Abstract
Background and Aims
Many orchid flowers have glands called elaiophores and these reward pollinating insects with oil. In contrast to other reward-producing structures such as nectaries, the anatomy of the elaiophore and the process of oil secretion have not been extensively studied. In this paper, elaiophore structure is described for two members of Oncidiinae, Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst.
Methods
Elaiophores of both species were examined using light microscopy, scanning electron microscopy and transmission electron microscopy.
Key Results and Conclusions
In flowers of Oncidium trulliferum and Ornithophora radicans, oil is secreted by morphologically distinct elaiophores associated with the labellar callus. However, in O. trulliferum, elaiophores also occur on the lateral lobes of the labellum. In both these species, the epithelial elaiophores are composed of a single layer of palisade-like epidermal cells and a distinct subepithelial layer. Secretory elaiophore cells may contain numerous, starchless plastids, mitochondria and smooth endoplasmic reticulum profiles. In O. trulliferum, the cytoplasm contains myelin-like figures but these are absent from O. radicans. In the former species, cavities occur in the cell wall and these presumably facilitate the passage of oil onto the elaiophore surface. In O. radicans, the accumulation of oil between the outer tangential wall and the cuticle causes the latter to become distended. Since it is probable that the full discharge of oil from the elaiophores of O. radicans occurs only when the cuticle is ruptured by a visiting insect, this may contribute towards pollinator specificity. The structure of the elaiophore in these species resembles both that found in previously investigated species of Oncidiinae and that of certain members of the Malpighiaceae.
Key words: Elaiophore, Oncidium trulliferum, Ornithophora radicans, Orchidaceae, oil secretion, pollination
INTRODUCTION
In Orchidaceae, floral rewards including nectar, fragrances, oils and a number of other compounds, such as resin-like substances and waxes, all play an important role in pollination (van der Pijl and Dodson, 1969; Proctor and Yeo, 1975; Dressler, 1990; Proctor et al., 1996). In contrast to many of these, nectar and oil belong to a class of rewards characterized by their great nutritional value.
In many orchids, oil is produced in specialized, secretory, floral glands called elaiophores. Floral oils are known to be secreted by certain African and Neotropical orchids and many of these species share a number of floral features such as yellow-green or yellow and pink colouration, often with brown markings and either the complete absence of fragrance or the presence of a sharp, pungent scent. Oils secreted by New World orchids are usually gathered by bees of the genera Tetrapedia, Paratetrapedia and Centris (Buchmann, 1987; Singer and Cocucci, 1999), whereas African oil-producing orchids are mainly visited by Rediviva spp. (Steiner and Whitehead, 1991; Steiner, 1989, 1993, 1998; Pauw, 2006). Once gathered, floral oil is either mixed with pollen and fed to developing larvae (Michener, 2000) or used in the nest to seal and waterproof cells (Cane et al., 1983; Endress, 1994; Silvera, 2002, and references therein; Singer et al., 2006). In orchids, floral elaiophores are located upon the petals (e.g. Ornithocephalinae; Toscano de Brito, 2001), on the labellar callus (e.g. Oncidium Sw; Buchmann, 1987; Toscano de Brito, 2001; Stpiczyńska et al., 2007), the lip-appendage (e.g. Pterygodium Sw; Pauw, 2006) or the column (e.g. Grobya amherstiae Lindl; Mickeliunas et al., 2006).
Elaiophores are of two types; trichomal and epithelial elaiophores (Vogel, 1974). Trichomal elaiophores occur in Disperis Sw. (Buchmann, 1987), certain species of Ornithocephalus Hook. (Toscano de Brito, 2001), Grobya amherstiae (Mickeliunas et al., 2006), Zygostates Lindl. (Toscano de Brito, 2001) and Phymatidium delicatulum Lindl. (Reis et al., 2006). In trichomal elaiophores, oil is secreted by specialized hairs and in some orchids, such as Phymatidium falcifolium Lindl., these trichomes are capitate. Epithelial elaiophores are more common and occur in many species of Oncidium, where their resemblance to those of Malpighiaceae is thought to be due to convergence (Singer and Cocucci, 1999; van der Cingel, 2001; Singer et al., 2006; Stpiczyńska et al., 2007; Davies and Stpiczyńska, 2008).
The chemical composition of the oily secretion is well known for some species (Reis et al., 2000, 2003, 2006). For example, analysis of oil derived from flowers of certain orchids belonging to the ‘Gomesa R. Br. clade’, a monophyletic Oncidiinae group restricted to eastern Brazil and north-eastern Argentina (Reis et al., 2000; Singer et al., 2006), which includes both Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst, revealed the presence of diacylglycerols. Moreover, similar compounds occur in species formerly assigned to Ornithocephalinae, such as Phymatidium tillandsioides Barb. Rodr. (syn. Phymatidium falcifolium) and Zygostates lunata Lindl. (Reis et al., 2000, 2003). The major component of the floral oil of Ornithophora radicans, oncidinol, has been identified as (2S,3′R,7′R)-1-acetyl-2-(3′,7′-diacetoxy-eicosanoyl)-glycerol (Reis et al., 2003). Similarly, in Oncidium loefgrenii Cogn. and Gomesa, also members of the ‘Gomesa clade’, the secreted oil is thought to contain acyl glycerols (R. B. Singer, pers. comm.). Yet, despite rigorous analyses of orchid elaiophore secretions, the anatomy of the elaiophore itself, with few exceptions (Singer and Cocucci, 1999; Stpiczyńska et al., 2007; Davies and Stpiczyńska, 2008), has been largely neglected. Indeed, detailed ultrastructural studies of the orchid elaiophore are known for only three species, namely Trichocentrum cavendishianum (Bateman) M. W. Chase & N. H. Williams, Gomesa recurva R. Br. and Oncidium loefgrenii (now transferred to Menezesiella Chiron & V. P. Castro; Chiron and Castro Neto, 2006).
It is thought that the structure of the elaiophore and the chemical composition of its secretion, like that of the nectary (Smets, 1986; Smets et al., 2000; Rudall, 2002), may have taxonomic significance (Toscano de Brito, 2001; Singer et al., 2006, and references therein). The aim of this study is to compare the structure of elaiophores in two contrasting species of the ‘Gomesa clade’ of Oncidiinae, namely, Ornithophora radicans and Oncidium trulliferum.
MATERIALS AND METHODS
Fresh flowers of Oncidium trulliferum Lindl. (Accession No. S20060407) and Ornithophora radicans (Rchb.f.) Garay & Pabst (Accession No. S19960344) were obtained from the Swansea Botanical Complex, Swansea, UK. Both are Brazilian species and belong to the ‘Gomesa clade’ of Oncidiinae.
The flower of O. trulliferum is greenish-yellow or golden with chestnut markings. The labellum is 3-lobed. The lower edge of each of the golden, lateral lobes of the labellum is thickened and marked chestnut and this coloration continues onto the mid-lobe resulting in the formation of a V-shaped, chestnut band. The rounded mid-lobe of the labellum is itself 3-lobed. The callus is complex, butterfly-shaped, lobed and spotted chestnut. The column is yellow with a prominent tabula infrastigmatica.
Ornithophora Barb. Rodr. is a monotypic genus. Flowers of O. radicans have greenish-yellow or whitish-green tepals. The labellum is 3-lobed, has a slender claw and the white lamina is transversely lunate or sagittate to semi-rotund with a pair of acute, backwardly pointing auricles. The yellow callus, borne on the claw, comprises fleshy lobules, whereas the disc is 2–6-cristate at its base. The column is purple and, like that of O. trulliferum, bears a tabula infrastigmatica.
The position of the elaiophore in fresh, intact flowers of Oncidium trulliferum and Ornithophora radicans was determined using an Olympus SZX12 stereo-microscope. Hand-cut sections through fresh elaiophore tissue were tested for starch and lipids using IKI and a saturated alcoholic solution of Sudan III or 0·3 % ethanolic Sudan Black, respectively. Pieces of elaiophore tissue (approx. 1 mm thick) were fixed in 2·5 % glutaraldehyde/4 % formaldehyde in phosphate buffer (pH 7·4; 0·1 m) for 4 h at 4 °C, washed in phosphate buffer and post-fixed in 1 % osmium tetroxide at 0 °C for 1·5 h. The fixed material was then dehydrated using a graded ethanol series and infiltrated and embedded in Spurr's resin. Semi-thin sections (0·7–1 µm thick) were stained for general histology using 0·25 % toluidine blue O in 0·25 % (w/v) aqueous sodium tetraborate solution (TBO). Micrometry and photomicrography of the elaiophores were undertaken using a Nikon Eclipse 600 microscope with screen measurement version 4·21 software. Transmission electron microscopy (TEM) sections were cut at 60 nm using a Reichert Om U-3 ultramicrotome and a glass knife. Sections were stained with uranyl acetate (40 min) and lead citrate (10 min) (Reynolds, 1963) and examined using a TESLA BS-340 transmission electron microscope at an accelerating voltage of 60 kV. For SEM, fixed pieces of labellum were dehydrated and subjected to critical-point drying using liquid CO2. They were then sputter-coated with gold and examined by means of a TESLA BS-300 at an accelerating voltage of 25 kV.
RESULTS
Oncidium trulliferum
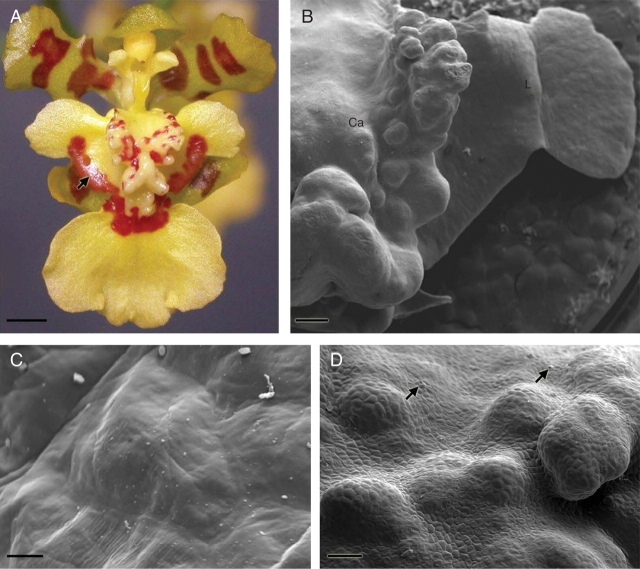
The elaiophores of Oncidium trulliferum are located symmetrically on the lower margins of the lateral lobes of the labellum and also on the callus (Fig. 1). Elaiophore activity commences at the beginning of anthesis and continues throughout the whole lifespan of the flower, by which time the labellum is completely covered with secretion. Small droplets of secretion occur on the callus and between the callus and the elaiophores at the base of the lateral lobes of the labellum, where they sometimes take on a stringy appearance (Fig. 1A and C). The whole of that area, including callus, as well as the pollinaria and the margins of tepals, stain with Sudan III and Sudan Black. Oily droplets within the elaiophore cells stain intensely with Sudan III (Fig. 2A).
Fig. 1.
Flower and elaiophore surface (SEM) of Oncidium trulliferum. (A) Flower with surface of elaiophore on lateral lobe of labellum covered with oil (arrow). Scale bar = 1·6 mm. (B) Part of callus (Ca) and lateral lobe of labellum (L) – the sites of floral oil secretion in this species. Scale bar = 1·0 mm. (C) Folds of cuticle covering epithelial cells of lateral lobe elaiophore. Notice the absence of pores or breaks in the cuticle. Scale bar = 10 µm. (D) Labellar callus showing position of stomata (arrows). Scale bar = 200 µm.
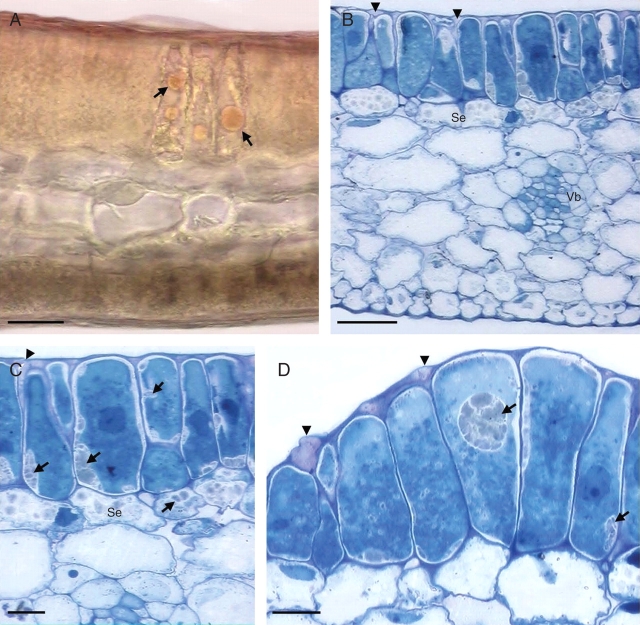
Fig. 2.
Sections through elaiophores (A–C, lateral lobe elaiophore; D, callus elaiophore) of Oncidium trulliferum (LM). (A) Fresh, hand-sectioned elaiophore tissue showing palisade-like secretory cells containing lipid droplets (arrows) stained with Sudan III. Scale bar = 20 µm. (B) Section of elaiophore tissue showing secretory epithelium and subepithelial layer (Se). Note the position of cavities in the cell wall (arrow heads) and the vascular bundle (Vb). Scale bar = 50 µm. (C) Detail of secretory epithelium and subepithelial cells (Se) of elaiophore tissue showing position of myelin-like figures (arrows). The position of the cavity in the cell wall is indicated by an arrowhead. Scale bar = 20 µm. (D) Detail of secretory epithelium showing myelin-like figures (arrows) and cell wall cavities (arrow heads). Scale bar = 20 µm.
Elaiophores on both the callus and lateral lobes of the labellum consist of a single layer of secretory epithelium beneath which occurs a single layer of small, subepithelial cells (Fig. 2B–D). Epithelial cells from both types of elaiophore have mean dimensions of 51·44 µm × 27·78 µm and a palisade-like arrangement, whereas the subepithelial layer has a mean thickness of 26·72 µm.
The secretory tissue of the lateral lobe elaiophore has a vascular supply (Fig. 2B) but only the glandular epidermis covering the callus bears stomata (Fig. 1D). The outer tangential walls of secretory epidermal cells have a mean thickness of 2·41 µm, whereas radial walls are thinner and have a mean thickness of 0·68 µm. Staining with TBO showed that the walls mainly consist of cellulose, and TEM observations revealed that cellulose microfibrils in the outer tangential wall are loosely arranged. The matrix thus formed contains cavities of irregular shape (Fig. 3A). These cavities vary in size from 0·68 µm to 2·29 µm. The cuticle does not become detached from the cell wall as secreted oil accumulates between it and the secretory epithelium (Figs 1C, D, 2B–D and 3A).
Fig. 3.
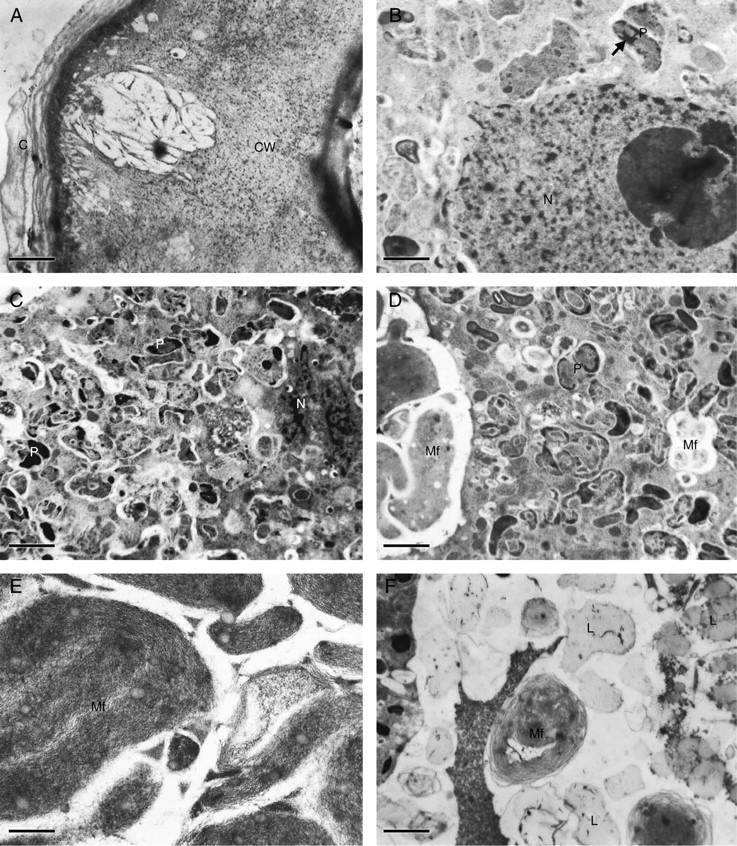
Elaiophore cells (A, D–F, lateral lobe elaiophore; B and C, callus elaiophore) of Oncidium trulliferum (TEM). (A) Outer tangential cell wall (CW) with reticulum of cellulose microfibrils and associated cavity. Note also the lamellate cuticle (C) on cell wall surface. Scale bar = 1 µm. (B) Epithelial cell with nucleus (N) and perinuclear plastids (P) containing darkly stained material (arrow). Scale bar = 1 µm. (C) Nucleus (N) surrounded by numerous plastids (P) containing irregularly distributed, darkly stained material. Scale bar = 1 µm. (D) Epithelial cell showing plastids (P) and myelin-like figures (Mf). Scale bar = 1 µm. (E) Detail of myelin-like figures (Mf) containing osmiophilic globules. Scale bar = 0·5 µm. (F) Subepithelial cell containing lipid droplets (L) and myelin-like figures (Mf). Scale bar = 2·5 µm.
Cellulose microfibrils within the distal part of radial walls are also loosely arranged but here, in contrast to the outer tangential wall, cavities are absent. Epithelial cells possess a prominent, centrally placed nucleus (Figs 2C and 3B) and electron-dense, highly granular cytoplasm with numerous mitochondria and plastids. Plastids are irregularly shaped and contain unevenly distributed osmiophilic material (Fig. 3C, D). Myelin-like, membranous configurations occur both in the cytoplasm and in vacuoles, especially in the parietal cytoplasm, the basal part of the cell and in the periplasmic space (Figs 2C, D and 3D, E). These membranous figures enclose small osmiophilic globules. Myelin-like structures also occur within larger vacuoles of subepithelial cells (Figs 2C and 3F) and these cells also contain large lipid droplets (Fig. 3F).
Ornithophora radicans
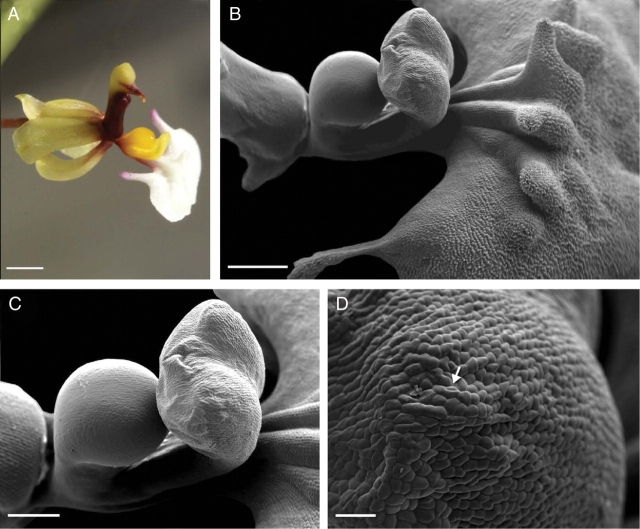
In Ornithophora radicans, the floral elaiophore is located on the complex labellar callus, just behind the labellar crest (Fig. 4A–D). The white lamina of the labellum stained with Sudan III and Sudan Black but the parts that stained most intensely were the auricles and the spherical lobule and associated callus structures just above the ‘waist’ of the labellum. As in the previous species, the callus functions as an epithelial elaiophore and the secretory epithelium is composed of a single layer of cells (mean cellular dimensions 47·34 µm × 15·39 µm) in palisade arrangement (Fig. 5A–C). Secretory epithelial cells cover the whole surface of the callus outgrowths. The cuticle covering the outer tangential wall becomes detached as secreted oil accumulates beneath its surface (Fig. 5A). Under the secretory epithelium occur two or three layers of cells with large amyloplasts (Fig. 5B, C). The underlying cells of the ground parenchyma also contain amyloplasts but in smaller numbers. Idioblasts with raphides occur amongst the innermost parenchyma cells. The secretory tissue has a vascular supply consisting of both xylem and phloem (Fig. 5B). Epithelial cells have relatively thin, outer tangential and radial walls (mean thickness, 0·75 µm and 0·5 µm, respectively) and thick cuticle (Figs 5C and 6A). Cavities are absent from the outer tangential cell wall. Dissolution of the middle lamella of radial walls results in the formation of intercellular spaces and frequently these contain darkly stained, osmiophilic material (Figs 5C and 6B–E). The centrally located nucleus is surrounded by numerous, small, starchless plastids with poorly developed lamellae and sparse plastoglobuli (Fig. 6D–F). Smooth endoplasmic reticulum and mitochondria predominate in the densely stained, granular cytoplasm (Fig. 6B–F) and, in contrast to O. trulliferum, no myelin-like structures were observed.
Fig. 4.
Flower and elaiophore surface (SEM) of Ornithophora radicans. (A) Flower with yellow callus, the site of the labellar elaiophore. Scale bar = 1·7 mm. (B) Labellum showing labellar crest and callus outgrowths bearing elaiophore tissue. Scale bar = 900 µm. (C) Lobed, labellar elaiophore showing wrinkled surface. Scale bar = 500 µm. (D) Detail of elaiophore surface showing distended cuticle (arrow). Scale bar = 50 µm
Fig. 5.
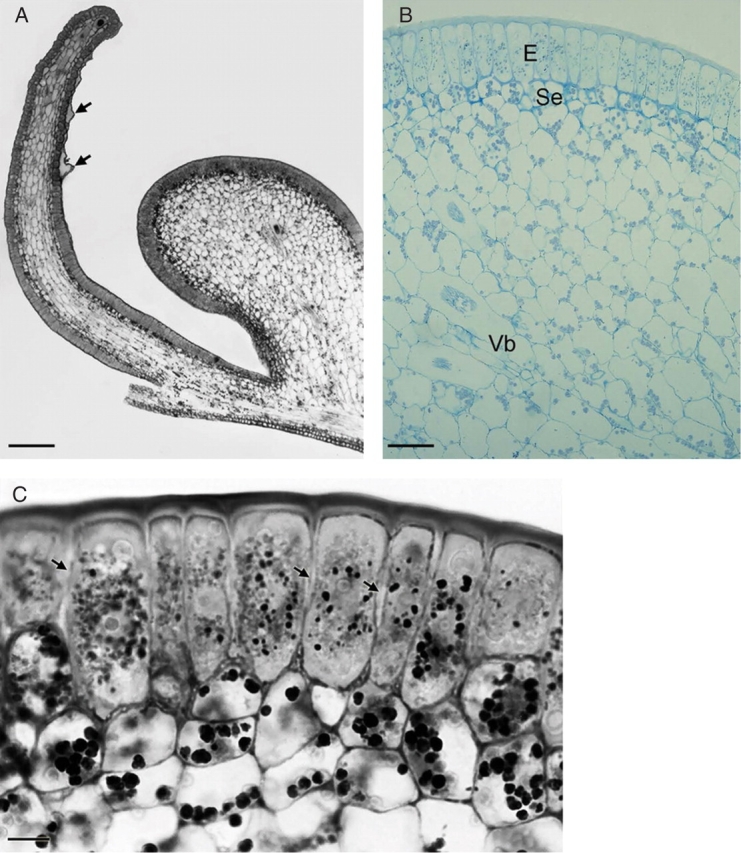
Sections of elaiophore tissue of Ornithophora radicans (LM). (A) Section of callus outgrowths showing position of elaiophore epithelium and distended cuticle (arrows). Scale bar = 195 µm. (B) Section through callus showing palisade-like, secretory epithelium (E), subepithelial cells (Se) and vascular bundle (Vb) embedded in ground parenchyma. Scale bar = 10 µm. (C) Epithelial cells in palisade arrangement with intercellular spaces (arrows) and centrally placed nuclei surrounded by darkly stained plastids. Beneath this secretory tissue occur subepithelial cells with amyloplasts. Scale bar = 15 µm.
Fig. 6.
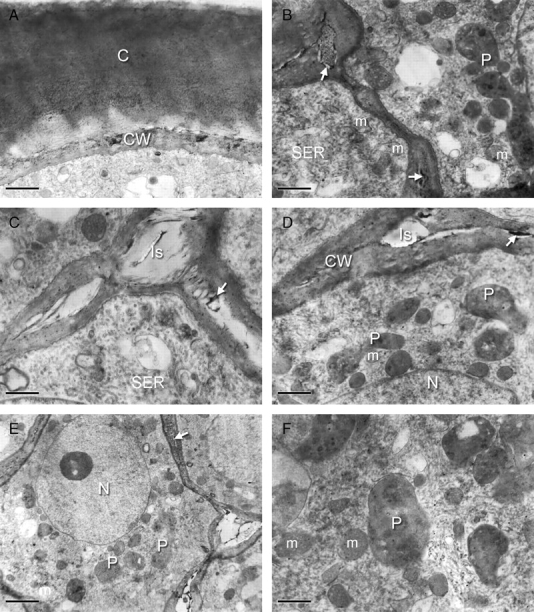
Ultrastructure of elaiophore epithelial cells of Ornithophora radicans (TEM). (A) Outer cell wall (CW) with dark deposits covered with a thick layer of reticulate cuticle (C). Scale bar = 0·7 µm. (B) Intercellular spaces with osmiophilic content (arrows). Note that the cytoplasm mainly contains smooth endoplasmic reticulum (SER), plastids (P) and mitochondria (m). Scale bar = 0·7 µm. (C) Large intercellular spaces (Is) with dark inclusions (arrow) and cytoplasm with numerous smooth endoplasmic reticulum (SER) profiles. Scale bar = 1 µm. (D) Secretory cytoplasm with small, starchless plastids (P) and mitochondria (m) associated with the nucleus (N). Note the cell wall (CW) with dark inclusion (arrow). Scale bar = 1 µm. (E) Nucleus (N) with associated plastids (P) and mitochondria (m). Note the large intercellular spaces and the presence of dark inclusions in the cell wall (arrow). Scale bar = 3 µm. (F) Mitochondria (m) and typical, starchless plastids (P) with few plastoglobuli. Scale bar = 0·5 µm.
DISCUSSION
Elaiophores of O. trulliferum and O. radicans are structurally well defined. In O. radicans, this gland is particularly elaborate and obvious. Elaiophores of certain other oil-producing orchids such as Gomesa recurva show no obvious morphological differentiation, even though oil is copiously produced (Stpiczyńska et al., 2007). In both O. radicans and O. trulliferum, elaiophores are located on the labellar callus although, in the latter species, they also occur on the lateral lobes of the labellum. The callus is also responsible for oil secretion in G. recurva and O. loefgrenii, but not in O. paranaense Kraenzl. and T. cavendishianum, where the elaiophores occur solely on the lateral lobes (Singer and Cocucci, 1999; Stpiczyńska et al., 2007). That the callus is involved in oil secretion in O. trulliferum, O. loefgrenii, G. recurva and O. radicans is noteworthy since these four taxa, unlike T. cavendishianum, belong to the ‘Gomesa clade’. Even so, the diverse degree of callus complexity found in this group would suggest that, even within the clade, the callus-elaiophore may have evolved along a number of lines in response to pollinator selection. Moreover, the likelihood that these plants are pollinated by different insect species is further supported by the presence of a tabula infrastigmatica in some, such as O. trulliferum and O. radicans, but not others. Another member of this clade, Oncidium pubes Lindl., was reported to be pollinated by female Tetrapedia spp. (cited in Singer et al., 2006). Although R. B. Singer (pers comm.) observed Trigona spp. visiting flowers of G. recurva, he nevertheless doubts that they are responsible for pollinating this orchid as they are too small to dislodge its pollinaria. Instead, he proposes that G. recurva and O. loefgrenii are pollinated by species of Monoeca and Tetrapedia, respectively. Although there is currently no concrete evidence, it is thought that O. radicans may also be pollinated by a species of Tetrapedia (R. B. Singer, pers comm.).
In O. trulliferum and O. radicans, the elaiophore is of the epithelial type and possesses a single layer of palisade-like secretory cells. The single-layered subepithelium of O. trulliferum contains lipid bodies and this perhaps indicates that it too is involved in elaiophore activity. Similar anatomical organization is found in T. cavendishianum and O. loefgrenii but here, the subepithelial layer is less distinct (Stpiczyńska et al., 2007). Conversely, a prominent subepithelial layer occurs in the elaiophores of some malpighiaceous taxa such as Hiptage sericea Hook. (Arumugasamy et al., 1993) and Banisteriopsis variabilis B. Gates (Attala and Machado, 2003).
The elaiophore cuticle of O. radicans becomes distended as secreted oil accumulates beneath its surface. It is probable that, in this species, full discharge of oil is not achieved until the cuticle is ruptured by a visiting insect and this may contribute towards pollinator specificity. Distension of the cuticle is also known to occur in T. cavendishianum (Stpiczyńska et al., 2007; Davies and Stpiczyńska, 2008) and Dinemandra ericoides A. Juss. (Malpighiaceae), which is pollinated by species of Centris (Cocucci et al., 1996). On the other hand, the cuticle of O. trulliferum, O. loefgrenii and Gomesa, which is smooth, shows no evidence of distension in response to oil accumulation (Stpiczyńska et al., 2007).
In O. trulliferum, the outer tangential cell walls contain cavities. Walls of similar structure also occur in T. cavendishianum and O. loefgrenii but not G. recurva. It is thought that these cavities may aid the transport of hydrophobic moieties across the hydrated cell wall, much in the same way that hydrophobic compounds are transported during the formation of cuticle (Kunst and Samuels, 2003, and references therein). Similar cavities also occur in elaiophore cell walls of the malpighiaceous Dinemandra ericoides (Cocucci et al., 1996). Intercellular spaces between the radial cell walls of O. radicans may also be involved in the transport or accumulation of oily secretions.
Epithelial cells of both O. trulliferum and O. radicans contain several small vacuoles and a centrally placed nucleus but, whereas smooth endoplasmic reticulum predominates in O. radicans, this is not the case for O. trulliferum. Nevertheless, numerous mitochondria and chromoplasts that lack starch but contain unevenly distributed osmiophilic material are present in both species. In O. radicans, the subsecretory parenchyma contains starch. Although no starch was observed for O. trulliferum during the secretory stage, this does not preclude the possibility that it may be present during the pre-secretory stage.
Osmiophilic globules occur as enclaves within myelin-like bodies of O. trulliferum or between the concentric membranes themselves. Similar membranous whorls were observed in epithelial cells of T. cavendishianum (Stpiczyńska et al., 2007) and in Hiptage sericea (Arumugasamy et al., 1993). Myelin-like structures containing dense, globular material also occur as a thylakoid plexus in the epidermal chromoplasts of the petals of Narcissus pseudonarcissus (Whatley and Whatley, 1987). They are also found in the periplasmic space and in vacuoles of other types of secretory tissue, such as nectaries (Eriksson, 1977; Schnepf and Deichgräber, 1984; Wist and Davis, 2006), gum-resin ducts (Bhatt, 1987; Fahn, 2000) and leaf secretory-trichomes (Valkama et al., 2003) and are frequently considered to have originated from the endoplasmic reticulum. Plastids and smooth endoplasmic reticulum membranes are known to be involved in plant lipid synthesis and this may explain their frequency in the elaiophore secretory cells of O. radicans, T. cavendishianum, G. recurva and O. loefgrenii. Smooth endoplasmic reticulum, however, does not feature prominently in elaiophore secretory cells of O. trulliferum. In this species, lipid droplets collect in vacuoles of subepithelial cells. Since the latter are also involved in the synthesis of oil, this may explain the absence of smooth endoplasmic reticulum from epithelial cells. Recently, Singer and co-workers (Singer et al., 2006) have reported the presence of epithelial elaiophores on the labella of a number of Brazilian species of Lockhartia Hook. (Oncidiinae). Elaiophores probably also occur on the labella of Rudolfiella aurantiaca (Lindl.) Hoehne and R. picta (Schltr.) Hoehne (Bifrenariinae; Singer et al., 2006; K. L. Davies, unpubl. res. 2006, respectively) and Grobya galeata Lindl. (Catasetinae sensu Chase et al., 2003; Singer et al., 2006). This indicates that elaiophores may occur more widely than previously thought. The occurrence of elaiophores of diverse form in Neotropical Oncidiinae and various other orchid subtribes suggests that they have arisen on a number of occasions and have developed along a number of evolutionary lines in response to pollinator selection.
The present study indicates that the elaiophores of Oncidiinae species investigated thus far share structural features with those of Malpighiaceae. Furthermore, Reis et al. (2007) have recently demonstrated similarities between oily secretions, namely oncidinol and byrsonic acid, produced by flowers of these two unrelated taxa. Thus, convergence expressed at both anatomical and molecular levels is probably largely responsible for the successful pollination of Oncidiinae by bees that have primarily evolved to pollinate Malpighiaceae.
ACKNOWLEDGEMENTS
K.L.D. is grateful to the Stanley Smith (UK) Horticultural Trust for their generous grant. The authors thank Dr Michal Rudaś and Inż. Marek Wróbel (CLA Agricultural University, Lublin) for help with TEM and SEM and Mgr Agata Pacek (Department of Botany, Agricultural University, Lublin) and Alan Gregg (Swansea Botanical Complex, Swansea, UK) for help in preparing figures and manuscript, respectively.
LITERATURE CITED
- Arumugasamy K, Udaiyan K, Manian S, Sugavanam V. Ultrastructure and oil secretion in Hiptage sericea Hook. Acta Societatis Botanicorum Poloniae. 1993;62:17–20. [Google Scholar]
- Attala NC, Machado SR. Anatomy and ultrastructure of Banisteriopsis variabilis Gates (Malpighiaceae) calyx glands. Acta Microscopica. 2003;12(Suppl. B):635–636. [Google Scholar]
- Bhatt JR. Development and structure of primary secretory ducts in the stem of Commiphora wightii (Burseraceae) Annals of Botany. 1987;60:405–416. [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–396. [Google Scholar]
- Cane JH, Eickwort GC, Wesley FR, Spielholz J. Foraging, grooming and mate-seeking behaviors of Macropis nuda (Hymenoptera, Melittidae) and use of Lysimachia ciliata (Primulaceae) oils in larval provisions and cell linings. American Midland Naturalist. 1983;110:257–264. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon K M, editor. Orchid conservation. Sabah, Malaysia: Kota Kinabalu; 2003. pp. 69–89. Natural History Publications. [Google Scholar]
- Chiron GR, Castro Neto V. Menezesiella (Orchidaceae, Oncidiinae), un nouveau genre pour des espèces brésiliennes bien connues. Richardiana. 2006;6:99–106. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination; America, Africa, Asia and Australia. Rotterdam: AA. Balkema; 2001. [Google Scholar]
- Cocucci AA, Holgado A, Anton A. Estudio morfológico y anatómico de los eleóforos pedicelados de Dinemandra ericoides, Malpigiácea endémica del desierto de Atacama. Darwiniana. 1996;34:189–192. [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Teixeira da Silva J, editor. Floriculture, ornamental and biotechnology: advances and topical issues. V. Isleworth, Middlesex: Global Science Books (in press); 2008. [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Havard University Press; 1990. [Google Scholar]
- Eriksson M. The ultrastructure of the nectary of red clover (Trifolium pratense) Journal of Apicultural Research. 1977;16:184–193. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Fahn A. Structure and function of secretory cells. Advances in Botanical Research. 2000;31:37–75. [Google Scholar]
- Kunst L, Samuels AL. Biosynthesis and secretion of plant cuticular wax. Progress in Lipid Research. 2003;42:51–80. doi: 10.1016/s0163-7827(02)00045-0. [DOI] [PubMed] [Google Scholar]
- Michener CD. The bees of the world. Baltimore, MD: John Hopkins University Press; 2000. [Google Scholar]
- Mickeliunas L, Pansarin ER, Sazima M. Biologia floral, melitofilia e influência de besouros Curculionidae no sucesso reproductivo de Grobya amherstiae Lindl. (Orchidaceae: Cyrtopodiinae) Revista Brasileira de Botânica. 2006;29:251–258. [Google Scholar]
- Pauw A. Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi, Melittidae) in a guild of South African orchids (Coryciinae) American Journal of Botany. 2006;93:917–926. doi: 10.3732/ajb.93.6.917. [DOI] [PubMed] [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Proctor M, Yeo P. The pollination of flowers. London: Collins; 1975. [Google Scholar]
- Proctor M, Yeo P, Lark A. The natural history of pollination. London: Harper Collins; 1996. [Google Scholar]
- Reis MG, de Faria AD, Bittrich V, Amaral MCE, Marsaioli AJ. The chemistry of flower rewards – Oncidium (Orchidaceae) Journal of the Brazilian Chemical Society. 2000;11:600–608. [Google Scholar]
- Reis MG, de Faria AD, Amaral MCE, Marsaioli AJ. Oncidinol – a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Letters. 2003;44:8519–8523. [Google Scholar]
- Reis MG, Singer RB, Gonçalves R, Marsaioli AJ. The chemical composition of Phymatidium delicatulum and P. tillandsioides (Orchidaceae) floral oils. Natural Products Communications. 2006;1:757–761. [Google Scholar]
- Reis MG, de Faria AD, Alves dos Santos I, Amaral MCE, Marsaioli AJ. Byrsonic acid – the clue to floral mimicry involving oil-producing flowers and oil-collecting bees. Journal of Chemical Ecology. 2007;33:1421–1429. doi: 10.1007/s10886-007-9309-y. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall P. Homologies of inferior ovaries and septal nectaries in Monocotyledons. International Journal of Plant Sciences. 2002;163:261–276. [Google Scholar]
- Schnepf E, Deichgräber G. Electron microscopical studies of nectaries of some Euphorbia species. Akademie der Wissenschaften und der Literatur, Mainz. Tropische und subtropische Pflanzenwelt. 1984;45:55–93. [Google Scholar]
- Silvera KI. Adaptive radiation of oil-rewarding compounds among Neotropical orchid species (Oncidiinae) University of Florida; 2002. MSc Thesis. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. The ecology and chemistry of pollination in Brazilian orchids: recent advances. In: Teixeira da Silva J, editor. Floriculture, ornamental and plant biotechnology. IV. Isleworth, Middlesex: Global Science Books; 2006. pp. 569–582. [Google Scholar]
- Smets E. Localization and systematic importance of the floral nectaries in the Magnoliatae (Dicotyledons) Bulletin du Jardin Botanique National de Belgique – Bulletin van de Nationale Plantentuin van België. 1986;56:51–76. [Google Scholar]
- Smets EF, Ronse Decraene L-P, Caris P, Rudall PJ. Floral nectaries in monocotyledons: distribution and evolution. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Victoria: CSIRO: Collingwood; 2000. pp. 230–240. [Google Scholar]
- Steiner KE. The pollination of Disperis (Orchidaceae) by oil-collecting bees in southern Africa. Lindleyana. 1989;4:164–183. [Google Scholar]
- Steiner KE. Oil orchids and oil bees in southern Africa –Disperis and Rediviva. South African Orchid Journal. 1993;42:2–5. [Google Scholar]
- Steiner KE. The evolution of beetle pollination in a South African orchid. American Journal of Botany. 1998;85:1180–1193. [PubMed] [Google Scholar]
- Steiner KE, Whitehead VB. Oil flowers and oil bees: further evidence for pollinator adaptation. Evolution. 1991;45:1493–1501. doi: 10.1111/j.1558-5646.1991.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Elaiophore diversity in three contrasting members of the Oncidiinae Benth. (Orchidaceae) Botanical Journal of the Linnean Society. 2007;155:135–148. [Google Scholar]
- Toscano de Brito AVL. Systematic review of the Ornithocephalus group (Oncidiinae; Orchidaceae) with comments on Hofmeisterella. Lindleyana. 2001;16:157–217. [Google Scholar]
- Valkama E, Salminen J-P, Koricheva J, Pihlaja K. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Annals of Botany. 2003;91:643–655. doi: 10.1093/aob/mcg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Abhandlungen Akademie Wissenschaften Mathematisch-Naturwissenschaften Klasse Tropische und Subtropische Pflanzenwelt. 1974;7:1–267. [Google Scholar]
- Whatley JM, Whatley FR. When is a chromoplast? New Phytologist. 1987;106:667–678. doi: 10.1111/j.1469-8137.1987.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Wist TJ, Davis AR. Floral nectar production and nectary anatomy and ultrastructure of Echinacea purpurea (Asteraceae) Annals of Botany. 2006;97:177–193. doi: 10.1093/aob/mcj027. [DOI] [PMC free article] [PubMed] [Google Scholar]