Abstract
Background and Aims
Stomata play an important role in both the CO2 assimilation and water relations of trees. Therefore, stomatal traits have been suggested as criteria for selection of clones or genotypes which are more productive and have larger water-use efficiency (WUE) than others. However, the relationships between plant growth, WUE and stomatal traits are still unclear depending on plant material (genus, species, families, genotypes) and, more precisely, on the strength of the relationships between the plants. In this study, the correlations between these three traits categories, i.e. plant growth, WUE and stomatal traits, were compared in two related poplar families.
Methods
Stomatal traits (stomatal density, length and ratio adaxial : abaxial stomatal densities) of a selection of F1 genotypes and the parents of two hybrid poplar families Populus deltoides ‘S9-2’ × P. nigra ‘Ghoy’ (D × N family, 50 F1) and P. deltoides ‘S9-2’ × P. trichocarpa ‘V24’ (D × T family, 50 F1) were measured, together with stem height and circumference. Carbon isotope discrimination (Δ) was determined and used as an indicator of leaf-level intrinsic WUE.
Key Results
Leaves of hybrids and parents were amphistomatous, except for the P. trichocarpa parent. Both families displayed high values of heritability for stomatal traits and Δ. In the progeny, the relationship between stem circumference and Δ was weak for the D × N family, while abaxial and total stomatal density were positively associated with stem dimensions for the D × T family only.
Conclusions
Genetic variation in stomatal traits and Δ was large within as well as between the different poplar species and their hybrids, but there were no direct relationships between stomatal traits and plant growth or Δ. As already noticed in various poplar hybrids, the absence of, or the weak, relationship between Δ and plant growth allows the possibility of selecting poplar genotypes combining high productivity and high WUE. In this study, stomatal traits are of limited value as criteria for selection of genotypes with good growth and large WUE.
Key words: Adaxial and abaxial stomatal density, stomatal length, heritability, water-use efficiency (WUE), F1 hybrids, breeding, Populus deltoides, Populus nigra, Populus trichocarpa
INTRODUCTION
In Europe, there is considerable interest in fast-growing trees such as poplar, which produce large amounts of biomass as a source of renewable energy (Bradshaw et al., 2000). Poplars have the capacity to grow in a wide range of environments (Dickmann and Stuart, 1983) and tolerate coppicing (Ceulemans and Deraedt, 1999), but require large amounts of water (Ceulemans et al., 1988; Barigah et al., 1994). In water-limited conditions, the ratio of total biomass production to water used (i.e. integrated water-use efficiency, WUE) becomes a crucial trait to be taken into account in the tree selection process. Genotypes of poplars producing more biomass per unit of water consumed would represent a considerable advantage. Selection of such genotypes has, however, been slow. Useful selection criteria include intrinsic water-use efficiency (WUEi), i.e. the ratio of net carbon dioxide assimilation to stomatal conductance of leaves. It can be indirectly estimated through carbon isotope discrimination (Δ) of leaves, as they are linearly and negatively correlated in many species (Farquhar et al., 1989; Ponton et al., 2001; Brendel et al., 2002) including poplar (Ripullone et al., 2004; Monclus et al., 2006).
Large genetic variation in Δ has been reported within various species, both in controlled environments and under field conditions (Brugnoli and Farquhar, 2000; Condon et al., 2002; Rebetzke et al., 2002; Monclus et al., 2005, 2006). Various relationships between Δ and productivity have been reported for a wide range of species (Brugnoli and Farquhar, 2000; Condon et al., 2002). In poplar, positive correlation occurred in P. × interamericana (Rae et al., 2004) and P. davidiana (Zhang et al., 2004) while no correlation was found for P. × euramericana (Marron et al., 2005; Monclus et al., 2005, 2006). Selection for low Δ (or high WUEi) may result in the selection of trees with reduced or higher productivity according to water availability and to the predominant trait (i.e. stomatal conductance or CO2 assimilation) accounting for the variability in Δ of a specific species (Farquhar et al., 1989; Condon et al., 2002). Nevertheless, few studies were conducted with the specific aim of clarifying or determining if a single, clear trend exists between productivity and WUEi in woody species.
Both CO2 uptake and water losses are affected by stomatal dimensions and aperture. Consequently, stomatal traits such as stomatal density, length and responsiveness are considered key determinants of plant growth and water balance. Evidently, environmental factors (light, temperature, vapour pressure deficit, etc.) also play a role. Large differences in stomatal traits occur between poplar species and genotypes (Pallardy and Kozlowski, 1979; Orlović et al., 1998; Dunlap and Stettler, 2001; Al Afas et al., 2005; Pearce et al., 2005) suggesting that they may offer good selection criteria for high-yielding genotypes. However, the links between stomatal traits and plant growth are not obvious and apparently dependent on the particular poplar species or hybrids. Positive correlations between number of stomata per unit leaf area and productivity occurred in poplar (Barigah et al., 1994; Orlović et al., 1998; Al Afas et al., 2005) and other tree species (Wang et al., 1995). Negative correlations occurred for P. × euramericana hybrids (Monclus et al., 2006), while no correlations were found in various poplar hybrids by Ceulemans et al. (1987).
In summary, the relationships between growth, WUE and stomatal traits are still very unclear and no general trend has been observed even within the Populus genus. Previous studies with P. × euramericana hybrids (Marron et al., 2005; Monclus et al., 2005, 2006) and P. × interamericana hybrids (Rae et al., 2004) assessed the relationships: Rae et al. (2004) found a weak but positive relationship between Δ and productivity while no correlation was observed by Marron et al. (2005) and Monclus et al. (2005, 2006). A negative relationship occurred between stomatal density and productivity, while Δ was positively associated with stomatal density for P. × euramericana hybrids (Monclus et al., 2006). However, both hybrid combinations (P. × euramericana and P. × interamericana) have never been studied together under similar growth conditions. In the present study, two poplar families sharing the same female parent P. deltoides ‘S9-2’ × P. nigra ‘Ghoy’ and P. deltoides ‘S9-2’ × P. trichocarpa ‘V24’ are described. The objectives were: (a) to test whether relationships between plant growth, Δ (as an indicator of WUEi) and stomatal size and density can be established in the two poplar families and; (b) to study the extent of genotypic variation in stomatal morphological traits of a subset of the two families to evaluate the value in the selection of more productive, as well as water-use efficient, genotypes.
MATERIALS AND METHODS
Plant material and experimental design
Two full-sib poplar families resulting from controlled crosses sharing the same female parent were investigated. One family consisted of 180 F1 genotypes from an interspecific cross between P. deltoides (Bartr. ex Marsh.) ‘S9-2’ and P. nigra (L.) ‘Ghoy’ (P. × euramericana, D × N family) (Cervera et al., 1996, 2001). The second family included 182 F1 genotypes from an interspecific cross of P. deltoides ‘S9-2’ and P. trichocarpa (Torr. and Gray) ‘V-24’ (P. × interamericana, D × T family). Both crosses were made by the Research Institute for Nature and Forest (INBO, Geraardsbergen, Belgium) in 1987 and repeated in 1995 to increase the number of progeny.
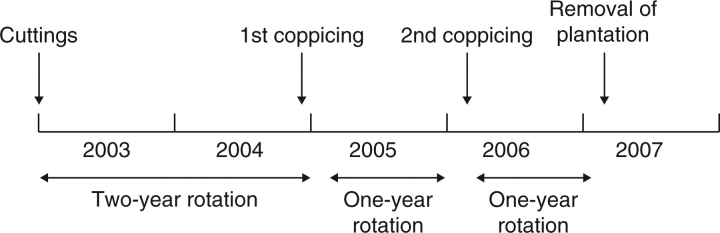
The experimental plantation in central France (Ardon; 47°46′N, 1°52′E) was established in April 2003 from 25-cm, uniform hardwood cuttings. The initial spacing was 0·75 m × 2 m giving 6670 trees ha−1. Randomized block design (Marron et al., 2006) was used with six blocks: one replicate of each F1 genotype and each parent was randomly allocated to each block. For each family, 50 genotypes were selected to be representative of the genetic variation in productivity based on circumference measurements. Three out of six replicates for each of the 50 selected genotypes were selected, based on their closest performance to the genotypic mean in terms of circumference (the genotypic mean was calculated on six replicates). To reduce border effects (Zavitkovski, 1981; Van Hecke et al., 1995), a double row of P. × euramerciana cv. ‘Robusta’ was planted around the plantation. Plantation management included irrigation and the use of insecticides and fungicides as needed throughout the four growing seasons (April 2003 to November 2006). All trees were coppiced in December 2004 and February 2006, after two and one growing seasons, respectively, and in early spring all shoots, except the largest one, were removed from each stump (Fig. 1; Marron et al., 2006; Dillen et al., 2007).
Fig. 1.
Coppice regime and rotation cycles of the high density poplar plantation in Ardon (France). In April 2003, the plantation was established with hardwood cuttings.
Growth measurements
At the end of the first growing season of the second coppice rotation (February 2006, just before the second harvest), stem height and circumference were assessed for all trees of both families. Stem height was measured (accuracy 1 cm) with an extendable pole, and stem circumference at 1 m above the ground with a tape (accuracy 1 mm).
Sampling procedure for leaf-related measurements
During the first growing season of the third coppice rotation (August 2006), the first fully developed leaf starting from the top and the following (older) leaf on the stem were collected from the 50 selected genotypes of each family. Excised leaves were put in plastic bags and imprints of both adaxial and abaxial leaf epidermises were immediately made in a field laboratory (150 first fully developed leaves for each family). The leaves following the first fully developed leaf were oven-dried at 80 °C to constant weight for carbon isotope discrimination determination.
Stomatal measurements
Adaxial and abaxial imprints of epidermis at maximum leaf width near the central vein of the first fully expanded leaf were made using clear nail varnish and adhesive cellophane tape (Ceulemans et al., 1995). All imprints were fixed onto glass microscope slides and examined at ×200 magnification using a digital camera (Olympus Camedia C-4040 Zoom, Japan) attached to an Olympus BX50 microscope (Japan). At least ten microscopic fields from each imprint of the adaxial and abaxial surfaces were randomly sampled and photographed. The average number of stomata was calculated for each imprint and then for each replicate. Stomatal density was defined as the number of stomata per unit of leaf area (mm−2) and was determined separately on the adaxial and abaxial leaf surfaces, and combined as total stomatal density. The ratio adaxial : abaxial stomatal densities was calculated. Stomatal lengths were measured with the ImageJ program (Abramoff et al., 2004). Stomatal length was defined as the length of the stomatal complex (Pallardy and Kozlowski, 1979; Ceulemans et al., 1984) and per imprint, 30 randomly sampled stomata were measured.
Carbon isotope discrimination
Oven-dried foliar samples (leaves following the first fully expanded leaf, 150 leaves for each family) were ground to fine powder for analysis of carbon isotope (13C) composition using a continuous flow isotope ratio mass spectrometer (20-20 mass spectrometer, PDZEuropa, Northwich, UK) at the UC Davis Stable Isotope Facility (California, USA). Carbon isotope composition (δ) was calculated relative to the international Pee Dee Belemnite standard (Farquhar et al., 1989): δplant = (Rsa – Rsd)/Rsd × 1000[‰] where Rsa and Rsd are the 13C : 12C ratios of the sample and the standard, respectively (Craig, 1957). Carbon isotope discrimination (Δ), a factor related to isotope fractionation by the photosynthetic process relative to the source carbon was estimated as: Δ = (δair – δplant)/(1 + δplant/1000) where δair is the 13C composition of atmospheric CO2, which is assumed to be –8·0 ‰ (Farquhar et al., 1989; Guehl et al., 1995).
Data analysis
Statistical analyses were performed with the R software (version 6·2·1, A Language and Environment Copyright, 2007). Assumptions on distributions of residuals of the linear models were checked with the Shapiro–Wilk statistic. When necessary, original values were transformed according to the Box-Cox procedure (Venables and Ripley, 2000). The following fixed models were used.
For analysis of block effects: Yij = μ+ Bi + εij where μ is the general mean and Bi is the effect of block i considered as fixed assuming that the three selected replicates of each genotype were randomly distributed over the six blocks. Bi was calculated, for each family, as the difference between the mean of each of the three blocks and the general mean of the whole family.
For comparison between families: Y′ijk = μ+ Gj(k) + Famk + εijk where Y′ijk are individual values adjusted for the block effects (Y′i = Y − Bi), Gj(k) is the genotype effect (random; nested in Famk) and Famk is the family effect (fixed).
For comparison between parental performances: Y′ij = μ+ Pj + εij where Pj is the effect of parent species j considered as fixed. The Scheffé method was chosen as post-hoc analysis due to different numbers of replicates for the three parents.
Differences between means were considered as significant when the P-value of the ANOVA F-test was <0·05. To characterize genetic variation present in each family separately, the following random model was used:
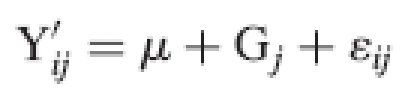 |
where Gj was the effect of genotype j considered as random. Variance components of random effects (σ2G and σ2 ε) were calculated by equating observed mean squares to expected mean squares and solving the resulting equations according to the Henderson III procedure (Henderson, 1953). Broad-sense heritability (H2) was estimated on an individual basis as H2 = σ2G/(σ2G + σ2 ε) where σ2G is the variance component of the genotype effect and σ2 ε is the variance component of the residual effect (Hanson, 1963; Nyquist, 1991; Holland et al., 2003). The standard errors of broad-sense heritability were calculated as in Singh et al. (1993).
Linear correlations between traits were estimated with Pearson's correlation coefficients (rp) on a genotypic-mean basis. Genetic correlations among traits (rg) were calculated from the variance-covariance matrices obtained from a MANOVA as rg = CovG(x,y)/√σ2G(x) × σ2G(y) where CovG(x, y) is the genetic covariance between traits x and y estimated by equating the mean co-products with their expected values according to the Henderson III procedure (Becker, 1984).
RESULTS
Parental variability
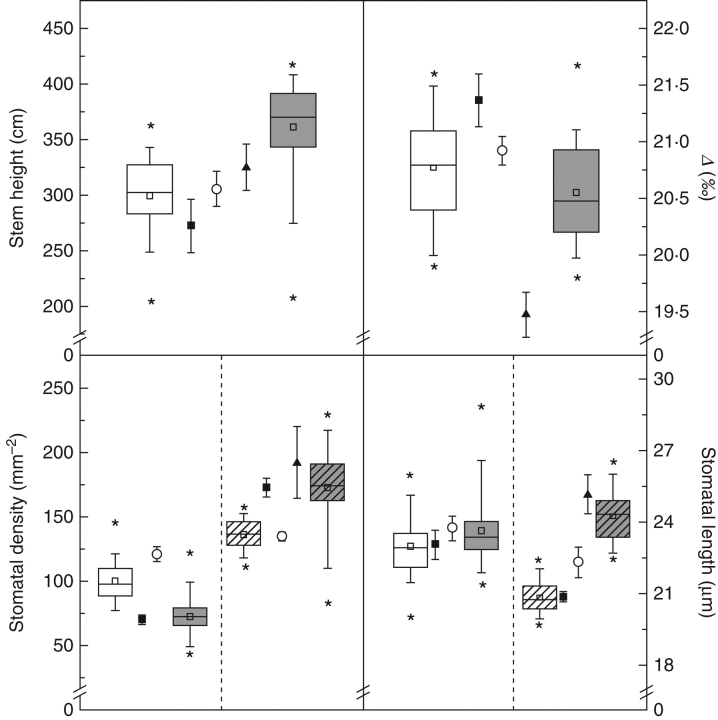
Stomatal densities and ratios differed significantly among parents of both families (Fig. 2 and Table 1). Stomata were absent, or nearly so, from the adaxial leaf surface of the P. trichocarpa ‘V24’ parent. In contrast, abaxial stomatal density of P. trichocarpa ‘V24’ was greater than other parents resulting in a significantly smaller ratio of adaxial : abaxial stomatal density compared with the P. deltoides ‘S9-2’ and P. nigra ‘Ghoy’ parents which were amphistomatous (Fig. 2 and Table 1). Populus deltoides ‘S9-2’ combined a relatively large adaxial with a relatively small abaxial density (118 cf. 134 stomata mm−2), while P. nigra ‘Ghoy’ displayed intermediate values for both ad- and abaxial stomatal densities (69 cf. 172 stomata mm−2). Thus, a relatively large ratio of adaxial : abaxial density occurred in P. deltoides ‘S9-2’, and an intermediate ratio in P. nigra ‘Ghoy’. Stomata on the abaxial leaf surface were significantly larger for P. trichocarpa ‘V24’ than for P. nigra ‘Ghoy’ (Table 1).
Fig. 2.
Stem height (cm), carbon isotope discrimination (Δ, ‰) and stomatal traits [stomatal density (mm−2) and length (μm) at the adaxial (empty) and abaxial (hatched) leaf surface] for selected F1 genotypes of the D × N (deltoides × nigra; white, 50 F1) and D × T (deltoides × trichocarpa; grey, 50 F1) families. The box plots are based on genotypic means (open squares) and are divided at the median; the height of the box represents the interquartile range. Parental means (± s.e.) for considered traits are indicated by the symbols as follows: Populus deltoides ‘S9-2’ (open circles), P. nigra ‘Ghoy’ (closed squares), P. trichocarpa ‘V24’ (closed triangles).
Table 1.
General means (± s.e.) and levels of significance of differences among parents for stomatal traits (adaxial, abaxial and total stomatal densities, adaxial and abaxial stomatal lengths, and ratio adaxial : abaxial densities) and carbon isotope discrimination (Δ) for Populus deltoides ‘S9-2’, P. nigra ‘Ghoy’ and P. trichocarpa ‘V24’
| P. nigra ‘Ghoy’ | P. deltoides ‘S9-2’ | P. trichocarpa ‘V24’ | F-test for differences among parents | |
|---|---|---|---|---|
| Stomatal traits | ||||
| Adaxial density (mm−2) | 69 ± 4a | 118 ± 5b | 0 | *** |
| Abaxial density (mm−2) | 172 ± 7b | 134 ± 3a | 193 ± 27b | ** |
| Total density (mm−2) | 241 ± 9ab | 252 ± 7b | 193 ± 27a | * |
| Ratio | 0·40 ± 0·02a | 0·88 ± 0·04b | 0 | *** |
| Adaxial length (μm) | 23·0 ± 0·6a | 23·8 ± 0·5a | n.s. | |
| Abaxial length (μm) | 20·8 ± 0·2a | 22·2 ± 0·6ab | 25·0 ± 0·8b | * |
| Δ (‰) | 21·4 ± 0·24b | 20·9 ± 0·13b | 19·5 ± 0·21a | ** |
Significant differences between parents are indicated by different letters and *** = P ≤ 0·001; ** = P ≤ 0·01; * = P ≤ 0·05; n.s. = non-significant.
Furthermore, P. trichocarpa ‘V24’ was characterized by a significantly smaller Δ compared with the P. nigra ‘Ghoy’ and the P. deltoides ‘S9-2’ parents suggesting that P. trichocarpa ‘V24’ was most efficient in its water use (Fig. 2 and Table 1).
Interfamily variability
All hybrids were amphistomatous with a larger stomatal density on the abaxial surface than on the adaxial. Significant differences between means of the two hybrid families occurred in all traits (P ≤ 0·001) (Table 2). On the adaxial surface, stomatal density was largest for the D × N family, while on the abaxial surface, the D × T family had most stomata per unit area. This resulted in a higher ratio of adaxial : abaxial stomatal densities for the D × N family than for the D × T family (Fig. 2 and Table 2). The total number of stomata per unit area on both leaf surfaces was slightly, but significantly, higher for the D × T family than for the D × N family. The D × N family had the smallest stomata on both leaf surfaces.
Table 2.
Family means and genotypic range of stomatal traits (adaxial, abaxial and total stomatal densities, adaxial and abaxial stomatal lengths, and ratio adaxial : abaxial densities) and carbon isotope discrimination (Δ) of the selected F1 genotypes of the D × N (50 F1) and the D × T (50 F1) families
| D × N family |
D × T family |
||||
|---|---|---|---|---|---|
| Mean (± s.e.) | Genotypic range | Mean (± s.e.) | Genotypic range | F-test for differences between families (D × N vs. D × T)* | |
| Stomatal traits | |||||
| Adaxial density (mm−2) | 99 ± 1 | 76–146 | 75 ± 1 | 47–124 | > |
| Abaxial density (mm−2) | 137 ± 1 | 112–157 | 173 ± 3 | 82–230 | < |
| Total density (mm−2) | 235 ± 2 | 198–286 | 250 ± 3 | 152–345 | < |
| Ratio | 0·72 ± 0·01 | 0·59–0·95 | 0·43 ± 0·01 | 0·31–0·67 | > |
| Adaxial length (μm) | 23·0 ± 0·1 | 20·0–26·0 | 23·8 ± 0·1 | 21·5–29·0 | < |
| Abaxial length (μm) | 21·0 ± 0·1 | 19·9–22·6 | 24·2 ± 0·1 | 22·5–26·6 | < |
| Δ (‰) | 20·79 ± 0·04 | 19·89–21·60 | 20·54 ± 0·04 | 19·81–21·69 | > |
* D = Populus deltoides ‘S9-2’; N = P. nigra ‘Ghoy’; T = P. trichocarpa ‘V24’. All differences between families were significant at P ≤ 0·001.
The D × N family showed a significantly larger Δ than the D × T family (Fig. 2 and Table 2). Thus, D × N hybrids appeared to have a lower WUEi than D × T hybrids.
Intrafamily variability
The genetic variability of stomatal traits was larger in the D × T family than in the D × N family, except for adaxial stomatal density and for the ratio adaxial : abaxial stomatal densities (Fig. 2 and Table 2). Moderate to high H2 were obtained for both families (Table 3). The highest heritability was for Δ of the D × N family (H2 = 0·61), and for adaxial and abaxial lengths of the D × T family (H2 = 0·77 and 0·67, respectively).
Table 3.
Broad-sense heritabilities (± s.e.) on an individual basis (H2) for stomatal traits (adaxial, abaxial and total stomatal densities, adaxial and abaxial stomatal lengths, and ratio adaxial : abaxial densities) and carbon isotope discrimination (Δ) of the selected F1 genotypes of the D × N and the D × T families (both 50 F1)
| D × N family* | D × T family* | |
|---|---|---|
| Stomatal traits | ||
| Adaxial density (mm−2) | 0·53 ± 0·07 | 0·49 ± 0·07 |
| Abaxial density (mm−2) | 0·28 ± 0·07 | 0·62 ± 0·06 |
| Total density (mm−2) | 0·37 ± 0·07 | 0·59 ± 0·06 |
| Ratio | 0·50 ± 0·07 | 0·37 ± 0·07 |
| Adaxial length (μm) | 0·46 ± 0·07 | 0·77 ± 0·04 |
| Abaxial length (μm) | 0·19 ± 0·06 | 0·67 ± 0·05 |
| Δ (‰) | 0·61 ± 0·06 | 0·37 ± 0·07 |
* D = Populus deltoides ‘S9-2’; N = P. nigra ‘Ghoy’; T = P. trichocarpa ‘V24’.
Relationships among traits
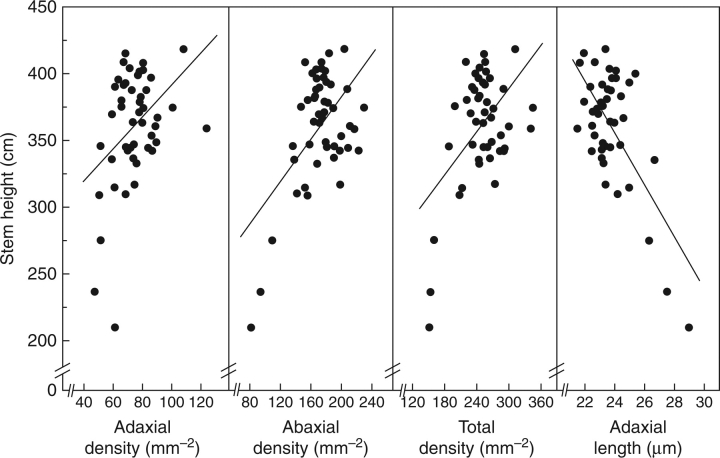
For the D × T family only, small but significant positive correlations occurred between stem height and stomatal densities (ad- and abaxial as well as total stomatal densities). Adaxial stomatal length showed a negative correlation with stem height (Fig. 3 and Table 4). The relationship between stomatal density and length on the same leaf surface was significant and negative in each family (Fig. 4 and Table 4). Weak but significant, positive, correlation between stem circumference and Δ was obtained for the D × N family only (rp = 0·31). For the D × T family, however, small but significantly negative correlations were found between Δ and abaxial, as well as total stomatal densities; Δ was also slightly associated with adaxial stomatal length. Strong genetic correlations were observed between stem dimensions (rg ranging from 0·87 to 0·92) and between several stomatal characteristics (rg ranging from 0·63 to 0·85; Table 5).
Fig. 3.
Relationships between stem height at the end of the first growing year of the second rotation and stomatal traits (adaxial, abaxial and total stomatal density, and adaxial stomatal length) during the first growing season of the third rotation on a genotypic-mean basis for 50 selected F1 genotypes of the D × T (deltoides × trichocarpa) family. Lines of best fit are indicated; corresponding Pearson's coefficients (rp) are shown in Table 4.
Table 4.
Pearson's correlation coefficients (rp) calculated from genotypic means between stem dimensions (stem circumference and height) and stomatal traits, and carbon isotope discrimination (Δ), of the selected F1 genotypes of the D × N (deltoides × nigra, above the diagonal) and the D × T (deltoides × trichocarpa, below the diagonal) families (both 50 F1)
| Stem circumference | Stem height | Adaxial density | Abaxial density | Total density | Ratio | Adaxial length | Abaxial length | Δ | |
|---|---|---|---|---|---|---|---|---|---|
| Stem dimensions | |||||||||
| Stem circumference | – | 0·88*** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0·31* |
| Stem height | 0·90*** | – | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Stomatal traits | |||||||||
| Adaxial density | n.s. | 0·40** | – | 0·63*** | 0·89*** | 0·79*** | −0·44** | −0·37** | n.s. |
| Abaxial density | 0·35* | 0·54*** | 0·69*** | – | 0·89*** | n.s. | n.s. | −0·36* | n.s. |
| Total density | 0·29* | 0·50*** | 0·84*** | 0·95*** | – | 0·46** | −0·31* | −0·42** | n.s. |
| Ratio | n.s. | −0·28* | 0·41** | −0·35* | − | −0·47*** | n.s. | n.s. | |
| Adaxial length | −0·53*** | −0·64*** | −0·49*** | −0·76*** | 0·65*** | 0·39** | − | 0·55*** | n.s. |
| Abaxial length | n.s. | n.s. | −0·45*** | −0·52*** | −0·49*** | n.s. | 0·59*** | – | n.s. |
| Δ | |||||||||
| n.s. | n.s. | n.s. | −0·30* | −0·27* | n.s. | 0·36** | n.s. | – |
Significant correlations are indicated as: *** = P ≤ 0·001; ** = P ≤ 0·01; * = P ≤ 0·05; n.s. = non-significant.
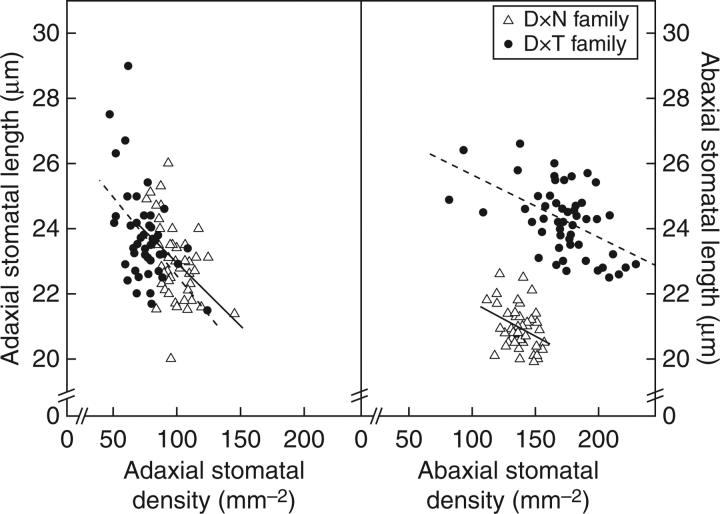
Fig. 4.
Relationships between stomatal length and density for both leaf surfaces (adaxial and abaxial) on a genotypic-mean basis for the selected F1 genotypes of the D × N (deltoides × nigra) and the D × T (deltoides × trichocarpa) families (both 50 F1). Lines of best fit are shown (full lines for D × N and dotted lines for D × T). See Table 4 for the corresponding Pearson's coefficients (rp).
Table 5.
Genetic correlations (rg) between stem dimensions (stem circumference and height), stomatal traits (adaxial, abaxial and total stomatal densities, adaxial and abaxial stomatal lengths, and ratio adaxial/abaxial densities) and carbon isotope discrimination (Δ) for the selected F1 genotypes of the D × N (deltoides × nigra, above the diagonal) and the D × T (deltoides × trichocarpa, below the diagonal) families (both 50 F1)
| Stem circumference | Stem height | Adaxial density | Abaxial density | Ratio | Adaxial length | Abaxial length | Δ | |
|---|---|---|---|---|---|---|---|---|
| Stem dimensions | ||||||||
| Stem circumference | – | 0·92 | −0·12 | −0·17 | −0·06 | 0·24 | 0·06 | 0·36 |
| Stem height | 0·87 | – | −0·06 | −0·15 | 0·02 | 0·17 | −0·15 | 0·09 |
| Stomatal traits | ||||||||
| Adaxial density | 0·03 | 0·24 | – | 0·63 | 0·85 | −0·35 | −0·49 | −0·24 |
| Abaxial density | –0·31 | 0·01 | 0·75 | − | 0·12 | 0·14 | −0·17 | −0·25 |
| Ratio | 0·33 | 0·32 | 0·78 | 0·17 | – | −0·54 | −0·52 | −0·14 |
| Adaxial length | −0·03 | −0·23 | −0·44 | −0·50 | −0·16 | − | 0·81 | 0·33 |
| Abaxial length | −0·27 | −0·36 | −0·27 | −0·51 | 0·11 | 0·71 | − | 0·23 |
| Δ | ||||||||
| 0·09 | −0·11 | 0·01 | −0·18 | 0·18 | 0·33 | 0·44 | – |
DISCUSSION
Phenotypic variability
In line with previous field studies, very few or no stomata were observed on the adaxial leaf surface of P. trichocarpa (i.e. hypostomatous leaves) while many large stomata occur on the abaxial surface (Ceulemans et al., 1988; Ferris et al., 2002; Al Afas et al., 2005), but in contrast, under greenhouse conditions, low adaxial stomatal densities have been observed (Braatne et al., 1992; Radoglou and Jarvis, 1990). Leaves of P. deltoides had more stomata than P. trichocarpa in agreement with Al Afas et al. (2005) and Pearce et al. (2005). Higher densities were often associated with higher stomatal conductance, which is in turn related to lower WUE (Drew and Chapman, 1992; Hetherington and Woodward, 2003; Pearce et al., 2005; Monclus et al., 2006). Indeed, P. deltoides displayed higher values of Δ, and thus a lower WUEi, as compared with P. trichocarpa. However, a higher conductance is not necessarily disadvantageous and could be an adaptive benefit in drier conditions through cooling of the leaves and rapid CO2 uptake (Pearce et al., 2005). Data concerning P. nigra are rare in literature: in this study, its leaves displayed small stomata and a high total stomatal density and, similar to P. deltoides, this parent was less water-use efficient than P. trichocarpa, as suggested by significantly higher Δ values.
Stomatal traits of the F1 hybrids appeared to be a combination of the stomatal traits from their parents. The D × T family had significantly larger stomata and a higher total stomatal density than the D × N family. However, the distribution of the stomata over the two leaf surfaces differed greatly, as the ratio adaxial : abaxial stomatal densities was much smaller for the D × T than for the D × N family (0·43 and 0·72, respectively). For both families, the genotypic ranges for Δ were comparable to the ranges found by Monclus et al. (2005) for 31 P. × euramericana clones, suggesting that the D × T family might be representative of the variability in terms of Δ (and WUEi) within P. × euramericana hybrids. Highly significant differences in Δ were observed between both hybrid families; the D × N hybrids were less efficient in their water use than the D × T hybrids. Thus, at family level, a higher total stomatal density for the D × T family did not lead to higher Δ or lower WUEi as often observed (Abrams, 1994; Pearce et al., 2005; Monclus et al., 2006). This study confirmed that differences in Δ (as a measure of WUEi) among poplar clones could not be adequately explained by any single variable such as stomatal density or size. Blake et al. (1984) hypothesized that differences in WUEi are best explained by different combinations of variables such as total stomatal density, ad- or abaxial stomatal density, stomatal responsiveness, stomatal dimensions, net photosynthesis and the presence of trichomes on the leaf surfaces, and that it is difficult to define the relative contribution of each of these variables. Both kinds of F1 hybrids appeared to have intermediate Δ compared with the two parents, and did not display high heterosis for this trait. Generally, the response of WUEi of the interspecific hybrids under similar conditions ranged from intermediacy to transgressive segregation, i.e. phenotypes that exceed those of the parental lines (Schulte et al., 1987; Hinckley et al., 1989, 1992; Braatne et al., 1992; de Vincente and Tanksley, 1993).
Leaf characterisics such as stomatal traits (Al Afas et al., 2005) and Δ (Livingston et al., 1998; Duursma and Marshall, 2006) often differ in relation to position of leaves in the canopy. Consequently, the position and number of leaves sampled should be well considered when comparing genotypes or species. In the present case, however, only leaves fully exposed to sun and from similar ranks on the stem were collected.
Genetic variability
Broad-sense heritability was generally high, indicating a large genetic variability for stomatal traits (Orlović et al., 1998; Al Afas et al., 2005; Monclus et al., 2006) as well as for Δ (Ponton et al., 2001; Lauteri et al., 2004; Monclus et al., 2005, 2006) and a low sensitivity to micro-environmental differences in the two families. However, heritabilities vary with environmental sampling and substantial care should be taken when extrapolating results beyond the environment in which they were obtained (Lynch and Walsh, 1998). For Δ and various earlier studied traits (i.e. tree dimensions, canopy architecture and leaf characteristics), values of heritability were mostly higher for the D × N family than for the D × T family (Marron et al., 2006, 2007). For stomatal traits, however, no clear trend was observed between families. For abaxial density and length, larger genetic variability was reported for the D × T family than for the D × N family due to the considerable differences in stomatal traits between the two parents of the D × T family, P. trichocarpa and P. deltoides.
With regard to the second aspect of the definition of a valuable determinant [traits have to be (a) closely correlated, (b) heritable and (c) environmentally stable; Tuberosa et al. 2002], the high values of heritability highlight the potential of using stomatal traits as selection criteria for genotypic discrimination of more productive or more water-use efficient trees in the genus (Radoglou and Jarvis, 1990; Tognetti et al., 2004; Al Afas et al., 2005; Monclus et al., 2006).
Relationships among traits
Weakly positive relationship between Δ and stem circumference (rp = 0·31) was observed for the D × N family only: faster plant growth was moderately associated with high Δ and, consequently, with lower WUEi. The weak correlation between Δ and plant growth observed for the D × N family, as well as the absence of a correlation for the D × T family, highlights the potential to select for genotypes which combine high productivity with a low Δ or high WUEi (Monclus et al., 2005, 2006). Due to the absence or weakness of the correlation between Δ and stem circumference, the measure of productivity, some genotypes of both families do combine good growth with low Δ under sufficient water availability. In this case, however, no extrapolation can be made from one trait to deduce the other one; all genotypes need to be measured separately in order to select those which simultaneously display high productivity and low Δ (or large WUEi).
Stomatal density and length were inversely correlated for both families as observed earlier (Hetherington and Woodward, 2003; Pearce et al., 2005). Among the D × T hybrids only, a poor and negative correlation was observed between Δ and abaxial as well as total stomatal density. In addition, Δ was positively associated with adaxial stomatal length implying that D × T genotypes with smaller but more stomata were more efficient in their water use. This contradicts the previous results for parents showing once more that differences in WUEi cannot be explained by one single variable. Genetic correlations between stomatal traits and plant growth as well as with Δ were weak, highlighting that indirect selection for high productivity, respectively, high water-use efficiency using stomatal traits could be of limited value as compared with growth, tree architecture or other leaf traits in both families (Marron et al., 2006, 2007). Improved understanding is necessary to estimate their potential as physiological selection criteria in breeding programmes.
To conclude, (a) this study has demonstrated a large genetic variability in stomatal traits and Δ within, as well as between, related poplar families; (b) direct relationships between plant growth and these traits, however, were weak confirming the absence of a tight link between productivity and WUEi for P. × euramericana as well as P. × interamericana hybrids grown in similar conditions; (c) stomatal traits are poor selection criteria for genotypes with good growth and large WUEi.
ACKNOWLEDGEMENTS
All plant materials were kindly provided by Marijke Steenackers (INBO, Geraardsbergen, Belgium). We gratefully acknowledge Dr Catherine Bastien and the INRA staff (Orléans, France) for logistic help during the measuring period as well as Prof. Louis Beyens and Dr Pieter Ledeganck (University of Antwerp, Belgium) for the use of the microscope infrastructure. We also thank Dr Didier Le Thiec (INRA Nancy, France) and two anonymous reviewers for useful comments on an earlier version of the manuscript. S. Y. Dillen is a Research Assistant of the Fund for Scientific Research-Flanders (F.W.O.-Vlaanderen, Belgium).
LITERATURE CITED
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Abrams MD. Genotypic and phenotypic variation as stress adaptations in temperate tree species: a review of several case studies. Tree Physiology. 1994;14:833–842. doi: 10.1093/treephys/14.7-8-9.833. [DOI] [PubMed] [Google Scholar]
- Al Afas N, Marron N, Ceulemans R. Clonal variation in stomatal characteristics related to biomass production of 12 poplar (Populus) clones in a short rotation coppice culture. Environmental and Experimental Botany. 2005;58:279–286. [Google Scholar]
- Barigah TS, Saugier B, Mousseau M, Guittet J, Ceulemans R. Photosynthesis, leaf area and productivity of 5 poplar clones during their establishment year. Annals of Forest Science. 1994;51:613–625. [Google Scholar]
- Becker WA. Manual of quantitative genetics. Washington, DC: Pullman Academic Enterprises; 1984. [Google Scholar]
- Blake TJ, Tschaplinski TJ, Eastham A. Stomatal control of water use efficiency in poplar clones and hybrids. Canadian Journal of Botany. 1984;62:1344–1351. [Google Scholar]
- Braatne JH, Hinckley TM, Stettler RF. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, P. deltoides and their F1 hybrids. Tree Physiology. 1992;11:325–339. doi: 10.1093/treephys/11.4.325. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Jr, Ceulemans R, Davis J, Stettler R. Emerging model systems in plant biology: poplar (Populus) as a model forest tree. Journal of Plant Growth Regulation. 2000;19:306–313. [Google Scholar]
- Brendel O, Pot D, Plomion C, Rozenberg P, Guehl JM. Genetic parameters and QTL analysis of delta 13C and ring width in maritime pine. Plant, Cell and Environment. 2002;25:945–953. [Google Scholar]
- Brugnoli E, Farquhar GD. Photosynthesis fractionation of carbon isotopes. In: Leegood RC, Sharkey TD, Von Caemmerer S, editors. Photosynthesis: physiology and metabolism. Dordrecht. The Netherlands: Kluwer Academic Publishers; 2000. pp. 399–434. [Google Scholar]
- Cervera MT, Gusmão J, Steenackers M, Peleman J, Storme V, Vanden Broeck A, et al. Identification of AFLP molecular markers for resistance against Melampsora larici-populina in Populus. Theoretical Applied Genetics. 1996;93:733–737. doi: 10.1007/BF00224069. [DOI] [PubMed] [Google Scholar]
- Cervera MT, Storme V, Ivens B, Gusmão J, Liu BH, Hostyn V, et al. Dense genetic linkage maps of three Populus species (Populus deltoides, P. nigra and P. trichocarpa) based on AFLP and microsatellite markers. Genetics. 2001;158:787–809. doi: 10.1093/genetics/158.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans R, Deraedt W. Production physiology and growth potential of poplars under short rotation forestry culture. Forest Ecology and Management. 1999;121:9–23. [Google Scholar]
- Ceulemans R, Impens I, Steenackers V. Stomatal and anatomical leaf characteristics of 10 Populus clones. Canadian Journal of Botany. 1984;62:513–518. [Google Scholar]
- Ceulemans R, Impens I, Steenackers V. Variation in photosynthetic, anatomical and enzymatic leaf traits and correlations with growth in recently selected Populus hybrids. Canadian Journal of Forest Research. 1987;17:273–283. [Google Scholar]
- Ceulemans R, Impens I, Steenackers V. Genetic variation in aspects of leaf growth of Populus clones, using the leaf plastochron index. Canadian Journal of Forest Research. 1988;18:1069–1077. [Google Scholar]
- Ceulemans R, Van Praet L, Jiang XN. Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus) New Phytologist. 1995;131:99–107. doi: 10.1111/j.1469-8137.1995.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Improving intrinsic water-use efficiency and crop yield. Crop Science. 2002;42:122–131. doi: 10.2135/cropsci2002.1220. [DOI] [PubMed] [Google Scholar]
- Craig H. Isotopic standards for carbon and oxygen and correction factors for mass spectrometric analysis of carbon dioxide. Geochimica et Cosmochimica Acta. 1957;12:133–149. [Google Scholar]
- Dickmann DI, Stuart KW. The culture of poplars in eastern North America. East Lansing, MI: Michigan State University Publications; 1983. [Google Scholar]
- Dillen SY, Marron N, Bastien C, Ricciotti L, Salani F, Sabatti M, et al. Effects of environment and progeny on biomass estimations of five hybrid poplar families grown at three contrasting sites across Europe. Forest Ecology and Management. 2007;252:12–23. [Google Scholar]
- Drew AP, Chapman JA. Inheritance of temperature adaptation in intra- and inter-specific Populus crosses. Canadian Journal of Forest Research. 1992;22:62–67. [Google Scholar]
- Dunlap JM, Stettler RF. Variation in leaf epidermal and stomatal traits of Populus trichocarpa from two transects across the Washington Cascades. Canadian Journal of Botany. 2001;79:528–536. [Google Scholar]
- Duursma RA, Marshall JD. Vertical canopy gradients in δ13C correspond with leaf nitrogen content in a mixed-species conifer forest. Trees. 2006;20:496–506. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Ferris R, Long L, Bunn SM, Robinson KM, Bradshaw HD, Rae A, et al. Leaf stomatal and epidermal cell development: identification of putative quantitative trait loci in relation to elevated carbon dioxide in poplar. Tree Physiology. 2002;22:633–640. doi: 10.1093/treephys/22.9.633. [DOI] [PubMed] [Google Scholar]
- Guehl JM, Fort C, Ferhi A. Differential response of leaf conductance, carbon isotope discrimination and water use efficiency to nitrogen deficiency in maritime pine and penduculate oak plants. New Phytologist. 1995;131:149–157. [Google Scholar]
- Hanson WD. Heritability. In: Hanson WD, Robinson HF, editors. Statistical genetics and plant breeding. Washington, DC: National Academy of Sciences – National Research Council; 1963. pp. 125–140. Publication no. 982. [Google Scholar]
- Henderson CR. Estimation of variance and co-variance components. Biometrics. 1953;9:226–252. [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hinckley TM, Ceulemans R, Dunlap JM, Figliola A, Heilman PE, Isebrands JG, et al. Physiological, morphological and anatomical components of hybrid vigor in Populus. In: Kreeb KH, Richter H, Hinckley TM, editors. Structural and functional responses to environmental stresses. The Hague, The Netherlands: SPB Academic Publishers; 1989. pp. 199–217. [Google Scholar]
- Hinckley TM, Braatne JH, Bradshaw HD, Ceulemans R, Clum P, Dunlap J, et al. Growth dynamics and canopy structure of fast-growing trees. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW, editors. Plant physiological ecology: field methods and instrumentation. New York, NY: Chapman and Hall; 1992. pp. 1–34. [Google Scholar]
- Holland JB, Nyquist WE, Cervantes-Martinez CT. Estimating and interpreting heritability for plant breeding: an update. Plant Breeding Reviews. 2003;22:9–112. [Google Scholar]
- Lauteri M, Pliura A, Monteverdi C, Brugnoli E, Villani F, Eriksson G. Genetic variation in carbon isotope discrimination in six European plantations of Castanea sativa Mill. originating from contrasting localities. Journal of Evolutionary Biology. 2004;17:1286–1296. doi: 10.1111/j.1420-9101.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- Livingston NJ, Whitehead D, Kelliher FM, Wang YP, Grace JC, Walcroft AS, et al. Nitrogen allocation and carbon isotope fractionation in relation to intercepted radiation and position in a young Pinus radiata D. Don. tree. Plant, Cell and Environment. 1998;21:795–803. [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer; 1998. pp. 170–176. [Google Scholar]
- Marron N, Villar M, Dreyer E, Delay D, Boudouresque E, Petit J-M, et al. Diversity of leaf traits related to productivity in 31 Populus deltoides × Populus nigra clones. Tree Physiology. 2005;25:425–435. doi: 10.1093/treephys/25.4.425. [DOI] [PubMed] [Google Scholar]
- Marron N, Bastien C, Sabatti M, Taylor G, Ceulemans R. Plasticity of growth and sylleptic branchiness in two poplar families grown in three sites across Europe. Tree Physiology. 2006;26:935–946. doi: 10.1093/treephys/26.7.935. [DOI] [PubMed] [Google Scholar]
- Marron N, Dillen SY, Ceulemans R. Evaluation of leaf traits for indirect selection of high yielding poplar hybrids. Environmental and Experimental Botany. 2007;61:103–116. [Google Scholar]
- Monclus R, Dreyer E, Delmotte FM, Villar M, Delay D, Boudouresque E, et al. Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoides × P. nigra clones. New Phytologist. 2005;167:53–62. doi: 10.1111/j.1469-8137.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit J-M, et al. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × P. nigra. New Phytologist. 2006;169:765–777. doi: 10.1111/j.1469-8137.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- Nyquist WE. Estimation of heritability and prediction of selection response in plant populations. Critical Reviews in Plant Sciences. 1991;10:235–322. [Google Scholar]
- Orlović S, Guzina V, Merkulov L. Genetic variability in anatomical, physiological and growth characteristics of hybrid poplar (Populus × euramericana Dode (Guinier)) and eastern cottonwood (Populus deltoides Bartr.) clones. Silvae Genetica. 1998;47:183–190. [Google Scholar]
- Pallardy SG, Kozlowski TT. Frequency and length of stomata of 21 Populus clones. Canadian Journal of Botany. 1979;57:2519–2523. [Google Scholar]
- Pearce DW, Millard S, Bray DF, Rood SB. Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiology. 2005;26:211–218. doi: 10.1093/treephys/26.2.211. [DOI] [PubMed] [Google Scholar]
- Ponton S, Dupouey J-L, Bréda N, Feuillat F, Bodénès C, Dreyer E. Carbon isotope discrimination and wood anatomy variations in mixed stands of Quercus robur and Quercus petraea. Plant, Cell and Environment. 2001;24:861–868. [Google Scholar]
- Radoglou KM, Jarvis PG. Effects of CO2 enrichment on four poplar clones. II. Leaf surface properties. Annals of Botany. 1990;65:627–632. [Google Scholar]
- Rae AM, Robinson KM, Street NR, Taylor G. Morphological and physiological traits influencing biomass productivity in short-rotation coppice poplar. Canadian Journal of Forest Research. 2004;34:1488–1489. [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science. 2002;42:739–745. [Google Scholar]
- Ripullone F, Lauteri M, Grassi G, Amato M, Borghetti M. Variation in nitrogen supply changes water-use efficiency of Pseudotsuga menziesii and Populus × euramericana; a comparison of three approaches to determine water-use efficiency. Tree Physiology. 2004;24:671–679. doi: 10.1093/treephys/24.6.671. [DOI] [PubMed] [Google Scholar]
- Schulte PJ, Hinckley TM, Stettler RF. Stomatal responses of Populus to leaf water potential. Canadian Journal of Botany. 1987;65:255–260. [Google Scholar]
- Singh M, Ceccarelli S, Hamblin J. Estimation of heritability from varietal trials data. Theoretical and Applied Genetics. 1993;86:437–441. doi: 10.1007/BF00838558. [DOI] [PubMed] [Google Scholar]
- Tognetti R, Sebastiani L, Minnocci A. Gas exchange and foliage characteristics of two poplar clones grown in soil amended with industrial waste. Tree Physiology. 2004;15:665–671. doi: 10.1093/treephys/24.1.75. [DOI] [PubMed] [Google Scholar]
- Tuberosa R, Salvi S, Sanguineti MC, Landi P, MacCaferri M, Conti S. Mapping QTLs regulating morpho-physiological traits and yield: case studies, shortcomings and perspectives in drought-stressed maize. Annals of Botany. 2002;89:941–963. doi: 10.1093/aob/mcf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke P, Moermans R, Mau F, Guittet J. Border effects and size inequality in experimental even-aged stands of poplar clones (Populus) Annals of Forest Science. 1995;52:193–200. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th edn. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- de Vincente MC, Tanksley SD. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics. 1993;134:585–596. doi: 10.1093/genetics/134.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Tigerstedt PMA, Vihera-Aarnio A. Photosynthesis and canopy characteristics in genetically defined families of silver birch (Betula pendula) Tree Physiology. 1995;15:665–671. doi: 10.1093/treephys/15.10.665. [DOI] [PubMed] [Google Scholar]
- Zavitkovski J. Small plots with unplanted plot border can distort data in biomass production studies. Canadian Journal of Forest Research. 1981;11:9–12. [Google Scholar]
- Zhang X, Zhang R, Li C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Science. 2004;166:791–797. [Google Scholar]