Abstract
Background and Aims
The deployment of temporally separated carboxylation pathways for net CO2 uptake in CAM plants provides plasticity and thus uncertainty on how species with this photosynthetic pathway will respond to life in a higher-CO2 world. The present study examined how long-term exposure to elevated CO2 influences the relative contributions that C3 and C4 carboxylation make to net carbon gain and to establish how this impacts on the availability of carbohydrates for export and growth and on water use efficiency over the day/night cycle.
Methods
Integrated measurements of leaf gas exchange and diel metabolite dynamics (e.g. malate, soluble sugars, starch) were made in leaves of the CAM bromeliad Aechmea ‘Maya’ after exposure to 700 µmol mol−1 CO2 for 5 months.
Key Results
There was a 60 % increase in 24-h carbon gain under elevated CO2 due to a stimulation of daytime C3 and C4 carboxylation in phases II and IV where water use efficiency was comparable with that measured at night. The extra CO2 taken up under elevated CO2 was largely accumulated as hexose sugars during phase IV and net daytime export of carbohydrate was abolished. Under elevated CO2 there was no stimulation of dark carboxylation and nocturnal export and respiration appeared to be the stronger sinks for carbohydrate.
Conclusions
Despite the increased size of the soluble sugar storage pool under elevated CO2, there was no change in the net allocation of carbohydrates between provision of substrates for CAM and export/respiration in A. ‘Maya’. The data imply the existence of discrete pools of carbohydrate that provide substrate for CAM or sugars for export/respiration. The 2-fold increase in water-use efficiency could be a major physiological advantage to growth under elevated CO2 in this CAM bromeliad.
Key words: Aechmea ‘Maya’, carbon budgets, elevated CO2, gas exchange, metabolite dynamics, PEPC, photosynthetic plasticity, Rubisco
INTRODUCTION
The prospect of global changes in the earth's climate has stimulated extensive research into assessing the impacts of elevated CO2 on different vegetation types. The pre-industrial atmospheric CO2 concentration of approx. 280 ppm has risen steadily to a present-day concentration of 380 ppm (Körner, 2006). As predicted by carbon-balance models a doubling in this CO2 concentration could be expected by the middle of this century due to the effects of deforestation, land-use changes and the burning of fossil fuels (Neftel et al., 1985; Schimel et al., 1996). The effects of CO2 enrichment on plant growth and physiological performance are diverse and complex, depending on the carbon fixation pathway, the exposure duration and the accompanying environmental conditions (Körner, 2001; Bazzaz and Catovsky, 2002). According to the carbon fixation pathway, three functional groups can be distinguished, i.e. C3, C4 and CAM. Numerous reports agree that elevated CO2 mainly increases net CO2 uptake and growth of C3 plants, reflecting the increased efficiency of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) for fixing CO2 at higher CO2:O2 ratios (Isdo and Kimball, 1992; Bowes, 1993; Poorter and Navas, 2002). After a short-term exposure of 4–6 weeks, acclimation to increased CO2 can occur, returning net CO2 uptake and growth to previous lower rates (Sage et al., 1989; Stitt, 1991). The situation for C4 plants is more uncertain and complex. In these plants Rubisco is localized in the chloroplasts of the bundle sheath cells where the internal CO2 concentration is 3–6 times higher than in the atmosphere. The C4 carbon-concentrating mechanism is a consequence of the prior uptake of CO2 mediated via phosphoenolpyruvate carboxylase (PEPC) which results in an effective draw down of CO2 in the mesophyll cells and produces C4 acids that are subsequently decarboxylated in the bundle sheath cells (Bowes, 1993; von Caemmerer and Furbank, 2003). C4 plants avoid photorespiration due to the saturation of Rubisco with CO2 and, theoretically would not be expected to display greater rates of photosynthesis in a higher CO2 environment. However, because of the typical reduction of stomatal conductance in plants exposed to elevated atmospheric CO2 (Ainsworth and Long, 2005), yield improvements could be achieved in a higher CO2 world through an amelioration of short-term drought stress via the conservation of soil moisture (Ghannoum et al., 2000; Leakey et al., 2004).
In comparison to C3 and C4 plants, relatively few studies have addressed the effects of elevated CO2 on gas exchange characteristics in CAM plants which use both Rubisco and PEPC to take up CO2. Different conclusions have been reached on how CAM species might be expected to respond to life in a higher CO2 world, in terms of net carbon gain, growth and water use efficiency (WUE) (Drennan and Nobel, 2000). Specifically, a doubling of the atmospheric CO2 concentration was found to have no effect on diel CO2 uptake by the CAM species Kalanchoë daigremontiana (Osmond and Björkman, 1975; Holtum et al., 1983); night-time CO2 uptake was decreased in Clusia uvitana and Portulacaria afra (Huerta and Ting, 1988; Winter et al., 1992) yet night-time CO2 uptake was enhanced by elevated CO2 in Agave vilmoriniana (Szarek et al., 1987) and a tropical orchid Mokara Yellow (Gouk et al., 1997; Li et al., 2002). In other CAM species, e.g. Agave deserti, Ananas comosus, Opuntia ficus-indica and Stenocereus queretaroensis, elevated CO2 was shown to lead to an increase in both daytime and night-time CO2 uptake (Nobel and Israel, 1994; Graham and Nobel, 1996; Nobel, 1996; Wang and Nobel, 1996; Zhu et al., 1999). Such contrasting reports on the impacts of elevated CO2 on CAM plants may in part relate to the inherent plasticity afforded by the existence of temporally separated C3 and C4 carboxylation processes which serves to optimize carbon gain and WUE under a changing environment. Thus, a better understanding is required on how extended exposure to elevated CO2 affects the relative contributions from PEPC- and Rubisco-mediated net CO2 uptake over the day/night cycle.
Plasticity in the deployment of C3 and C4 carboxylation processes in CAM plants can be analysed by monitoring the shifts in net CO2 uptake and metabolite levels that define the four phases of carbon supply and demand over a 24-h cycle (Osmond, 1978). Phase I represents the dark period when stomata are open and atmospheric and/or respiratory CO2 is fixed in the cytosol by PEPC, resulting in malate formation. The CO2 acceptor phosphoenolpyruvate (PEP) is formed by glycolysis of storage polysaccharides (e.g. starch) or soluble sugars (e.g. glucose, fructose, sucrose) assembled during the previous day. Phase II at dawn indicates the switch between C4 and C3 carboxylation but atmospheric CO2 uptake still largely appears to be dominated by PEPC (Griffiths et al., 1990). Rubisco remains at low activation state until PEPC is inactivated by dephosphorylation (Maxwell et al., 1999). During the middle of the day (phase III) gas exchange is curtailed by stomatal closure as a result of high intercellular partial pressure of CO2 generated by malate decarboxylation. The released CO2 is fixed through Rubisco via the Calvin cycle into triose phosphates that have to be partitioned between the provision of carbohydrates for growth and as substrates for the production of PEP for phase I carboxylation. At the end of the light period, decarboxylation of malate has ceased causing CO2 partial pressure to drop and stomates to reopen. During this phase IV, CO2 is mainly fixed and assimilated via Rubisco with a major proportion of carbon fixed partitioned for export and growth (Borland et al., 1994). Over the latter part of this phase C4 carboxylation by PEPC can be detected (Griffiths et al., 1990; Borland and Griffiths, 1996). The relative contribution that each phase makes to net carbon gain over a 24-h CAM cycle is determined by an interplay of circadian and metabolite control (Borland and Taybi, 2004). Control by a circadian clock sets up the diel phases of CAM to achieve appropriate synchronization of metabolic and transport processes. An additional layer of control over the CAM cycle is exerted by the day/night reciprocating accumulation/degradation of carbohydrates and malate which facilitates photosynthetic plasticity by entraining output from the clock to fluctuations in the environment (Borland et al., 1999; Borland and Taybi, 2004). By serving as an internal gauge of external environmental conditions, the reciprocating pools of organic acids and carbohydrates are central to the metabolic control of CAM. The day/night patterns and availability of carbohydrates in particular play a key role in setting the phases of CAM, by determining both the magnitude of dark CO2 uptake and the amount of carbohydrate available for export and growth. Thus, it can be hypothesized that any change in carbohydrate or malate content elicited by growth under elevated CO2 will result in a shift in the relative contributions that C3 and C4 carboxylation make to 24-h carbon gain and WUE.
In the present study, integrated measurements of leaf gas exchange and diel metabolite dynamics (e.g. malate, citrate, glucose, fructose, sucrose and starch) were made in leaves of the CAM bromeliad Aechmea ‘Maya’ after exposure to 700 µmol mol−1 CO2 for 5 months. Specific questions that were addressed included: (a) how does long-term exposure to elevated CO2 influence the magnitude and duration of C3 and C4 carboxylation processes over the four phases of CAM; (b) how do shifts in the proportions of C3 and C4 carboxylation impact on leaf WUE over a 24-h light dark cycle; (c) how does carbohydrate availability influence the magnitude of CAM under elevated CO2; and (d) how are carbohydrates partitioned between the potentially competing fates of dark carboxylation and export for growth under contrasting regimes of CO2 supply.
MATERIALS AND METHODS
Plant material
Aechmea ‘Maya’ is a spineless cultivar resulting from a cross between A. tessmannii and A. fasciata. These aechmeas are CAM bromeliads and belong to the subfamily of the Bromelioideae (Londers et al., 2005; Benzing, 2000). Twelve-month-old vegetative A. ‘Maya’ plants with 27 (±5) leaves were equally divided between two greenhouse compartments (Leuven, Belgium). Control plants were grown under ambient atmospheric CO2 concentrations (approx. 380 µmol mol−1). The CO2-treated plants were exposed to approx. 700 µmol mol−1. This was achieved by automated continuous injection of pure CO2 each time until the low set point (700 µmol mol−1) was reached. All other environmental conditions in both compartments were identical. During day time a minimum temperature of 21 °C was maintained while at night a minimum of 19·5 °C was achieved. Between 0600 h and 2200 h artificial lighting was provided (PAR = 30 µmol m−2 s−1). Plants were watered once weekly with a conventional nutrient solution of 1 mS cm−1.
Gas exchange measurements
After 5 months, leaf CO2 and water vapour exchange data were collected on the youngest fully expanded leaf from plants under ambient and elevated CO2. An LCi portable photosynthesis system (ADC BioScientific Ltd, UK) was used. The top part of the leaf was enclosed in a broad leaf chamber (6·25 cm2) and, to buffer short-term fluctuations in the CO2 concentration, the incoming air was passed through a 25-L bottle. Gas exchange data were collected over a 24-h period using a 15-min interval (n = 5 plants). Total net gas exchange was calculated for specific periods during the 24-h time course by integrating specific areas under the CO2 exchange curves (Griffiths et al., 1986).
Chemical analyses of metabolites
During a cycle of 24 h starting from 0600 h leaf samples were taken from the upper part of young fully expanded leaves (n = 10 plants) from plants under both CO2 regimens every 3 h. These were immediately frozen in liquid nitrogen to arrest any enzyme activity.
Extraction of organic acids was performed as earlier described by Ceusters et al. (2008). Quantification of malic and citric acid was accomplished using high performance liquid chromatography (Waters 510, Waters, Milford, MA, USA) with detection at 210 nm (Waters 484) using an aminex HPX87-H (300 mm × 7·8 mm) resin-based column from Bio-Rad (Hercules, CA, USA).
Soluble sugars (glucose, fructose and sucrose) were extracted, subjected to enzymatic treatment (Enzytec, Scil Diagnostics GmbH, Germany) and analysed at 340 nm using a spectrophotometer (DU-65, Beckman, Fullerton, USA). Starch content was determined as glucose equivalents following digestion with amyloglucosidase (Enzytec, Scil Diagnostics GmbH, Germany). The procedures were conducted as described by Farrar (1993).
Carbon budgets
Carbon budgets were calculated and adapted from a previously described model (Borland, 1996; Borland and Dodd, 2002) which quantitatively describes: (a) the source of carbon in the leaf, i.e. C3 (Rubisco-mediated CO2 uptake) or C4 (from breakdown of malate where 1 mol malate = 4 mol C); (b) the partitioning of this carbon between starch or soluble sugars (sucrose, glucose and fructose) with the excess going to daytime export where 1 mol Glc eq = 6 mol C; (c) the partitioning of carbohydrates between the generation of substrates for CAM, respiratory CO2 or dark export. The budgets presented are net carbon flows, thus taking into account any respiratory losses of carbon during the light period. All numbers presented on budgets are mmol C m−2 d−1.
Data analysis
Where appropriate, the data were analysed using the statistical software package SAS Enterprise Guide 4·0. Before carrying out statistical tests normality of the data was checked by means of the Kolmogorov–Smirnoff statistic (P > 0·05). Means are compared by two-sample t-test (α = 0·05).
RESULTS
Leaf gas exchange
After exposure to 700 µmol mol−1 for 5 months, diel gas exchange patterns were recorded and integrated CO2 uptake was calculated for each of the different CAM phases over a 24-h period (Fig. 1 and Table 1). CO2 enrichment significantly increased both the rate of CO2 assimilation and total assimilation during the diel cycle (Fig. 1 and Table 1). More specifically, after a cycle of 24 h at elevated CO2 60 % more CO2 was taken up compared with total uptake by plants kept at ambient CO2 (Table 1). Night-time CO2 uptake did not account for this substantial difference because neither the maximum rate of uptake (Fig. 1) nor the total amount of net CO2 uptake (Table 1) during phase I were influenced by elevated CO2. However, in the light (phases II and IV) net CO2 uptake was significantly higher for plants grown at elevated CO2 with a 3-h extended period of phase IV net CO2 exchange compared with plants in ambient CO2. The maximum rate of net CO2 uptake during phase IV even exceeded uptake during the night under CO2 enrichment (Fig. 1).
Fig. 1.
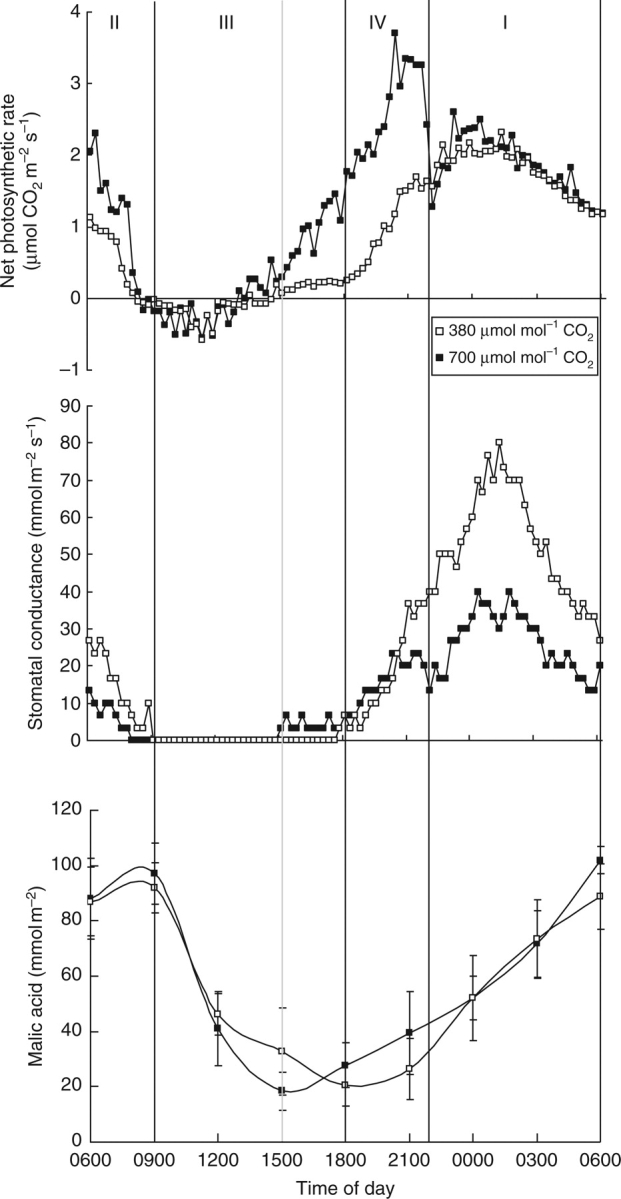
Net 24 h CO2 uptake (μmol m−2s−1), diel stomatal conductance (mmol m−2 s−1) and diel malic acid (mmol m−2) pattern for young fully expanded leaves of Aechmea ‘Maya’ at ambient (380 µmol mol−1) and elevated (700 µmol mol−1) CO2. Gas exchange curves are representative of five replicate runs with s.e. <10 %. Malic acid data are means ± s.e. (n = 10 plants). The four different phases of CAM are indicated and the grey line shows a phase shift caused by CO2 elevation.
Table 1.
Integrated net CO2 uptake for the times of each of the four CAM phases (mmol CO2 m−2 phase−1) by young fully expanded leaves of Aechmea ‘Maya’ under ambient (380 μmol mol−1) and elevated (700 μmol mol−1) CO2 concentrations
| CO2 (μmol mol−1) | Phase I 2200–0600 | Phase II 0600–0900 | Phase III 0900–1800* | Phase IV 1800–2200* | Total 24 h 1200–1200 |
|---|---|---|---|---|---|
| 380 | 52·5 ± 6·1 | 7·2 ± 0·6 | −2·5 ± 4·7 | 10·5 ± 2·0 | 67·7 ± 6·9 |
| 700 | 55·4 ± 2·6 | 16·1 ± 1·3 | −3·7 ± 2·5 | 42·5 ± 3·7 | 109·5 ± 10·5 |
| P-value | >0·05 | <0·05 | >0·05 | <0·05 | <0·05 |
The data are means ± s.e. for five leaves, each from a separate plant and were compared for each phase between the two treatments by two-sample t-test (α = 0·05).
*Due to elevated CO2 phase IV extended by starting 3 h earlier at 1500 h compared with controls; concomitantly phase III reduced by the same extent.
The diel courses of stomatal conductance (gs) showed maximum values at night for plants under both CO2 treatments (Fig. 1). However, in plants grown under elevated CO2 lower values of gs were observed simultaneously with a higher rate of CO2 uptake in phase IV compared with phase I. Moreover, for an equal amount of CO2 uptake during phase I, the values of gs were 2-fold higher in ambient CO2 compared with those under elevated CO2 (Fig. 1), resulting in a nightly transpiration reduction of 50 % for plants under elevated CO2 (Table 2). As a consequence WUE was 2-fold higher during the night under elevated CO2. During the daytime phases II and IV, a 3- to 4-fold increase in WUE was recorded in plants under elevated CO2. The resulting 24 h WUE of 15·3 mmol mol−1 under elevated CO2 was significantly higher than 7·4 mmol mol−1 under ambient conditions (Table 2).
Table 2.
Mean photosynthetic water use efficiency (mmol CO2 mol−1 H2O) for young fully expanded leaves of Aechmea ‘Maya’ under ambient (380 μmol mol−1) and elevated (700 μmol mol−1) CO2 concentrations
| CO2 (μmol mol−1) | Phase I 2200–0600 | Phase II 0600–0900 | Phase IV 1800–2200* | Total 24 h 1200–1200 |
|---|---|---|---|---|
| 380 | 13·1 ± 1·3 | 5·7 ± 0·8 | 6·6 ± 1·2 | 7·4 ± 1·2 |
| 700 | 24·7 ± 3·0 | 16·6 ± 1·0 | 24·6 ± 4·2 | 15·3 ± 2·8 |
| P-value | <0·05 | <0·05 | <0·05 | <0·05 |
Because no net CO2 uptake occurred during phase III, WUE was not calculated for this period. The data are means ± s.e. for five leaves, each from a separate plant and were compared for each phase between the two treatments by two-sample t-test (α = 0·05).
*Due to elevated CO2 phase IV extended by starting 3 h earlier at 1500 h compared with controls.
Metabolite dynamics
Under both CO2 concentrations the overall day/night changes in malic acid content were similar (Fig. 1; P > 0·05). However, malic acid started to accumulate 3 h earlier in the afternoon, at 1500 h, in plants grown under elevated CO2 compared with plants in ambient CO2 where malic acid did not start to accumulate until 1800 h. As the beginning of malic acid accumulation indicates the start of phase IV (Osmond, 1978), the earlier suggested phase extension from this phase with 3 h and an associated reduction of phase III is hereby confirmed. Lowest values of malic acid were reached at the end of the respective phase III (19 ± 5 mmol m−2), while the highest values were recorded in the morning, at the end of phase II (94 ± 11 mmol m−2) (Fig. 1). Citric acid levels did not show a varying diel trend or significant differences concerning CO2 concentration (P > 0·05) and varied around 40 ± 11 mmol m−2 throughout the whole cycle (results not shown).
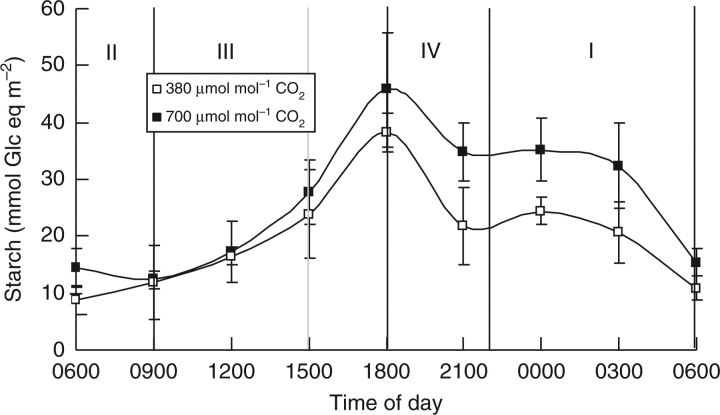
The diel cycle of starch content under both CO2 treatments varied reciprocally to that of malic acid and was not significantly altered by CO2 enrichment (Fig. 2; P > 0·05). In the morning during phase II the mean minimum starch concentration was 12 ± 5 mmol Glc eq m−2. Around 1800 h the maximal starch content was achieved for leaves from plants under both ambient and enhanced CO2 with an average value of 43 ± 9 mmol Glc eq m−2. Starch degradation commenced during phase IV and continued during phase I (Fig. 2).
Fig. 2.
Diel pattern of starch (mmol Glc eq m−2) for young fully expanded leaves of Aechmea ‘Maya’ at ambient (380 µmol mol−1) and elevated (700 µmol mol−1) CO2. The four different phases of CAM are indicated and the grey line shows a phase shift caused by CO2 elevation. Data are means ± s.e. (n = 10 plants).
Sucrose showed a diel pattern of accumulation and depletion analogous to starch and reciprocal to malic acid and remained unaffected by CO2 enhancement (Fig. 3; P > 0·05). The maximum concentrations of sucrose (13 ± 2 mmol m−2) were reached around 1800 h and nocturnal breakdown of sucrose during phase I yielded 9 ± 1 mmol m−2 (Fig. 3). Hexose sugars (glucose and fructose) fluctuated around 8 ± 2 mmol m−2 and they did not participate in the diel carbohydrate flux to sustain CAM under ambient CO2 conditions (Fig. 3). However, under elevated CO2, substantial accumulation of glucose and fructose was noted over the latter part of the day. Analogous to starch the lowest concentrations for both glucose and fructose were present in the morning at the end of phase II. At the start of the extended phase IV under elevated CO2, concentrations of hexose sugars started to rise to achieve significantly different (P < 0·05) maximum values from those in plants grown under ambient CO2 (i.e. 18 ± 5 mmol m−2 for glucose and 31 ± 5 mmol m−2 for fructose at the end of phase IV). In contrast to starch, however, only limited net nocturnal degradation of both glucose and fructose took place during phase I under elevated CO2 (Fig. 3; P > 0·05) and as such did not appear to contribute to the nocturnal provision of PEP.
Fig. 3.
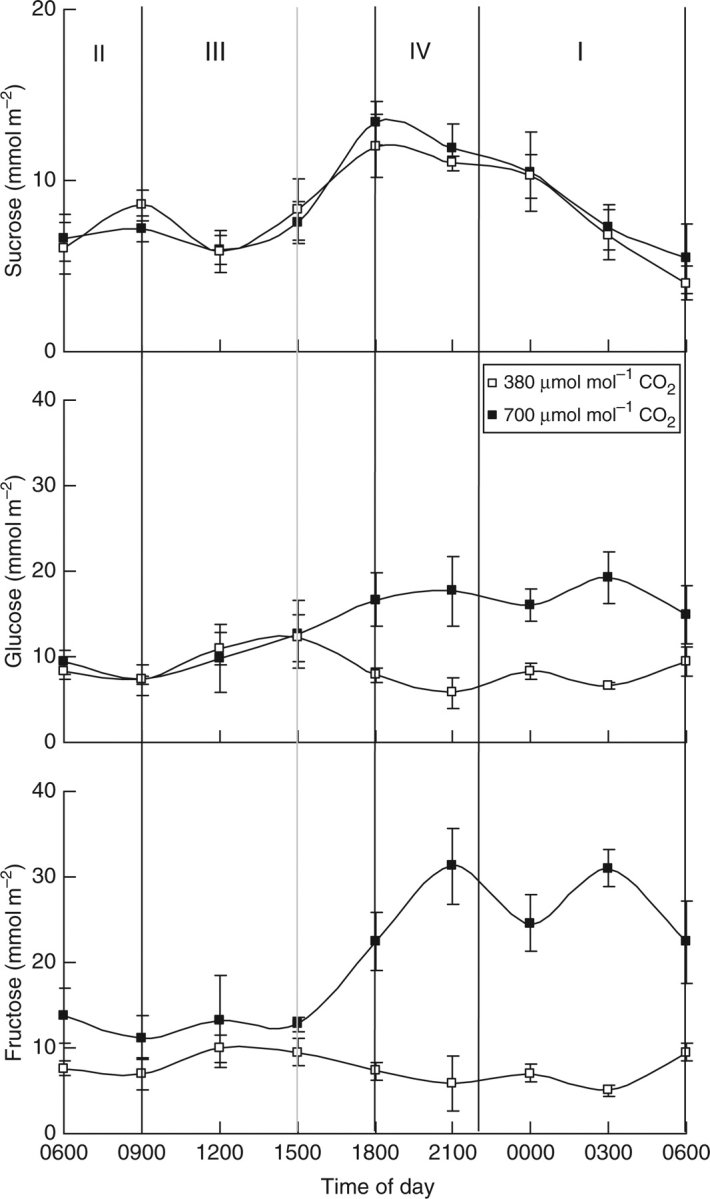
Diel patterns of sucrose, glucose and fructose (mmol m−2) for young fully expanded leaves of Aechmea ‘Maya’ at ambient (380 µmol mol−1) and elevated (700 µmol mol−1) CO2. The four different phases of CAM are indicated and the grey line shows a phase shift caused by CO2 elevation. Data are means ± s.e. (n = 10 plants).
Carbon budgets
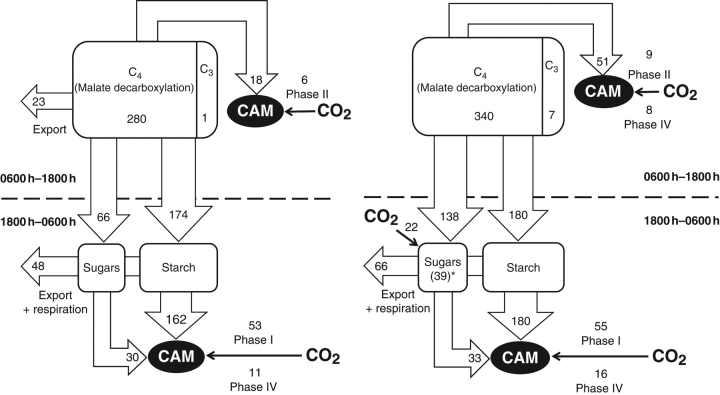
To integrate gas exchange and carbohydrate measurements leaf carbon budgets were calculated for plants under ambient and elevated CO2 (Fig. 4). Doubling of the CO2 concentration resulted in an increase of daily carbon input by 35 %, mainly due to enhanced atmospheric CO2 uptake during the transitional phases II and IV. In ambient conditions, CO2 uptake in phases II and IV was almost entirely mediated via PEPC activity (Table 3). Under elevated CO2 a 2- to 4-fold increase in CO2 acquisition in phases II and IV, respectively (Table 1) was mediated by stimulation in both C4 and C3 carboxylation. As such, 60 % of CO2 uptake during the translational phases was mediated by PEPC while 40 % was taken up through direct assimilation by Rubisco in a high CO2 environment (Table 3). For both CO2 treatments the major sink for the acquired carbon was the daytime accumulation of starch. Under ambient CO2, a further 23 % of fixed carbon was allocated to the accumulation of soluble sugars, allowing 8 % of total acquired carbon to be exported. Under elevated CO2 the net flux of carbon into soluble sugars doubled over that in ambient conditions. Net flux to starch accumulation remained the same under elevated and ambient CO2 but daytime net export of sugars was abolished under elevated CO2 (Fig. 4). Starch degradation was the major source of carbon skeletons for the synthesis of PEP for C4 carboxylation under both CO2 regimes (Fig. 2). Additionally, limited breakdown of soluble sugars, especially sucrose occurred during the period of malate formation (Figs 1 and 3). In total, 80 % of carbohydrate degradation was used to fuel PEPC uptake resulting in 20 % of carbon available for export/respiration during the period of malate formation under ambient CO2. Under elevated CO2, 65 % of previously accumulated carbohydrates were used to provide substrate for PEPC-mediated CO2 uptake, 20 % of carbohydrates were exported/respired and 15 % was retained in the form of fructose and glucose (Figs. 3 and 4). Overall, the total net export of carbon during the diel cycle was similar under both CO2 treatments.
Fig. 4.
Diel net carbon budgets for young fully expanded leaves of Aechmea ‘Maya’ at ambient (380 µmol mol−1) (left) and elevated (700 µmol mol−1) (right) CO2. Fluxes shown above the horizontal line mainly coincide with degradation of malic acid and concomitant accumulation of carbohydrate reserves while those under the horizontal line are characterized by the reverse process. Gas exchange data were calculated as the integrated mean from five replicate leaves and metabolites were sampled from ten replicate leaves on individual plants. Export includes growth and maintenance and all units are mmol C m−2 d−1. * accumulated hexose carbon which is not degraded or exported overnight.
Table 3.
Relative contribution of PEPC- and Rubisco-mediated CO2 uptake during translational phases II and IV for young fully expanded leaves of Aechmea ‘Maya’ under ambient (380 μmol mol−1) and elevated (700 μmol mol−1) CO2 concentrations
| % C4 carboxylation |
% C3 carboxylation |
|||
|---|---|---|---|---|
| CO2 (μmol mol−1) | Phase II 0600–0900 | Phase IV 1800–2200* | Phase II 0600–0900 | Phase IV 1800–2200* |
| 380 | 85 | 100 | 15 | 0 |
| 700 | 56 | 60 | 44 | 40 |
Percentage of C4 carboxylation was quantified by comparison of malic acid build up and total net CO2 uptake in each period whereby 1 mol malic acid corresponds to 1 mol CO2. The remaining CO2 uptake was attributed to C3 carboxylation through Rubisco activity.
*Due to elevated CO2 phase IV extended by starting 3 h earlier at 1500 h compared with controls.
DISCUSSION
Impact of elevated CO2 on C3 and C4 carboxylation processes
Extended exposure of A. ‘Maya’ to elevated CO2 resulted in a 60 % increase in 24-h net carbon gain, but the stimulation was due entirely to an increase in the direct uptake of CO2 during the day and nocturnal CO2 uptake remained unchanged. Although Ting (1994) previously postulated that exposure to elevated CO2 will result in a minimal increase in dark CO2 uptake due to carbon saturation of PEPC at current atmospheric CO2 concentrations, CAM species like Hylocereus undatus (Raveh et al., 1995), Opuntia ficus-indica (Cui et al., 1993) and Ananas comosus (Zhu et al., 1999) have shown significant increases in CO2 uptake during phase I in response to CO2 enrichment. The present results for A. ‘Maya’ might be explained by an average stomatal conductance that was approx. 50 % lower under CO2 enrichment, which would imply carbon saturation of PEPC at ambient CO2 levels. It is also possible that dark CO2 uptake could have been limited by vacuolar storage capacity for malic acid given that the leaves of A. ‘Maya’ are less succulent than many other CAM species, e.g. pineapple (Popp et al., 2003). Another potential limiting factor for dark CO2 uptake could be carbonic anhydrase (CA) activity. In the C4 plant Flaveria bidentis it has been reported that leaf CA activity is in excess and does not limit CO2 assimilation under normal conditions (Cousins et al., 2006) but in several other C4 species it would appear that CA activity is only just sufficient to support photosynthetic rates, especially in monocots (Hatch and Burnell, 1990; Gillon and Yakir, 2000). The situation in CAM plants with regards to CA activity and the allocation of CA between cytosol (to support PEPC-mediated CO2 uptake) and chloroplast (to support Rubisco-mediated CO2 uptake) remains to be resolved.
Although there was no difference in the amount of CO2 taken up at night in A. ‘Maya’ under ambient or elevated CO2, there did appear to be a stimulation of net C4 carboxylation during phase II and, particularly during late afternoon, phase IV under elevated CO2, even although stomatal conductance was lower than that measured in plants grown under ambient CO2. On the assumption that PEPC was carbon saturated, there are two possible explanations for the CO2-induced increase in daytime net C4 carboxylation: (1) given reports that PEPC may be limited by availability of PEP under ambient conditions, exposure to elevated CO2 may have resulted in an increased flux into the production of PEP during the day; or (2) since the contribution from PEPC to net carbon balance was assessed in terms of net malate accumulation, it is possible that exposure to elevated CO2 affected malate turnover. Thus, PEPC activity in phase II may have been similar under ambient and elevated CO2, but malate breakdown may have been delayed/curtailed at the start of the day in plants exposed to elevated CO2. Although the factors that regulate the timing and rate of malate decarboxylation in CAM plants remain unclear, exposure to elevated CO2 resulted in an increased rate of malate decarboxylation during phase III so that stomata re-opened and phase IV commenced some 3 h earlier than in plants under ambient CO2. Thus, there was a significant increase in direct C3-mediated net carbon gain over the latter part of the day in plants grown under elevated CO2, despite the lower stomatal conductance compared with plants in ambient conditions. The typically thick, succulent leaves of CAM plants are believed to impose significant diffusional limitations to CO2 transfer from the atmosphere to Rubisco in the chloroplast, with the consequence that Rubisco may be severely CO2 limited during the latter part of the photoperiod when malate decarboxylation has reached completion (Maxwell et al., 1997). Thus, as shown here, it seems likely that direct daytime C3 photosynthesis will show substantial and sustained stimulation in CAM plants growing in a higher CO2 world.
In total, approx. 50 % of the 24 h net CO2 uptake occurred during the day in A. ‘Maya’ grown under elevated CO2, representing a larger proportion of net carbon gain than previously reported for CAM plants with a comparable daytime contribution of approx. 22 % under ambient conditions. Under CO2 enrichment, Agave deserti (Nobel and Hartsock, 1986), Ananas comosus (Zhu et al., 1999) and Hylocereus undatus (Raveh et al., 1995) still showed a dominating night-time CO2 uptake of approx. 65 % of total diel uptake. Despite the relative increase in daytime to night-time CO2 uptake and the concomitant stimulation in C3 carboxylation in A. ‘Maya’, diel WUE increased from 7·4 to 15·3 mmol mol−1 under CO2 enrichment. Comparable findings have been obtained for the CAM plants Ananas comosus and Opuntia ficus-indica (Cui et al., 1993; Zhu et al., 1999). By restricting most transpirational water loss to the cooler nocturnal period, high WUE values are usually attributed to PEPC-mediated CO2 uptake during phase I of the CAM cycle (Black, 1973; Lüttge, 2004), a statement which holds under ambient conditions for A. ‘Maya’. However, under CO2 enrichment high values of WUE (25 mmol mol−1) were established during both phases I and IV. Compared with plants under ambient CO2, the gain in WUE during phase I under elevated CO2 was achieved by a 50 % reduction of stomatal conductance without a concomitant reduction in CO2 uptake. However, during the daytime phases both increased Rubisco, and PEPC-mediated CO2 uptake resulted in high WUE values, confirming the statement of Eller and Ferrari (1997) that high WUE is not exclusively associated with performing C4 carboxylation at night.
Impact of elevated CO2 on carbohydrate partitioning
In accordance with gas exchange analyses the diel pattern of accumulation/degradation of malate was hardly affected by elevated CO2. Similarly, CO2 enrichment did not alter the dynamics of the reciprocating carbohydrates. Stoichiometric considerations indicated that under both CO2 regimes starch appeared to be the predominant substrate for nocturnal carboxylation while sucrose also accumulated during daytime. Comparable results were obtained under ambient CO2 for pineapple by Antony et al. (2008). These observations confirm that bromelioids accumulate both sugar and starch to fuel the dark reactions of CAM (Christopher and Holtum, 1998; Popp et al., 2003). Soluble hexose sugars (glucose and fructose) did not fluctuate over the diel cycle under ambient CO2 in A. ‘Maya’. However, under elevated CO2 glucose and fructose started to accumulate to levels 3- and 5-fold higher, respectively, compared with controls during phase IV, utilizing the carbon gained during the period of malate decarboxylation and abolishing net daytime export of sugars. Since there was no net sugar accumulation during the day under ambient CO2, 8 % of the carbon gained over the photoperiod could be exported for growth and maintenance. During the subsequent period of malate formation, approx. 80 % of accumulated carbohydrates were degraded to provide substrate for PEPC-mediated CO2 uptake, while a further 20 % of carbohydrates were depleted and used for export/respiration. Under elevated CO2 a hexose (glucose and fructose) pool of 32 ± 5 mmol Glc eq m−2 was available at the start of phase I and thus constituted a pool of carbon skeletons almost equivalent to those available from starch and which could potentially be degraded to support night-time CO2 uptake and malate formation. However, in contrast to starch, only limited net nocturnal degradation of either glucose or fructose took place, thereby withholding 25 % of accumulated sugar carbon from the competing fates of PEPC-mediated CO2 uptake and export for growth. Carbohydrate availability is considered to be a major limiting factor for the magnitude of dark CO2 uptake in CAM plants (Borland and Dodd, 2002). Moreover, the restoration of nocturnal malate accumulation in a starch-deficient mutant of Mesembryanthemum crystallinum by feeding leaves with glucose or sucrose has implied flexibility of the CAM pathway in terms of utilizing different carbohydrate sources to generate substrate for nocturnal carboxylation (Cushman et al., 2008). However, the results obtained here indicate that for A. ‘Maya’ under elevated CO2, PEPC was not carbohydrate limited or else the glucose and fructose reserves, presumably stored in the vacuole, were unavailable for the generation of PEP. Previous considerations of 13C signatures of different carbohydrate species have implied the existence of discrete pools of storage carbohydrates destined for CAM or export/respiration (Borland and Dodd, 2002). The data obtained here for A. ‘Maya’ are consistent with this suggestion and could provide a means by which the potentially competing fates of CAM and export/respiration for growth could be regulated independently, thereby facilitating photosynthetic plasticity in a changing environment. As a consequence of hexose accumulation under elevated CO2, the total net export of carbon for growth and maintenance during the diel cycle in A. ‘Maya’ was similar in both regimes of CO2 supply, although a shift occurred from combined daytime and night-time export to exclusive night-time export of carbohydrates under elevated CO2.
In conclusion, elevation of CO2 was found to exert a pronounced influence on the magnitude and duration of both C3 and C4 carboxylation processes during the day while nocturnal PEPC-mediated CO2 uptake remained unaffected. This impacted on the synchronization of both carboxylation processes by altering the balance of almost exclusive C4 carboxylation under ambient CO2 to approx. 60 % PEPC- and approx. 40 % Rubisco-mediated uptake over the day under elevated CO2. Nevertheless, WUE doubled under elevated CO2, demonstrating that WUE is not always related to specific CAM phases. The extra gain of carbon under high CO2 was largely accumulated as hexose sugars during phase IV, abolishing any daytime net export of carbon. Around 25 % of the sugars that accumulated under elevated CO2 were not degraded or exported overnight, indicating that factors other than carbohydrate pool size might limit dark carboxylation. Consequently, total net export of carbon for growth and maintenance was similar under the contrasting CO2 regimes. The data indicate that whilst some CAM species may not show enhanced biomass production in a higher CO2 world, productivity could be maintained with reduced inputs of water. Such findings indicate a major ecophysiological advantage for CAM plants under a changing climate and suggest that CAM species should also be considered in an agronomic context as potential sources of biomass production on arid, marginal lands.
ACKNOWLEDGEMENTS
The authors wish to thank Deroose Plants NV for supplying plant material. This research was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
LITERATURE CITED
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Antony E, Taybi T, Courbot M, Mugford S, Smith JAC, Borland AM. Cloning, localization and expression analysis of vacuolar sugar transporters in the CAM plant Ananas comosus (pineapple) Journal of Experimental Botany. 2008;59:1895–1908. doi: 10.1093/jxb/ern077. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA, Catovsky S. Impact of global environmental change on plants: from cells to ecosystems. In: Mooney HA, Canadell JG, editors. Encyclopedia of global environmental change. Vol. 2. Chichester: Wiley; 2002. pp. 94–111. [Google Scholar]
- Benzing DH. Bromeliaceae – profile of an adaptive radiation. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Black CC. Photosynthetic carbon fixation in relation to net CO2 uptake. Annual Review of Plant Physiology. 1973;24:253–286. [Google Scholar]
- Borland AM. A model for the partitioning of photosynthetically fixed carbon during the C3-CAM transition in Sedum telephium. New Phytologist. 1996;134:433–444. [Google Scholar]
- Borland AM, Dodd AN. Carbohydrate partitioning in CAM plants: reconciling potential conflicts of interest. Functional Plant Biology. 2002;29:707–716. doi: 10.1071/PP01221. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H. Variations in the phases of CAM and regulation of carboxylation patterns determined by carbon isotope discrimination techniques. In: Winter K, Smith JAC, editors. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag; 1996. pp. 230–246. [Google Scholar]
- Borland AM, Taybi T. Synchronization of metabolic processes in plants with Crassulacean acid metabolism. Journal of Experimental Botany. 2004;55:1255–1265. doi: 10.1093/jxb/erh105. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Broadmeadow MSJ, Fordham MC, Maxwell C. Carbon-isotope composition of biochemical fractions and the regulation of carbon balance in leaves of the C3-crassulacean acid metabolism plant Clusia minor L. growing in Trinidad. Plant Physiology. 1994;106:493–501. doi: 10.1104/pp.106.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG. Metabolite control overrides circadian regulation of PEPc kinase and CO2 fixation in crassulacean acid metabolism (CAM) Plant Physiology. 1999;121:889–896. doi: 10.1104/pp.121.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. Facing the inevitable: plant and increasing atmospheric CO2. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:309–332. [Google Scholar]
- von Caemmerer S, Furbank RT. The C-4 pathway: an efficient CO2 pump. Photosynthesis Research. 2003;77:191–207. doi: 10.1023/A:1025830019591. [DOI] [PubMed] [Google Scholar]
- Ceusters J, Londers E, Verdoodt V, Ceusters N, De Proft MP. Seasonal impact on physiological leaf damage risk of Aechmea hybrid under greenhouse conditions. 2008 Scientia Horticulturae (in press) [Google Scholar]
- Christopher JT, Holtum JAM. Carbohydrate partitioning in the leaves of Bromeliaceae performing C3 photosynthesis or Crassulacean acid metabolism. Australian Journal of Plant Physiology. 1998;25:371–376. [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from anti-sense RNA in Flaveria bidentis. Plant Physiology. 2006;141:232–242. doi: 10.1104/pp.106.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Miller PM, Nobel PS. CO2 exchange and growth of the Crassulacean acid metabolism plant Opuntia ficus-indica under elevated CO2 in open-top chambers. Plant Physiology. 1993;103:519–524. doi: 10.1104/pp.103.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Agarie S, Albion S, Elliot SM, Taybi T, Borland AM. Isolation and characterization of mutants of common ice plant deficient in Crassulacean acid metabolism. Plant Physiology. 2008;147:228–238. doi: 10.1104/pp.108.116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan PM, Nobel PS. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant, Cell and Environment. 2000;23:767–781. [Google Scholar]
- Eller BM, Ferrari S. Water use efficiency of two succulents with contrasting CO2 fixation pathways. Plant, Cell and Environment. 1997;20:93–100. [Google Scholar]
- Farrar JF. Carbon partitioning. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and production in a changing environment. London: Chapman & Hall; 1993. pp. 232–246. [Google Scholar]
- Ghannoum O, von Caemmerer S, Ziska LH, Conroy JP. The growth response of C4 plants to rising atmospheric CO2 partial pressure: a reassessment. Plant, Cell and Environment. 2000;23:931–942. [Google Scholar]
- Gillon JS, Yakir D. Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant, Cell and Environment. 2000;20:1217–1230. [Google Scholar]
- Gouk SS, Yong JWH, Hew CS. Effects of superelevated CO2 on the growth and carboxylating enzymes in an epiphytic CAM orchid plantlet. Journal of Plant Physiology. 1997;151:129–136. [Google Scholar]
- Graham EA, Nobel PS. Long-term effects of a doubled atmospheric CO2 concentration on the CAM species Agave deserti. Journal of Experimental Botany. 1996;47:61–69. [Google Scholar]
- Griffiths H, Lüttge U, Stimmel K-H, Crook CE, Griffiths NM,, Smith JAC. Comparative ecophysiology of CAM and C3 bromeliads. III. Environmental influences on CO2 assimilation and transpiration. Plant, Cell and Environment. 1986;9:385–393. [Google Scholar]
- Griffiths H, Broadmeadow MSJ, Borland AM, Hetherington CS. Short-term changes in carbon isotope discrimination identify transitions between C3 and C4 carboxylation during Crassulacean acid metabolism. Planta. 1990;181:604–610. doi: 10.1007/BF00193017. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Burnell JN. Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiology. 1990;93:825–828. doi: 10.1104/pp.93.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, O'Leary MH, Osmond CB. Effect of varying CO2 partial pressure on photosynthesis and on carbon isotope composition of carbon-4 of malate from the Crassulacean acid metabolism plant Kalanchoë daigremontiana Hamet et Perr. Plant Physiology. 1983;71:602–609. doi: 10.1104/pp.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta AJ, Ting IP. Effects of various levels of CO2 on the induction of Crassulacean acid metabolism in Portulacaria afra (L.) Jacq. Plant Physiology. 1988;88:183–188. doi: 10.1104/pp.88.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isdo SB, Kimball BA. Above-ground inventory of sour orange trees exposed to different atmospheric CO2 concentrations for three full years. Agricultural and Forest Meteorology. 1992;60:145–151. [Google Scholar]
- Körner C. Biosphere responses to CO2 enrichment. Ecological Applications. 2001;10:1590–1619. [Google Scholar]
- Körner C. Plant CO2 responses: an issue of definition, time and resource supply. New Phytologist. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP. Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE) Global Change Biology. 2004;10:951–962. [Google Scholar]
- Li CR, Gan LJ, Xia K, Zhou X, Hew CS. Responses of carboxylating enzymes, sucrose metabolising enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant, Cell and Environment. 2002;25:369–377. [Google Scholar]
- Londers E, Ceusters J, Vervaeke I, Deroose R, De Proft MP. Organic acid analysis and plant water status of two Aechmea cultivars grown under greenhouse conditions: implications on leaf quality. Scientia Horticulturae. 2005;105:249–262. [Google Scholar]
- Lüttge U. Ecophysiology of Crassulacean Acid Metabolism (CAM) Annals of Botany. 2004;93:629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR. Is a low internal conductance to CO2 diffusion a consequence of succulence in plants with Crassulacean acid metabolism? Australian Journal of Plant Physiology. 1997;24:777–786. [Google Scholar]
- Maxwell K, Borland AM, Haslam RP, Helliker BR, Roberts A, Griffiths H. Modulation of Rubisco activity during the diurnal phases of the crassulacean acid metabolism plant Kalanchoë daigremontiana. Plant Physiology. 1999;121:849–856. doi: 10.1104/pp.121.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neftel A, Moor E, Oeschger H, Stauffer B. Evidence from polar ice cores for the increase in atmospheric CO2 in the past two centuries. Nature. 1985;315:45–47. [Google Scholar]
- Nobel PS. Responses of some North American CAM plants to freezing temperatures and doubled CO2 concentrations: implications of global change for extending cultivation. Journal of Arid Environments. 1996;34:187–196. [Google Scholar]
- Nobel PS, Hartsock TL. Short-term and long-term responses of crassulacean acid metabolism plants to elevated CO2. Plant Physiology. 1986;82:604–606. doi: 10.1104/pp.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS, Israel AA. Cladode development, environmental responses of CO2 uptake, and productivity for Opuntia ficus-indica under elevated CO2. Journal of Experimental Botany. 1994;45:295–303. [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annual review of Plant Physiology. 1978;29:379–414. [Google Scholar]
- Osmond CB, Björkman O. Pathways of CO2 fixation in the CAM plant Kalanchoë daigremontiana. II. Effects of O2 and CO2 concentration on light and dark CO2 fixation. Australian Journal of Plant Physiology. 1975;2:155–162. [Google Scholar]
- Poorter H, Navas ML. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist. 2002;157:175–198. doi: 10.1046/j.1469-8137.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- Popp M, Janett H-P, Lüttge U, Medina E. Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytologist. 2003;157:649–656. doi: 10.1046/j.1469-8137.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- Raveh E, Gersani M, Nobel PS. CO2 uptake and fluorescence for a shade-tolerant cactus Hylocereus undatus under current and doubled CO2 concentrations. Physiologia Plantarum. 1995;93:505–511. [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiology. 1989;89:590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimel D, Ives D, Enting I, Heimann M, Joos F, Raynaud D, et al. CO2 and the carbon cycle. In: Houghton JT, Meira Filho LG, Callendar BA, Harris N, Kattenberg A, Maskell K, editors. Climate change 1995. Cambridge: Cambridge University Press; 1996. pp. 65–131. [Google Scholar]
- Stitt M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant, Cell and Environment. 1991;14:741–762. [Google Scholar]
- Szarek SR, Holthe PA, Ting IP. Minor physiological response to elevated CO2 by the CAM plant Agave vilmoriniana. Plant Physiology. 1987;83:938–940. doi: 10.1104/pp.83.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting IP. CO2 and Crassulacean acid metabolism plants: a review. In: Tolbert NE, Preiss J, editors. Regulation of atmospheric CO2 and O2 by photosynthetic carbon metabolism. New York, NY: Oxford University Press; 1994. pp. 176–183. [Google Scholar]
- Wang N, Nobel PS. Doubling the CO2 concentration enhanced the activity of carbohydrate-metabolism enzymes, source carbohydrate production, photoassimilate transport and sink strength for Opuntia ficus-indica. Plant Physiology. 1996;110:893–902. doi: 10.1104/pp.110.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Zotz G, Baur B, Dietz KJ. Light and dark CO2 fixation in Clusia uvitana and the effects of plant water status and CO2 availability. Oecologia. 1992;91:47–51. doi: 10.1007/BF00317239. [DOI] [PubMed] [Google Scholar]
- Zhu J, Goldstein G, Bartholomew DP. Gas exchange and carbon isotope composition of Ananas comosus in response to elevated CO2 and temperature. Plant, Cell and Environment. 1999;22:999–1007. [Google Scholar]