Abstract
Background and Aims
Ranunculaceae has a prominent phylogenetic position in Ranunculales which appears at the base of eudicots. The aims of the present paper are to reveal the features of ovule morphogenesis in different taxa and gain a better understanding of the systematics of Ranunculaceae.
Methods
Flowers of 17 species from three subfamilies, nine tribes and 16 genera of Ranunculaceae, at successive developmental stages, were collected in the wild and studied with a scanning electron microscope.
Key Results
The integuments in the unitegmic ovules in Helleborus, Ranunculus and Oxygraphis, as well as the inner integuments in the bitegmic genera, initiate annularly and eventually become cup-shaped. However, the integuments in the unitegmic ovules in Anemone and Clematis, as well as the outer integuments in the bitegmic genera, arise semi-annularly and eventually become hood-shaped. Different kinds of appendages appear on the ovules during development. In Coptis of subfamily Coptidoideae, a wrap-shaped appendage arises outside the ovule and envelopes the ovule entirely. In the genera of subfamily Thalictroideae and tribe Anemoneae of subfamily Ranunculoideae, appendages appear on the placenta, the funicle or both. In tribe Helleboreae of subfamily Ranunculoideae, an alary appendage is initiated where the integument and the funicle join and becomes hood-shaped.
Conclusions
Ovule morphogenesis characteristics are significant in classification at the levels of subfamilies and tribes. The initiation patterns of the integuments and the development of appendages show diversity in Ranunculaceae. The present observations suggest that the bitegmic, hood-shaped outer integument and endostomic micropyle are primitive while the unitegmic, cupular-shaped outer integument and bistomic micropyle are derivative.
Key words: Ranunculaceae, ovule, morphogenesis, systematics
INTRODUCTION
The ovule is vital in the angiosperm as a sexual reproductive organ. Many significant events in the plant life cycle, such as the initiation and development of the female gametophyte, guidance for the pollen tube, the double fertilization and the development of the new sporophyte, are achieved in the ovule. The angiosperm ovule consists of a central body, which is enclosed more or less completely by one or two integuments and supported on a funicle. The central body consists of a nucellus, in which sporogenous tissue is borne, and the chalaza, where funicle, integuments and nucellus merge (Eames, 1961). Three features of the ovule in angiosperms are characteristic: the number of integuments, thickness of the nucellus and the curvature. Bitegmic and anatropous ovules are common in basal angiosperms and are usually considered to be primitive in flowering plants (Stebbins, 1974; Bouman, 1984; Cronquist, 1988; Takhtajan, 1991; Johri et al., 1992; Endress and Igersheim, 2000; Yamada et al., 2001b; Yamada et al., 2003). However, a few authors regard anatropous ovule as derived and orthotropous ovule as ancestral (Bocquet and Bersier, 1960; Taylor, 1991).
The homology between the inner integument of angiosperms and the single cupular integument of gymnosperms suggests that the outer integument is a novel organ of angiosperms (Meeuse and Bouman, 1974; Stewart and Rothwell, 1993; Endress, 2001). It is widely believed that the outer integument is cupular, either symmetric or asymmetric, and entirely encloses the inner integument and nucellus (Bouman, 1984). However, in the anatropous ovules of many primitive, non-eudicot angiosperms (e.g. Cabombaceae, Austrobaileyaceae, Trimeniaceae, Schisandraceae, Winteraceae, Aristolochiaceae, Lactoridaceae, Degeneriaceae, Annonaceae, Idiospermaceae, most of Monimiaceae, Lauraceae, Hernandiaceae and many basal monocots), the outer integument is lacking on the funicle (Yamada et al., 2001a, b, 2003); in other words, it is semi-annular (Endress and Igersheim, 1997; Igersheim and Endress, 1997, 1998; Igersheim et al., 2001). It is believed that ovules with a cupular outer integument are primitive, while those lacking outer integuments on the concave side of their funicle are extremely derivative (Eames, 1961; Bouman, 1984; Cronquist, 1988; Fahn, 1990; Johri et al., 1992), but, recently, developmental studies have indicated that the outer integument is semi-annular at initiation and subsequently becomes hood-shaped in Cabombaceae, Austrobaileyaceae, Trimeniaceae, Annonaceae, Degeneriaceae, Eupomatiaceae and Winteraceae (Matsui et al., 1993; Umeda et al., 1994; Imaichi et al., 1995; Yamada et al., 2001a, b, 2003). Furthermore, Matsui et al. (1993), Umeda et al. (1994), Imaichi et al. (1995) and Yamada et al. (2001a, b, 2003) suggested that anatropous ovules with a hood-shaped outer integument are primitive in angiosperms.
The micropyle may be bistomic or endostomic in ovules. The wide occurrence of endostomic ovules in basal angiosperms and the character phylogeny of the micropyle obtained suggested that the endostomic ovule is primitive (Johri et al., 1992; Imaichi et al., 1995; Endress and Igersheim, 1997; Igersheim and Endress, 1997, 1998; Tobe et al., 2000; González and Rudall, 2003; Yamada et al., 2001a, b, 2003).
Ranunculales is one of the most important groups in basal eudicots (APG, 1998, 2003). Ranunculaceae, a core family in Ranunculales, varies in morphological characteristics such as habitat and form of leaves and flowers, carpels and ovules. The variety of the ovules within Ranunculaceae may provide useful information for understanding better the classification and phylogeny of the group, as well as the evolution of ovules. Ranunculaceae has been often divided into many-seeded and one-seeded groups since Adanson (1763). Hutchinson (1923) put both groups in subfamilial rank, i.e. Helleboroideae with follicles (many-seeded) and Ranunculoideae with achenes (one-seeded). Langlet (1932) recognized two chromosome types, R(anunculus)-type and T(halictrum)-type, in Ranunculaceae and proposed the subdivision of the family into Ranunculoideae with R-type chromosomes and Thalictroideae with T-type ones. Jensen (1968) divided the family into subfamily Hydrastidoideae and subfamily Ranunculoideae based on serological studies. Wang (1980) divided the family into three subfamilies, i.e. Thalictroideae with T-type chromosomes and follicles, Helleboroideae with R-type chromosomes and follicles, and Ranunculoideae with R-type chromosomes and achenes. Tamura (1966) divided the Ranunculaceae into six subfamilies, i.e. Helleboroideae, Ranunculoideae, Isopyroideae, Thalictroideae, Coptidoideae and Hydrastidoideae. Accordingly the numerical variation becomes continuous in this group and Tamura (1993, 1995) reduced the subfamily Coptidoideae to a tribe Coptideae under the Isopyroideae, but the molecular phylogeny studies showed that Ranunculaceae consists of three (Hoot et al., 1995, excluding Hydrastis) or four (Ro et al., 1997; Wang et al., 2005, including Hydrastis) subfamilies, i.e. Hydrastidoideae (including Hydrastis), Coptidoideae (including Coptis and Xanthorhiza), Thalictroideae (including other T-type chromosomes genera) and Ranunculoideae (including all R-type chromosomes genera). There are some similarities and differences in molecular and non-molecular systematic studies. The research carried out on ovule morphogenesis of Ranunculaceae may help us to understand the subfamilial relationships in Ranunculaceae.
MATERIALS AND METHODS
Flowers of 17 species were collected from Taibai Mountain, Shaanxi province, in successive developmental stages, and fixed in FAA. The vouchers were deposited in Herbaria of Shaanxi Normal University (SANU) (Table 1).
Table 1.
List of taxa, source of samples and voucher specimen
| Taxon | Elevation | Voucher |
|---|---|---|
| Subfamily Coptidoudeae | ||
| Coptis chinensis Linn. | 350 m, cultivated | Gu Tian-qi SN001 |
| SubfamilyThalictroideae | ||
| Thalictrum przewalskii Maxim. | 2800–3100 m | Zhao Liang N14-w-103 |
| Dichocarpum fargesii (Franch.) W. T. Wang et P. K. Hsiao | 1300–1600 m | Li Hai-ning TK05065 |
| Aquilegia yabeana Kitag. | 900–2200 m | Bai Genlu 2004005 |
| Aquilegia ecalcarata Maxim. | 1200 m | Ren Yi N14-w-23 |
| Subfamily Ranunculoideae | ||
| Caltha palustris Linn. | 2018 m | Song Ping 20050517 |
| Trollius buddae Schipcz. | 2299 m | Song Ping 20050725 |
| Helleborus thibetanus Franch. | 1100 m | Bai Gen-lu 2004036 |
| Beesia calthaefolia (Maxim.) Ulbr. | 1300–1400 m | Yang Rui 20070727 |
| Actaea asiatica Hara | 2500–2800 m | Bai Gen-lu 010 |
| Aconitum taipeicum Hand.-Mazz. | 2800–3500 m | Bai Gen-lu 2004011 |
| Anemone taipaiensis W. T. Wang | 3100–3600 m | Bai Gen-lu 2004018 |
| Clematis montana Buch. -Ham. | 600–1750 m | Bai Gen-lu 2004004 |
| Oxygraphis glacialis (Fisch.) Bge. | 3000–3700 m | Bai Gen-lu 2004006 |
| Ranunculus sieboldii Miq. | 1800 m | Ren Yi 244 |
| Adonis sutchuenensis Franch. | 2500–3200 m | Bai Gen-lu 2004007 |
| Callianthemum taipaicum W. T. Wang | 3500–3600 m | Bai Gen-lu 2004008 |
For observation with the scanning electron microscope (Hitachi S-570), the materials were dehydrated in an absolute ethanol series, CO2 critical-point dried and coated with gold.
RESULTS
Coptis chinensis (subfamily Coptidoideae)
The ovule primordium is shortly digitate and upright (Fig. 1A). As the ovule primordium elongates, the inner integument is initiated annularly in the middle of the primordium (Fig. 1B). The inner integument is annular throughout the developmental stages and eventually becomes cup-shaped (Fig. 1C, E). After a while, with the ovule primordium curving inward slightly (Fig. 1C), the outer integument arises semi-annularly below the inner integument (Fig. 1D) and eventually becomes hood-shaped (Fig. 1E). The brim of the inner integument becomes slightly lobed when it encloses the nucellus (Fig. 1F) and the lobes become obvious later (Fig. 1I). When some longitudinal veins appear on the surface of the outer integument, a wrap-shaped appendage arises outside the outer integument and the funicle (Fig. 1G). This appendage grows and enlarges (Fig. 1H) and eventually is very obvious (Fig. 1J). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle (endostomic) is formed by a four-lobed inner integument (Fig. 1I, J).
Fig. 1.
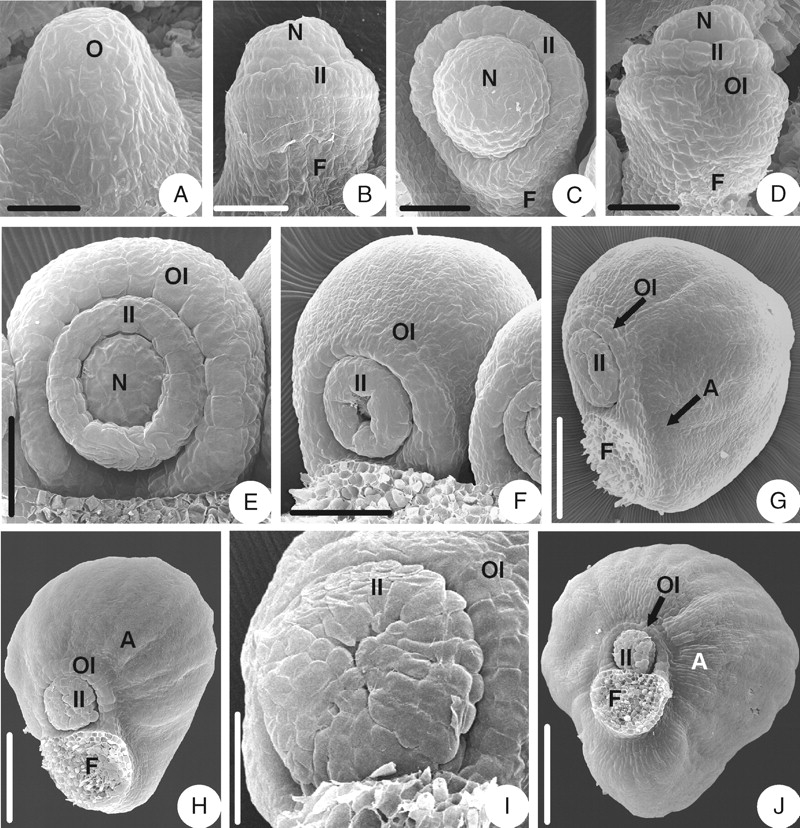
Ovule morphogenesis in Coptis chinensis: (A) ovule primordium; (B) inner integument is initiated annularly; (C) incurved ovule with developing inner integument and funicle; (D) outer integument is initiated semi-annularly; (E) hood-shaped outer integument and cup-shaped inner one; (F) the brim of the inner integument becomes slightly lobed; (G) initiation of the appendage; (H) the appendage grows and enlarges; (I) micropyle (endostomic) with lobed inner integument; (J) a mature ovule with appendage. A, Appendage; F, funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A = 25 µm; B, C = 30 µm; D = 38 µm; E = 50 µm; F = 75 µm; G = 120 µm; H = 150 µm; I = 100 µm; J = 200 µm.
Thalictrum przewalskii (subfamily Thalictroideae)
The initiation and early development of the ovule in T. przewalskii are similar to that of Coptis chinensis (Fig. 2A–C), but there are some differences in later developmental stages. During the development of the outer integument, an appendage consisting of unicellular papillae appears on the placenta near the dorsal base of the funicle (Fig. 2D), extends upwards to the dorsal side of the funicle (Fig. 2E) and then to the ventral side (Fig. 2F). The appendage finally becomes lorate-shaped, surrounding the funicle (Fig. 2H). The appendage heightens later (Fig. 2I) and eventually becomes collar-shaped (Fig. 2J) with the opening in the dorsal side of the funicle (Fig. 2K). When the outer integument becomes hood-shaped, the brim of the inner integument becomes lobed (Fig. 2G). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle (endostomic) is formed by a four-lobed inner integument (Fig. 2J–L).
Fig. 2.
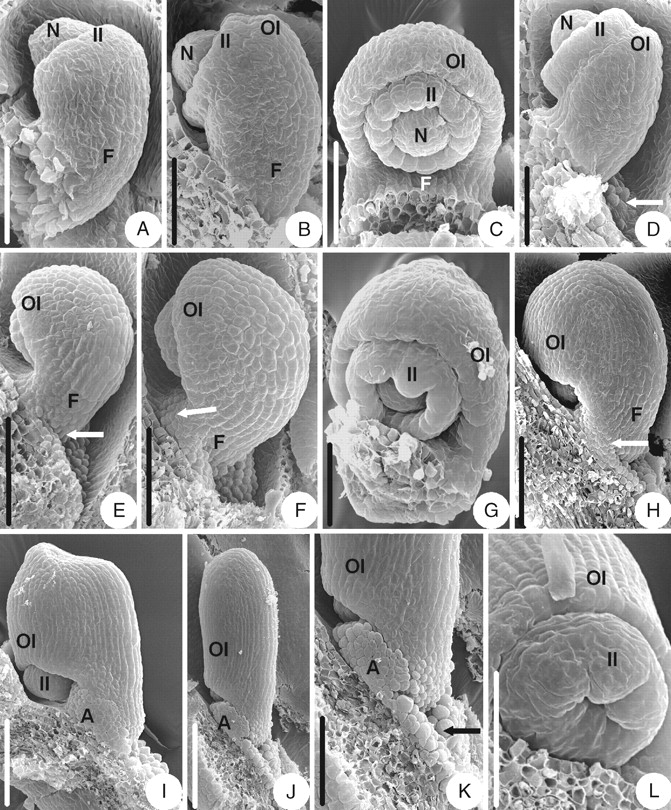
Ovule morphogenesis in Thalictrum przewalskii: (A) inner integument is initiated annularly; (B) outer integument is initiated semi-annularly; (C) developing semi-annular outer integument and cup-shaped inner one; (D) appendage (arrow) consisting of unicellular papillae is initiated on the placenta; (E) an appendage (arrow) appears on the dorsal side of the funicle; (F) an appendage (arrow) appears on the ventral side of the funicle; (G) the brim of the inner integument becomes lobed and the outer integument becomes hood-shaped; (H) lorate-shaped appendage (arrow); (I) the appendage keeps on growing and heightening; (J) a mature ovule with collar-shaped appendage; (K) magnified part of micropylar end shown in (J) – the appendage consists of unicellular papillae and the arrow indicates the opening of the collar-shaped appendage; (L) micropyle (endostomic) with lobed inner integument. A, Appendage; F, funicle; II, inner integument; N, nucellus; OI, outer integument. Scale bars: A, B, D, G = 50 µm; C = 60 µm; E, H, K = 100 µm; F, L = 75 µm; I = 120 µm; J = 200 µm.
Dichocarpum fargesii (subfamily Thalictroideae)
The ovule initiation and early development in D. fargesii are similar to that of Coptis chinensis (Fig. 3A–D), but there are some differences in later stages. When the semi-annular outer integument becomes hood-shaped, the funicle elongates (Fig. 3F, G). With the development of the ovule, the brim of the inner integument becomes slightly lobed (Fig. 3E) and the lobes become obvious in maturity (Fig. 3L). When some longitudinal veins appear on the surface of the outer integument, an appendage arises on the upper part of the ventral side of the funicle (Fig. 3H). The appendage extends to the dorsal side of the funicle (Fig. 3I) and eventually becomes ring-shaped (Fig. 3J, K). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses with about two-thirds of the funicle. The micropyle (endostomic) is formed by a four-lobed inner integument (Fig. 3J, L).
Fig. 3.
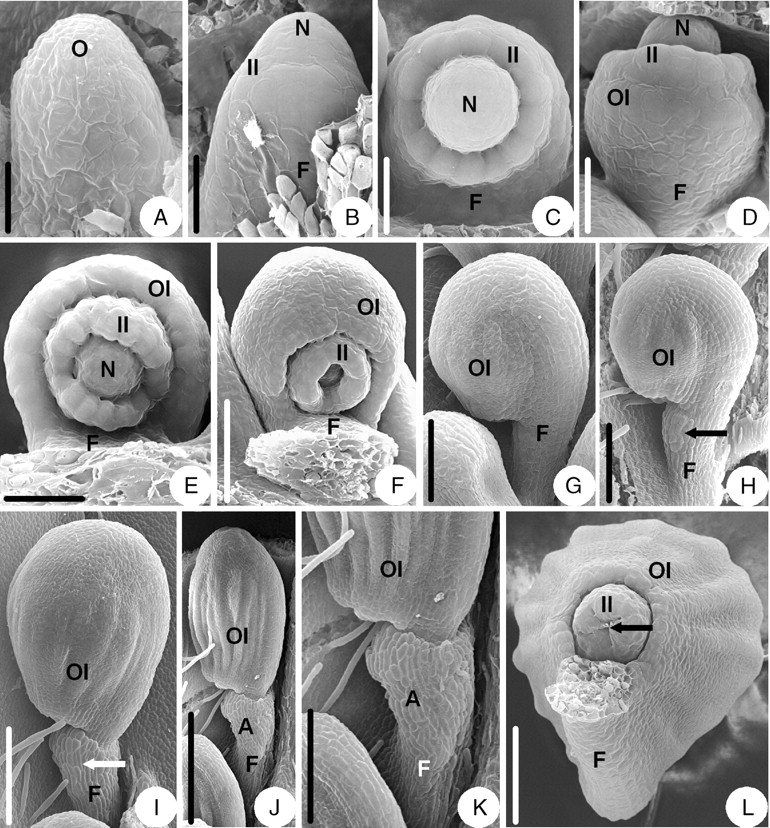
Ovule morphogenesis in Dichocarpum fargesii: (A) ovule primordium; (B) inner integument is initiated annularly; (C) incurved ovules with developing inner integument and funicle; (D) outer integument is initiated semi-annularly; (E) the brim of inner integument becomes slightly lobed; (F) the funicle elongates; (G) ovule with hood-shaped outer integument and a long funicle; (H) an appendage (arrow) arises on the ventral side of the funicle; (I) the appendage (arrow) extends to the dorsal side of the funicle; (J) a mature ovule with ring-shaped appendage; (K) magnified part of the micropylar end shown in (J); (L) a mature ovule – the arrow indicates the micropyle (endostomic) with the lobed inner integument. A, Appendage; F, funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A = 23·1 µm; B = 25 µm; C, D = 30 µm; E = 43 µm; F = 75 µm; G = 100 µm; H = 120 µm; I = 150 µm; J, K = 0·3 mm; L = 100 µm.
Aquilegia yabeana (subfamily Thalictroideae)
The initiation and early development of the ovule in A. yabeana are similar to that of Coptis chinensis (Fig. 4A–D), but there are some differences in later stages. As the outer integument becomes hood-shaped, an appendage consisting of unicellular papillae appears at the base of the ventral side of the funicle and on the placenta (Fig. 4E). Later, the appendage extends along the lateral sides of the funicle and heightens on the placenta (Fig. 4F). The appendage of each ovule enlarges (Fig. 4G), fuses with that of the neighbouring ovule and, finally, becomes a raphe-shaped appendage on the placenta and around the funicles (Fig. 4H, I). The appendage covers about half of the micropyle (Fig. 4J). The brim of the inner integument becomes slightly lobed in the above-mentioned stages (Fig. 4F, G). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle (endostomic) is formed by a four-lobed inner integument and the brim of the outer integument is slightly lobed too (Fig. 4J, K).
Fig. 4.
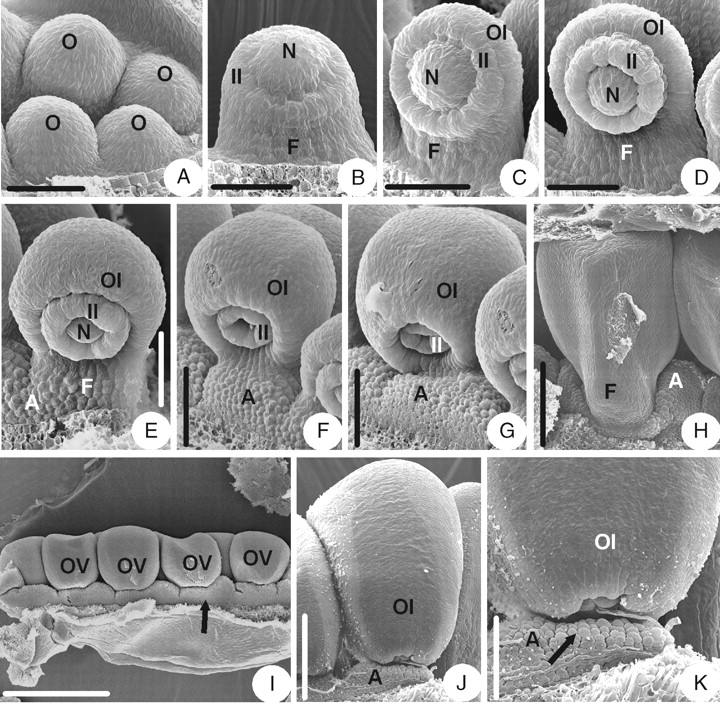
Ovule morphogenesis in Aquilegia yabeana: (A) ovule primordium; (B) inner integument is initiated annularly; (C) outer integument is initiated semi-annularly; (D) developing semi-annular outer integument and cup-shaped inner one; (E) an appendage consisting of unicellular papillae is initiated at the ventral base of the funicle and placenta; (F) slightly lobed brim of inner integument and the appendage extends along the lateral sides of the funicle and heightens on the placenta; (G) ovule with hood-shaped outer integument and lobed inner integument – the appendage enlarges; (H) raphe-shaped appendages; (I) same as in (H), but frontal view, arrow indicates appendage; (J) a mature ovule; (K) magnified part of micropylar end shown in (J), showing micropyle (endostomic) and slightly lobed outer integument – the arrow indicates the appendage which consists of unicellular papillae. A, Appendage; F, funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A, C, D = 43 µm; B = 38 µm; E = 60 µm; F, G, K = 75 µm; H = 0·38 mm; I = 136 µm; J = 176 µm.
Aquilegia ecalcarata (subfamily Thalictroideae)
The initiation and development of the ovule in A. ecalcarata are similar to that of A. yabeana (Fig. 5A–E), but there are some differences between the two species in later stages. The outer integument is semi-annular in the early developmental stages (Fig. 5E) but becomes cupular-shaped when it wraps round the inner integument (Fig. 5J). Before the outer integument becomes as long as the inner integument, an appendage consisting of unicellular papillae appears on the placenta between two ovules (Fig. 5F, G). The appendage extends from the placenta to the base of the ventral side of the funicle (Fig. 5H), and, finally, the appendage fuses with that of the neighbouring ovules and becomes raphe-shaped (Fig. 5K and L). The appendage covers about half of the micropyle (Fig. 5M). With the development of the ovule, the brim of the inner integument becomes lobed (Fig. 5I, J). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle (bistomic) is formed by both a four-lobed inner integument and a slightly lobed outer integument (Fig. 5M, N).
Fig. 5.
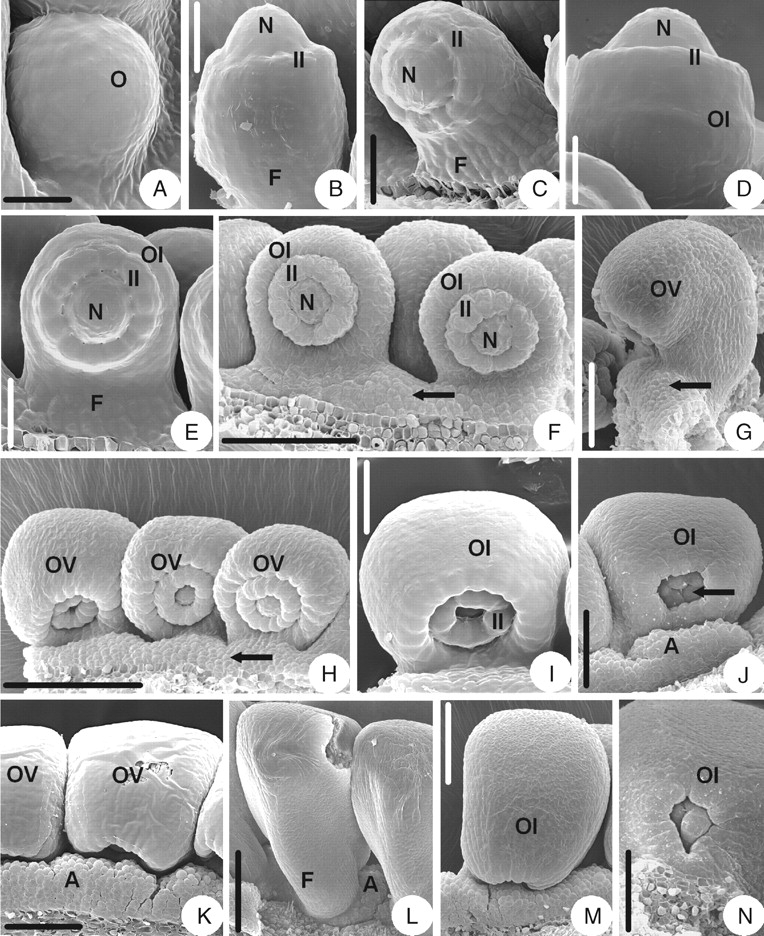
Ovule morphogenesis in Aquilegia ecalcarata: (A) ovule primordium; (B) inner integument is initiated annularly; (C) incurved ovules with developing inner integument and funicle; (D) outer integument is initiated semi-annularly; (E) developing semi-annular outer integument and cup-shaped inner one; (F) the arrow indicates where the appendage is initiated on the placenta; (G) same as in (F), but a lateral view; (H) the appendage (arrow) extends from the placenta to the base of the ventral side of the funicle; (I) slightly lobed brim of the inner integument; (J) cupular-shaped outer integument with the lobed inner integument indicated (arrow); (K) appendages of neighbouring ovules fuse to form a raphe-shaped larger appendage; (L) same as in (K), but dorsal view; (M) a mature ovule; (N) magnified part of micropylar end shown in (M), showing micropyle (biostomic) and slightly lobed outer integument. A, Appendage; F, funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument; OV, ovule. Scale bars: A, D = 17·6 µm; B = 25 µm; C = 27 µm; E = 30 µm; F, M = 86 µm; G, K = 75 µm; H= 136 µm; I = 38 µm; J, N = 60 µm; L = 120 µm.
Caltha palustris (subfamily Ranunculoideae)
The ovule primordium is shortly digitate (Fig. 6A). As the ovule primordium elongates, the inner integument initiates annularly in the middle of the primordium (Fig. 6B). The inner integument eventually becomes cup-shaped (Fig. 6E). After a while, with the ovule primordium curving slightly inward (Fig. 6C), the outer integument arises semi-annularly below the inner integument (Fig. 6D) and eventually becomes hood-shaped (Fig. 6F). The mature ovule is anatropous and bitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle (endostomic) is formed by a three-lobed inner integument. There is no appendage on the ovule (Fig. 6G, H).
Fig. 6.
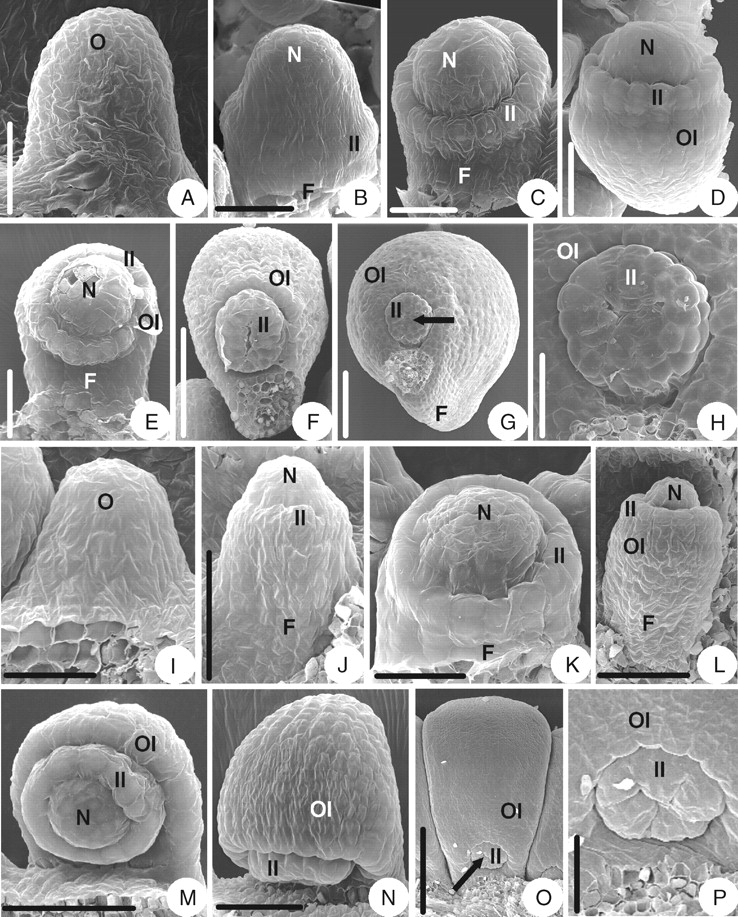
Ovule morphogenesis in Caltha palustris and Trollius buddae. (A–H) Caltha palustris: (A) ovule primordium; (B) inner integument is initiated annularly; (C) incurved ovules with developing inner integument and funicle; (D) outer integument is initiated semi-annularly; (E) developing semi-annular outer integument and cup-shaped inner one; (F) hood-shaped outer integument; (G) a mature ovule with an arrow indicating the micropyle (endostomic). (H) magnified part of the micropylar end shown in (G), showing the lobed inner integument. (I–P) Trollius buddae: (I) ovule primordium; (J) inner integument is initiated annularly; (K) developing inner integument and funicle; (L) outer integument is initiated semi-annularly; (M) developing semi-annular outer integument and cup-shaped inner one; (N) hood-shaped outer integument; (O) a mature ovule with an arrow indicating the micropyle (endostomic); (P) magnified part of the micropylar end shown in (O), showing the lobed inner integument. F, Funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A–C = 38 µm; D = 43 µm; E, P = 50 µm; F = 136 µm; G = 150 µm; H, J, L, N = 60 µm; I, K = 30 µm; M = 75 µm, O = 200 µm.
Trollius buddae (subfamily Ranunculoideae)
The ovule morphogenesis in T. buddae is similar to that of Caltha palustris (Fig. 6I–P).
Aconitum taipeicum (subfamily Ranunculoideae)
The ovule morphogenesis in A. taipeicum is similar to that of Caltha palustris (Fig. 7A–G).
Fig. 7.
Ovule morphogenesis in Aconitum taipeicum, Actaea asiatica and Beesia calthaefolia. (A–G) Aconitum taipeicum: (A) ovule primordium; (B) inner integument is initiated annularly; (C) ovule with cup-shaped inner integument; (D) ovule with hood-shaped integument; (E) lateral view of ovule, showing no appendage presents; (F) a mature ovule with an arrow indicating the micropyle (endostomic); (G) micropylar end showing lobed inner integument. (H–J) Actaea asiatica: (H) developing ovule with cup-shaped inner integument and hood-shaped outer one; (I) a mature ovule with an arrow indicating the micropyle (endostomic); (J) magnified part of the micropylar end shown in (I), showing lobed inner integument. (K–M) Beesia calthaefolia: (K) developing ovule with cup-shaped inner integument and hood-shaped outer one; (L) a mature ovule with an arrow indicating the micropyle (endostomic); (M) magnified part of the micropylar end shown in (L), showing lobed inner integument. F, Funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A, B, J, M = 50 µm; C = 120 µm; D, G, I, K = 200 µm; E = 0·30 mm; F = 231 µm; H, L = 150 µm.
Actaea asiatica (subfamily Ranunculoideae)
The ovule morphogenesis in A. asiatica is similar to that of Aconitum taipeicum (Fig. 7H–J).
Beesia calthaefolia (subfamily Ranunculoideae)
The ovule morphogenesis in B. calthaefolia is similar to that of Aconitum taipeicum (Fig. 7K–M).
Helleborus thibetanus (subfamily Ranunculoideae)
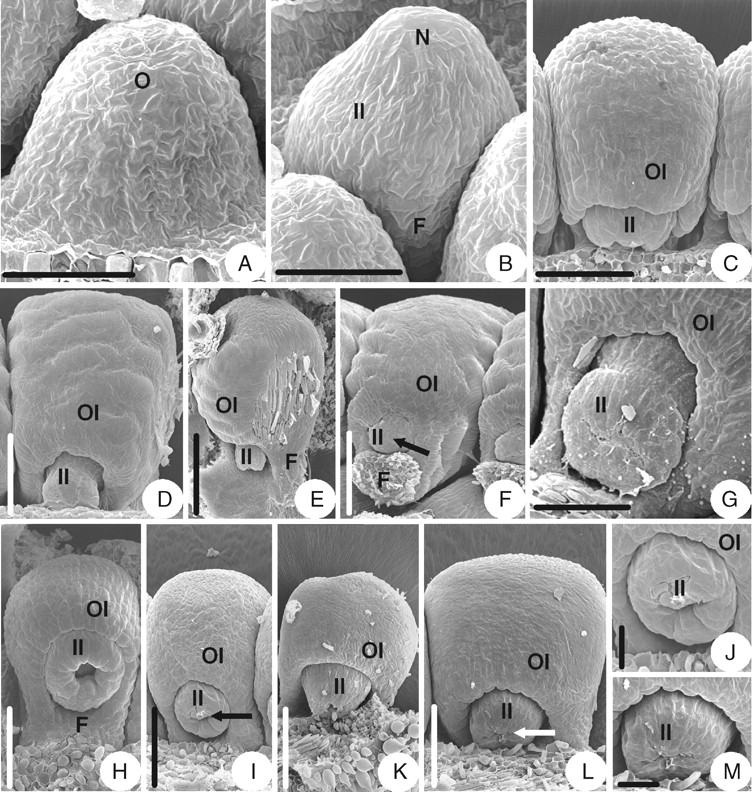
The ovule primordium is shortly digitate (Fig. 8A). As the ovule primordium elongates, the integument is initiated annularly in the middle of primordium (Fig. 8B) and eventually becomes cup-shaped (Fig. 8C, D). When the integument overtops the nucellus, an alary appendage is initiated where the integument and the funicle join (Fig. 8D). The alary appendage grows (Fig. 8E) and converges on the dorsal side of the funicle (Fig. 8F, G), finally becoming hood-shaped (Fig. 8H). The appendage forms an aril in the seed. The mature ovule is anatropous and unitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle is formed by a four- or five-lobed integument (Fig. 8H–J).
Fig. 8.
Ovule morphogenesis in Helleborus thibetanus: (A) ovule primordium; (B) integument is initiated annularly; (C) developing ovule with annular integument; (D) ovule with cup-shaped integument – the arrow indicates where an appendage is initiated where the integument and the funicle join; (E) growing appendage (arrow); (F) the appendage (arrow) converges at the dorsal side of the funicle; (G) the appendage keeps on enlarging; (H) a mature ovule with a hood-shaped appendage enclosing the funicle – an arrow indicates the lobed integument; (I) four-lobed integument; (J) five-lobed integument. A, Appendage; F, funicle; I, integument; N, nucellus; O, ovule primordium. Scale bars: A = 43 µm; B, J = 50 µm; C, I = 60 µm; D, E = 86 µm; F = 120 µm; G = 150 µm; H = 250 µm.
Anemone taipaiensis (subfamily Ranunculoideae)
The integument is initiated semi-annularly on the upper part of the ovule primordium (Fig. 9A). Then the integument grows (Fig. 9B, C) and becomes hood-shaped later (Fig. 9D). In the later developmental stages, the part of the integument that is opposite to the funicle grows faster and becomes a middle lobe (Fig. 9E). After that, two additional lateral lobes appear (Fig. 9F–H). In that process, an appendage consisting of unicellular papillae appears at the ventral base of the funicle and covers the micropyle together with the integument lobes (Fig. 9G). The mature ovule is anatropous and unitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle is formed by a three-lobed integument (Fig. 9F, H).
Fig. 9.
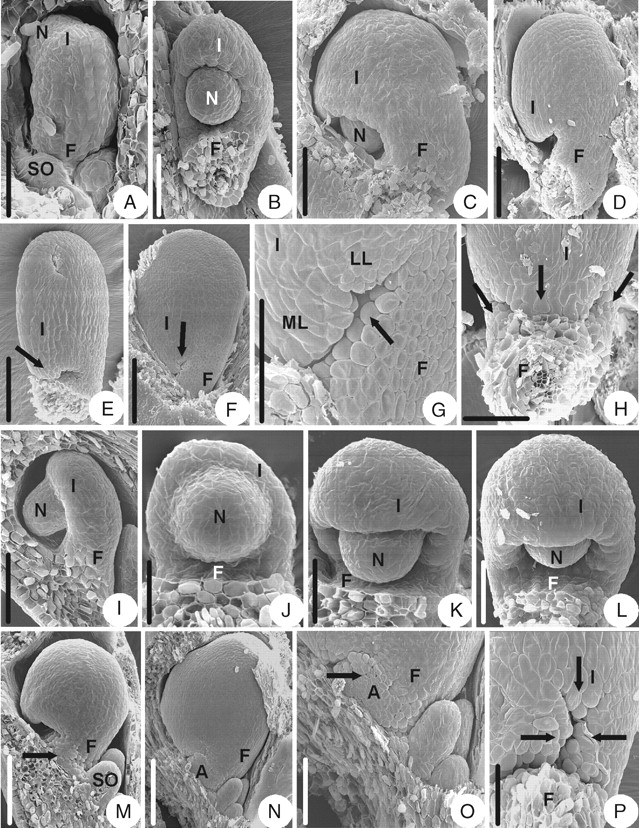
Ovule morphogenesis in Anemone taipaiensis and Clematis montana. (A–H) Anemone taipaiensis: (A) the integument is initiated semi-annularly; (B) developing ovule with semi-annular integument; (C) the integument keeps on enlarging; (D) ovule with hood-shaped integument; (E) the arrow indicates a middle lobe of the integument; (F) a mature ovule with an arrow indicating the lobed integument; (G) appendage (arrow) consisting of unicellular papillae appears at base of the ventral side of funicle; (H) ovule with three-lobed integument (arrows). (I–P) Clematis montana: (I) integument is initiated semi-annularly with incurving ovule – lateral view; (J) same as (I), but frontal view; (K) developing ovule with semi-annular integument; (L) ovule with hood-shaped integument; (M) the appendage (arrow) arises on the funicle; (N) a mature ovule with an appendage; (O) magnified part of the micropylar end shown in (N) with an arrow indicating the appendage which consists of unicellular papillae; (P) ovule with three-lobed integument (arrows). A, Appendage; F, funicle; I, integument; LL, lateral integument lobe; ML, middle integument lobe; N, nucellus; SO, sterile ovule. Scale bars: A, I, L, P = 75 µm; B, C = 100 µm; D, E = 176 µm; F, N = 0·30 mm; G = 120 µm; H = 100 µm; J = 38 µm; K = 50 µm; M, O = 150 µm.
Clematis montana (subfamily Ranunculoideae)
The ovule morphogenesis in C. montana is similar to that of Anemone taipaiensis (Fig. 9I–P), but the appendage is larger than that in A. taipaiensis (Fig. 9N, O).
Oxygraphis glacialis (subfamily Ranunculoideae)
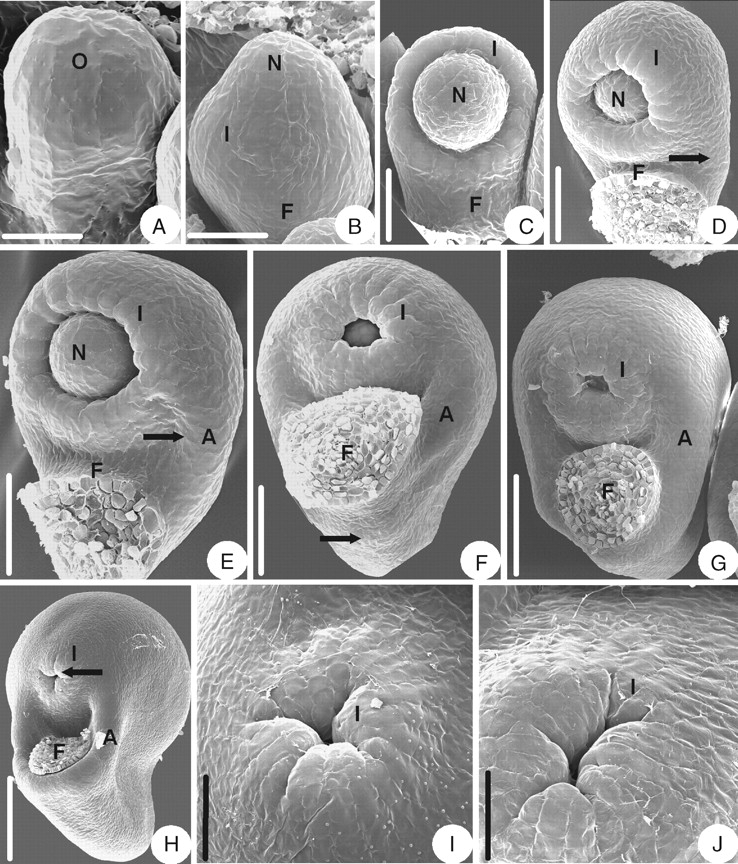
The ovule primordium is shortly digitate (Fig. 10A). The integument is initiated annularly in the middle of the primordium (Fig. 10B). The integument grows faster on the dorsal and lateral sides than on the ventral side (Fig. 10C–E), but it grows almost equally in the later stages (Fig. 10F) and eventually becomes cup-shaped (Fig. 10G, H). The mature ovule is anatropous and unitegmic. The ovule body is almost parallel to the funicle and fuses completely with it. The micropyle is formed by a slightly lobed integument (Fig. 10G–I).
Fig. 10.

Ovule morphogenesis in Oxygraphis glacialis and Ranunculus sieboldii. (A–I) Oxygraphis glacialis: (A) ovule primordium; (B) the integument is initiated annularly; (C) ovule with asymmetrical annular integument, frontal view; (D) same as (C), but lateral view; (E) asymmetrical annular integument; (F) the integument grows almost equally on both sides in the later stage; (G) a mature ovule with symmetrical annular integument, lateral view; (H) same as in (G), but frontal view; (I) micropylar end, showing lobed integument. (J–M) Ranunculus sieboldii: (J) ovule primordium; (K) the integument is initiated annularly; (L) developing ovule with cup-shaped integument; (M) a mature ovule with lobed integument. F, Funicle; I, integument; N, nucellus; O, ovule. Scale bars: A, E = 75 µm; B = 43 µm; C, D, I, J, K = 60 µm; F = 176 µm; G = 150 µm; H, M = 120 µm; L= 86 µm.
Ranunculus sieboldii (subfamily Ranunculoideae)
The ovule morphogenesis in R. sieboldii is similar to that of Oxygraphis glacialis (Fig. 10J–M) but it seems that the mature ovule is hemitropous (Fig. 10M).
Adonis sutchuenensis (subfamily Ranunculoideae)
The ovule morphogenesis in A. sutchuenensis is similar to that of Caltha palustris (Fig. 11A–G), but the outer integument is initiated annularly below the inner integument (Fig. 11C, D) and eventually becomes cupular-shaped (Fig. 11G). The mature ovule is hemitropous (Fig. 11E, F).
Fig. 11.

Ovule morphogenesis in Adonis sutchuenensis and Callianthemum taipaicum. (A–G) Adonis sutchuenensis: (A) ovule primordium; (B) incurved ovule with developing annular inner integument and funicle; (C) outer integument is initiated annularly; (D) same as in (C), but frontal view; (E) a mature ovule; (F) a mature ovule, showing no appendage; (G) micropyle (arrow), showing a four-lobed inner integument and cupular-shaped outer integument. (H–N) Callianthemum taipaicum: (H) ovule primordium; (I) inner integument is initiated annularly; (J) outer integument is initiated semi-annularly; (K) ovule with hood-shaped outer integument and slightly lobed inner one; (L) magnified part of micropylar end shown in (K); (M) a mature ovule with lamellar appendage; (N) magnified part of micropylar end shown in (M), showing the lamellar appendage. A, Appendage; F, funicle; II, inner integument; N, nucellus; O, ovule primordium; OI, outer integument. Scale bars: A–D = 75 µm; E = 150 µm; F, M = 250 µm; G, J = 60 µm; H = 43 µm; I = 50 µm; K = 0·30 mm; L = 120 µm; N = 86 µm.
Callianthemum taipaicum (subfamily Ranunculoideae)
The ovule morphogenesis in C. taipaicum is similar to that of Caltha palustris (Fig. 11H–N), but the ovule of the former has a lamellar appendage on the ventral side of the funicle (Fig. 11M, N).
DISCUSSION
Ovule morphogenesis and the subdivision of Ranunculaceae
The subfamily level
The molecular systematic studies showed that Ranunculaceae could be divided into four subfamilies, i.e. Hydrastidoideae, Coptidoideae, Thalictroideae and Ranunculoideae (Ro et al., 1997; Wang et al., 2005). In the present studies, it was found that the characteristics of ovule morphogenesis in different genera are similar in the early stages, whereas differences could be found in the later stages in subfamilies Coptidoideae, Thalictroideae and Ranunculoideae.
In Coptis of subfamily Coptidoideae, an appendage appears outside the outer integument and the funicle after the ovule body fuses with the funicle. Finally, it covers the ovule completely. This kind of appendage was not found in other genera in the family. In Aquilegia, Dichocarpum and Thalictrum of subfamily Thalictroideae, the appendage arises from the funicle (Dichocarpum) or from the placenta and the funicle (Thalictrum and Aquilegia). The appendages are collar-shaped, or raphe-shaped, or ring-shaped and surrounding the funicles anyhow. In subfamily Ranunculoideae, the ovules of most genera have no appendage except Helleborus, Anemone, Clematis and Callianthemum. In Helleborus, an alary appendage arises at the joint of the integument and the funicle. In Anemone, Clematis and Callianthemum, the appendages arise on the ventral side of the funicle. The ovule morphogenesis studies support the subdivision of the family based on molecular systematics (Jensen et al., 1995; Ro et al., 1997; Wang et al., 2005).
The tribe level: in subfamily Thalictroideae
Based on molecular systematic studies, subfamily Thalictroideae includes the genera with T-type chromosomes (excluding Coptis and Xanthorhiza). Many authors have proposed different ideas on the subdivision of this subfamily. It has been considered that Aquilegia and Dichocarpum are closely related genera (Wang, 1980; Jensen et al., 1995; Tamura, 1995; Ro et al., 1997) and are placed in the same tribe (Wang, 1980; Jensen et al., 1995), but Tamura (1993) placed Aquilegia and Dichocarpum in different tribes in subfamily Isopyroideae and Thalictrum in Thalictroideae. Recently, molecular systematic studies recognized three major groups in Thalictroideae and Thalictrum, Aquilegia and Dichocarpum were included in these three groups, respectively (Wang et al., 2007).
The ovule morphogenesis studies showed that Dichocarpum is obviously different from Aquilegia and Thalictrum. In Dichocarpum, the appendage appears on the upper part of the ventral side of the funicle, and about two-thirds of the funicle fuses with the ovule body, but in Aquilegia and Thalictrum the appendages appear from the placenta and the funicles, and the funicle fuses completely with the ovule body. Therefore, we agreed with the opinion that the genetic relationships of Thalictrum, Dichocarpum and Aquilegia are equal (Wang et al., 2005; Wang and Chen, 2007).
The tribe level: in subfamily Ranunculoideae
Based on the molecular systematic studies (Jensen et al., 1995; Ro et al., 1997; Wang et al., 2005), subfamily Ranunculoideae includes the genera that were traditionally placed in different subfamilies according to fruit types, i.e. achene or follicle (Tamura, 1966, 1993, 1995; Wang, 1980). There is low relativity between the ovule morphogenesis characteristics and the fruit types. The follicular genera, except Helleborus, have bitegmic ovules without an appendage, while the achenial genera have unitegmic ovules with or without appendages. The exceptions are Adonis, which has a bitegmic ovule without an appendage, and Callianthemum, which has a bitegmic ovule with an appendage. Adonis was placed in tribe Ranunculeae based on non-molecular data (Wang, 1980, Tamura, 1993, 1995). However, molecular systematic studies showed that this genus is closely related to Trollius that has follicles (Johansson and Jansen, 1993; Jensen et al., 1995; Johansson, 1995; Hoot et al., 1995; Ro et al., 1997; Wang et al., 2005). The ovule morphogenesis characteristics weakly support the affinity between Adonis and other follicular genera. Callianthemum was considered to have a close affinity with Adonis, based on non-molecular data (Tamura, 1993, 1995), but up to now there have been no molecular data to support this opinion. With respect to ovule morphogenesis, a well-developed funicle appendage appears on the ovules of Callianthemum but not in Adonis. Based on the present studies, it seems that Callianthemum is not related to Adonis.
The ovule morphogenesis characteristics have systematic significance at the tribe or subtribe level. Based on molecular data, Helleborus was placed in a tribe by itself by Jensen et al. (1995). With respect to ovule morphogenesis, Helleborus is special not only in subfamily Ranunculoideae but also in the family because an alary appendage is initiated where the integument and the funicle join. Three genera, Anemone, Clematis and Callianthemum, have funicle appendages. Anemone and Clematis were placed in tribe Anemoneae (Jensen et al., 1995) but in different subtribes. The ovule morphogenesis characteristics support the close affinity between Anemone and Clematis. However, the funicle appendage is well developed in Clematis but is weakly developed in Anemone. This result indicated that it is preferable to place these two genera in separate subtribes.
The relationship of unitegmic and bitegmic ovules
It has been considered that the bitegmic ovules clearly represent a more primitive structure than unitegmic ones, and that the unitegmic ovules are derived from bitegmic ones (Stebbins, 1974; Bouman, 1984; Cronquist, 1988; Takhtajan, 1991; Johri et al., 1992), but the path taken is uncertain. Coulter and Chamberlain (1903) believed that the indifferentiation of the primordium of the two integuments results in the derivation of unitegmic ovule, but Joshi (1939), Endress and Igersheim (2000), González and Rudall (2003) and Kimoto and Tobe (2003) considered that the unitegmic situation was derived from a fusion of two integuments or one of the integuments becoming obsolete. In Ranunculaceae, most of the genera observed have bitegmic ovules, with the exception of Helleborus, Anemone, Clematis, Oxygraphis and Ranunculus. In Helleborus, Oxygraphis and Ranunculus, the morphogenesis of the single integuments is similar to that of the inner integument in bitegmic genera, i.e. the integument is initiated as a ring and develops to a cup-shaped structure. However, the morphogenesis of the single integuments in Anemone and Clematis is similar to the outer integument in the bitegmic genera, i.e. the integument is initiated as a semi-annular ring and develops to a hood-shaped structure. As no trace of the fusion can be found, we considered that the unitegmic ovule might not be derived from the fusion of two integuments. Considering the similarities of the initiation patterns between unitegmic and bitegimic ovules, it is assumed that the unitegmic ovule may be derived from the bitegmic ones through the loss of the outer or the inner integument.
ACKNOWLEDGEMENTS
This project was supported by the National Nature Science Foundation of China (No. 30370095).
LITERATURE CITED
- Adanson M. Familles des plantes. 2 vols. Paris: Vincent; 1763. [Google Scholar]
- APG. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Bocquet G, Bersier JD. La valeur systématique de l'ovule: développements tératologiques. Archives des Sciences. 1960;13:475–496. [Google Scholar]
- Bouman F. The ovule. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer-Verlag; 1984. pp. 123–157. [Google Scholar]
- Coulter JM, Chamberlain CJ. Morphology of angiosperms. New York, NY: Appleton; 1903. [Google Scholar]
- Cronquist A. The evolution and classification of flowering plants. 2nd edn. New York, NY: New York Botanical Garden; 1988. [Google Scholar]
- Eames AJ. Morphology of the angiosperms. New York, NY: McGraw-Hill; 1961. [Google Scholar]
- Endress PK. Origins of flower morphology. In: Wagner GP, editor. The character concept in evolutionary biology. San Diego, CA: Academic Press; 2001. pp. 495–512. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium diversity and systematics of the Laurales. Botanical Journal of the Linnean Society. 1997;125:93–168. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium structure and evolution in basal angiosperms. International Journal of the Plant Sciences. 2000;161(Suppl. 6):S211–S223. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Fahn A. Plant anatomy. 4th edn. London: Pergamon Press; 1990. [Google Scholar]
- González F, Rudall PJ. Structure and development of the ovule and seed in Aristolochiaceae, with particular reference to Saruma. Plant Systematics and Evolution. 2003;241:223–244. [Google Scholar]
- Hoot SB, Johansson JT, Kosuge K. Phylogeny of the Ranunculaceae based on preliminary atpB, rbcL and 18S nuclear ribosomal DNA sequence data. Plant Systematics and Evolution. 1995;9(Suppl.):241–251. [Google Scholar]
- Hutchinson J. Contributions towards a phylogenetic classification of flowering plants 1. Kew Bulletin. 1923;1923:65–89. [Google Scholar]
- Igersheim A, Endress PK. Gynoecium diversity and systematics of the Magnoliales and winteroids. Botanical Journal of the Linnean Society. 1997;124:213–271. [Google Scholar]
- Igersheim A, Endress PK. Gynoecium diversity and systematics of the paleoherbs. Botanical Journal of the Linnean Society. 1998;127:289–370. [Google Scholar]
- Igersheim A, Buzgo M, Endress PK. Gynoecium diversity and systematics of basal monocots. Botanical Journal of the Linnean Society. 2001;136:1–65. [Google Scholar]
- Imaichi R, Kato M, Okada H. Morphology of the outer integument in three primitive angiosperm families. Canadian Journal of Botany. 1995;73:1242–1249. [Google Scholar]
- Jensen U. Serologishes Beiträge zur Systematik der Ranunculaceae. Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie. 1968;88:204–310. [Google Scholar]
- Jensen U, Hoot SB, Johansson JT, Kosuge K. Systematics and phylogeny of the Ranunculaceae – a revised family concept on the basis of molecular data. Plant Systematics and Evolution. 1995;9(Suppl.):273–280. [Google Scholar]
- Johansson JT. A revised chloroplast DNA phylogeny of the Ranunculaceae. Plant Systematics and Evolution. 1995;9(Suppl.):253–261. [Google Scholar]
- Johansson JT, Jansen RK. Chloroplast DNA variation and phylogeny of the Ranunculaceae. Plant Systematics and Evolution. 1993;187:29–49. [Google Scholar]
- Johri BM, Ambegaokar KB, Srivastava PS. Comparative embryology of angiosperms. 1 and 2. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Joshi AC. Morphology of Tinospora cordifolia, with some observations on the origin of the single integument, nature of synergidae, and affinities of the Menispermaceae. American Journal of Botany. 1939;26:433–439. [Google Scholar]
- Kimoto Y, Tobe H. Embryology of Siparunaceae (Laurales): characteristics and character evolution. Journal of Plant Research. 2003;116:281–294. doi: 10.1007/s10265-003-0091-9. [DOI] [PubMed] [Google Scholar]
- Langlet O. Über Chromosomenverhältnisse und Systematik der Ranunculaceae. Svensk Botanisk Tidskrift. 1932;26:381–400. [Google Scholar]
- Matsui M, Imaichi R, Kato M. Ovular development and morphology of some Magnoliaceae species. Journal of Plant Research. 1993;106:297–304. [Google Scholar]
- Meeuse ADJ, Bouman F. The inner integument – its probable origin and homology. Acta Botanica Neerlandica. 1974;23:237–249. [Google Scholar]
- Ro KE, Keener CS, Mcpheron BA. Molecular phylogenetic study of the Ranunculaceae: utility of the nuclear 26S Ribosomal DNA in inferring intrafamilial relationships. Molecular Phylogenetics and Evolution. 1997;8:117–127. doi: 10.1006/mpev.1997.0413. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge: Belknap Press of Harvard University Press; 1974. [Google Scholar]
- Stewart WN, Rothwell GW. Paleobotany and the evolution of plants. 2nd edn. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Takhtajan A. Evolutionary trends in flowering plants. New York, NY: Columbia University Press; 1991. [Google Scholar]
- Tamura M. Morphology, ecology and phylogeny of the Ranunculaceae. VI. Science Reports Osaka University. 1966;15:13–35. [Google Scholar]
- Tamura M. Ranunculaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants. Vol. 2. Berlin: Springer-Verlag; 1993. pp. 563–583. [Google Scholar]
- Tamura M. Ranunculaceae. In: Engler A, Prantl K, Hiepko P, editors. Die natürlichen pflanzenfamilien. Berlin: Duncker & Humblot; 1995. pp. 1–555. 17 a IV. [Google Scholar]
- Taylor WD. Angiosperm ovules and carpels: their characters and polarities, distribution in basal clades, and structural evolution. Postilla. 1991;208:1–40. [Google Scholar]
- Tobe H, Jaffré T, Raven PH. Embryology of Amborella (Amborellaceae): descriptions and polarity of character states. Journal of Plant Research. 2000;113:271–280. [Google Scholar]
- Umeda A, Imaichi R, Kato M. Ovular development and morphology of the outer integument of Magnolia grandiflora (Magnoliaceae) American Journal of Botany. 1994;81:361–367. [Google Scholar]
- Wang WC. Flora Reipublicae Popularis Sinicae. Vol. 28. Beijing: Science Press; 1980. pp. 240–241. [Google Scholar]
- Wang W, Chen ZD. Generic level phylogeny of Thalictroideae (Ranunculaceae) – implications for the taxonomic status of Paropyrum and petal evolution. Taxon. 2007;56:811–821. [Google Scholar]
- Wang W, Li RQ, Chen ZD. Systematic position of Asteropyrum (Ranunculaceae) inferred from chloroplast and nuclear sequences. Plant Systematics and Evolution. 2005;255:41–54. [Google Scholar]
- Yamada T, Imaichi R, Kato M. Developmental morphology of ovules and seeds of Nymphaeales. American Journal of Botany. 2001;a 88:963–974. [PubMed] [Google Scholar]
- Yamada T, Tobe H, Imaichi R, Kato M. Developmental morphology of the ovules of Amborella trichopoda (Amborellaceae) and Chloranthus serratus (Chloranthaceae) Botanical Journal of the Linnean Society. 2001;b 137:277–290. [Google Scholar]
- Yamada T, Imaichi R, Kato M. The outer integument and funicular outgrowth complex in the ovule of Magnolia grandiflora (Magnoliaceae) Journal of Plant Research. 2003;116:189–198. doi: 10.1007/s10265-003-0086-6. [DOI] [PubMed] [Google Scholar]


