Abstract
Background and Aims
The volume of tree stems is made up of three components: solid wood, gas and water. These components have important consequences for the construction costs, strength and stability of trees. Here, the importance of stem components for sapling growth and survival in the field was investigated, and then these stem components were related to two important life history axes of variation: the light requirements for regeneration and the adult stature of the species.
Methods
Stem fractions of wood, gas and water were determined for saplings and adults of respectively 30 and 58 Bolivian tropical moist-forest species. Sapling height growth and survival were monitored for 2 years in the field as indicators of sapling performance.
Key Results
Sapling stems consisted of 26 % wood (range 7–36 % for species), 59 % water (range 49–88 %), and 15 % gas (range 0–38 %) per unit volume. The wood fraction was the only determinant of sapling performance and was correlated with increased survival and decreased growth rate across species. The wood fraction decreased with light requirements of the species, probably because a high wood fraction protects shade-tolerant species against pathogens and falling debris. The gas fraction increased with the light requirements and adult stature of the species; probably as an aid in realizing a rapid height growth and accessing the canopy in the case of light-demanding species, and for rapidly attaining stability and a large reproductive size in the case of tall species. The water fraction was not correlated with the life history variation of tree species, probably because it leads to increased stem loading and decreased stability.
Conclusions
The wood fraction might partially explain the growth–survival trade-off that has been found across tropical tree species. The wood and gas fractions are closely related to the regeneration light requirements of the species. Tall species have a high gas fraction, probably not only because gas is a cheap filler, but also because it might lead to an increased stability of these tall trees.
Key words: Adult stature, biomechanics, Bolivia, shade tolerance, second moment of area, tropical rain forest, wood density, wood specific gravity
INTRODUCTION
Woody cells and perennial above-ground structures are the main features that set trees apart from herbaceous plants. Tree species vary tremendously in their wood density, which has important consequences for the dimensions and architecture of their perennial stems (van Gelder et al., 2006). Wood density is also thought to play a central role in the life history variation of trees; low-density wood is cheap to construct, thus allowing for fast growth (King et al., 2006) and a rapid completion of the life cycle, whereas high-density wood results in persistent stems, thus allowing for an increased survival (Muller-Landau, 2004) and long life-span. Wood density may therefore be an important functional trait that underlies the growth–survival trade-off that is frequently observed among temperate and tropical tree species (e.g. Kobe et al., 1995; Davies, 2001; Poorter and Bongers, 2006) and that contributes to species coexistence (Kitajima and Poorter, 2008).
Basic wood density (henceforth referred to as ‘wood density’) is defined as stem dry mass over fresh volume, and is determined by the amount of solid wood per unit stem volume (i.e. the volumetric wood fraction). The remainder of the stem volume is filled up by water and gas. For temperate tree species, on average as little as 30 % of the volume of tree stems is made up of solid wood material, 40 % is occupied by water and as much as 30 % by gas (Gartner et al., 2004). Water is localized in the protoplasm and the vessel, fibre and tracheid lumens. Gas may be localized in intercellular spaces, pores within the cell walls, parenchyma rays, and vessel, fibre and tracheid lumens.
There is an accumulating body of evidence for the importance of wood material for plant growth and survival (e.g. ter Steege, 2003: King et al., 2006; Alvarez-Clare and Kitajima, 2007). Investment in solid wood material leads to a better protection against pathogens (Schwarze and Spycher, 2005; Alvarez-Clare and Kitajima, 2007) and to an enhanced stem toughness (Niklas, 1992; van Gelder et al., 2006; Alvarez-Clare and Kitajima, 2007), and hence a better resistance against stem breakage by animals and falling debris. Such a strategy would be beneficial for shade-tolerant and small trees because they spend a longer time in the damp, lower strata of the forest and are more likely to suffer from pathogens and falling debris.
The importance of gas for biomechanics and tree performance has largely been overlooked in the biological literature, probably because most people assume that the internal tree processes take place in an aqueous environment (Gartner et al., 2004). Biomechanical theory states that the ability of a stem to resist compression or bending (i.e. the flexural stiffness, EI) is the product of the modulus of elasticity (E) and the second moment of area (I; Niklas, 1992). The modulus of elasticity indicates the amount of deflection a tree stem realizes under a given bending force and is a material property that is strongly determined by the wood fraction (Niklas, 1992; van Gelder et al., 2006). The second moment of area of a circular cross-section is defined as πD4/64 and is a structural property that is determined by the stem diameter (D). Trees may enhance their stem diameter by having gas-filled voids, which would lead to a higher I, a better stem resistance against bending forces (Gartner et al., 2004) and probably a better survival. Gas is a cheap filler, with the additional advantage that it does not lead to an increased stem mass and loading stress, as is the case for water (Gartner et al., 2004). Species show large interspecific variation in the stem volume occupied by gas, which ranges from 1–60 % (Gartner et al., 2004). Such variation might be closely related to the performance of species in different environments, and hence might have led to important life-history adaptations. Gas allows trees to realize a large volume gain for the same biomass investment, while at the same time having relatively stable stems because of reduced stem loads and increased second moment of area. Such a strategy would be especially beneficial for light-demanding species that establish in gaps and need to grow rapidly in height and attain a position in the canopy before the gap is closed. It would also be beneficial for tall species that need to grow rapidly in height to attain their large reproductive size (Thomas, 1996). Alternatively, water could be used as a cheap filler instead of gas, but has a disadvantage in that it leads to additional stem loading (Niklas, 1992; Gartner et al., 2004); apart from this, the water fraction may have similar consequences for life history variation as the gas fraction. To my knowledge, no study so far has evaluated the importance of the volumetric gas and water fraction for plant performance and life history variation.
Presented here are data on sapling and adult wood characteristics (volumetric fractions of wood, water and gas) of 30 and 58 tropical tree species, respectively. First, the implications of wood characteristics for sapling growth and survival are tested, and then these wood characteristics are related to two important life history axes of variation (cf. Loehle, 2000; Turner, 2001): the light requirements for regeneration and the adult stature (Hmax) of the species. It is hypothesized that: (1) a high wood fraction leads to a high survival- and a low growth rate, whereas a high gas and water fraction lead to a high survival and high growth rate; and 2) the wood fraction decreases with the light requirement and adult stature of the species, whereas the gas and water fraction increase with the light requirement and adult stature of the species.
MATERIALS AND METHODS
Research was conducted in the lowland tropical moist-forest of La Chonta, Bolivia. Annual precipitation is 1580 mm year−1 with a dry season (<100 mm month−1) from April until October. The forest is semi-evergreen, has a canopy height of 27 m, a basal area of 19·3 m2 ha−1 and a stem density of 367 ha−1 (stems >10 cm diameter at breast height, DBH; Peña-Claros et al., 2008). In this forest the Instituto Boliviano de Investigación Forestal (IBIF) administers 320 ha of permanent sample plots in which trees >10 cm DBH are monitored in a nested design.
Fifty-eight of the most abundant species that differed in adult stature and light requirements were selected (Table 1). Adult stature (Hmax) is defined as the potential height that a species can attain when it attains its maximal diameter. For each species this potential height was calculated using species-specific allometric relationships between height and diameter (Poorter et al., 2006) and the diameter of the third-thickest tree in the permanent sample plots. The third-thickest rather than the maximal tree diameter was used, thus avoiding outliers. Species were subjectively classified into shade-tolerants that can establish and survive in the shade, and pioneers that need high light as found in gaps for successful survival (Poorter et al., 2006). This two-group classification is merely used to give the reader a first feeling as to which strategy a species adopts; for the formal statistical analysis an objective and continuous measure of the regeneration light requirements of the species is used. In a separate study the regeneration light requirements were determined by analysing for each species the crown exposure in relation to the height of individual trees (Poorter et al., 2006). An average number of 612 individuals (median 283, range 41–9319) per species were measured over their whole size range for their height and crown exposure (CE; Dawkins and Field, 1978). For eight of the small (<8 m) or rare species, fewer than 100 individuals were used. The crown exposure is categorized as 1 if a tree does not receive any direct light, 2 if it receives lateral light, 3 if it receives overhead light on part of the crown, 4 if it receives full overhead light on the whole crown, and 5 if it has an emergent crown that receives light from all directions. The crown exposure can be measured repeatably (average difference between two independent observers is 0·1 ± 0·01 s.e.), and there is a good relation between CE and both canopy openness and incident radiation (Davies et al., 1998; Clark et al., 1993; Keeling and Phillips, 2007). For each species the CE was related to tree height using a multinomial regression analysis (Poorter et al., 2006; cf. Poorter et al., 2005). Using the regression equation, the average population-level crown exposure at a standardized height of 2 m (juvenile crown exposure: CEjuv) was calculated. In principle, 2-m tall saplings can be found under a wide range of crown exposures, but the value of CEjuv indicates the average CE at the population level for this plant size and is a good indicator of the regeneration light requirements (i.e. the inverse of the shade tolerance) of the species.
Table 1.
Overview of the species included in the study, their juvenile crown exposure (CEjuv), maximum adult stature (Hmax), and stem volumetric fractions of wood, gas and water for saplings and adults
| Saplings (n = 30) |
Adults (n = 58) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Family | CEjuv | Hmax (m) | Wood (%) | Gas (%) | Water (%) | Wood (%) | Gas (%) | Water (%) |
| Alibertia verrucosa | Rubiaceae | 1·35 | 13 | 36 | 9 | 55 | 51 | 13 | 36 |
| Ampelocera ruizii | Ulmaceae | 1·35 | 36 | 34 | 11 | 55 | 49 | 21 | 29 |
| Aspidosperma cylindrocarpon | Apocynaceae | 1·75 | 28 | 28 | 12 | 60 | 46 | 24 | 30 |
| Aspidosperma rigidum | Apocynaceae | 1·56 | 30 | 30 | 14 | 55 | 46 | 28 | 26 |
| Casearia sp. | Flacourtiaceae | 1·39 | 7 | 36 | 5 | 59 | 44 | 10 | 45 |
| Cecropia concolor | Cecropiaceae | 2·44 | 33 | 10 | 38 | 52 | 24 | 12 | 64 |
| Centrolobium microchaete | Fabaceae | 1·94 | 26 | 21 | 29 | 50 | 42 | 27 | 31 |
| Cupania cinerea | Sapindaceae | 1·79 | 22 | 33 | 16 | 51 | 50 | 14 | 36 |
| Erythrochiton fallax | Rutaceae | 1·27 | 5 | 29 | 14 | 57 | 44 | 19 | 37 |
| Eugenia florida | Myrtaceae | 1·52 | 8 | 36 | 9 | 56 | 46 | 9 | 45 |
| Gallesia integrifolia | Phytolaccaceae | 1·84 | 42 | 23 | 17 | 60 | 27 | 29 | 44 |
| Guarea guidonia | Meliaceae | 1·33 | 9 | 27 | 10 | 63 | 37 | 22 | 41 |
| Heliocarpus americanus | Tiliaceae | 2·36 | 22 | 13 | 32 | 55 | 19 | 42 | 39 |
| Hirtella triandra | Chrysobalanaceae | 1·28 | 11 | 35 | 7 | 58 | 42 | 17 | 42 |
| Hura crepitans | Euphorbiaceae | 1·62 | 44 | 16 | 29 | 55 | 28 | 30 | 42 |
| Jacaratia spinosa | Caricaceae | 2·02 | 21 | 7 | 5 | 88 | 6 | 18 | 76 |
| Licaria triandra | Lauraceae | 1·35 | 14 | 31 | 10 | 60 | 43 | 21 | 36 |
| Myrciaria sp. | Myrtaceae | 1·45 | 25 | 35 | 10 | 55 | 44 | 20 | 36 |
| Ocotea guianensis | Lauraceae | 1·54 | 25 | 26 | 24 | 50 | 35 | 30 | 35 |
| Ocotea sp.1 | Lauraceae | 1·37 | 27 | 21 | 15 | 64 | 36 | 34 | 30 |
| Peschiera australis | Apocynaceae | 1·38 | 16 | 22 | 12 | 66 | 32 | 20 | 48 |
| Pouteria macrophylla | Sapotaceae | 1·55 | 29 | 27 | 8 | 66 | 48 | 12 | 41 |
| Pouteria nemorosa | Sapotaceae | 1·57 | 35 | 29 | 13 | 58 | 41 | 20 | 38 |
| Pseudolmedia laevis | Moraceae | 1·32 | 35 | 29 | 9 | 62 | 42 | 20 | 38 |
| Sapium glandulosum | Euphorbiaceae | 2·23 | 31 | 17 | 18 | 65 | 25 | 37 | 38 |
| Stylogyne ambigua | Myrsinaceae | 1·46 | 10 | 28 | 0 | 72 | 38 | 13 | 48 |
| Sweetia fruticosa | Fabaceae | 1·91 | 34 | 35 | 16 | 49 | 59 | 11 | 30 |
| Terminalia oblonga | Combretaceae | 1·88 | 34 | 24 | 19 | 57 | 42 | 26 | 32 |
| Trema micrantha | Ulmaceae | 2·52 | 31 | 13 | 38 | 49 | 24 | 33 | 43 |
| Urera caracasana | Urticaceae | 1·99 | 13 | 11 | 12 | 76 | 20 | 4 | 76 |
| Acacia bonariensis | Mimosaceae | 1·59 | 28 | 46 | 25 | 29 | |||
| Batocarpus amazonicus | Moraceae | 1·35 | 26 | 38 | 17 | 45 | |||
| Caesalpinia pluviosa | Caesalpiniaceae | 1·87 | 27 | 50 | 11 | 39 | |||
| Cariniana estrellensis | Lecythidaceae | 1·40 | 42 | 39 | 21 | 40 | |||
| Cariniana ianeirensis | Lecythidaceae | 1·74 | 44 | 22 | 39 | 39 | |||
| Casearia gossypiosperma | Flacourtiaceae | 1·83 | 28 | 49 | 18 | 33 | |||
| Cavanillesia hylogeiton | Bombacaceae | 2·16 | 29 | 7 | 33 | 60 | |||
| Cedrela fissilis | Meliaceae | 1·98 | 29 | 29 | 31 | 40 | |||
| Ceiba pentandra | Bombacaceae | 45 | 21 | 21 | 57 | ||||
| Cordia alliodora | Boraginaceae | 1·95 | 26 | 32 | 36 | 32 | |||
| Dendropanax arboreus | Araliaceae | 1·46 | 25 | 24 | 31 | 44 | |||
| Ficus boliviana | Moraceae | 1·93 | 46 | 27 | 18 | 55 | |||
| Hymenaea courbaril | Caesalpiniaceae | 2·00 | 37 | 48 | 20 | 32 | |||
| Inga edulis | Mimosaceae | 1·72 | 25 | 39 | 19 | 41 | |||
| Maclura tinctoria | Moraceae | 1·83 | 30 | 44 | 15 | 40 | |||
| Margaritaria nobilis | Euphorbiaceae | 1·84 | 30 | 42 | 23 | 35 | |||
| Neea hermaphrodita | Nyctaginaceae | 1·45 | 13 | 20 | 7 | 73 | |||
| Ocotea sp.2 | Lauraceae | 1·34 | 20 | 37 | 38 | 25 | |||
| Ormosia nobilis | Fabaceae | 1·46 | 28 | 42 | 17 | 41 | |||
| Picramnia sellowii | Simaroubaceae | 1·34 | 8 | 41 | 13 | 45 | |||
| Pourouma cecropiifolia | Cecropiaceae | 1·38 | 20 | 27 | 21 | 52 | |||
| Sapindus saponaria | Sapindaceae | 1·63 | 22 | 46 | 19 | 35 | |||
| Schizolobium parahyba | Caesalpiniaceae | 2·39 | 35 | 28 | 14 | 58 | |||
| Simira rubescens | Rubiaceae | 1·63 | 23 | 40 | 18 | 42 | |||
| Spondias mombin | Anacardiaceae | 1·95 | 31 | 30 | 20 | 50 | |||
| Swietenia macrophylla | Meliaceae | 1·62 | 25 | 35 | 25 | 40 | |||
| Triplaris americana | Polygonaceae | 1·90 | 14 | 27 | 23 | 50 | |||
| Zanthoxylum sprucei | Rutaceae | 2·25 | 31 | 31 | 49 | 20 | |||
Hmax and CEjuv are positively correlated (r = 0·38, P < 0·05, n = 58), indicating that taller species are also more light demanding in the sapling stage. Yet, Hmax explains only 14 % of the variation in light requirements, which suggests that there is ample opportunity for independent effects of Hmax and CEjuv on stem traits.
Adult wood characteristics were determined for 58 species and sapling wood characteristics were determined for a subsample of 30 species (van Gelder et al., 2006). All samples were taken in the wet season. For adult wood, samples (approx. 2 × 2 × 2 cm) were taken from three trees per species, just interior to the cambium, and contained mostly sapwood. The trees were between 20 and 50 cm diameter for the tall species, and close to their maximal sizes for small species. Samples were stored for a week in plastic bags in a refrigerator. Fresh mass was determined with a balance, and fresh wood volume was measured using the water displacement method, after which the wood was oven-dried for at least 48 h at 70 ºC and then weighed. For saplings, the terminal meter of stem was sampled for ten individuals per species. This stem sample included the bark, because the intention was to relate the properties of the whole stem to sapling growth and survival (see below). It is acknowledged that wood and bark have different material properties, and hence different relationships between density and strength, but given the small amount of bark and the large interspecific differences in wood properties, such a whole-stem measurement gives a good estimate of the stem strength and rigidity of the species (van Gelder et al., 2006).
Saplings were on average 2·9 m tall (range 1·8–4). The green volume was calculated based on the length and diameters at the beginning and end of the sample, assuming the shape of a truncated cone. Fresh mass was measured on the day of collection, and dry mass after oven-drying for at least 48 h at 70 ºC. It should be noted that a direct comparison between sapling and adult stems is somewhat confounded by the difference in sampling methodology, as for the adults it strictly refers to the xylem, whereas for saplings it refers to the whole stem (including xylem, phloem and outer bark).
The volumetric percentages of wood, gas and water in the sample were calculated following Gartner et al. (2004). The percentage of stem volume in wood material was calculated as 100 × (dry mass/fresh volume) divided by the wood density of pure wood material (1·53 g cm−3; Kellog and Wangaard, 1969). The percentage of cell volume occupied by water was calculated as 100 × (fresh mass – dry mass)/cell volume. The percentage of cell volume of gas was calculated as (100 – percentage wood material – percentage water). The three stem volume components might be negatively correlated, simply because of the fact that they should add up to 100 %. The stem volume of wood sets an upper limit to the volume that can be occupied by the fillers water and gas. To separate out the role of gas versus water, the gas fraction of the filler was also calculated: 100 × gas percentage/(gas percentage + water percentage).
For the analysis it was presumed that all dry matter was in wood. However, some species also contain latex and resins, thereby potentially over-estimating the percentage wood and under-estimating the percentage water and gas. For the saplings there were nine species that produced latex or resins, and for the adults there were 11 species. Their wood fraction was not significantly higher than non-latex/resin-producing species (t-test: P = 0·29 for saplings and P = 0·60 for adults), suggesting that it did not bias the results to a significant extent.
For each species approx. 16 additional saplings between 0·5 and 2 m tall were sampled in the forest understorey, or under as shaded conditions as possible. Height and survival were monitored several times a year for a 2-year period: see Poorter and Bongers (2006) for a description of the calculation of annual height growth and survival rates. It is acknowledged that the survival rates provide a first estimation of the population-level survival rates of the species, especially for the species with very low inherent mortality rates, as mortality is a stochastic process and the sample size is relatively small.
Wood characteristics were related to CEjuv, Hmax, and sapling performance [log10(height growth), survival] using Pearson correlations. Height growth and survival rates were related to the percentage wood material and gas fraction of the filler using a forward multiple regression. All statistical analyses were carried out using SPSS 12 (SPSS Inc. Chicago, IL, USA). Ontogenetic consistency in characteristics of sapling and adult wood was evaluated with standard major axis regression using SMATR (Falster et al., 2006).
RESULTS
Stem fractions
Sapling stems consisted, on average, of 26 % (range 7–36 %) wood, 59 % (range 49–88 %) water and 15 % (range 0–38 %) gas (Table 1). In adult stems the corresponding figures were 36 % (6–59 %) wood, 42 % (20–76 %) water and 22 % (4–49 %) gas. For the 30 species for which saplings and adults were measured, there was a strong ontogenetic consistency in the wood fraction (r = 0·90, P < 0·001), and to a lesser extent in the water fraction (r = 0·65, P < 0·001) and the gas fraction (r = 0·52, P < 0·01; Fig. 1). This weaker ontogenetic consistency for gas and water could largely be attributed to the outlier Cecropia concolor that had a relatively high gas fraction as a sapling and a relatively high water fraction as an adult. If Cecropia was removed, then there were good relationships between adult and sapling stems for the wood fraction (standard major axis regression: y = 2·05 + 1·39x, r2 = 0·81, P < 0·001, n = 29), the water fraction (y = –39·7 + 1·34x, r2 = 0·61, P < 0·001) and the gas fraction (y = 6·30 + 1·05x, r2 = 0·48, P < 0·001; Fig. 1). All intercepts were significantly different from zero, and the slope was significantly different from 1 for the wood and water fractions.
Fig. 1.
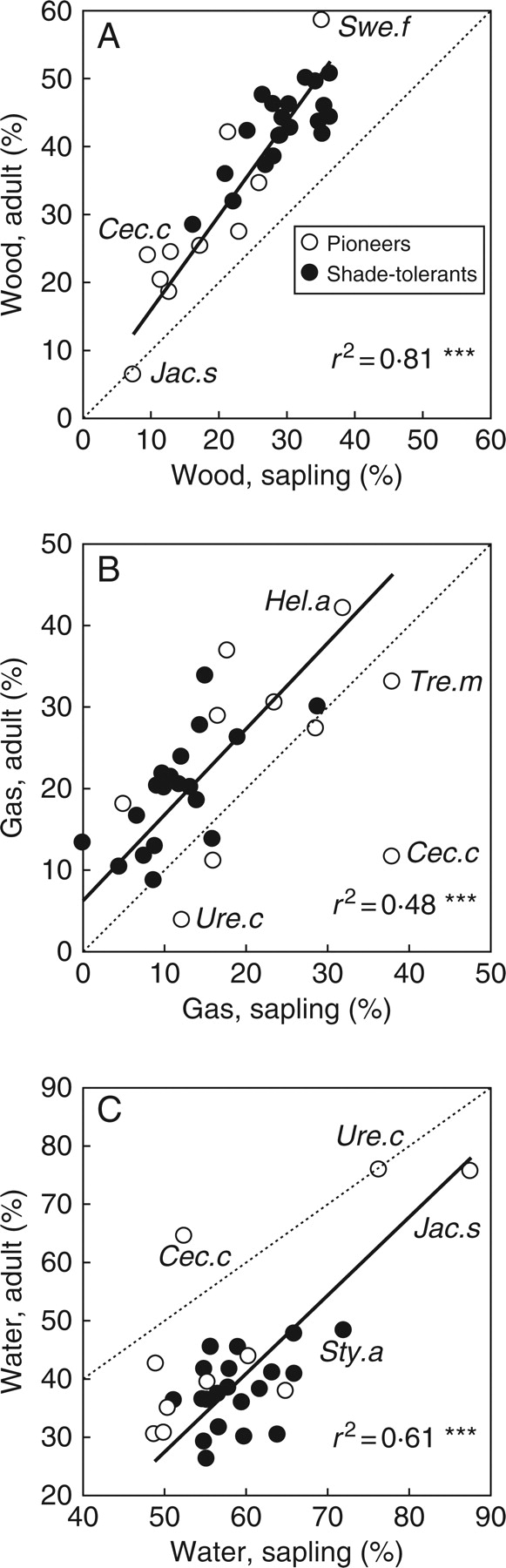
Ontogenetic consistency of adult versus sapling stem traits of 30 tropical tree species: (A) volumetric wood fraction, (B) volumetric gas fraction, and (C) volumetric water fraction. Pioneers and shade-tolerants are indicated and the dashed line indicates the trait values where y = x. The results of a standard major axis regression, coefficients of determination and significance levels are shown; the regression is done without the outlying species Cecropia concolor. Individual species are indicated by the first three letters of the genus name followed by one letter of their species' name; see Table 1. ***, P < 0·001.
The three stem fractions were negatively correlated amongst themselves, which was to be expected as the three together should add up to 100 %; what is of interest is which stem fractions show the strongest trade-offs. For saplings the wood fraction was most strongly correlated with the gas fraction (r = –0·57, P < 0·001) and to a lesser extent with the water fraction (r = –0·38, P < 0·05). The gas and water fractions were negatively associated between themselves (r = –0·54, P < 0·01; Table 2). For adults the wood fraction was most strongly correlated with the water fraction (r = –0·67, P < 0·001), and less strongly correlated with the gas fraction (r = –0·34, P < 0·01).
Table 2.
Pearson correlations between wood-, water- and gas fraction for saplings and adults of Bolivian tree species (this study), tropical timber species and temperate hard- and softwood species: data for the latter three groups come from Gartner et al. (2004)
| Bolivian tree species |
|||||
|---|---|---|---|---|---|
| Relationship | Saplings | Adults | Tropical timber | Hardwood | Softwood |
| Wood–Gas | −0·57 *** | −0·34 ** | −0·64 *** | −0·30 ns | 0·01 ns |
| Wood−Water | −0·38 * | −0·67 *** | −0·08 ns | −0·37 * | −0·26 ns |
| Gas–Water | −0·54 ** | −0·47 *** | −0·71 *** | −0·78 *** | −0·97 *** |
| n | 30 | 58 | 52 | 34 | 28 |
| Range WD (g cm−3) | 0·11−0·56 | 0·10−0·90 | 0·31−0·92 | 0·32−0·66 | 0·31−0·54 |
n refers to the number of species and ‘Range WD’ to the minimum and maximum basic wood densities included in the comparison. Similar correlation coefficients are obtained for Bolivian adult trees even if the analysis is confined to only the same 30 species for which saplings were measured. ***, P < 0·001; **, P < 0·01; *, P < 0·05, ns, P > 0·05.
Stem characteristics versus sapling performance
Sapling growth and survival rate were negatively correlated (r = –0·76, P < 0·001), suggesting that there is a trade-off between growth and survival. Height growth rate of saplings was negatively correlated with the wood fraction (r = –0·69, P < 0·001), positively correlated with the gas fraction (r = 0·58, P < 0·001) and not significantly correlated with the water fraction of the saplings (r = 0·06, P > 0·05; Fig. 2). Height growth was also positively correlated with the gas fraction of the filler (r = 0·51, P < 0·01). Multiple regression analysis showed that the wood fraction was the only stem characteristic contributing significantly (P < 0·001) to interspecific variation in growth rate.
Fig. 2.
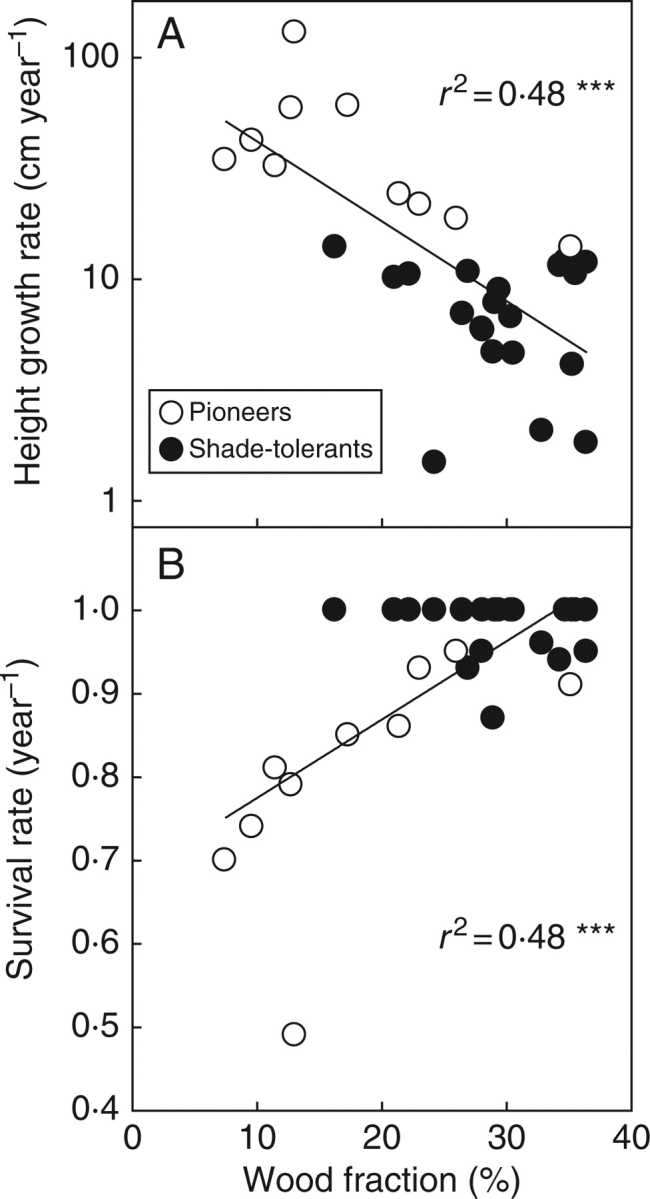
Annual height growth rate and survival rate versus volumetric stem wood fraction of saplings of 30 tropical tree species. Pioneers and shade-tolerants are indicated, and regression lines, coefficients of determination and significance levels are given. ***, P < 0·001. Note that many shade-tolerant species have an annual survival rate of 1 because the population of monitored saplings was relatively small (n = 16 per species) and no mortality occurred in the 2-year evaluation period.
Sapling survival rate showed the opposite pattern, and was positively correlated with the wood fraction (r = 0·69, P < 0·001), negatively correlated with the gas fraction (r = –0·58, P < 0·001), not correlated with the water fraction (r = –0·05, P > 0·05), and negatively correlated with the gas fraction of the filler (r = –0·51, P < 0·01). A forward multiple regression indicated that the wood fraction was the only stem characteristic that contributed significantly (P < 0·001) to interspecific variation in survival.
Stem characteristics in relation to CEjuv and Hmax
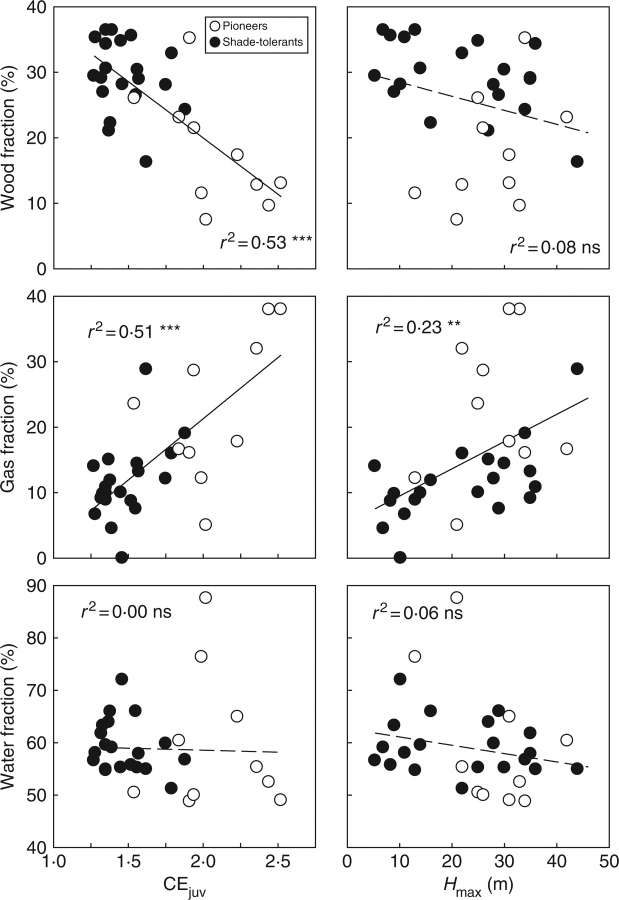
For saplings, the wood fraction was negatively related, the gas fraction was positively related, and the water fraction was not significantly related to the juvenile crown exposure (Fig. 3). The gas fraction of the filler was also positively related to the juvenile crown exposure (Table 3). The wood and water fractions were not significantly related to Hmax, whereas the gas fraction and the gas fraction of the filler were positively related to Hmax (Fig. 3, Table 3). The same relationships between stem traits, CEjuv and Hmax were found for adult trees (Table 3), although the correlations were somewhat weaker.
Fig. 3.
Relationships between sapling stem characteristics (volumetric wood, water and gas fractions), and the juvenile crown exposure (CEjuv) and maximum adult stature (Hmax) of 30 tropical tree species. Pioneers and shade-tolerants are indicated, and regression lines, coefficients of determination and significance levels are given. Continuous regression lines indicate a significant relationship, broken lines indicate a non-significant relationship. ***, P < 0·001; **, P < 0·01; ns, P > 0·05.
Table 3.
Pearson correlations between stem characteristics of saplings and adults and the juvenile crown exposure (CEjuv) and maximum adult stature (Hmax) of tree species
| Saplings (n = 30) |
Adults (n = 58) |
|||
|---|---|---|---|---|
| CEjuv | Hmax | CEjuv | Hmax | |
| Wood fraction | −0·73 *** | −0·28 ns | −0·45 *** | −0·13 ns |
| Gas fraction | 0·71 *** | 0·48 ** | 0·27 ** | 0·34 ** |
| Water fraction | −0·06 ns | −0·25 ns | 0·20 ns | −0·14 ns |
| Gas fraction of filler | 0·65 *** | 0·50 ** | 0·11 ns | 0·32 * |
***, P < 0·001; **, P < 0·01; *, P < 0·05; ns, P > 0·05.
DISCUSSION
Components of stem volume
Stems of saplings and adult trees consist mostly of water, to a lesser extent of wood and, least of all, of gas. Despite differences in stem sampling methodology (for adults only xylem, for saplings the whole stem), there was a strong ontogenetic consistency in stem traits (Fig. 1). All stem volume that is not wood can be filled up with either gas or water. For saplings, the wood fraction was most closely and negatively correlated with the gas fraction (Table 2), probably because saplings may have a relatively large pith with many parenchyma cells (that lack a secondary cell wall) and many intercellular, gas-filled spaces, or because for saplings the bark was included, and the bark may contain more gas-filled spaces. For adults, the wood fraction was most closely and negatively correlated with the water fraction (Table 2), probably because for adult trees the wood samples were taken from the sapwood, and included little or no heartwood.
Gartner et al. (2004) provided data on volumetric stem fractions for a large number of tropical tree species and temperate soft-and hardwood species. Remarkably, the trade-off between gas and wood fractions was only observed for the tropical species and not for the temperate species (Table 2). This is probably because the temperate species covered a smaller range in wood density (0·31–0·66 g cm−3) compared with the tropical species (0·11–0·92 g cm−3).
Stem traits and sapling performance
The wood and gas fractions showed almost equally strong correlations with sapling performance in the field. However, a multiple regression analysis indicated that sapling performance was determined only by the wood fraction; a high wood fraction was associated with an increased survival rate and a decreased growth rate (Fig. 2), as predicted with the first hypothesis (see Introduction). The gas and water fractions did not have a significant additional effect on sapling performance, contrary to the hypothesis.
The wood fraction (and hence wood density) had a positive relationship with survival, probably because it is closely associated with the stiffness (modulus of elasticity) and strength (modulus of rupture) of the wood (Forest Product Laboratory, 1999; van Gelder et al., 2006). This higher amount of material per volume makes the structure stronger because there is more material in a given volume to transfer the forces. Thus, denser material will help plants avoid buckling under their own weight, and confer on them a higher resistance against bending forces or breakage due to wind or falling debris (Putz et al., 1983; van Gelder et al., 2006). As much as 25 % of the plants in the understorey are hit annually by falling debris (Clark and Clark, 1989), many of them dying immediately or in the longer term. It is therefore not surprising that an enhanced wood density pays off in terms of an increased survival. Other studies have also found a positive relationship between wood density and survival rate for seedlings growing under controlled conditions (Kitajima, 1994), and for seedlings (Alvarez-Clare and Kitajima, 2007) saplings (Muller-Landau, 2004) and adult trees (Nascimento et al., 2005; King et al., 2006; Osunkoya et al., 2007; Poorter et al., 2008) growing in the field.
The wood fraction had a negative relationship with sapling growth. Carbon gain proceeds at slow rates in the shaded understorey of tropical rain forests, and there is thus a premium on the efficient use of carbon. By having a low wood fraction, more stem volume is created per unit biomass invested, which leads to increased height growth. The remainder of the stem volume can be filled up with cheap fillers such as gas or water. Gartner et al. (2004) showed with a modelling approach that gas has an advantage over water as a filler because it leads to an increased stem volume without adding on extra mass, and results in biomechanically more stable trees that can grow efficiently in height. This could explain why the gas fraction is significantly and positively correlated with sapling growth rate, whereas the water fraction is not. Negative relationships between growth rates and wood density of trees have also been reported from tropical rain forest species from Guyana (Arets et al., 2003), Surinam (ter Steege et al., 2003), the Amazon (Nascimento et al., 2005), the neotropics (Poorter et al., 2008) and Borneo (Verburg and Eijk-Bos, 2003).
The wood fraction is part of a suite of correlated stem, leaf and root traits (Reich et al., 2003), all of which may contribute to the trade-off between growth and survival (Kitajima and Poorter, 2008). The leaf mass per unit area, for example, contributes to the growth–survival trade-off as it enhances the lifespan of leaves and plants whereas it comes at the expense of a reduced photosynthetic capacity and, hence, growth (Reich et al., 1992; Poorter and Bongers, 2006; Sterck et al., 2006). As is the case with stems, a high leaf mass per unit area is often associated with a high cell wall fraction and a low gas and water fraction (Niinemets, 1999). The wood fraction of sapling stems and leaf mass per unit area of sapling leaves are closely correlated (data from Poorter and Bongers, 2006: r = 0·68, P < 0·001, n = 30 species), suggesting that they are part of the same plant syndrome that determines the fast–slow continuum in growth and survival (cf. Westoby et al., 2002; Wright et al., 2007; Alvarez-Clare and Kitajima, 2007). Investment in a high cell wall fraction might not only enhance tissue longevity but it might also serve a hydraulic function, as it allows stems and leaves to resist the high tension that comes along with low water potentials during dry conditions (e.g. Hacke et al., 2001).
Stem traits in relation to light requirement and adult stature
The gas fraction increased with the juvenile light requirement (CEjuv) and Hmax of the species (Fig. 3), in line with the second hypothesis proposed in the Introduction. This is not merely because the gas fraction is the complement of wood fraction; the gas fraction of the filler showed the same positive correlations with juvenile light requirement and Hmax (Table 3). A greater gas fraction increases the stem volume, which allows species to realize a rapid height growth and get access to the canopy in the case of light-demanding species, and to rapidly attain their large reproductive size in the case of tall species. An additional advantage of a greater gas fraction for tall species is that it allows them to make wide stems, which enhance the second moment of area (I), and hence flexural stiffness (EI) and stability. For saplings, a substantial amount of the gases might be found in the pith of the young stems. From a mechanical point of view, columns are stiffer if most material is displaced towards the periphery of the column, rather than uniformly distributed; a wide pith therefore contributes to an increased stem stiffness (Niklas, 1997). Such a strategy is, for the species studied here, taken to the extreme by Cecropia concolor, whose saplings have a hollow stem. For adult trees, most gas is likely to be found in pore spaces, or embolized vessel or tracheid lumens (Gartner et al., 2004).
The wood fraction decreased with light requirement, in line with the second hypothesis (Fig. 3). A high wood fraction is advantageous for species that mainly regenerate in the shade, because it protects these species better against pathogens and falling debris, which are important mortality agents in a humid forest understorey (Augspurger, 1984; Gartner, 1989; Alvarez-Clare and Kitajima, 2007). Of course, apart from the wood fraction, other stem features such as decay-resisting compounds in the heartwood and latex and resins contribute to stem protection against pathogens. It was also hypothesized that the wood fraction would decrease with Hmax, because small species spend a longer part of their life in the lowest forest strata and run a higher life-time risk of being hit by falling debris. This prediction breaks down because pioneers and shade tolerants show opposite relationships between the wood fraction and Hmax (cf. Falster and Westoby, 2005). If shade-tolerant species are considered alone, then there is indeed a significant negative relationship between the wood fraction and Hmax (Fig 3: r = –0·46, P < 0·05, n = 20; cf. Thomas, 1996; Falster and Westoby, 2005). However, the pioneers tend to show a positive relationship (r = 0·44, P > 0·21, n = 10), probably because early in succession small, highly light-demanding, short-lived pioneer species are gradually replaced by rather taller, less light-demanding and longer-lived pioneers. The short-lived pioneers have a low wood density in order to realize fast height growth and to rapidly complete their life cycle, whereas the long-lived pioneers have a higher wood density because they run a higher risk of being hit by falling debris in the closing vegetation, and need well-protected stems to persist for a long time.
It was hypothesized that the water fraction would increase with the juvenile light requirement and Hmax of the species because water is a cheap filler that is readily available. This hypothesis was rejected; in contrast to gas, stem water leads to increased self-loading and a decreased stability. The water fraction was therefore neither correlated with the sapling performance, nor with the life history variation of tree species (Table 3, Fig. 3). When only shade-tolerant species are considered is there then a significant negative correlation between water fraction and Hmax (Fig. 3; cf. Osunkoya et al., 2007). Tall trees have their crowns exposed in the dry conditions of the canopy and it might be difficult to meet these higher transpirational demands through an increased water uptake, because of the negative effects of gravity and an enhanced path length. Instead, tall trees may use water stored in the stems, leading to a lower water fraction. Such a relationship has been found within species (Phillips et al., 2003) but it is not clear whether the same mechanism holds across species.
Conclusions
In this study, the wood fraction partially explains the growth–survival trade-off that has been found for tropical tree species. The wood and gas fractions are also closely related to the regeneration light requirement of the species. Interestingly, the gas fraction was the only stem trait related to the adult stature of the species, and this pattern was found both for saplings as well as for adult trees. Tall species have a high gas fraction, not only because gas is a cheap filler, but probably also because it leads to an increased stability in these tall species.
ACKNOWLEDGEMENTS
Jose Chuviña and Victor Hugo Hurtado are thanked for their help with the wood sample collection, and the staff and personnel of the Instituto Boliviano de Investigación Forestal (IBIF) for their logistic support. Arnold van Gelder kindly provided data on sapling wood characteristics. Barb Lachenbruch, Marielos Peña-Claros, Jack Putz, Frank Sterck and two anonymous reviewers provided insightful comments on the manuscript. LP was supported by Veni grant 863·02·007 from the Netherlands Organisation of Scientific Research (NWO), and a fellowship from the Wageningen graduate school Production Ecology and Resource Conservation. This research was partially supported by the Inter-American Institute for Global Change Research (IAI) CRN 2015, which is supported by the US National Science Foundation (Grant GEO-0452325).
LITERATURE CITED
- Alvarez-Clare S, Kitajima K. Physical defence traits enhance seedling survival of neotropical tree species. Functional Ecology. 2007;21:1044–1054. [Google Scholar]
- Arets E, van der Hout P, Zagt R. Responses of tree populations and forest composition to selective logging in Guyana. In: ter Steege H., editor. Long-term changes in tropical tree diversity. Studies from the Guiana Shield, Africa, Borneo, and Melanesia. Wageningen: Tropenbos International; 2003. pp. 95–116. Tropenbos Series 22. [Google Scholar]
- Augspurger CK. Seedling survival of tropical tree species; interactions of dispersal distance, light gaps, and pathogens. Ecology. 1984;65:1705–1712. [Google Scholar]
- Clark DA, Clark DB. The role of physical damage in the seedling mortality regime of a Neotropical rain forest. Oikos. 1989;55:225–230. [Google Scholar]
- Clark DB, Clark DA, Rich PM. Comparative analysis of microhabitat utilization by saplings of nine tree species in Neotropical rain forest. Biotropica. 1993;25:397–407. [Google Scholar]
- Davies SJ. Tree mortality and growth in 11 sympatric Macaranga species in Borneo. Ecology. 2001;82:920–932. [Google Scholar]
- Davies SJ, Palmiotto PA, Ashton PS, Lee HS, LaFrankie JV. Comparative ecology of 11 sympatric species of Macaranga in Borneo: tree distribution in relation to horizontal and vertical resource heterogeneity. Journal of Ecology. 1998;86:662–673. [Google Scholar]
- Dawkins HC, Field DRB. A long-term surveillance system for British woodland vegetation. Oxford: Department of Forestry, Oxford University; 1978. [Google Scholar]
- Falster DS, Westoby M. Alternative height strategies among 45 dicot rain forest species from tropical Queensland, Australia. Journal of Ecology. 2005;93:521–535. [Google Scholar]
- Falster DS, Warton DI, Wright IJ. SMATR: Standardised Major Axis Tests & Routines Version 2. 2006. http://www.bio.mq.edu.au/ecology/SMATR/
- Forest ProductsLaboratory. Wood handbook. Wood as an engineering material. Madison, WI: US Department of Agriculture, Forest Service, Forest Products Laboratory; 1999. General Technical Report 113. [Google Scholar]
- Gartner BL. Breakage and regrowth of Piper species in rain forest understorey. Biotropica. 1989;21:303–307. [Google Scholar]
- Gartner BL, Moore JR, Gardiner BA. Gas in stems: abundance and potential consequences for tree biomechanics. Tree Physiology. 2004;24:1239–1250. doi: 10.1093/treephys/24.11.1239. [DOI] [PubMed] [Google Scholar]
- van Gelder HA, Poorter L, Sterck FJ. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phytologist. 2006;171:367–378. doi: 10.1111/j.1469-8137.2006.01757.x. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- Keeling HC, Phillips OL. A calibration method for the crown illumination index for assessing forest light environments. Forest Ecology and Management. 2007;242:431–437. [Google Scholar]
- Kellog RM, Wangaard FF. Variation in the cell-wall density of wood. Wood Fiber Science. 1969;1:180–204. [Google Scholar]
- King DA, Davies SJ, Tan S, Boor NSM. The role of wood density and stem support costs in the growth and mortality of tropical trees. Journal of Ecology. 2006;94:670–680. [Google Scholar]
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Poorter L. Functional basis for resource niche differentiation by tropical trees. In: Carson WP, Schnitzer SA, editors. Tropical forest community ecology. Oxford: Blackwell Publishing, in press.; 2008. [Google Scholar]
- Kobe RK, Pacala SW, Silander JA, Canham CD. Juvenile tree survivorship as a component of shade tolerance. Ecological Applications. 1995;5:517–532. [Google Scholar]
- Loehle C. Strategy space and the disturbance spectrum: a life-history model for tree species coexistence. American Naturalist. 2000;156:14–33. doi: 10.1086/303369. [DOI] [PubMed] [Google Scholar]
- Muller-Landau HC. Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica. 2004;36:20–32. [Google Scholar]
- Nascimento HEM, Laurence WF, Condit R, Laurence SG, D'Angelo S, Andrade AC. Demographic and life history correlated for Amazonian trees. Journal of Vegetation Science. 2005;16:625–634. [Google Scholar]
- Niinemets ü. Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist. 1999;144:35–57. [Google Scholar]
- Niklas KJ. Plant biomechanics. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Niklas KJ. Relative resistance of hollow, septate internodes to twisting and bending. Annals of Botany. 1997;80:275–287. [Google Scholar]
- Osunkoya OO, Sheng TK, Mahmud N, Damit N. Variation in wood density, wood water content, stem growth and mortality among twenty-seven tree species in a tropical rainforest on Borneo Island. Austral Ecology. 2007;32:191–201. [Google Scholar]
- Phillips NG, Ryan MG, Bond BJ, McDowell NG, Hinckley TM, Cermak J. Reliance on stored water increases with tree size in three species in the Pacific Northwest. Tree Physiology. 2003;23:237–245. doi: 10.1093/treephys/23.4.237. [DOI] [PubMed] [Google Scholar]
- Peña-Claros M, Fredericksen TS, Alarcón A, Blate GM, Choque U, Leaño C, et al. Beyond reduced impact logging; silvicultural treatments to increase growth rates of tropical trees. Forest Ecology and Management. 2008 In press. doi:10.1016/j.foreco.2007.11.013. [Google Scholar]
- Poorter L, Bongers F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology. 2006;87:1733–1743. doi: 10.1890/0012-9658(2006)87[1733:ltagpo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F, Sterck FJ, Wöll H. Beyond the regeneration phase: differentiation of height-light trajectories among tropical tree species. Journal of Ecology. 2005;93:256–267. [Google Scholar]
- Poorter L, Bongers L, Bongers F. Architecture of 54 moist forest tree species: traits, trade-offs, and functional groups. Ecology. 2006;87:1289–1301. doi: 10.1890/0012-9658(2006)87[1289:aomtst]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Poorter L, Paz H, Wright SJ, Ackerly DD, Condit R, Ibarra-Manríquez G, et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology. 2008;89:1908–1920. doi: 10.1890/07-0207.1. [DOI] [PubMed] [Google Scholar]
- Putz FE, Coley PD, Lu K, Montalvo A, Aiello A. Uprooting and snapping of trees: structural determinants and ecological consequences. Canadian Journal of Forest Research. 1983;13:1011–1020. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs. 1992;62:365–392. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences. 2003;164:143–164. [Google Scholar]
- Schwarze FWMR, Spycher M. Resistance of thermo-hygro-mechanically densified wood to colonisation and degradation by brown-rot fungi. Holzforschung. 2005;59:358–363. [Google Scholar]
- ter Steege H. Long-term changes in tropical tree diversity. Studies from the Guiana Shield, Africa, Borneo, and Melanesia. Wageningen: Tropenbos International; 2003. Tropenbos Series 22. [Google Scholar]
- ter Steege H, Laumans B, Laumans-Bus D, Zondervan G, Bongers F. Long-term effect of timber harvesting in Mapana, North Suriname. In: ter Steege H, editor. Long-term changes in tropical tree diversity. Studies from the Guiana Shield, Africa, Borneo, and Melanesia. Wageningen: Tropenbos International; 2003. pp. 79–94. Tropenbos Series 22. [Google Scholar]
- Sterck FJ, Poorter L, Schieving F. Leaf traits determine the growth–survival trade-off across rain forest tree species. The American Naturalist. 2006;167:758–765. doi: 10.1086/503056. [DOI] [PubMed] [Google Scholar]
- Thomas SC. Asymptotic height as a predictor of growth and allometric characteristics in Malaysian rain forest trees. American Journal of Botany. 1996;83:556–566. [Google Scholar]
- Turner IM. The ecology of trees in the tropical rainforest. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Verburg R, van Eijk-Bos C. Temporal changes in species diversity, composition, and plant functional types after logging in a Bornean rain forest. In: ter Steege H, editor. Long-term changes in tropical tree diversity. Studies from the Guiana Shield, Africa, Borneo, and Melanesia. Wageningen: Tropenbos International; 2003. pp. 117–134. Tropenbos Series 22. [Google Scholar]
- Westoby M, Falster DS, Moles TA, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Wright IJ, Ackerly DD, Bongers F, Harms KE, Ibarra-Manriquez G, Martinez-Ramos M, et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Annals of Botany. 2007;99:1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]