in south-western China.
The main objectives of this study were: (1) to determine the natural pollinator(s) of I. arguta; (2) to document the pollination mechanism; (3) to document the reproductive significance of the novel anther configuration and appendages in the process of pollination; and (4) to evaluate the pollen-dispensing efficiency in terms of male reproductive success.
MATERIALS AND METHODS
Study area
The study was carried out in Sichuan province of south-western China. Five populations of Incarvillea arguta Royle were sampled (Table 1). Populations ranged in size from approximately 50 to 500 individuals and were 100–500 m−2 in area. Habitats for I. arguta are rocky slopes or roadside thickets. Field observations and floral manipulations were conducted during the flowering period from July to August in 2005 and 2006. Voucher specimens of I. arguta are deposited in the Herbarium of College of Life Sciences, Wuhan University (WH).
Table 1.
Details of the five Incarvillea arguta study sites
| Population | Altitude | Location | Habitat |
|---|---|---|---|
| Wenchuan | 1336 m | 31°27·836′N, 103°34·281′E | Sandy soil |
| Lixian | 1854 m | 31°26·371′N, 103°9·901′E | Rocky limestone |
| Ma'erkang | 2548 m | 31°54·625′N, 102°11·959′E | Sandy soil |
| Kangding | 2704 m | 30°8·992′N, 101°55·515′E | Sandy soil |
| Yajiang | 2558 m | 30°0·988′N, 101°0·701′E | Rocky limestone |
Floral maturation
Observations on flower maturation were conducted during the entire 2-month flowering period in the five populations. To estimate foraging opportunities available to floral visitors and maturation patterns, the sequence and number of flowers opening on 30 inflorescences from 30 individuals in each population was recorded. In addition, for individual flowers on each inflorescence the time of corolla opening and wilting was measured in order to ascertain the rate of anthesis under natural conditions.
Flower–pollinator interactions
In order to document flower–pollinator interactions, floral visitor activity in three populations was observed (Lixian, Ma'erkang and Yajiang). Observations were made from 0600 h to 1800 h (dawn to dusk) and the pollinator visitation frequency was recorded in the Ma'erkang population. Two 1 × 1 m plots (20 m apart) containing two individuals were selected. The total number of open flowers was recorded in each plot and a record was made of the time each potential pollinator entered the plot and the number of flowers it visited. Floral visitations per hour were calculated as the total number of flowers visited in 1 h divided by total number of open flowers. The visitation frequency experiment was conducted for a total of 36 h over 3 d in the field in July, 2005.
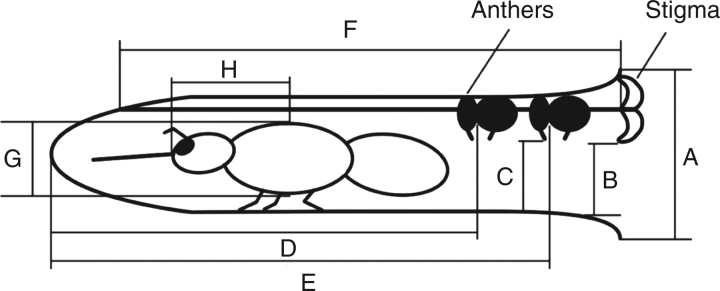
Insect visitors were collected in order to measure the height of the thorax (Fig. 1, G) and the distance between head and thorax (Fig. 1, H). The voucher specimens were sent to entomologists for identification at the Institute of Zoology, Chinese Academy of Sciences, and are deposited in the Laboratory of Plant Systematics and Evolutionary Biology, Wuhan University.
Fig. 1.
Diagram of the flower and pollinator of Incarvillea arguta, indicating parts measured in this study. A, corolla tube width (mean ± s.e. = 6·66 ± 0·61 mm, n = 31); B, distance between lower stigmatic lobe and floral tube (=4·11 ± 1·10 mm, n = 73); C, distance between anther appendage and floral tube (=4·20 ± 1·06 mm, n = 73); D, distance between inner anther whorl and base of floral tube (=18·00 ± 0·99 mm, n = 30); E, distance between outer anther whorl and base of floral tube (=21·47 ± 1·32 mm, n = 30); F, length of style (=24·64 ± 1·20 mm, n = 31); G, height of insect thorax (=5·52 ± 0·59 mm, n = 39); H, distance between head and thorax (=10·89 ± 2·17 mm, n = 39).
In order to ascertain whether the pollinator could touch the anther appendages during foraging, open flowers (n = 100) were chose randomly in order to measure the size and distance between the different floral structures (Fig. 1). The data collected included maximum corolla tube width (at mouth; Fig. 1, A); distance between lower stigmatic lobe and floral tube (B); distance between anther appendages and floral tube (C); distance between inner anther whorl and bottom of floral tube (D); distance between outer anther whorl and bottom of floral tube (E); and length of style (F).
Pollen production, removal and deposition
In order to confirm the role of the anther appendages in pollination, anthers were collected for pollen grain counts following different treatments. First, to estimate the pollen production per flower, 30 buds were randomly collected from ten individuals, placed in 1·5-mL Eppendorf tubes and brought back to the laboratory. Each of pollen sac was split separately to suspend all the pollen grains in 5 mL of water. Pollen production was then determined by counting the number of mature and aborted pollen grains in ten drops (0·5 mL) of the pollen solution; these counts were extrapolated to ascertain the total number of grains in the 5 mL volume. Similarly, flowers were collected randomly after corolla abscission in order to count the pollen grains remaining in each anther sac. The corresponding stigmas were cut off separately and fixed in standard FAA solution (formalin : acetic acid : 70 % ethanol at a ratio of 5 : 6 : 89 by volume). Pollen grains deposited on the stigmas were counted under a fluorescence microscope.
In addition, in order to estimate the contribution of anther appendages in the pollen-dispensing mechanism, counts of pollen grains were made subsequent to an initial, secondary and tertiary visit by pollinators. Ninety-nine individual flowers just prior to opening from 20 plants were bagged with mesh bags (1 × 1 mm pore size) to exclude visitors. When the bilobed stigma lobes reflexed (meaning that the stigma was receptive) the bag was removed and the first visit by a pollinator was awaited; the stigma lobes close after a floral visitation. One-third of the flowers were removed, fixed in FAA solution and brought back to the laboratory in order to count the pollen grains remaining in the pollen sacs and those deposited on the stigmas. The remaining two-thirds of the flowers were again bagged until the stigma lobes reflexed and became receptive once more. The flower collection and bagging steps were repeated until a total of three visits had occurred. The entire operation was accomplished in a single day, with each phase being accomplished over an approx. 2-h period. Lastly, 40 flowers were randomly chose after corolla abscission in order to count the pollen left in the pollen sacs and those deposited on stigma. Pollen efficiency (Kearns and Inouye, 1993) was calculated by dividing the proportion of pollen grains deposited on stigma by the number of pollen grains removed by the insect.
In addition, dissecting needles were used in order to manually stimulate the anther appendages. One hundred and five virgin flowers were carefully cut open along the bottom of floral tube, and the anthers exposed. A dissecting needle was used to manipulate all the anther appendages once in the direction so as to simulate an insect entering the flower, and the same manipulation was repeated in the opposite direction so as to simulate an insect exiting the flower. Thirty-five anthers were then removed and placed separately into Eppendorf tubes in order to count the pollen grains left in each pollen sac. This process was repeated in order to simulate second and third visits to the flowers, with the remaining pollen grains being counted for 35 anthers in each case.
RESULTS
Floral maturation and anatomy
The flowers of Incarvillea arguta are borne on a racemose inflorescence that produces 5–20 flowers during August. Each day (1–)2–3(–5) flowers open per inflorescence. The inflorescence is indeterminate, maturing from the base to the tip. The flowers last 1–3 d, but bad weather can shorten longevity.
The stamens, style and stigma are located near the posterior wall of the corolla tube (Fig. 1). The style is longer than the stamens, so that the stigma is positioned nearest to the mouth of the corolla. The lobes of the stigma, which are weakly strigous on their inner surface, fold together after stimulation to conceal the receptive surfaces. A nectar reward is produced at the base of tubular corolla.
The androecium is didynamous and consists of four stamens. The anthers are arranged in weakly fused pairs on either side of the style, and each anther consists of an anterior, conspicuously larger pollen sac and a posterior, smaller pollen sac (Fig. 1). In I. arguta, as in other species of Incarvillea, the anther lobes are parallel to the filament and each has a trigger appendage that protrudes at a right-angle to the anther surface, downwards into the tube of the corolla. The suture (line of anther dehiscence) runs from the appendage to the distal end of each anther lobe. Between the trigger appendage and the connective tissue, the lobes are hollow and the appendage itself is hinged to thin-walled cells.
Flower–pollinator interactions
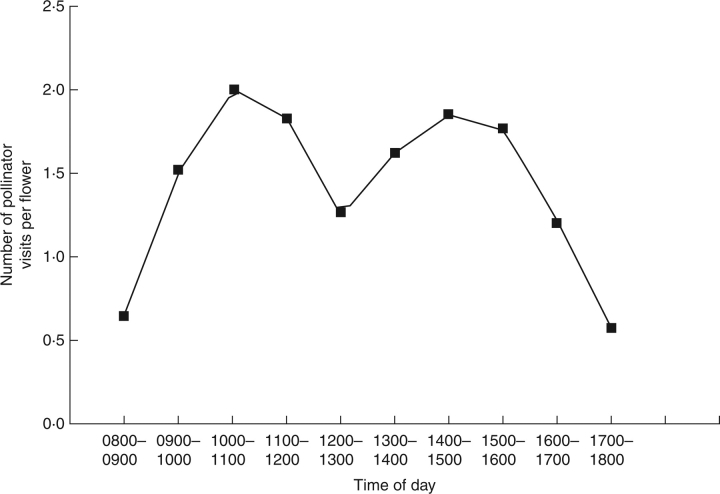
The pollinator observations confirmed that the flower–pollinator interaction in I. arguta is similar to Cutting's (1921) suppositions based on the floral mechanisms of I. delavayi. When an insect of the proper size enters a flower, the thorax first touches against the bilobed stigma and deposits pollen from another flower; the stigma lobes then fold together. As the insect moves down the floral tube it reaches the first whorl of stamens and the thorax contacts the first pair of anther appendages on the larger pollen sacs. However, the contact does not trigger the opening of the anther; on the contrary, they will remain shut tightly because the pressure on the trigger is in an ineffectual direction. When the insect touches the next pair of appendages (on the smaller anther sacs), it triggers anther dehiscence and pollen is shed on the thorax of the insect. As the insect passes the next whorl of stamens an identical trigger procedure and deposition takes place. Thus, as an insect enters a flower only the smaller anther sacs shed pollen. When the insect exits the flower after obtaining nectar, the reverse process takes place and only the larger anther sacs open and shed pollen. Our observations of floral visitors in the natural populations showed that visits occurred between 0800 h and 1800 h, with two peaks in visitation frequency at 1000–1100 h and 1400–1500 h (Fig. 2). The two higher visitation frequencies values were 1·99 and 1·85 times per flower h−1, respectively, and the mean frequency of floral visitations over an entire day was 1·42 ± 0·50 times per flower h−1 (± s.e., n = 30). These data illustrate that I. arguta populations experience a relatively high mean frequency of floral visitors, and the interval between visits to individual plants was short.
Fig. 2.
Pollinator visitation frequency for Incarvillea arguta.
The floral visitors included bumble-bees, smaller bees (Halictus and Tetralonia) and ants. In all populations of I. arguta, the insects observed that would serve as effective pollinators belonged to the family Apidae, bumble-bees (Bombus friseanus and Bombus picipes). The smaller bees (Halictus and Tetralonia) may serve as incidental pollinators, occasionally triggering pollen-shed and deposition, but they do not consistently contact the anther appendages or the stigma during visits. Ants are merely opportunistic nectar robbers, and only on rare occasions may serve as a pollinator.
The floral measurement data (Fig. 1) clarified the assumed of flower–pollinator interactions. The corolla tube width and height exceeded the size of all floral visitors and made it possible for the putative pollinators to land and enter the flower. The main reward for the insect visitors is nectar produced at the base of floral tube. Because the distance from head (including mouth parts) to thorax of the insect are shorter than the corolla tube (distances H and F in Fig. 1), it is inevitable that the pollinator touches the two whorls of anthers. There is no significant difference in the height of the lobe of the stigma and the anther appendages (distances B and C in Fig. 1; F = 0·003, d.f. = 144, P = 0·956), which means that pollen deposition and discharge can be accomplished during a single visit. The height of the thorax of pollinators (Fig. 1, G) are greater than the distance of the anther appendages (Fig. 1, C) and stigma (Fig. 1, B) to the anterior corolla tube wall. Therefore, the effective pollinators should consistently contact with these structures while entering and exiting the flowers. The data that was collected from bumble bees attests to this supposition (Fig. 1, G = 5·43 ± 0·59 mm, n = 10; H = 8·61 ± 0·63 mm, n = 8 for B. picipes; and see Fig. 1G and H for data of B. friseanus).
Pollen production, removal and deposition
Anthers were removed prior to pollination in order to measure pollen production in the outer whorl-large, outer whorl-small, inner whorl-large, and inner whorl-small anther sacs. The mean number of pollen grains per anther sac type was 8647 ± 1630 (n = 10) in the large outer sacs, 6188 ± 1662 (n = 10) in the small outer sacs, 8735 ± 2435 (n = 10) in the large inner sacs, and 4972 ± 1244 (n = 10) in the small inner sacs. The larger anther sacs contained significantly more pollen grains than the smaller ones (17382 vs. 11160; F = 21·55, P = 0·0002). Insects trigger the smaller anther sacs whilst entering the flower and trigger the larger sacs whilst exiting, thus receiving more pollen grains whilst departing the flower.
In flowers of I. arguta, the total number of pollen grains in the four anthers was 34375 ± 3665 (n = 30). Interestingly, the total number of pollen grains removed increased with each subsequent floral visit (Fig. 3A). During the first visit the mean number of pollen grains shed was 9408 ± 3875 (n = 29), and this increased to 11974 ± 3460 (n = 33) and 13466 ± 4997 (n = 31) in the second and third visits, respectively. The proportion of remaining pollen grains removed from the anthers in the three sequential visits decreased with each visit (0·274 ± 0·110, n = 29; 0·103 ± 0·139, n = 33; and 0·067 ± 0·223, n = 31; Fig. 3B]. In contrast, the amount of out-cross pollen deposited on the stigma increased with each floral visit (454 ± 246, n = 31; 861 ± 300, n = 32; and 1243 ± 455, n = 35; Fig. 3C).
Fig. 3.
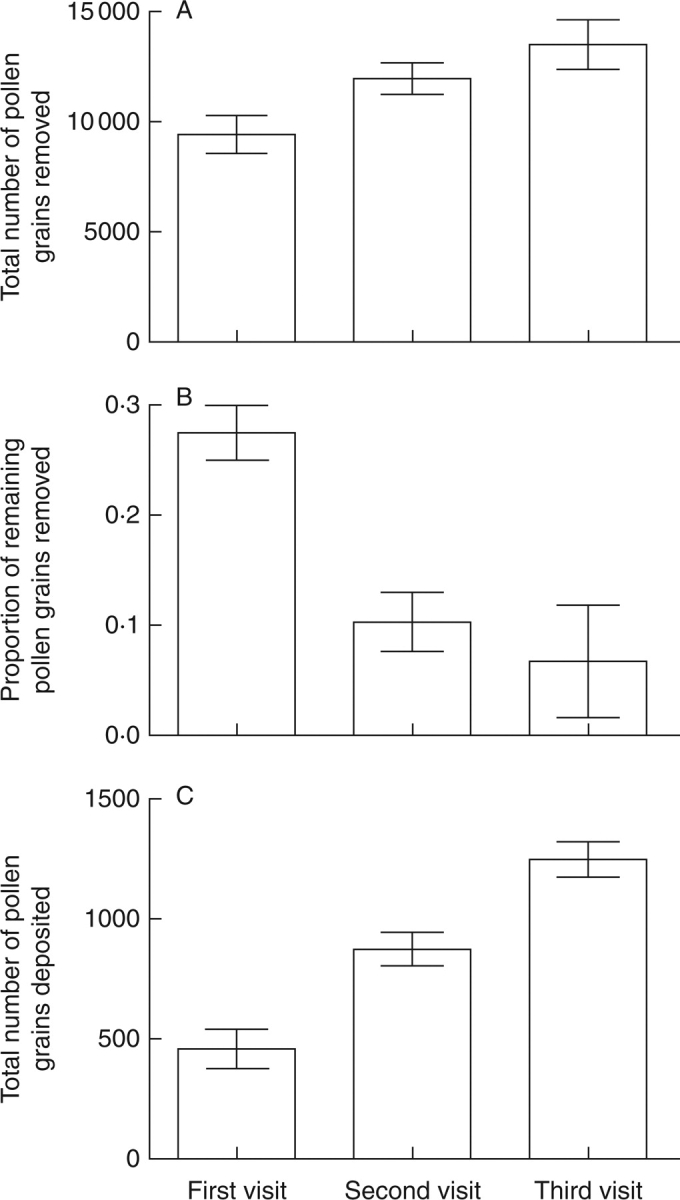
Pollen removal and deposition for three successive floral visitations to a flower of Incarvillea arguta in the field. (A) Pollen removal per visit, (B) the proportion of the remaining pollen removed, and (C) pollen deposition per visit. Data are means ± s.e.
The data collected during the manual simulations showed similar results, with the percentage of the pollen load discharged increasing over the three sequential visits: 17·1 % (5890 ± 2421, n = 31), 19·4 % (6662 ± 2815, n = 30) and 21·2 % (7174 ± 2257, n = 30), respectively. Correspondingly, the proportion of remaining pollen grains discharged by this manual mimicry was 17·4 %, 2·8 % and 2·2 %, respectively. Thus, both under artificial conditions in the laboratory and under natural conditions in the field plants of I. arguta exhibited increasing pollen discharge and diminishing proportions of remaining pollen discharged during three successive floral visitations. In the field, as a result of the complicated foraging behaviour of bumble-bees (for example, brushing and squeezing, or even buzzing), the amount of pollen discharged was more than under the artificial conditions. On the other hand, the simulation experiments indicated that only a single touch of an anther appendage will result in pollen discharge.
The pollination efficiency calculated for I. arguta was 2·2 %; the mean total number of pollen grains removed from the flower anthers was 24504 ± 2736 (n = 30), and the number deposited on stigma was 539 ± 315 (n = 40). For species with granular pollen, typically <1 % of the pollen removed from anthers reaches a stigma (Thomson and Thomson, 1989; Galen, 1992; Stanton et al., 1992; Harder, 2000; Johnson et al., 2005), so the value of 2·2 % for I. arguta is relatively high compared with other studies.
DISCUSSION
The pollination mechanism and stamen structures in Incarvillea are unique among flowering plants. An early paper by Cutting (1921) provided a general description of the pollination mechanism of the genus, whilst Knapp (1954) revisited this topic by examining the floral structure from an anatomical point of view. A more recent study of Incarvillea sinensis (Qu et al., 2007) revealed a new mechanism of delayed self-pollination, facilitated by wind-driven corolla abscission. However, in all these studies there were not sufficient data or observations to document the function of the anther-appendage trigger mechanism or to link the unique mechanism and floral morphology with native pollinators. Based on our field observations of five natural populations of I. arguta, it was found that the anther appendages act as a trigger mechanism that are activated by pollinator movements in order to ensure pollen loads are released both on entry and exit from the flower. The success of pollen discharge, trigger activation and pollen deposition onto the receptive stigmatic surfaces were found in this study to be linked to insect body size (Fig. 1).
An optimal pattern of pollen packaging and dispensing would maximize pollen dispersal by animal-pollinated plants, and consequently result in an increase of male reproductive success (Lloyd and Yates, 1982; Harder and Thomson, 1989; Harder and Wilson, 1994). Packaging arises in I. arguta because only a few flowers open at once. For many species, a sequential pollen-dispensing mechanism can enhance male fitness by governing the proportion of available pollen removed by each pollinator (Harder and Thomson, 1989). In case of I. arguta, the anther appendages function as switches to control the discharge of pollen grains when a pollinator is visiting the flower. The anther appendages ensure that only a small proportion of pollen is removed by individual pollinators. Effective allocation and dispensing strategies in I. arguta, which operate simultaneously as in Lupinus (Dunn, 1956) and Lobelia (Devlin and Stephenson, 1985), allow all of the pollinators visiting a plant to participate in pollen dispersal, thus potentially increasing pollen dispersal. This pollen-dispensing mechanism, directly triggered by the anther appendages of I. arguta, differs from previous reports on dispensing mechanisms, where the mechanisms were commonly found as characteristics of nectar production, secondary pollen presentation and poricidal anthers (Harder, 1990; Harder and Barrett, 1993; Harder and Barclay, 1994; LeBuhn and Anderson, 1994).
The results showed that pollen deposition on individual stigmas increased steadily over three floral visitations (Fig. 3C). The mean total pollen deposition reached 1243 ± 455 grains, which exceeds the estimate for the minimum number of pollen grains required for complete ovule fertilization (197 ± 32·4; Han et al., unpubl. res.). In fact, a single visit would probably ensure female reproductive success (Bell, 1985; Spira et al., 1996; Bell and Cresswell, 1998). The excessive number of pollen grains deposited in Incarvillea may provide a selective advantage by enhancing genetic variability through increasing the probability of xenogamous over geitonogamous fertilization. Even more importantly, a large pollen load on stigma can produce competition among male genotypes, and result in increased seed set (Marshall et al., 2007).
The proportion of remaining pollen grains removed declined with successive visits, which is in agreement with previous studies (Galen and Stanton, 1989; Harder, 1990; Young and Stanton, 1990). Harder and Wilson (1994) ascribed the decline in the proportion of pollen removal to unnaturally long periods preceding the first visit and short inter-visit intervals for subsequent visits. The short intervals between floral visits in I. Arguta (Fig. 2) probably reduce the impact of time-dependent fertilization problems. Thus there appears to be little selective advantage in releasing a large proportion of the pollen in an initial visit. In fact, in I. arguta nearly 60 % of the total pollen remained in the flower after three floral visitations (Fig. 3A). Pollen availability may be restricted by the anther appendages in order to increase the number of pollinators that can remove pollen from a flower (Lloyd and Yates, 1982; Queller, 1983; Harder and Thomson, 1989; Robertson and Lloyd, 1993), which leads to an attenuated male phase. Thus, I. arguta flowers may initially have both female and male function, but after a single pollination visit the flower will function primarily as a male pollen donor (Bell, 1985).
This precise pollen-dispensing mechanism triggered by the anther appendages of I. arguta minimizes the waste of resources and extends male function in four aspects. First, anther appendages control the opening of anther sacs and reduce pollen loss when effective pollinators are not present. Second, the trigger appendages reduce pollen predation and transport loss (Harder and Barrett, 2006) caused by ineffective visitors, for example by small bees and ants. Third, the directional activation of the anther appendages ensures that the majority of the pollen grains are deposited during the departure from the flower and thus reduces pollen loss during the foraging phase of visitation (Harder and Barrett, 2006). Lastly, the anther appendages prevent the total removal of pollen by an individual pollinator, and therefore many pollinators can be involved in pollen dispersal; as a result, male function and duration are enhanced.
ACKNOWLEDGMENTS
We sincerely thank Spencer C. H. Barrett, Lawrence D. Harder and two anonymous reviewers for critical comments and suggestions to improve the manuscript. We thank Hong Liu and Tao Wan for their help during the fieldwork, and Jian Yao and Yan-Ru Wu for identification of insect samples. This study was supported by grants from the National Natural Science Foundation of China (No. 30500032 and 30670362) and the New Century Program for Outstanding Talents in Universities (from Ministry of Education, People's Republic of China) granted to WQF (NCET-05-0619).
LITERATURE CITED
- Armstrong JE. Lever action anthers and the forcible shedding of pollen in Torenia (Scrophulariaceae) American Journal of Botany. 1992;79:34–40. [Google Scholar]
- Augspurger CK. Mass flowering of a tropical shrub (Hybanthus prunifolius): influence on pollinator attraction and movement. Evolution. 1980;34:475–488. doi: 10.1111/j.1558-5646.1980.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Beattie AJ. Pollination of mechanisms in Viola. New Phytologist. 1971;70:343–360. [Google Scholar]
- Beattie AJ. Floral evolution in Viola. Annals of the Missouri Botanical Garden. 1974;61:781–793. [Google Scholar]
- Bell G. On the function of flowers. Proceedings of the Royal Society of London Series B. 1985;224:223–265. [Google Scholar]
- Bell SA, Cresswell JE. The phenology of gender in homogamous flowers: temporal change in the residual sex function of flowers of oil-seed rape (Brassica napus) Functional Ecology. 1998;12:298–306. [Google Scholar]
- Brantjes NBM. Regulated pollen issue in Isotoma, Campanulaceae, and evolution of secondary pollen presentation. Acta Botanica Neerlandica. 1983;32:213–222. [Google Scholar]
- Buchmann SL. Buzz pollination in angiosperms. In: Jones CE, Little RJ, editors. Handbook of experimental pollination. New York: Scientific and Academic Editions; 1983. pp. 73–113. [Google Scholar]
- Charlesworth D, Charlesworth B. Allocation of resources to male and female function in hermaphrodites. Biological Journal of the Linnean Society. 1981;15:57–74. [Google Scholar]
- Charnov EL. Simultaneous hermaphroditism and sexual selection. Proceedings of the National Academy of Sciences of the USA. 1979;76:2480–2484. doi: 10.1073/pnas.76.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL. The theory of sex allocation. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Cutting EM. On the pollination mechanism of Incarvillea delavayi Franch. Annals of Botany. 1921;35:63–71. [Google Scholar]
- Devlin B, Stephenson AG. Sex differential floral longevity, nectar section, and pollinator foraging in a protandrous species. American Journal of Botany. 1985;72:303–310. [Google Scholar]
- Dunn DB. The breeding system of Lupinus, group Micranthi. American Midland Naturalist. 1956;55:443–472. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Galen C. Pollen dispersal dynamics in an alpine wildflower, Polemonium viscosum. Evolution. 1992;46:1043–1051. doi: 10.1111/j.1558-5646.1992.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Galen C, Stanton ML. Bumble bee pollination and floral morphology: factors influencing pollen dispersal in the alpine sky pilot, Polemonium viscosum (Polemoniaceae) American Journal of Botany. 1989;76:419–426. [Google Scholar]
- Harder LD. Pollen removal by bumble bees and its implications for pollen dispersal. Ecology. 1990;71:1110–1125. [Google Scholar]
- Harder LD. Pollen dispersal and the floral diversity of Monocotyledons. In: Wilson KL, Morrison D, editors. Monocots: systematics and evolution. Melbourne, Australia: CSIRO Publishing; 2000. pp. 243–257. [Google Scholar]
- Harder LD, Barclay RMR. The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Functional Ecology. 1994;8:509–517. [Google Scholar]
- Harder LD, Barrett SCH. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialization. Ecology. 1993;74:1059–1072. [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal pollinated plants. The American Naturalist. 1989;133:323–344. [Google Scholar]
- Harder LD, Wilson WG. Floral evolution and male reproductive success: optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evolutionary Ecology. 1994;8:542–559. [Google Scholar]
- Hermann PM, Palser BF. Stamen development in the Ericaceae. I. Anther wall, microsporogenesis, inversion, and appendages. American Journal of Botany. 2000;87:934–957. [PubMed] [Google Scholar]
- Johnson SD, Neal PR, Harder LD. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society. 2005;86:175–190. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologist. Boulder, CO: University of Colorado Press; 1993. [Google Scholar]
- Knapp Staubblattbildung und Bestäubungsmechanismus von Incarvillea variabilis Batalin. Österreichische Botanische Zeitschrift. 1954;101:208–219. [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Lebuhn G, Anderson GJ. Anther tripping and pollen dispensing in Berberis thunbergii. American Midland Naturalist. 1994;131:257–265. [Google Scholar]
- Lloyd DG. Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhán J, editors. Perspectives on plant population ecology. Sunderland, MA: Sinauer Associates; 1984. pp. 277–300. [Google Scholar]
- Lloyd DG, Yates JMA. Intrasexual selection and the segregation of pollen and stigmas in hermaphrodite plants, exemplified by Wahlenbergia albomarginata (Campanulaceae) Evolution. 1982;36:903–913. doi: 10.1111/j.1558-5646.1982.tb05462.x. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Shaner MGM, Oliva JP. Effects of pollen load size on seed paternity in wild radish: the roles of pollen competition and mate choice. Evolution. 2007;61:1925–1937. doi: 10.1111/j.1558-5646.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Queller DC. Sexual selection in a hermaphroditic plant. Nature. 1983;35:706–707. [Google Scholar]
- Robertson AW, Lloyd DG. Rates of pollen deposition and removal in Myosotis colensoi. Functional Ecology. 1993;7:549–559. [Google Scholar]
- Qu R, Li X, Luo Y, Dong M, Xu H, Chen X, Dafni A. Wind-dragged corolla enhances self-pollination: a new mechanism of delayed self-pollination. Annals of Botany. 2007;100:1154–1164. doi: 10.1093/aob/mcm209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira TP, Snow AA, Puterbaugh MN. The timing and effectiveness of sequential pollinations in Hibiscus moscheutos. Oecologia. 1996;105:230–235. doi: 10.1007/BF00328551. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Snow AA, Handel SN. Floral evolution: attractiveness to pollinators increases male fitness. Science. 1986;232:1625–1627. doi: 10.1126/science.232.4758.1625. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Ashman T, Galloway LF, Young HJ. Estimating male fitness of plants in natural populations. In: Wyatt R, editor. Ecology and evolution of plant reproduction: new approaches. New York: Chapman & Hall; 1992. pp. 62–90. [Google Scholar]
- Sutherland S, Delph LF. On the importance of male fitness in plants: patterns of fruit-set. Ecology. 1984;65:1093–1104. [Google Scholar]
- Thomson JD, Barrett SCH. Temporal variation of gender in Aralia hispida Vent (Araliaceae) Evolution. 1981;35:1094–1107. doi: 10.1111/j.1558-5646.1981.tb04979.x. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Thomson BA. Dispersal of Erythronium grandiflorum pollen by bumble bees: implications for gene flow and reproductive success. Evolution. 1989;43:657–661. doi: 10.1111/j.1558-5646.1989.tb04261.x. [DOI] [PubMed] [Google Scholar]
- Willson MF, Price PW. The evolution of inflorescence size in Asclipias (Asclepiadaceae) Evolution. 1977;31:495–511. doi: 10.1111/j.1558-5646.1977.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Young HJ, Stanton ML. Influences of floral variation on pollen removal and seed production in wild radish. Ecology. 1990;71:536–548. [Google Scholar]