Abstract
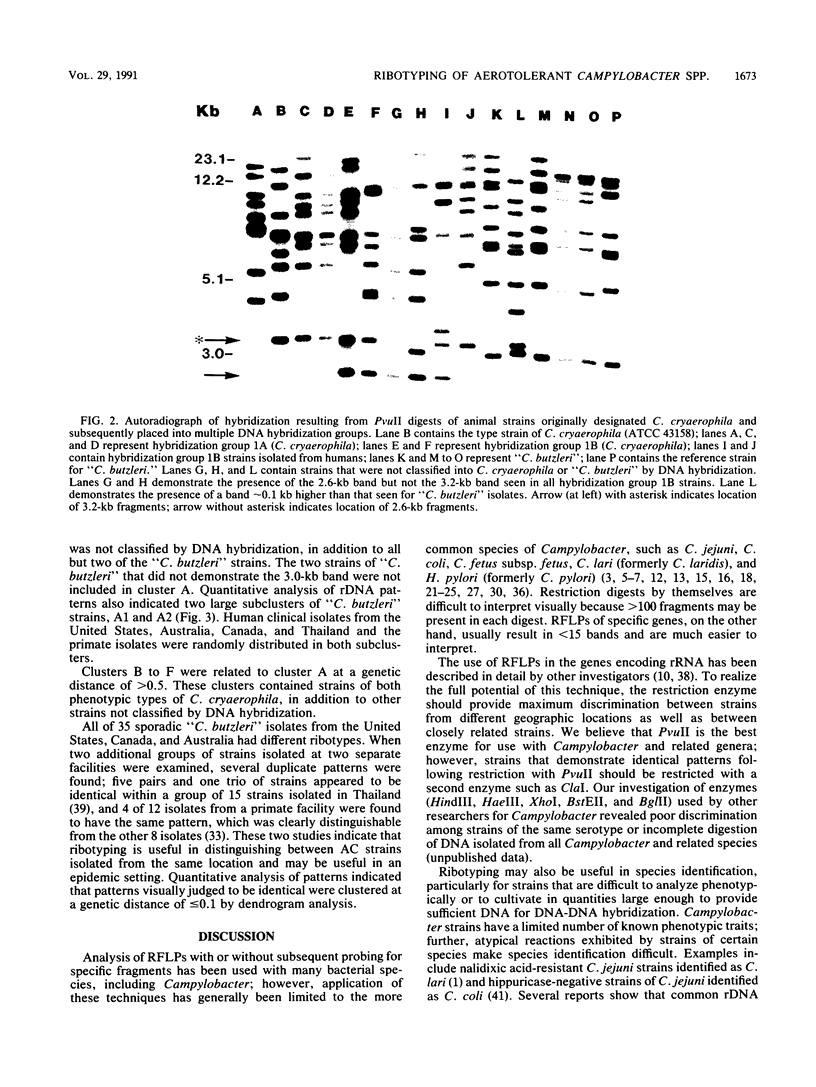
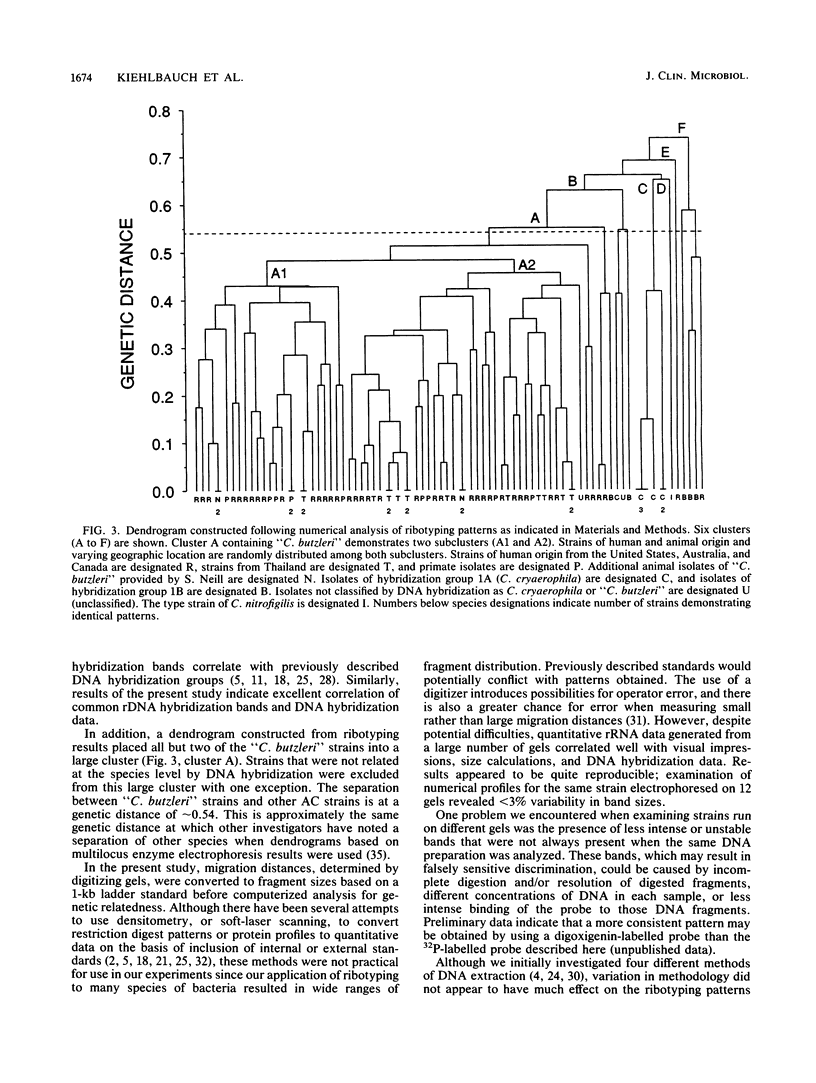
Whole-cell chromosomal digests of 84 strains of aerotolerant Campylobacter (AC) were examined by using PvuII restriction fragment length polymorphisms of rRNA genes followed by hybridization with Escherichia coli 16S and 23S rRNA (ribotyping). The AC strains belonged to Campylobacter cryaerophila (n = 13) and a newly defined species, "C. butzleri" (n = 64). Strains of C. cryaerophila belonged to two hybridization groups: DNA group 1A (including the type strain of C. cryaerophila) and DNA group 1B (J. A. Kiehlbauch, D. J. Brenner, M. A. Nicholson, C. N. Baker, C. M. Patton, A. G. Steigerwalt, and I. K. Wachsmuth, J. Clin. Microbiol. 29:376-385, 1991). Six AC strains not classified as C. cryaerophila or "C. butzleri" were also included. All 35 sporadic human and animal isolates of "C. butzleri" sent to the Centers for Disease Control for identification showed different ribotype patterns. However, most "C. butzleri" strains contained common bands at approximately 3.0, 6.2, 12.0, and 15.0 kb; the 3.0-kb band was present in all but four strains. An additional 23 strains of "C. butzleri," isolated as part of special studies, contained the 3.0-kb band. Thus, on the basis of visual identification of the 3.0-kb band, 94% of available strains were correctly identified as "C. butzleri." Ribotyping demonstrated that C. cryaerophila strains (DNA groups 1A and 1B) were different from C. butzleri strains. All C. cryaerophila strains demonstrated a common ribosomal DNA restriction fragment of 3.2 kb; DNA group 1B strains contained an additional common band at 2.6 kb. Ribotyping patterns of AC species were easily distinguished from patterns of other Campylobacter, Helicobacter, and Wolinella species.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altwegg M., Burnens A., Zollinger-Iten J., Penner J. L. Problems in identification of Campylobacter jejuni associated with acquisition of resistance to nalidixic acid. J Clin Microbiol. 1987 Sep;25(9):1807–1808. doi: 10.1128/jcm.25.9.1807-1808.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Mayer L. W. Bacterial molecular epidemiology based on a nonradioactive probe complementary to ribosomal RNA. Res Microbiol. 1989 May-Jun;140(4-5):325–333. doi: 10.1016/0923-2508(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Bradbury W. C., Pearson A. D., Marko M. A., Congi R. V., Penner J. L. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J Clin Microbiol. 1984 Mar;19(3):342–346. doi: 10.1128/jcm.19.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce D., Hookey J. V., Waitkins S. A. Numerical classification of campylobacters by DNA-restriction endonuclease analysis. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;269(3):284–297. doi: 10.1016/s0176-6724(88)80172-x. [DOI] [PubMed] [Google Scholar]
- Collins D. M., Ross D. E. Restriction endonuclease analysis of Campylobacter strains with particular reference to Campylobacter fetus ss. fetus. J Med Microbiol. 1984 Aug;18(1):117–124. doi: 10.1099/00222615-18-1-117. [DOI] [PubMed] [Google Scholar]
- De Lisle G. W., Pettett A. M., Wall E. P., Collins D. M. An examination of Campylobacter fetus subsp. fetus by restriction endonuclease analysis and serology. Vet Microbiol. 1987 May;14(1):53–60. doi: 10.1016/0378-1135(87)90052-6. [DOI] [PubMed] [Google Scholar]
- Grimont F., Chevrier D., Grimont P. A., Lefevre M., Guesdon J. L. Acetylaminofluorene-labelled ribosomal RNA for use in molecular epidemiology and taxonomy. Res Microbiol. 1989 Sep;140(7):447–454. doi: 10.1016/0923-2508(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Grimont F., Lefèvre M., Ageron E., Grimont P. A. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989 Nov-Dec;140(9):615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- Kakoyiannis C. K., Winter P. J., Marshall R. B. Identification of Campylobacter coli isolates from animals and humans by bacterial restriction endonuclease DNA analysis. Appl Environ Microbiol. 1984 Sep;48(3):545–549. doi: 10.1128/aem.48.3.545-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoyiannis C. K., Winter P. J., Marshall R. B. The relationship between intestinal Campylobacter species isolated from animals and humans as determined by BRENDA. Epidemiol Infect. 1988 Jun;100(3):379–387. doi: 10.1017/s0950268800067133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Brenner D. J., Nicholson M. A., Baker C. N., Patton C. M., Steigerwalt A. G., Wachsmuth I. K. Campylobacter butzleri sp. nov. isolated from humans and animals with diarrheal illness. J Clin Microbiol. 1991 Feb;29(2):376–385. doi: 10.1128/jcm.29.2.376-385.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Moureau P., Derclaye I., Gregoire D., Janssen M., Cornelis G. R. Campylobacter species identification based on polymorphism of DNA encoding rRNA. J Clin Microbiol. 1989 Jul;27(7):1514–1517. doi: 10.1128/jcm.27.7.1514-1517.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Beck A., Borman P. Restriction endonuclease digest patterns of chromosomal DNA from nitrate-negative Campylobacter jejuni-like organisms. Eur J Epidemiol. 1985 Dec;1(4):281–287. doi: 10.1007/BF00237103. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Beck A., Dayal P. A., Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988 Oct;26(10):2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Costas M., Dawson C. Application of different chromosomal DNA restriction digest fingerprints to specific and subspecific identification of Campylobacter isolates. J Clin Microbiol. 1989 Oct;27(10):2338–2343. doi: 10.1128/jcm.27.10.2338-2343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C. M., Wachsmuth I. K., Evins G. M., Kiehlbauch J. A., Plikaytis B. D., Troup N., Tompkins L., Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991 Apr;29(4):680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Mills S. D., Bradbury W. C. Application of serotyping and chromosomal restriction endonuclease digest analysis in investigating a laboratory-acquired case of Campylobacter jejuni enteritis. J Clin Microbiol. 1983 Dec;18(6):1427–1428. doi: 10.1128/jcm.18.6.1427-1428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis B. D., Carlone G. M., Edmonds P., Mayer L. W. Robust estimation of standard curves for protein molecular weight and linear-duplex DNA base-pair number after gel electrophoresis. Anal Biochem. 1986 Feb 1;152(2):346–364. doi: 10.1016/0003-2697(86)90420-3. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. D., Carlone G. M., Plikaytis B. B. Numerical analysis of normalized whole-cell protein profiles after sodium dodecyl sulphate-polyacrylamide gel electrophoresis. J Gen Microbiol. 1986 Sep;132(9):2653–2660. doi: 10.1099/00221287-132-9-2653. [DOI] [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Kiehlbauch J. A., Tee W., Pitarangsi C., Echeverria P. Isolation of group 2 aerotolerant Campylobacter species from Thai children with diarrhea. J Infect Dis. 1991 May;163(5):1062–1067. doi: 10.1093/infdis/163.5.1062. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Fennell C. L., Tenover F. C., Wezenberg J. M., Perine P. L., Stamm W. E., Holmes K. K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985 Jan;151(1):131–139. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Patton C. M., Tenover F. C., Barrett T. J., Stamm W. E., Steigerwalt A. G., Lin J. Y., Holmes K. K., Brenner D. J. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J Clin Microbiol. 1987 Sep;25(9):1747–1752. doi: 10.1128/jcm.25.9.1747-1752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]