Abstract
Background and Aims
Although a claim has been made that dormancy cycling occurs in seeds of Ipomoea lacunosa (Convolvulaceae) with physical dormancy, this would seem to be impossible since the water gap cannot be closed again after it opens (dormancy break). On the other hand, changes in sensitivity (sensitive ↔ non-sensitive) to dormancy-breaking factors have been reported in seeds of Fabaceae with physical dormancy. The primary aim of the present study was to determine if sensitivity cycling also occurs in physically dormant seeds of I. lacunosa.
Methods
Treatments simulating conditions in the natural habitat of I. lacunosa were used to break seed dormancy. Storage of seeds at temperatures simulating those in spring, summer, autumn and winter were tested for their effect on sensitivity change. Seeds made non-dormant were stored dry in different temperature regimes to test for dormancy cycling. In addition, seeds collected on different dates (i.e. matured under different climatic conditions) were used to test for maternal effects on sensitivity to dormancy-breaking factors.
Key Results
Sensitivity was induced by storing seeds under wet conditions and reversed by storing them under dry conditions at low (≤5 °C) or high (≥30 °C) temperatures, demonstrating that seeds of I. lacunosa can cycle between sensitive and insensitive states. Sensitive seeds required ≥2 h at 35 °C on moist sand for release of dormancy. However, there is no evidence to support dormancy cycling per se. Conceptual models are proposed for sensitivity cycling and germination phenology of I. lacunosa in the field.
Conclusions
Seasonal germination behaviour of physically dormant I. lacunosa seeds can be explained by sensitivity cycling but not by dormancy cycling per se. Convolvulaceae is only the second of 16 families known to contain species with physical dormancy for which sensitivity cycling has been demonstrated.
Key words: Convolvulaceae, germination phenology, Ipomoea, physical dormancy, sensitivity cycling
INTRODUCTION
Cycling between dormancy (D) and non-dormancy (ND) is a common phenomenon in seeds with non-deep physiological dormancy (Baskin and Baskin, 1985, 1998), i.e. dormancy caused by low growth potential of the embryo (Baskin and Baskin, 2004). Courtney (1968), Schafer and Chilcote (1970) and Taylorson (1970) presented the first evidence for dormancy cycling by exhuming and testing germination at regular intervals of seed samples that had been buried in soil. Dormancy cycling allows seeds to germinate only in a particular season (Baskin and Baskin, 1985). If D seeds become ND and do not germinate due to lack of favourable environmental conditions (e.g. light), they re-enter dormancy, i.e. secondary dormancy (Baskin and Baskin, 1998).
Cyclic patterns of germinability have been reported for several legumes with water-impermeable seed coats, i.e. physical dormancy (PY) (Taylor and Revell, 1999; Van Assche et al., 2003; Taylor, 2005) and for Cuscuta campestris (Benvenuti et al., 2005). Benvenuti et al. (2005) reported dormancy cycling in the physically dormant seeds of C. campestris (Convolvulaceae). However, they did not say that re-entrance into dormancy of seeds that became non-dormant (water-permeable) during burial in soil from late September to late April was due to reclosing of the water gap. Instead, seeds of C. campestris became permeable during the first year via opening of the water gap and then completed the two annual dormancy cycles by cycling between non-deep physiological dormancy (PD) and ND. In other words, the 2 years of annual dormancy cycling were PY → ND → PD → ND → PD.
To explain changes in germinability in seeds of legumes, Taylor (1981, 2005), Taylor and Revell (1999) and Van Assche et al. (2003) suggested sensitivity cycling (sensitive ↔ non-sensitive), i.e. seeds cycle between the capacity to respond and not to respond to dormancy-breaking treatment. Taylor (1981, 2005) proposed a two-stage model for dormancy break in seeds of Trifolium subterraneum and of Ornithopus compressus. Thus, water-impermeable seeds must first be preconditioned to make them latent soft (but still impermeable) and then subjected to a dormancy-breaking treatment to make them soft (water-permeable). To date, sensitivity cycling in physically dormant seeds has been reported only in Fabaceae species.
The maternal environment can have an effect on seed dormancy and germination. Thus, seeds that mature under different environmental conditions may exhibit different intensities of dormancy (e.g. Roach and Wulff, 1987; Bench-Arnold, 2004). Most studies on maternal effects of seed dormancy have been done on species with PD (Baskin and Baskin, 1998), and only a relatively few such studies have been carried out on seeds with PY. Seeds of the desert annual legumes (Fabaceae) Ononis sicula (Evenari et al., 1966; Gutterman and Evenari, 1972; Gutterman, 1973, 1993) and Trigonella arabica (Gutterman, 1978, 1993), matured in different day lengths, had different proportions of hard seeds. The proportion of hard (water-impermeable) seeds of Trifolium alexandrinum (El Bagoury and Niyazi, 1973) and Vicia faba (El Bagoury, 1975) changed with fertilization regime. Degree of hardseededness in Trifolium subterraneum (Aiken, 1939; Quinlivan and Millington, 1962; Quinlivan, 1965, 1966; Collins, 1981) and in annual Medicago species (Fabaceae) (Taylor, 1996) differed with level of moisture stress.
Ipomoea lacunosa belongs to the Convolvulaceae, the only family in the evolutionary advanced asterid clade that produces seeds with PY (Baskin et al., 2000). It is a summer annual vine native to eastern USA (Gleason and Cronquist, 1991; Elmore et al., 1990; Abel and Austin, 1981). Seeds of I. lacunosa buried in soil can remain viable for at least 39 years (Toole and Brown, 1946). Thus, the species can form a long-lived persistent soil seed bank. Egley and Chandler (1978, 1983) and Gomes et al. (1978) have shown that manually scarified I. lacunosa seeds germinate to high percentages, whereas non-scarified seeds germinate to much lower percentages. These studies suggest, but do not prove (see Baskin et al., 2006), that I. lacunosa seeds have PY. However, Jayasuriya et al. (2007) demonstrated conclusively that seeds of I. lacunosa have PY by showing that manually scarified seeds, as well as those in which the water gap was made to open by subjecting them to high temperature under wet conditions, took up (imbibed) water, whereas intact seeds did not. However, no complete study has been done on natural dormancy break in seeds of I. lacunosa. Ipomoea lacunosa is a troublesome weed in agricultural crops, especially in corn, cotton (Anonymous, 1995), soybeans (Anonymous, 2000) and rice (Anonymous, 2001). In Mississippi, Missouri, Arkansas and Louisiana, it has been listed as one of the ten most troublesome weeds and as one of the ten most common ones (Anonymous, 2001).
The present study had five objectives: (1) describe the germination phenology of I. lacunosa; (2) identify the natural seed dormancy-breaking mechanism; (3) determine if seeds cycle between sensitive and insensitive states and/or between PY and ND; (4) determine if there is a maternal effect on seed dormancy breaking and germination requirements; and (5) prepare conceptual models for sensitivity cycling and seed germination phenology in the field.
MATERIALS AND METHODS
Collection of seeds
Seeds were collected from numerous plants of Ipomoea lacunosa in a corn field at Spindletop Farm, University of Kentucky, Lexington, Kentucky, on 5 October 2005 (2005C1), 2 December 2005 (2005C2), 25 September 2006 (2006C1), 6 October 2006 (2006C2), 22 October 2006 (2006C3) and 3 November 2006 (2006C4). Each collection of seeds was stored separately in plastic bottles under ambient room conditions [22–24 °C, 50–60 % relative humidity (RH)] until used. The first experiments were started within 1 week after seeds were collected.
Germination of non-treated and of scarified seeds
Three replicates of 25 intact and of 25 manually scarified (individually with a scalpel) seeds of 2005C1, 2005C2 and 2006C2 were tested for germination at 35/20, 30/15, 25/15, 20/10 and 15/6 °C (14/10 h) in both light/dark (12/12 h) and dark regimes. Germination of 2006C1, 2006C3 and 2006C4 was tested only at 35/20, 25/15 and 15/6 °C. Seeds were incubated on wet sand in Petri dishes. Light was provided by cool white fluorescent tubes (approx. 40 µmol m−2 s−1, 400–700 nm) and darkness by wrapping Petri dishes with aluminium foil. Light/dark treatments were checked for germination at 3-d intervals, while germination of the dark treatments was checked only after 14 d, i.e. at end of incubation period. Radicle emergence was the criterion for germination.
Dormancy-breaking treatments
Six samples containing three replicates of 25 seeds each from 2005C2 were incubated at 35 °C on both wet sand and dry sand for 1, 2, 3, 6, 9 and 12 h. After the incubation period, seeds were transferred to wet sand in Petri dishes and tested for germination at the 25/15 °C light/dark regime. Four samples containing three replicates of 25 seeds each of 2005C2 were also incubated at 30, 25, 20 and 15 °C on wet sand for 3 h and then transferred to the 25/15 °C temperature regime. Three replicates of 25 seeds from other collections were stored separately at ambient laboratory conditions on wet sand for 2 months. These pre-treated samples were incubated at 35 °C on dry sand and on wet sand for 3 h, after which seeds were incubated on wet sand in the 25/15 °C light/dark regime for 30 d.
Germination requirements following dormancy break
Five samples of three replicates each of 25 non-dormant seeds (made non-dormant by incubation at 35 °C in >95 % RH for 3 h followed by 2 months wet storage at laboratory temperatures) from each collection were incubated at the 35/20, 30/15, 25/15, 20/10 and 15/6 °C temperature regimes in light/dark and in dark conditions. Germinated seeds in light/dark treatments were counted at 2-d intervals for 14 d, whereas those in the dark treatments were counted only at the end of the 2-week dark incubation period.
Effect of RH on dormancy break
RH conditions were provided by distilled water and saturated salt solutions. Six samples of three replicates each of 25 seeds from 2005C2 were incubated in sealed Petri dishes at 35 °C for 3 h at 100 (distilled water), 97 (K2SO4), 83 (KCl), 75 (NaCl), 65 (ambient in incubator) and 50 [Mg(NO3)2] % RH. After incubation, seeds were transferred to wet sand in Petri dishes and tested for germination at 25/15 °C in light/dark.
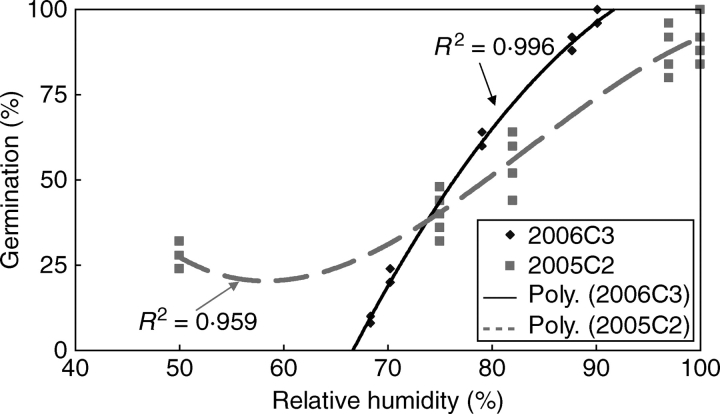
Five samples of three replicates of 25 2-month-wet-stored (at ambient laboratory temperatures) seeds each of 2006C3 were incubated in sealed Petri dishes at 35 °C for 3 h at 100 (distilled water), 97 (K2SO4), 83 (KCl), 75 (NaCl) and 65 (ambient in incubator) % RH. RH and temperature inside the container with seeds during incubation were monitored using sensors (Hygrochron iButtons, Embedded Data Systems, Lawrenceburg, KY, USA). Mean RH values (calculated from the data obtained by the sensor) during incubation were used in data analyses and in Fig. 6.
Fig. 6.
Germination of innately sensitive 2005C2 seeds and of induced-sensitive 2006C3 seeds of I. lacunosa at 25/15 °C following incubation at 35 °C for 3 h at different levels of RH.
Imbibition of seeds
Manually scarified 2005C2 seeds, non-treated 2005C2 seeds, 2005C2 seeds previously incubated 3-h wet and 2005C2 seeds previously incubated 3-h dry at 35 °C were tested for water uptake (imbibition) at ambient laboratory conditions. Samples of 25 seeds were weighed individually and placed separately on moistened filter paper in Petri dishes. At 1-h intervals for 16 h, seeds were taken from the dishes, blotted to remove peripheral water, re-weighed and returned to the moist filter paper. Water uptake was monitored until all seeds were fully imbibed.
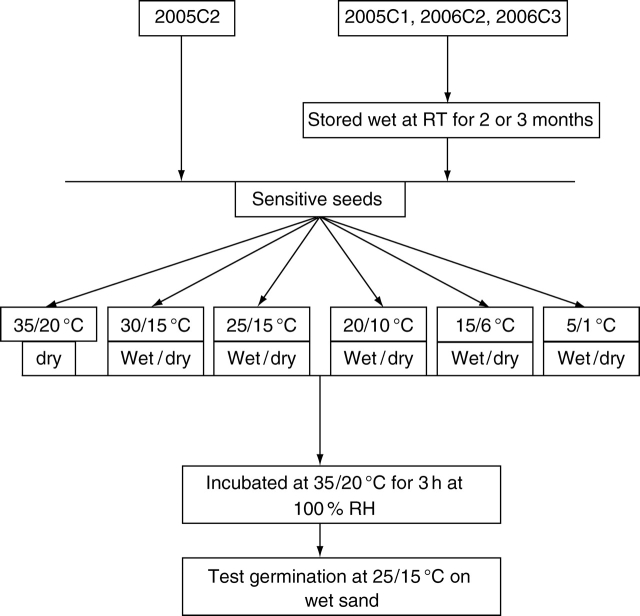
Sensitivity of seeds
The procedure (flow diagram) for this experiment is shown in Fig. 1. Twelve samples of three replicates of 25 seeds each of 2005C1, 2006C1 and 2006C3 were stored dry at 35/20 °C and both wet and dry at laboratory temperatures. Sixteen samples of three replicates of 25 seeds each of 2006C2 and 2006C4 were stored dry at 35/20 °C and both wet and dry at 15/6, 5/1 °C and laboratory temperatures. After storage for 1, 2, 3 and 6 months, seeds were tested for germination at 35/20 °C in light/dark. If a sample of a treatment germinated to 100 % at 35/20 °C, then another sample from the same treatment was tested at 25/15 °C, after seeds were incubated for 3 h at 35 °C on wet sand and without prior incubation.
Fig. 1.
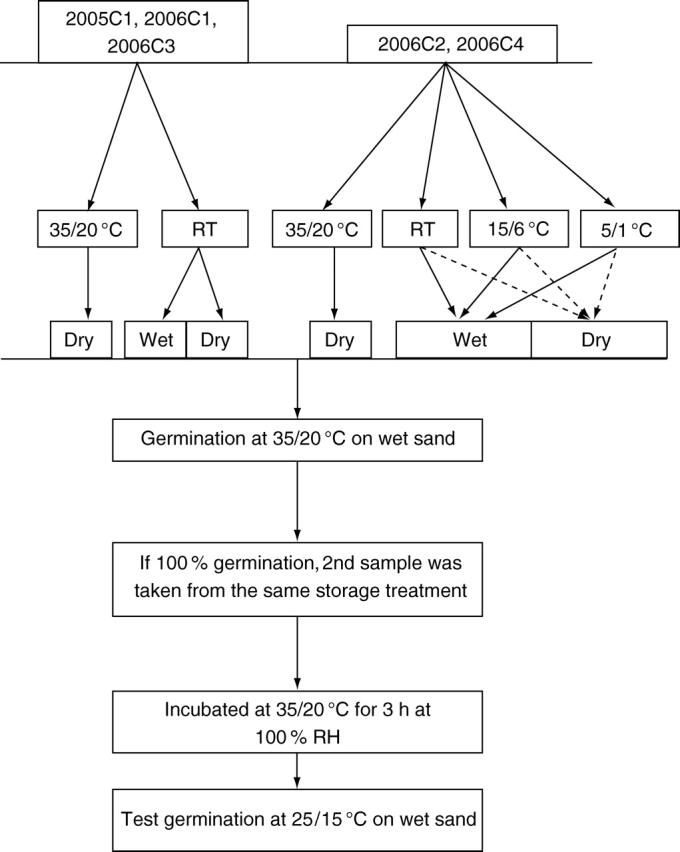
Outline of procedure for testing sensitivity of seeds of I. lacunosa. RT, Ambient room temperature.
Reversing the sensitivity of sensitive seeds
The procedure (flow diagram) for this experiment is shown in Fig. 2. Three samples each of three replicates of 25 (1) 2005C2 (non-treated) seeds, (2) 2-month-wet-stored (at ambient laboratory temperatures) 2005C1 seeds, (3) 2-month-wet-stored 2006C3 seeds and (4) 3-month-wet-stored (at ambient laboratory temperatures) 2006C2 seeds were stored dry (only) at 35/20 °C and both wet and dry at 30/15, 25/15, 20/10, 15/6 and 5/1 °C. After storage for 1·5, 3 and 6 (2005 collections only) months, seeds were incubated at 35 °C on wet sand for 3 h and then tested for germination at 25/15 °C in light/dark.
Fig. 2.
Outline of procedure for testing for reversion of sensitive seeds of I. lacunosa to non-sensitive seeds. RT, Ambient room temperature.
Cycling of sensitivity
The procedure (flow diagram) for this experiment is shown in Fig. 3. Four samples of three replicates each of 25 2005C1 seeds, previously stored wet for 2 months at ambient laboratory temperature, were then stored dry at 5/1 °C for 9 months. Three samples of 2-month-wet-stored (at ambient laboratory temperature) 2006C3 seeds were stored dry at 35/20 °C for 1·5 months, and another three similar samples were stored at 5/1 °C for 1·5 months. Germination of these samples was tested at 25/15 °C in light/dark followed by incubation at 35 °C on wet sand for 3 h. One of the other samples from each of the two collections was rewet after dry storage and incubated under laboratory conditions for 3 months, after which it was incubated for 3 h on wet sand at 35 °C and then tested for germination at 25/15 °C in light/dark. The other sample from each collection was stored dry at ambient laboratory temperatures and then incubated under the same conditions as the first sample.
Fig. 3.
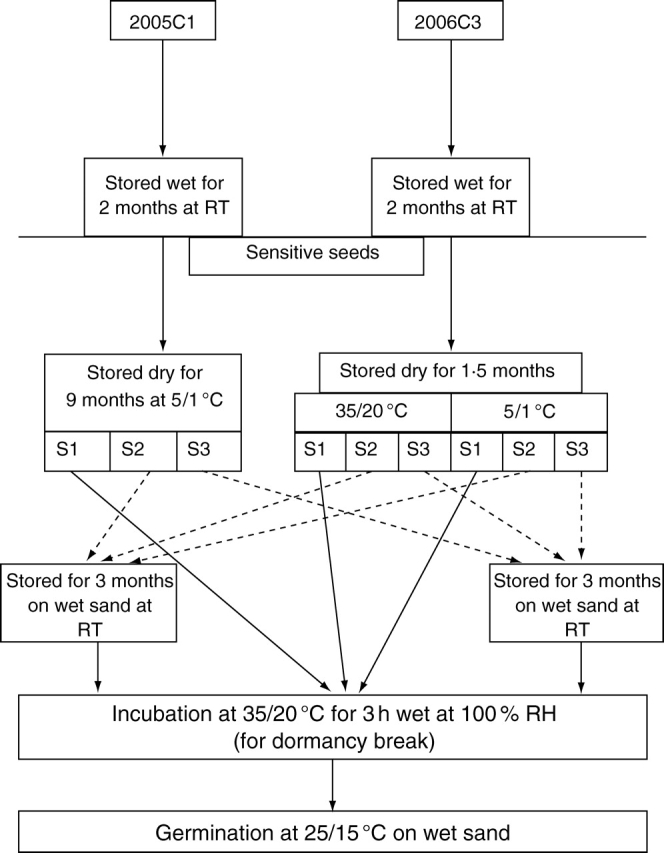
Outline of procedure for testing for sensitivity cycling in seeds of I. lacunosa. RT, Ambient room temperature.
Cycling of dormancy
Ten samples of three replicates each of 25 2005C2 seeds were incubated for 3 h on wet sand at 35 °C to break dormancy. Five samples then were stored dry at 35/20 °C. After 1, 2, 7, 14, 30, 90 and 180 d of storage, germination of one sample was tested at 25/15 °C in light/dark. The other five samples were stored dry at 35/20, 30/15, 25/15, 20/10 and 15/6 °C for 2 months, after which germination was tested at 25/15 °C in light/dark.
Maternal effects on sensitivity
Germination percentages of fresh seeds of different collections incubated at 35/20 °C for 7 d and the time required for seeds to become sensitive in wet storage at ambient laboratory temperature were used to measure the sensitivity of each collection. Temperature and rainfall data collected at the University of Kentucky Spindletop Farm during seed development were obtained from the University of Kentucky Climatic Data Collection Center.
Analysis of data
A completely randomized design was used in all experiments. Arcsine-transformed germination data were analysed using one-way ANOVA with SAS statistical software. Duncan's and Dunnet's mean separation procedures were used to compare treatments. To determine the effect of RH and of maternal effects on dormancy break, regression models were used with REG procedure.
RESULTS
Germination of non-treated and of scarified seeds
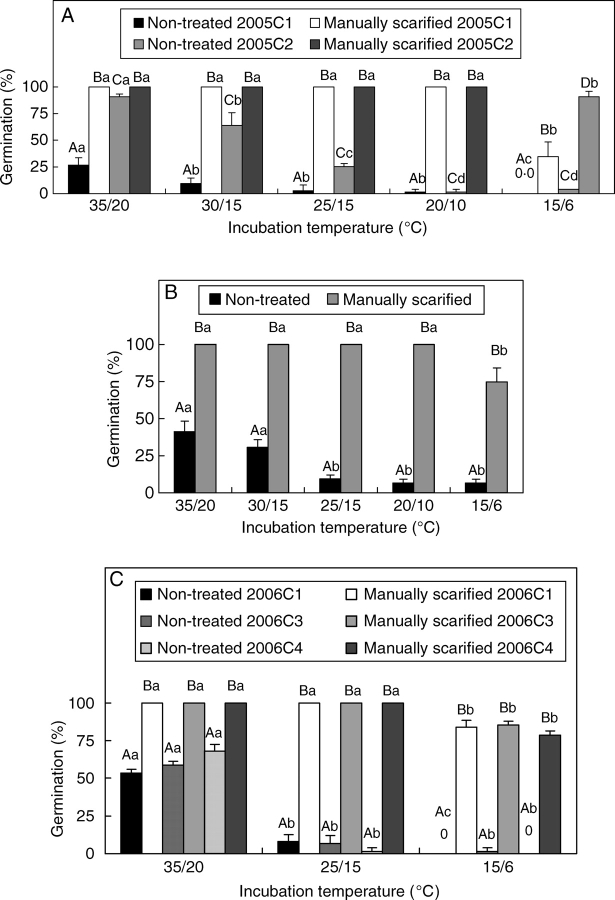
Scarified seeds of all collections germinated to >90 % at all temperature regimes tested except 15/6 °C (Fig. 4A–C). At 15/6 °C, all scarified seeds imbibed, but germination percentages were lower than those at the other temperature regimes. Non-treated 2005C2 seeds germinated to >90 % at 35/20 °C and to >60 % at 30/15 °C, whereas at the other temperature regimes they germinated to <25 % (Fig. 4A). Non-treated 2005C1 seeds germinated to <30 % at all temperature regimes. Germination of nontreated 2006C1, 2006C2, 2006C3 and 2006C4 seeds germinated to 52 %, 42 %, 63 % and 69 %, respectively, at 35/20 °C (Fig. 4B, C). At all other temperature regimes tested, seeds germinated to <25 %. There was no difference in germination percentages between light/dark and dark treatments (data not shown).
Fig. 4.
Germination at various temperature regimes of non-treated and of manually scarified seeds of I. lacunosa: (A) 2005C1 and 2005C2; (B) 2006C2; (C) 2006C1, 2006C3 and 2006C4. Different upper-case letters indicate significant differences between treatments within same temperature regimes and different lower-case letters significant differences between temperature regimes within the same treatment. Bars are +1 s.e.
Dormancy-breaking treatments
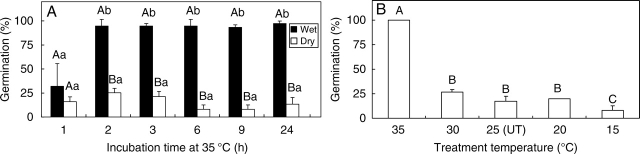
Seeds of 2005C2 incubated at 35 °C on dry sand germinated to <25 % at 25/15 °C regardless of incubation time (Fig. 5A). However, seeds incubated on wet sand at 35 °C for ≥2 h germinated to >95 % at 25/15 °C. Seeds treated at 30, 25, 20 and 15 °C did not germinate at 25/15 °C (Fig. 5B). Pre-treated (stored at ambient laboratory temperatures on wet sand for 2 months) 2005C1, 2006C1, 2006C2, 2006C3 and 2006C4 seeds also germinated to >95 % at 25/15 °C following incubation at 35 °C for 3 h on wet sand (data not shown). However, these seeds did not germinate at 25/15 °C without pre-treatment incubation at 35 °C on wet sand. Also, they did not germinate at 25/15 °C after incubation at 35 °C on dry sand.
Fig. 5.
Germination of I. lacunosa seeds of 2005 collection at 25/15 °C: (A) after incubation at 35 °C on wet and on dry sand for different periods of time, and (B) after incubation on wet sand at four temperatures for 3 h. UT, Untreated. Different upper-case letters indicate significant differences between treatments (wet or dry) within the same storage time and different lower-case letters significant differences between storage time within the same treatment (wet or dry). Bars are +1 s.e.
Germination requirements following dormancy break
Non-dormant seeds of all collections germinated at all of the temperature regimes in light/dark and in dark (data not shown). However, at 15/6 °C the germination rate was very slow; only after 45 d had 100 % of the seeds germinated at 15/6 °C (data not shown).
Effect of RH on dormancy break
Correlation between germination percentage (i.e. dormancy break) and RH in which seeds were incubated at 35 °C for 3 h was highly significant for fresh seeds of 2005C2 and for 2-month-wet-stored (at ambient laboratory temperature) seeds of 2006C3 (Fig. 6). Two-month-wet-stored (at ambient laboratory temperature) 2006C3 seeds were more sensitive to RH than those of 2005C2, with a dramatic increase in germination percentage occurring between 67 % and 90 % RH.
Imbibition of seeds
The mass of manually scarified seeds and of seeds incubated at 35 °C on wet sand for 3 h increased to >130 %, while non-treated intact seeds and seeds incubated at 35 °C on dry sand did not take up water at ambient laboratory temperatures (data not shown).
Sensitivity of seeds
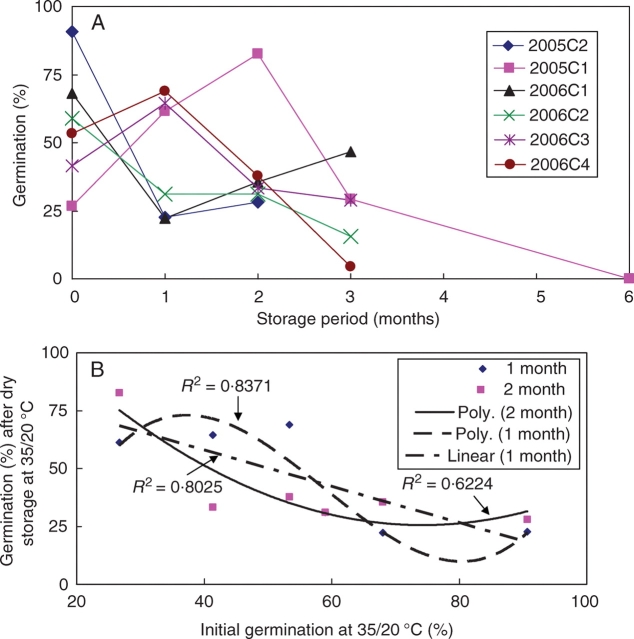
Seeds of 2005C1 germinated to 80 % at 35/20 °C after storage at ambient laboratory temperatures on wet sand for 1 month (Fig. 7A), but after 2 months of wet storage they germinated to >95 %. The germination percentage of 2005C1 seeds stored dry for 2 months at 35/20 °C increased gradually to 80 %, but germination of seeds stored 6 months decreased dramatically to 0%. The same trend was observed for 2006C1 seeds (Fig. 7B). Germination of 2005C1 seeds stored on dry sand at ambient laboratory temperatures decreased to 0 % after 1 month of storage. However, they gradually increased to 47 % after 3 months of storage, but then dropped to 25 % after storage for 6 months.
Fig. 7.
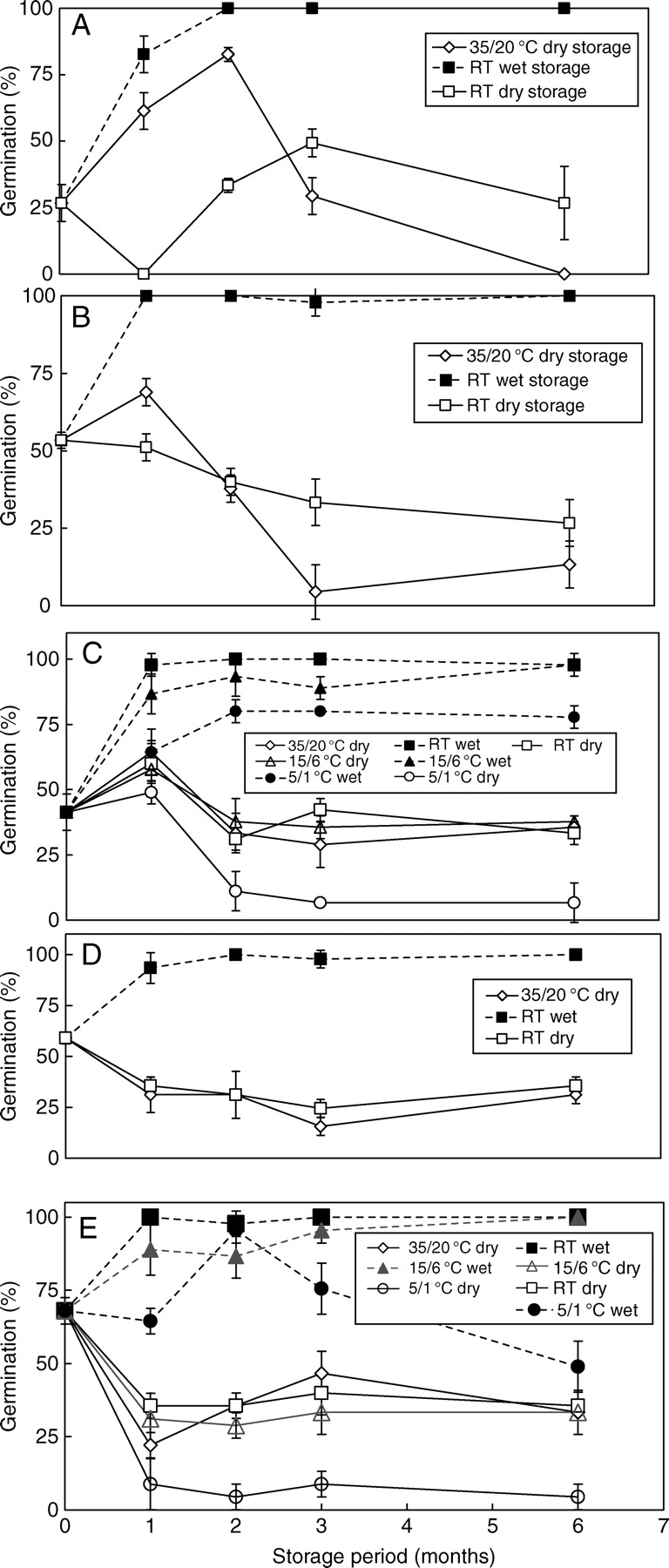
Germination of I. lacunosa seeds of (A) 2005C1, (B) 2006C1, (C) 2006C2, (D) 2006C3 and (E) 2006C4 at 35/20 °C following wet and dry storage: (A), (B) and (D) stored at 35/20 °C and ambient laboratory conditions only; (C) and (E) at 35/20 °C, ambient laboratory conditions, 15/6 °C and 5/1 °C. Bars are ±1 s.e.
Seeds of 2006C1, 2006C2, 2006C3 and 2006C4 stored on wet sand for >1 month germinated to >95 % at 35/20 °C (Fig. 7B–E). Germination percentages decreased after dry storage at 35/20 °C and at ambient laboratory temperature, except for dry-stored 2006C1 seeds at 35/20 °C. However, the germination percentage of 2006C2 seeds dry-stored at ambient laboratory temperature and of 2006C4 seeds dry stored at 35/20 °C increased after 3 months of storage. The germination percentage of 2006C4 seeds stored on dry sand at all temperatures tested decreased after 1 month of storage (Fig. 7E). However, the germination percentage of dry-stored 2006C2 seeds increased after 1 month of storage and then decreased (Fig. 7C).
The germination percentage of seeds stored at 35/20 °C for 1 and 2 months correlate significantly with the germination percentage of untreated seeds at 35/20 °C (Fig. 8A, B). Germination percentages of seed collections with high initial germination percentages decreased after 1 month of dry storage at 35/20 °C, whereas germination percentages of collections with low initial germination percentages increased after 1 month of dry storage at 35/20 °C.
Fig. 8.
(A) Germination percentage of I. lacunosa seeds with different initial sensitivities after dry storage at 35/20 °C for up to 6 months; (B) relationship between germination percentage of I. lacunosa seeds at 35/20 °C and wet storage at 35/20 °C for 1 and 2 months.
Germination percentages of wet-stored 2006C2 and 2006C4 seeds increased gradually (Fig. 7C, E). However, after 3 months of wet storage of 2006C4 seeds at 5/1 °C the germination percentage decreased (Fig. 7E). None of the pre-treated seeds imbibed or germinated at 25/15 °C (data not shown) or at any lower temperature (data not shown). After these wet-stored seeds were incubated on wet sand or in high RH (>90 %) at 35 °C for 3 h, they imbibed and germinated at 25/15 °C and at lower temperatures (25/15–15/6 °C; data not shown).
Reversing the sensitivity of sensitive seeds
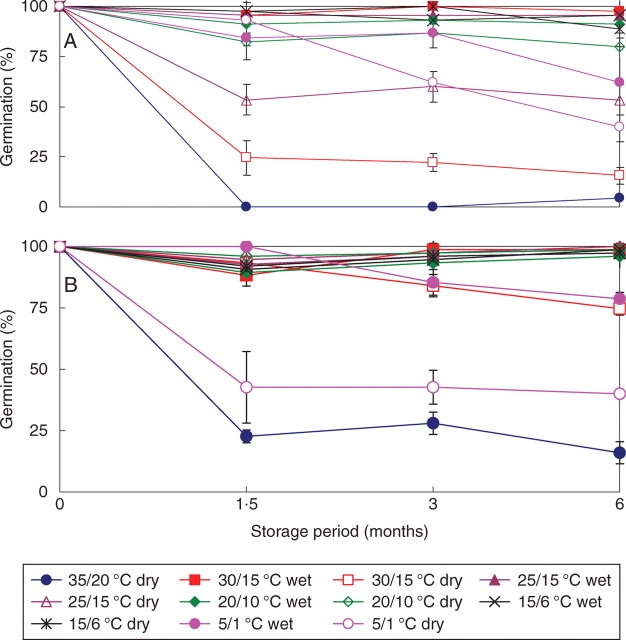
The germination percentage of 2005C1 sensitive seeds (made sensitive by storing wet at ambient room temperatures for 2 months) at 25/15 °C decreased from 100 % immediately following 3 h incubation at 35 °C on wet sand, to 0 % after they were stored dry for 1·5 months at 35/20 °C and then incubated at 35 °C on wet sand for 3 h (Fig. 9A). The germination percentage decreased from 100 % to 25 %, when seeds were stored dry at 30/15 °C for 1·5 months. A decline in germination also occurred in dry-stored seeds at 25/15 and 5/1 °C. However, germination percentages had not decreased after dry storage for 1·5 months at 5/1 °C, although a dramatic loss of germinability was observed after 3 months of dry storage at this temperature. After 6 months of dry storage (at 5/1 °C), the germination percentage was lower than that of seeds stored dry for 6 months at 25/15 °C. Germination percentages did not decrease for seeds stored wet at any of the temperature regimes except 5/1 °C, and even at this regime they decreased gradually.
Fig. 9.
Germination of sensitive (induced and inherent) I. lacunosa seeds of (A) 2005C1 and (B) 2005C2 at 25/15 °C following wet and dry storage for 0–6 months at different temperature regimes and then incubation at 35 °C for 3 h. Bars are ±1 s.e.
Seeds of 2005C2 showed the same trends in reversal of sensitivity as those of 2-month-wet-stored (in ambient laboratory conditions) 2005C1, with the exception that 2005C2 seeds were less sensitive to dry storage at all temperatures and to wet storage at 5/1 °C (Fig. 9B). Loss of germinability of wet-stored 2005C2 seeds was less than that of wet-stored 2005C1 seeds, when they received the same treatment. Similar trends were observed for wet-stored 2006C2 and 2006C3 seeds (data not shown).
Cycling of sensitivity
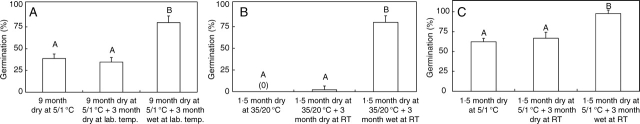
The germination percentage of 2005C1 sensitive seeds (made sensitive by 2 months wet storage at ambient laboratory temperatures) at 25/15 °C decreased from 100 % immediately following 3 h incubation at 35 °C on wet sand to 40 % after they were stored dry for 9 months at 5/1 °C and then incubated at 35 °C on wet sand. However, they regained the ability to germinate to high percentage following wet storage at ambient laboratory conditions for 3 months; 3 months of dry storage under the same conditions did not affect germinability (Fig. 10A). The same trend was observed for 2006C3 seeds. When stored dry at 35/20 °C for 1·5 months, they did not respond to 3 h incubation at 35 °C on wet sand for dormancy break. However, they regained sensitivity (80 %) after they were stored on wet sand for 3 months at ambient laboratory temperatures (Fig. 10B). Sensitivity of 2006C3 seeds also decreased (100 % to 62 %) after they were stored dry for 1·5 months at 5/1 °C. They also regained the ability to germinate after wet storage for 3 months at ambient laboratory temperatures (Fig. 10C).
Fig. 10.
Germination of sensitive (sensitivity induced by storage for 2 months at ambient laboratory temperatures on wet sand) of I. lacunosa seeds at 25/15 °C following various storage treatments and then incubation at 35 °C at 100 % RH to break dormancy: (A) 2005C1; (B) 2006C3; (C) 2006C3. Different upper-case letters indicate significant differences between treatments. Bars are +1 s.e.
Cycling of dormancy
Dry storage for 1, 2, 7, 14, 30, 90 and 180 d at 35/20 °C did not reduce germinability of non-dormant 2005C2 seeds incubated at 35 °C for 3 h on wet sand to break dormancy (data not shown). Also there was no decrease in germinability of non-dormant seeds stored on dry sand at 35/20, 30/15, 25/15, 20/10 or 15/6 °C for 2 months (data not shown). Thus, in contrast to its effect on sensitivity, dry storage of seeds at 35/20 and 30/15 °C did not cause non-dormant seeds to re-enter dormancy.
Maternal effects on sensitivity
There was a marginally significant (P = 0·069 and 0·076) relationship between germination percentage of seeds incubated at 35/20 °C for 7 d and temperature and rainfall during the maturation period (R2 = 76·4 %; Table 1).
Table 1.
Results of regression analysis showing relationship between sensitivity of seeds and climatic conditions (rainfall and temperature) during seed development
| Source | F value | P > F |
|---|---|---|
| Temperature | 7·68 | 0·069 |
| Rain | 7·03 | 0·076 |
| Model | 4·86 | 0·094 |
| R2 | 0·764 |
DISCUSSION
Ipomoea lacunosa seeds have physical dormancy (Jayasuriya et al., 2007). However, intact dormant 2005C2 seeds germinated to >95 % at 35/20 °C and to moderate percentage at 30/15 °C. Only a low percentage of seeds of this collection germinated or imbibed at the other temperature regimes. Dormant seeds of other collections germinated to moderate percentages at 35/20 °C and at 30/15 °C but to very low percentages at the lower temperatures. These results agree with those of Gomes et al. (1978), who showed that non-treated intact seeds of I. lacunosa germinated to high percentage at high temperatures. Thus, high temperature has an effect on physical dormancy-break of I. lacunosa seeds. Figure 5A shows that 2005C2 seeds can germinate to >95 % at 25/15 °C, after incubation at 35 °C on wet sand for ≥2 h. However, seeds incubated on dry sand, even for long periods of time, did not acquire the ability to germinate at 25/15 °C or at other lower temperatures. Incubation of seeds at lower temperatures (<30 °C) on wet sand for 3 h had no effect on breaking PY of sensitive seeds of I. lacunosa. Incubation of 2005C2 seeds at 35 °C on wet sand made seeds permeable, and therefore they could germinate at all temperatures tested. Seeds of the other collections did not respond to this treatment. Thus, they are insensitive to a 3-h wet treatment at 35 °C. Sensitivity of seeds of other collections was induced by storing them under wet conditions.
Sensitive seeds (non-treated 2005C2 and 2-month-wet-stored 2006C3) not only responded to the 35 °C wet-sand treatment, but they also were made permeable by incubating them at 35 °C for 3 h at high RH (>90 %). There was a highly significant 2nd degree polynomial correlation between RH and dormancy break (imbibition) of seeds at 25/15 °C following the 3 h at 35 °C treatment [2005C2 (non-treated) and wet stored (at ambient laboratory temperature) 2006C3 seeds]. A high percentage of the seeds became permeable at high RH, whereas a low percentage of seeds became permeable at low RH. Wet stored (at ambient laboratory temperatures) 2006C2 seeds are very sensitive to change in RH, and thus the percentage of permeable seeds decreased dramatically with a small decrease in RH. The effect of humidity on physical dormancy break has been reported in several other studies. High relative humidity can accelerate dormancy breaking (seed softening) of subterranean clover (Kirchner and Andrew, 1971; Taylor, 1981; Fairbrother, 1991), and Van Klinken and Flack (2005) showed that wet heat can break dormancy of physically dormant seeds of Parkinsonia aculeata. Physical dormancy of seeds of Cassia (Chamaecrista) nictitans and Cassia (Chamaecrista) aspera (Fabaceae) was released only by moist heat, whereas dormancy of seeds of several other leguminous species was broken by both dry and wet heat (Martin et al., 1975). The present study clearly shows that seeds of I. lacunosa require a high humidity/high temperature treatment to break PY.
For non-preconditioned seeds, the 35 °C wet treatment for 3 h was effective in breaking dormancy only in 2005C2 seeds. Seeds of the other collections were insensitive to this treatment. Sensitivity of the non-responsive seeds of these other collections could be induced by storing them under wet conditions. Rate of induction of sensitivity was higher at high than at low temperatures. However, prolonged storage (>3 months) at low temperatures (5/1 °C) under wet conditions decreased the sensitivity of seeds in some collections. The effect of storage under dry conditions depended on the initial level of sensitivity (at the beginning of storage) of seeds. Sensitivity of seeds with high initial sensitivity decreased in dry storage, while sensitivity of those with low initial sensitivity increased.
Preconditioning of seeds with low sensitivity increased their sensitivity to the physical dormancy-breaking treatment (35 °C incubation on wet sand or at high RH for 3 h). These results agree with those obtained by Taylor (1981) for seeds of Trifolium subterraneum, by Revell et al. (1999) for Ornithopus compressus and by Van Assche et al. (2003) on several other legumes. Taylor (1981) and Revell et al. (1999) suggested a two-stage model for dormancy break for Trifolium subterraneum and Ornithopus compressus. According to this model, hard (water impermeable) seeds must be preconditioned and made latent soft (i.e. sensitive to dormancy-breaking treatment but not permeable, i.e. the sensitive stage in the present study) first to make them sensitive to the dormancy-breaking treatment (60/15 °C).
Experiments with sensitive seeds showed that sensitivity can be reversed by storing seeds under dry conditions. The rate of sensitivity reversal is high at extreme temperatures (35 °C and 5 °C). Even under wet conditions at low temperatures (≤5 °C), sensitivity of the seeds was reversed after prolonged storage. Wet storage at any other temperature did not affect sensitivity. Seeds with induced sensitivity (wet-stored 2005C1, 2006C2 and 2006C3) lost sensitivity more rapidly than did those with innate sensitivity (2005C2). This suggests a dormancy-breaking model with sensitivity cycling (cycling of sensitivity/insensitivity to dormancy-breaking treatments). Revell et al. (1999) have shown that sensitivity of seeds of Ornithopus compressus can be reversed, and Taylor (2005) thought that reversing sensitivity of latent soft (=sensitive in the present study) seeds is also possible for other clover species. Van Assche et al. (2003) suggested the same type of mechanism for dormancy-break in several other legumes. Results of sensitivity cycling experiments in seeds of I. lacunosa show that it can be repeated.
Norsworthy and Oliveira (2007) concluded that dormancy fluctuates (increases ↔ decreases) in seeds of Ipomoea lacunosa in the field throughout a year. However, the present results do not support this claim that dormancy can be broken and then re-imposed in seeds of this species. Release of physical dormancy in seeds of I. lacunosa is due to formation of slits around the two bulges on the seed coat adjacent to the micropyle, and it does not seem that resealing of the slit would be anatomically possible (Jayasuriya et al., 2007). Dormancy of the seeds of I. lacunosa cannot be reversed by dry storage for 3 months at 35/20, 30/15, 25/15, 20/10 or 15/6 °C. However, dry storage at some of these temperatures can reverse the sensitivity of seeds (sensitive → nonsensitive) of this species. Permeability of I. lacunosa seeds cannot be reversed by storing them at 35/20 °C for long periods of time. According to Aiken (1939) hard seeds of Trifolium subterraneum become soft by splitting of the strophiole (lens) under pressure, and that ‘There is no reversion to hard on further drying, since a split between cells through the light line, once developed, is not sealed’. Hamly (1932) also stated that the lens in seeds of Melilotus alba that had been opened by percussion (impaction) could not be resealed. However, Hagon and Ballard (1970) reported that impermeability of Trifolium subterranean seeds made permeable by percussion, which opened the lens, the only site of water entry into the seed, could be re-imposed by storing them at low RH. Permeability of percussed seeds stored at high RH was not reversed. Thus, resealing of lens occurred only when seeds were subjected to conditions that were drier than those previously experienced by seeds before they softened (Hagon and Ballard, 1970).
The proportion of sensitive seeds and their sensitivity level were correlated with the seed maturation environment. There was a marginally significant (P = 0·069, 0·076) correlation between proportion of sensitive seeds and temperature and rainfall during the maturation period. This relationship was more pronounced in 2005 than in 2006. Thus, I. lacunosa seeds that matured at high temperatures were less sensitive than those that mature at low temperatures. Therefore, seeds that mature in early autumn are insensitive when they are dispersed, whereas those that mature in late autumn are sensitive. As discussed earlier, sensitive seeds can become permeable when they are exposed to high temperatures under wet conditions. These conditions can be met by seeds dispersed in early autumn. To avoid germination in autumn, they have to be insensitive at the time of dispersal. However, seeds dispersed in late autumn or early winter have no possibility of being exposed to high temperatures, and therefore they do not need to be insensitive.
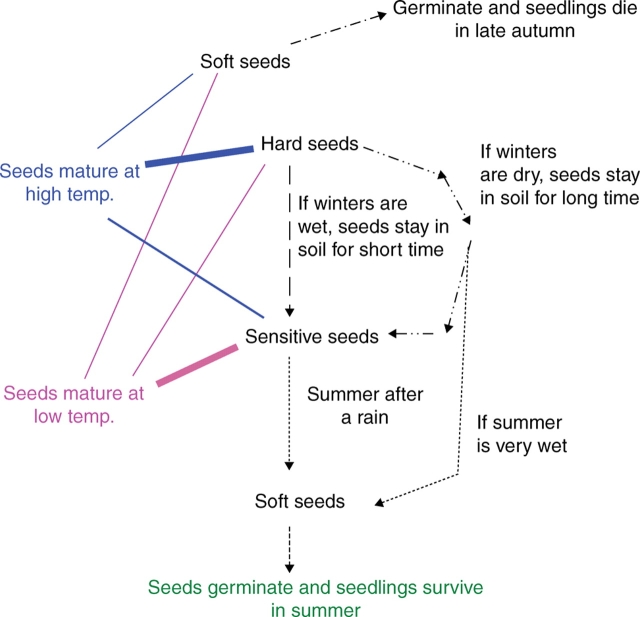
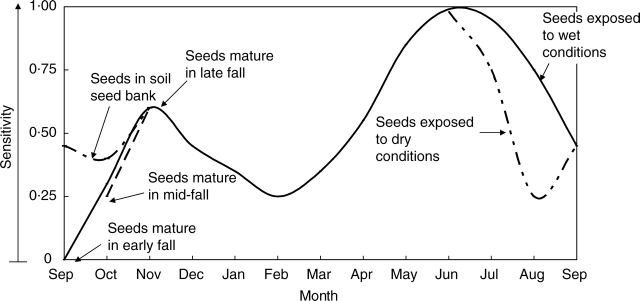
From these results, a conceptual model for dormancy break in I. lacunosa seeds was constructed (Fig. 11). According to this model, there is a continuum of sensitivity to the dormancy-breaking treatment, i.e. from completely insensitive (0) to the highest sensitivity (1·0). At the highest sensitivity, seeds can be made permeable by incubating them at 35 °C under high RH for <3 h. When sensitivity of the seeds is at a level close to insensitivity, a longer period or high temperature is required to break dormancy. Sensitivity can be increased when seeds are at high RH. Under high temperature conditions, the rate of increase of sensitivity is high compared with that at lower temperatures. Insensitive seeds in nature can become sensitive in spring or autumn, when soil moisture conditions are high (Figs 12 and 13). Sensitivity of insensitive seeds can also be increased at a slow rate under high temperature and dry conditions. Therefore, insensitive seeds can become sensitive in dry summer conditions (Figs 12 and 13) and thus become permeable and germinate in late summer. However, when seeds are close to their highest sensitivity, sensitivity decreases at high temperatures (≥30 °C) under dry conditions. This prevents germination of seeds in autumn (Figs 12 and 13). Sensitivity of sensitive seeds can also be reversed at slow rates at low temperatures (<5 °C) under dry conditions. At low temperatures (<5 °C) under wet conditions, sensitivity of the sensitive seeds can be reversed at a much slower rate.
Fig. 11.
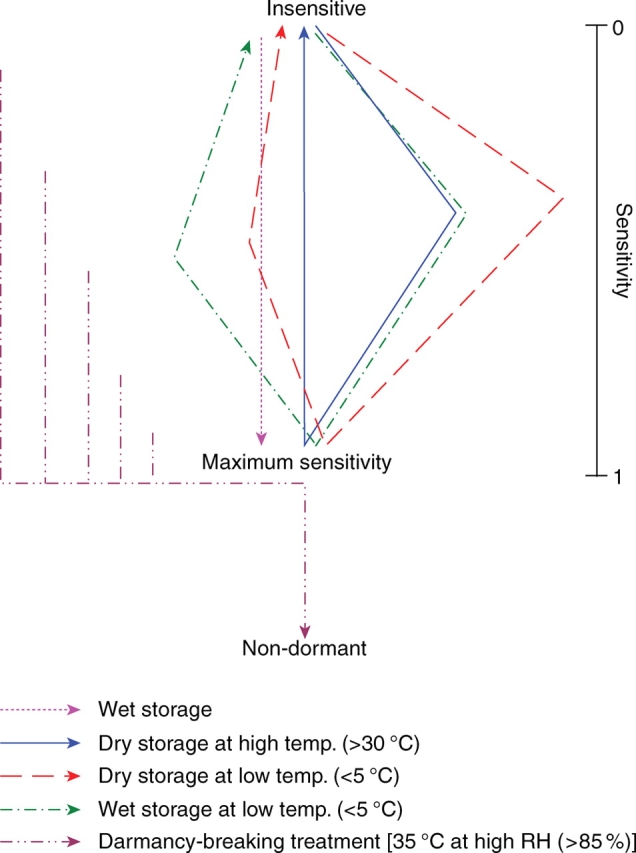
Model of dormancy-breaking requirements and sensitivity cycling of I. lacunosa seeds. The length of the arrows indicates relative lengths of the time period.
Fig. 12.
Model of the germination ecology of I. lacunosa seeds. The relative thickness of the continuous lines indicates proportion of seeds matured under different conditions.
Fig. 13.
Model of annual changes in sensitivity of I. lacunosa seeds.
According to this model, seeds will be sensitive or insensitive when they are dispersed. Seeds that mature in early autumn are more insensitive than those that mature in late autumn. When seeds go through the winter surrounded by wet soil, they will remain sensitive or will increase in sensitivity. However, if seeds are exposed to dry conditions in winter they will lose the sensitivity, but if they are exposed to wet condition in spring their sensitivity increases again. If seeds are exposed to dry conditions, they will not lose sensitivity, since spring temperatures are not high enough to reverse it. At the beginning of summer, sensitive seeds will become non-dormant after a period of rain. However, if they have not received appropriate conditions to become non-dormant their sensitivity starts to decrease and, in late summer temperatures, they will become insensitive again (Figs 12 and 13).
Seeds of Trifolium subterraneum (Taylor, 1981) and Ornithopus compressus (Revell et al., 1999) become sensitive (latent soft) when they are stored dry at 60 °C. After seeds become sensitive, they require a 60/15 °C alternating temperature regime to make them permeable (soft). However, response of sensitive seeds to 60/15 °C is slow, and thus they require at least 1 month at 60/15 °C to become permeable (soft). In contrast, seeds of I. lacunosa require wet storage to become sensitive, and after becoming sensitive they respond rapidly to the dormancy-breaking treatment. Ipomoea lacunosa seeds with maximum sensitivity require <3 h at 35 °C to become permeable.
Seeds of I. lacunosa require wet conditions to become sensitive or to maintain the sensitivity, and high temperatures in wet conditions to become permeable. High temperature together with high soil moisture content serves as the environmental cue for germination of I. lacunosa seeds. Seeds do not germinate in summer if they do not have the required amount of moisture. Therefore, this strategy seems to be very important for detecting the appropriate time for germination and subsequent growth of I. lacunosa plants. This strategy insures that both water and temperature are appropriate for seedling establishment, which is the most vulnerable stage of the life cycle.
LITERATURE CITED
- Abel WE, Austin DF. Introgressive hybridization between Ipomoea trichocarpa and I. lacunosa. Bulletin of the Torrey Botanical Club. 1981;108:231–239. [Google Scholar]
- Aiken Y. The problem of hard seeds in subterranean clover. Proceedings of the Royal Society of Victoria. 1939;51:187–213. [Google Scholar]
- Anonymous. Weed survey – Southern states, broad leaf crops subsection. Proceedings, Southern Weed Science Society. 1995;48:290–305. [Google Scholar]
- Anonymous. Weed survey – Southern states, grass crop subsection. Proceedings, Southern Weed Science Society. 2000;53:247–274. [Google Scholar]
- Anonymous. Weed survey – Southern states, broadleaf crops subsection. Proceedings, Southern Weed Science Society. 2001;54:244–259. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin CC, Thompson K, Baskin JM. Mistakes in germination ecology and how to avoid them. Seed Science Research. 2006;16:165–168. [Google Scholar]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. BioScience. 1985;35:492–498. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Benech-Arnold RL. Inception, maintenance, and termination of dormancy in grain crops: physiology, genetics, and environmental control. In: Benech-Arnold RL, Sanchez RA, editors. Handbook of seed physiology: applications to agriculture. Binghamton, NY: The Haworth Press; 2004. pp. 169–198. [Google Scholar]
- Benvenuti S, Dinelli G, Bonetti A, Catizone P. Germination ecology, emergence and host detection in Cuscuta campestris. Weed Research. 2005;45:270–278. [Google Scholar]
- Collins WJ. The effects of length of growing season, with and without defoliation, on seed yield and hard-seededness in swards of subterranean clover. Australian Journal of Agricultural Research. 1981;32:783–792. [Google Scholar]
- Courtney AD. Seed dormancy and field emergence in Polygonum aviculare L. Journal of Applied Ecology. 1968;5:675–684. [Google Scholar]
- Egley GH, Chandler JM. Germination and viability of weed seeds after 2·5 years in a 50-year buried seed study. Weed Science. 1978;26:230–239. [Google Scholar]
- Egley GH, Chandler JM. Longevity of weed seed after 5·5 years in the Stoneville 50-year buried seed study. Weed Science. 1983;31:264–270. [Google Scholar]
- El Bagoury OH. Effect of different fertilizers on the germination and hard seed percentage of broad bean seeds (Vicia faba) Seed Science and Technology. 1975;3:569–574. [Google Scholar]
- El Bagoury OH, Niyazi MA. Effect of different fertilizers on the germination and hard seed percentage of Egyptian clover seeds (Trifolium alexandrinum L.) Seed Science and Technology. 1973;1:773–779. [Google Scholar]
- Elmore CD, Hurst HR, Austin DF. Weed biology and principles of control of the weedy morningglories (Ipomoea spp.) and related species. Reviews of Weed Science. 1990;5:83–114. [Google Scholar]
- Evenari M, Koller D, Gutterman Y. Effects of the environment of the mother plant on germination by control of seed-coat permeability to water in Ononis sicula Guss. Australian Journal of Biological Sciences. 1966;19:1007–1016. [Google Scholar]
- Fairbrother TE. Effect of fluctuating temperatures and humidity on softening rate of subterranean clover (Trifolium subterraneum L.) Seed Science and Technology. 1991;19:93–105. [Google Scholar]
- Gleason HA, Cronquist A. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd edn. Bronx, NY: New York Botanical Garden; 1991. [Google Scholar]
- Gomes LF, Chandler JM, Vaughan CE. Aspects of emergence and seed production of three Ipomoea taxa. Weed Science. 1978;26:245–248. [Google Scholar]
- Gutterman Y. Differences in the progeny due to daylength and hormone treatment of the mother plant. In: Hedecker W, editor. Seed ecology. University Park and London: Pennsylvania State University Press; 1973. pp. 59–80. [Google Scholar]
- Gutterman Y. Seed coat permeability as a function of photoperiodical treatments of the mother plants during seed maturation in the desert annual plant: Trigonella arabica Del. Journal of Arid Environments. 1978;1:141–144. [Google Scholar]
- Gutterman Y. Seed germination in desert plants: adaptations of desert organisms. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Gutterman Y, Evenari M. The influence of day length on seed coat colour, an index of water permeability, of the desert annual Ononis sicula Guss. Journal of Ecology. 1972;60:713–719. [Google Scholar]
- Hagon MW, Ballard LAT. Reversibility of strophiolar permeability to water in seeds of subterranean clover (Trifolium subterraneum L.) Australian Journal of Biological Sciences. 1970;23:519–528. [Google Scholar]
- Hamly DH. Softening of the seeds of Melilotus alba. Botanical Gazette. 1932;93:345–375. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. Morphology and anatomy of physical dormancy in Ipomoea lacunosa: identification of the water gap in seeds of Convolvulaceae (Solanales) Annals of Botany. 2007;100:13–22. doi: 10.1093/aob/mcm070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner R, Andrew WD. Effect of various treatments on hardening and softening of seeds in pods of barrel medic (Medicago truncatula) Australian Journal of Experimental Agriculture and Animal Husbandry. 1971;11:536–540. [Google Scholar]
- Martin RE, Miller RL, Cushwa CT. Germination response of legume seeds subjected to moist and dry heat. Ecology. 1975;56:1441–1445. [Google Scholar]
- Norsworthy JK, Oliveira MJ. Role of light quality and temperature on pitted morningglory (Ipomoea lacunosa) germination with after-ripening. Weed Science. 2007;55:111–118. [Google Scholar]
- Quinlivan BJ. The influence of the growing season and the following dry season on the hardseededness of subterranean clover in different environments. Australian Journal of Agricultural Research. 1965;16:277–291. [Google Scholar]
- Quinlivan BJ. The relationship between temperature fluctuations and the softening of hard seeds of some legume species. Australian Journal of Agricultural Research. 1966;17:625–631. [Google Scholar]
- Quinlivan BJ, Millington AJ. The effect of a Mediterranean summer environment on the permeability of hard seeds of subterranean clover. Australian Journal of Agricultural Research. 1962;13:377–387. [Google Scholar]
- Revell CK, Taylor GB, Cocks PS. Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research. 1999;50:1211–1223. [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Schafer DE, Chilcote DO. Factors influencing persistence and depletion in buried seed populations. II. The effects of soil temperature and moisture. Crop Science. 1970;10:342–345. [Google Scholar]
- Taylor GB. Effect of consistent temperature treatments followed by fluctuating temperatures on the softening of hard seeds of Trifolium subterraneum L. Australian Journal of Plant Physiology. 1981;8:547–558. [Google Scholar]
- Taylor GB. Effect of the environment in which seeds are grown and softened on the incidence of autumn seed softening in two species of annual medics. Australian Journal of Agricultural Research. 1996;47:141–159. [Google Scholar]
- Taylor GB. Hardseedness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research. 2005;56:645–661. [Google Scholar]
- Taylor GB, Revell CK. Effect of pod burial, light, and temperature on seed softening in yellow serradella. Australian Journal of Agricultural Research. 1999;50:1203–1209. [Google Scholar]
- Taylorson RB. Phytochrome controlled changes in dormancy and germination of buried weed seeds. Weed Science. 1970;27:7–10. [Google Scholar]
- Toole EH, Brown E. Final results of the Duvel buried seed experiment. Journal of Agricultural Research. 1946;72:201–210. [Google Scholar]
- Van Assche JA, Debucquoy LA, Rommens AF. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae) New Phytologist. 2003;158:315–323. [Google Scholar]
- Van Klinken RD, Flack L. Wet heat mechanism for dormancy release and germination of seeds with physical dormancy. Weed Science. 2005;53:663–669. [Google Scholar]