Abstract
Background and Aims
Pilosella officinarum (syn. Hieracium pilosella) is a highly structured species with respect to the ploidy level, with obvious cytogeographic trends. Previous non-collated data indicated a possible differentiation in the frequency of particular ploidy levels in the Czech Republic and Slovakia. Therefore, detailed sampling and ploidy level analyses were assessed to reveal a boundary of common occurrence of tetraploids on one hand and higher ploids on the other. For a better understanding of cytogeographic differentiation of P. officinarum in central Europe, a search was made for a general cytogeographic pattern in Europe based on published data.
Methods
DNA-ploidy level and/or chromosome number were identified for 1059 plants using flow cytometry and/or chromosome counting on root meristem preparations. Samples were collected from 336 localities in the Czech Republic, Slovakia and north-eastern Hungary. In addition, ploidy levels were determined for plants from 18 localities in Bulgaria, Georgia, Ireland, Italy, Romania and Ukraine.
Key Results
Four ploidy levels were found in the studied area with a contrasting pattern of distribution. The most widespread cytotype in the western part of the Czech Republic is tetraploid (4x) reproducing sexually, while the apomictic pentaploids and mostly apomictic hexaploids (5x and 6x, respectively) clearly prevail in Slovakia and the eastern part of the Czech Republic. The boundary between common occurrence of tetraploids and higher ploids is very obvious and represents the geomorphologic boundary between the Bohemian Massif and the Western Carpathians with the adjacent part of Pannonia. Mixed populations consisting of two different ploidy levels were recorded in nearly 11% of localities. A statistically significant difference in a vertical distribution of penta- and hexaploids was observed in the Western Carpathians and the adjacent Pannonian Plain. Hexaploid populations tend to occur at lower elevations (usually below 500 m), while the pentaploid level is more or less evenly distributed up to 1000 m a.s.l. For the first time the heptaploid level (7x) was found on one site in Slovakia. In Europe, the sexual tetraploid level has clearly a sub-Atlantic character of distribution. The plants of higher ploidy level (penta- and hexa-) with mostly apomictic reproduction prevail in the northern part of Scandinavia and the British Isles, the Alps and the Western Carpathians with the adjacent part of Pannonia. A detailed overview of published data shows that extremely rare records on existence of diploid populations in the south-west Alps are with high probability erroneous and most probably refer to the closely related diploid species P. peleteriana.
Conclusions
The recent distribution of P. officinarum in Europe is complex and probably reflects the climatic changes during the Pleistocene and consequent postglacial migrations. Probably both penta- and hexaploids arose independently in central Europe (Alps and Carpathian Mountains) and in northern Europe (Scandinavia, Great Britain, Ireland), where the apomictic plants colonized deglaciated areas. We suggest that P. officinarum is in fact an amphidiploid species with a basic tetraploid level, which probably originated from hybridizations of diploid taxa from the section Pilosellina.
Key words: Amphidiploidy, apomixis, Asteraceae, flow cytometry, geographical parthenogenesis, Hieracium, postglacial migration, polyploidy
INTRODUCTION
The genus Pilosella Hill., often treated as a subgenus of Hieracium L. [syn. Hieracium subgen. Pilosella (Hill) Gray], is one of the taxonomically most intricate vascular plant groups of the temperate flora. The reticulate pattern of morphological variation reflected in several thousands of taxa described from the species level to the form (Zahn, 1921–1923) complicates taxonomic treatement. Widespread polyploidy, various modes of reproduction (sexuality, obligate and facultative apomixis of aposporous type, haploid parthenogenesis, vegetative propagation), inter- and intraspecific hybridization within the same and across different ploidy levels are the most important processes involved in microevolution of the genus (Krahulcová et al., 2000). The ploidy level occurring in natural populations varies from diploid (2n = 2x = 18) to octoploid (2n = 8x = 72). The most frequent cytotypes are tetraploids, pentaploids and hexaploids. Diploids are less frequent, and triploids, heptaploids, octoploids and aneuploids are rather rare (Schuhwerk and Lippert, 1997; Krahulcová et al., 2000). Higher ploidy levels up to dodecaploid (2n = 12x = 108) were found in plants obtained by experimental hybridization (Skalińska, 1976). In approximately half of the taxa of the genus Pilosella analysed more than one ploidy level was found, even in plants growing together in one locality (Schuhwerk, 1996 and references therein).
Sell and West (1976) recognize 63 ‘numbered’ species (including nothospecies) of Pilosella (treated as subgenus of Hieracium) in Europe. Six species having only one capitulum per stem (with the exception of hybrids) are members of the section Pilosellina Zahn. All but one are diploids occurring mainly in western, southern and central parts of Europe: P. argyrocoma (Fries) (southern and central Spain), P. castellana (Boiss. & Reuter) F. W. Schultz & Sch. Bip. (Spain and northern Portugal), P. hoppeana (Schult.) F. W. Schultz & Sch. Bip. (central and eastern Alps), P. pseudopilosella (Ten.) F. W. Schultz & Sch. Bip. (southern Europe, from Portugal and Spain to Bulgaria, Turkey and Romania), P. peleteriana (Mérat) F. W. Schultz & Sch. Bip. (northern and western Europe and the western part of central Europe). In addition, some authors distinguish a lowland form of P. hoppeana as a distinct diploid taxon – P. macrantha (Ten.) F. W. Schultz & Sch. Bip. (central and southern Europe) (e.g. Holub, 1986; Chrtek, 1998, 2002; Gottschlich, 1998; Schuhwerk and Fischer, 2003). The only almost exclusively polyploid species of section Pilosellina is Pilosella officinarum F. W. Schultz & Sch. Bip. (syn. Hieracium pilosella L.). It is distributed much wider than the diploid taxa mentioned above (cf. Hultén and Fries, 1986; Bräutigam, 1992). It extends from the British Isles across the whole of Europe, except the Arctic parts, to western Asia and north-western Siberia. Moreover, it has been introduced into New Zealand, North America and Patagonia, and has become an invasive and troublesome weed (Hultén and Fries, 1986; Chapman et al., 2000; Cárdenas Vergara, 2005; Wilson et al., 2006). Pillosella officinarum usually grows on dry, permeable and nutrient-poor soils from sea level to the sub-Alpine belt. The obligate heliofilous species occurs in tussock grassland communities usually with regular disturbance (grazing, mowing). Due to its low competitive ability, it tends to establish itself on open, sparsely vegetated, sites (roadside dykes, eroded slopes, landslides, etc.). Morphologically, P. officinarum may be distinguished from other representatives of Pilosellina section by long and slender stolons bearing small distant leaves decreasing in size towards the apex, involucral bracts (0·5–)1–2 mm wide, covered by stellate, glandular and eglandular trichomes (Sell and West, 1976). Despite its more or less easy identification in the field, a great phenotypic plasticity has been recorded within the species (Turesson and Turesson, 1960; Gadella, 1987, 1991). A high level of morphological variation is reflected in numerous infraspecific taxa described from the entire distribution range (Nägeli and Peter, 1885; Zahn, 1921–1923). The correlation between some phenotypic characters on one hand (e.g. rosette size, the number and length of stolons) and ploidy level on the other was revealed by Gadella (1991). In total, five cytotypes (2x, 4x, 5x, 6x and 7x) have been recorded in natural populations of P. officinarum (cf. Table 1). The mode of seed reproduction of each particular cytotype depends on the ploidy level. Diploids are sexual. Tetraploid plants reproduce sexually, but several apomictic populations deviate from this general rule (Gadella, 1984, 1987). Pentaploid P. officinarum is almost exclusively apomictic, although a rare sexual seed production was also reported (Turesson and Turesson, 1960; Turesson, 1972; Gadella, 1984). Facultative apomixis in pentaploids was later confirmed embryologically by Pogan and Wcisło (1995). Recently, two accessions of fully sexual pentaploids have been found in the Czech Republic (Krahulcová et al., 2000; Rotreklová et al., 2002). Hexaploids are either sexual or apomictic, while very rare heptaploids are either apomictic or sterile (Gadella, 1984, 1991). Vegetative reproduction by means of over-ground stolons is common for all cytotypes and, together with apomixis, it might contribute to the uniclonal structure of populations.
Table 1.
Ploidy levels found in Pilosella officinarum in Europe according to the literature and present records
Diploid plants of P. officinarum are rare and their distribution is considered to be of a relict character (e.g. Gadella, 1984). They were reported from the Valley of Aosta (Italy) (Gadella, 1972) and south-eastern France (Delcourt, 1972), respectively (but see the Discussion below). In most of Europe, the tetraploid and pentaploid populations of P. officinarum are by far the most common cytotypes (Gadella, 1984). Tetraploids are widespread in the lowlands of west and central Europe (e.g. Turesson and Turesson, 1960; Gadella, 1972, 1984; Pogan and Wcisło, 1989; Schuhwerk and Lippert, 1997, 2002; Krahulcová and Krahulec, 1999), while the pentaploids occur chiefly in regions that were covered by the Pleistocene glaciation – Scandinavia, the British Isles (Turesson and Turesson, 1960; Gadella, 1972, 1984, 1987; Finch, 2005; Watson, 2005). Several hexaploid populations of P. officinarum were found mainly in the Alps, Scandinavia, Balkan Peninsula (e.g. Turesson and Turesson, 1960, Gadella, 1972, 1984, 1991; Lavrenko and Sereditov, 1991; Schuhwerk and Lippert, 1997) and the Western Carpathians (see below). The rare occurrence of heptaploids was reported from only three localities in Sweden (Turesson and Turesson, 1960), one site in the Netherlands (Gadella, 1984) and one population in the Czech Republic (Košt'álová, 2004).
Four ploidy levels (tetra-, penta-, hexa- and heptaploid) have been recorded in the Czech Republic and Slovakia (Májovský et al., 1970; Uhríková and Feráková, 1977; Mičieta, 1982; Murín, 1986; Měsíček and Jarolímová, 1992; Krahulcová and Krahulec, 1999; Píšt'anský and Mičieta, 2000; Krahulcová et al., 2001; Rotreklová et al., 2002, 2005; Košt'álová, 2004). Recently, Píšt'anský and Mičieta (2000) recorded tetraploids in approx. 30 localities mainly in southern and western Slovakia, while other authors reported pentaploid and hexaploid plants mostly from eastern, northern and central Slovakia. Most of the chromosome counts coming from the Czech Republic that had been published indicated that the plants analysed were tetraploids.
Almost all published data on ploidy level of P. officinarum are based on classical chromosome counting. This precise method is, however, considerably time-consuming. Since routine introduction of the flow cytometry in plant science in the nineties of the last century (Doležal, 1991), this approach has rapidly became popular for estimating DNA-ploidy level (Doležel, 1991). This is mainly due to the very easy sample preparation and the possibility of screening large numbers of individuals in a very short time. Here, the search which was carried out for a boundary between the area of distribution of the tetraploid cytotype and the range of penta- and hexaploids of P. officinarum in the territory of Slovakia and the Czech Republic, using mostly a flow cytometric approach, is reported. Moreover, an attempt was made to find out if there was a correlation between the distribution of particular ploidy levels on one hand and the altitude on the other. To understand better the cytogeographic differentiation of P. officinarum in central Europe, a search, based on published data, was made for a general cytogeographic pattern in Europe.
The area studied
Research has been carried out in the area of the Czech and Slovak Republics with an adjacent part of north-eastern Hungary. The area studied belongs to two different biogeographic regions, the mountain range of the Western Carpathians and the Bohemian Massif. The border between both regions is situated in the eastern part of the Czech Republic, lying north-north-east to south-south west. These two regions differ in a variety of environmental and historical parameters. In this respect, differences in the cytotype distribution cannot be explained in any easy way. On the other hand, this area covering their border can show that the pattern in cytotype distribution can be very contrasting even across a very narrow zone.
The Bohemian Massif has an old Paleogenic relief, younger areas being only canyons, those areas with Tertiary volcanism in the northern part of Bohemia, and glacial cirques in the Sudetes and the Šumava Mountains. The highest point is Mt Sněžka (1602 m), the lowest is the valley of the River Elbe on the German border at 115 m. Mostly acid Varisian parts were later covered with Permian-Carboniferous or Mesozoic sediments. Base-rich bedrocks are concentrated at lower altitudes. Vegetation cover has a coarser grain (homogeneous on a larger scale) in comparison with the Carpathian Mountains.
The Western Carpathians, including the Intra-Carpathian (Pannonian) Basin, represent the north-west part of the Carpathian arc extending from north-east Austria and south-east Czech Republic to north-east Slovakia and south-east Poland. The relief is young, of Tertiary age, similar to the Alps. The highest point is Gerlach Peak (2655 m). The bedrock is more complicated, mostly of Mesozoic and Tertiary ages. Calcium-rich substrates occur from lowland to the high mountains; e.g. in central Slovakia almost consistant limestone substrates can be found from the xerothermic Slovak Karst to the highest altitudes of the Belaer Tatra with altitudes above 2000 m. Some areas are very continental, with climatic conditions which do not allow the growth of Fagus sylvatica as in the area between the High and Low Tatra Mountains. On the other hand, some not distant areas are more oceanic, as in north-west Slovakia. For all these reasons, the vegetation cover is fine-grained (homogeneous in small areas but, on a larger scale, heterogeneous). Large regions with homogeneous vegetation are rare.
The area of the Czech Republic has a rather uniform climate; the warmest month is July and it is also the month of highest rainfall. This contrasts with Slovakia, where the same condition applies only at higher altitudes. At lower altitudes, the warmest month is also July, but the highest rainfall is distributed from May to September, depending on the exact geographic position (Vesecký, 1961). In this way, the same area is rather oceanic in May–June and more continental in September and vice versa. Slovakia (the Carpathian Mountains with the Pannonian Plain) is therefore fine-grained and more diverse with respect to relief, bedrock and climate.
MATERIALS AND METHODS
Material collection
Plants of Pilosella officinarum F. W. Schultz & Sch. Bip. (syn. Hieracium pilosella L.) for the present study were collected in 2003–2006 in their natural habitats throughout Slovakia and the Czech Republic, to a lesser extent also in the north-eastern part of Hungary to cover all geographic regions. They were cultivated in pots in the Botanical Garden of P. J. Šafárik University, Košice and in the experimental field of the Institute of Botany, Academy of Sciences of the Czech Republic, Průhonice. For the complete list of localities see Supplementary Information 1 (available online). Besides the plants from the region above mentioned, some plants from a further 18 localities from different parts of Europe have also been analysed (Supplementary Information 4, available online).
As a rule, three or five plants from each population were sampled, three from the pure populations, five in the case of co-occurrence of other potentially hybridizing species of the genus Pilosella. Efforts were made to avoid collecting samples originating from one clone. If it was apparent that plants at the collecting site did originate from one plant, they belonged to one clone (usually plants growing very close together in a very small area of several square cm), only one individual plant per locality (population) was dug up. In some cases however, several cultivated plants died before analysis. For both these reasons, some populations are represented by only one plant. To determine the proportion of mixed cytotypes in populations, only those populations with two and more plants analysed were involved. Despite the fact that the ploidy level of only one plant had been estimated by us, some localities (marked in Supplementary Information 1, available online) can be considered as collecting sites with two or more analysed plants because the chromosome number of other plant/plants from the same locality was published earlier (see Supplementary Information 2, available online). Therefore, in addition to the data collected for this research, a few previous accounts from the literature (Rotreklová et al., 2005) were used to search for some localities consisting of two different ploidy levels. These plants were not included in the total number of plants analysed in this present study. The voucher specimens have been deposited recently in the herbarium of Patrik Mráz, at the Institute of Biology and Ecology, P. J. Šafárik University, Košice and in the herbarium of the Institute of Botany, Průhonice (PRA).
Chromosome counts
The chromosome counts are based on the somatic mitosis in the root-tip cuttings of pot-cultivated plants. The material was pre-treated at room temperature with a 0·5% solution of colchicine for 1·5–3 h and then fixed in a cold mixture of ethanol and acetic acid (3 : 1) for at least 1 h. The fixed material was stored in 70 % ethanol at 4 °C until processed. The root tips were macerated in 1 n HCl at 60 °C for 7–10 min. The squash and smear method with cellophane replacing the glass covers (Murín, 1960) and with Giemsa solution in a phosphate buffer was used. Selected permanent slides are deposited at the Institute of Biology and Ecology, P. J. Šafárik University in Košice.
Estimation of ploidy level
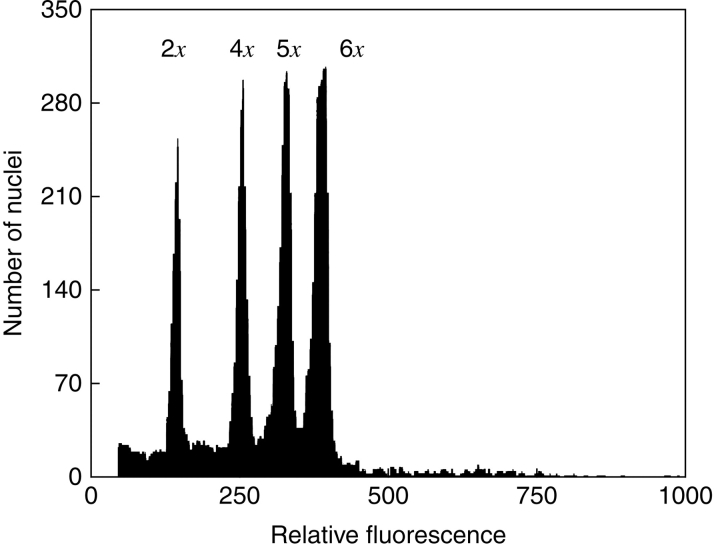
Flow cytometry was used to detect the DNA-ploidy level (Suda et al., 2006) for most of the plants. An analysis of relative DNA content was performed with a PA II ploidy analyser (Partec GmbH, Münster, Germany) equipped with an HBO-100 mercury arc lamp in the Flow Cytometry Laboratory, Institute of Botany, Academy of Sciences, Průhonice, Czech Republic and FACSCalibur instrument (Becton Dickinson, USA) equipped with an argon-ion laser excitation at 488 nm in the Flow Cytometry Laboratory, Institute of Biology and Ecology, P. J. Šafárik University, Košice. Sample preparations were carried out in a two-step procedure (Otto, 1990; Doležel and Göhde, 1995). Approximately 1 cm2 of leaf tissues from both the sample and the reference internal standard were ground together for about 30 s in a Petri dish containing 1 ml of ice-cold Otto I buffer (4·2 g citric acid monohydrate + 1 mL 0·5 % Tween 20 adjusted to 200 mL and filtered through a 42-μm filter). Filtration through a 42-μm nylon mesh was followed by centrifugation at 150 g for 5 min. The supernatant was removed and 100 µL of fresh Otto I buffer was added. The nuclei in the pellet were resuspended and stored for 30 min at room temperature for incubation. For DNA staining 1 mL of Otto II buffer (0·4 m disodium hydrogenphosphate dodekahydrate) including 50 µL of propidium iodide, 50 µL ribonuclease, 2 µL mercaptoethanol (FACSCalibur, Becton Dickinson) or DAPI (4′,6-diamidino-2-phenylindole) at a concentration of 4 µg ml−1 (PA II flow cytometer, Partec GmbH) was used. The clones of previously cytologically studied diploid (2n = 2x = 18) plants of Pilosella lactucella (Wallr.) P. D. Sell & C. West (Rotreklová et al., 2002, 2005) were used as an internal reference standard for the relative DNA content measurements. Moreover, one tetraploid and several pentaploid and hexaploid plants of P. officinarum with known chromosome numbers were used in separate and mixed flow cytometry analysis to determine the exact position of peaks of known polyploids in relation to the diploid standard peak (Fig. 1). Histograms were accumulated at a flow rate of about 20–50 particles per second for a total count of 3000–5000 nuclei. The resulting values were expressed as a peak ratio, which is a ratio of the mean position of the G0/G1 peak in the DNA histogram of the tested plant to the mean position of the G0/G1 peak in the histogram of the reference plant.
Fig. 1.
Histogram of relative DNA content of DAPI-stained nuclei from a diploid plant of Pilosella lactucella (2x) used as a reference plant, with tetraploid (4x), pentaploid (5x) and hexaploid (6x) plants of Pilosella officinarum.
Maps
The distribution maps of cytotypes/ploidy levels in the Czech Republic and Slovakia are based on the co-ordinates determined by a GPS receiver, or found ex post facto from the tourist maps at a scale of 1 : 50 000 (usually old literature data). For most references from Europe for which the appropriate geographical co-ordinates were not given in original sources, the geographical position of collecting sites was estimated using Microsoft Encarta World Atlas (1998 Edition) and GeoNet Name Server (http://gnswww.nga.mil/geonames/GNS/index.jsp; accessed in December 2005). However, for approx. 10 % of references, estimation of co-ordinates failed (marked by an asterisk in Supplementary Information 3, available online) usually due to the absence of the name of the nearest village/town, or the existence of two or more villages/towns with the same name. Most of the chromosome numbers of the plants from the British Isles were obtained from the online version of Cytological database of the Botanical Society of the British Isles (accessed in February 2005). Distributional maps were prepared using distribution mapping software DMAP (Morton, 2004).
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey's pairwise comparison (using Minitab for Windows Release 11) were applied to determine the significance (P < 0·05) of the difference in the altitudinal distribution between pentaploid, hexaploid and mixed populations in the West Carpathians and adjacent Pannonia (Slovakia and the eastern part of the Czech Republic).
RESULTS
Ploidy level distribution in the Czech Republic and Slovakia
The DNA-ploidy levels and/or chromosome numbers were detected for 1059 plants sampled at 336 localities throughout the Czech Republic and Slovakia. Some plants were sampled also in north-eastern Hungary, along the Slovak–Hungarian state border. In total, 1055 plants were analysed by flow cytometric analysis. For eight plants the ploidy level was found using two approaches – by classical counting and by flow cytometry – while another four plants were counted only (cf. Supplementary Information 1, available online).
Altogether, four ploidy levels, tetra-, penta-, hexa- and heptaploid, were revealed in the area on which the study focused. The tetraploid level (4x; altogether 426 plants which represent 40·2 % of all plants analysed) was found to be the most common, followed by pentaploid (5x; 389 plants, 36·7 %) and hexaploid (6x; 241 plants, 22·8 %). Three heptaploid plants (7x) were discovered in a mixed population with one pentaploid plant at only one site in western Slovakia (Fig. 2). The record of heptaploid ploidy level is the first for P. officinarum in the territory of the Western Carpathians. The effort made to determine the chromosome number of heptaploid plants was not successful (the plants died), thus the new ploidy level should be considered merely as a DNA-ploidy level, i.e. not based on an exact chromosome count. Estimations of ploidy levels given for the plants from the Hungarian part of the Western Carpathians are the first records of ploidy level for P. officinarum for this area. In 32 localities out of 302 (10·6 %), from which at least two plants were analysed, mixed populations consisting of two different ploidies were found.
Fig. 2.
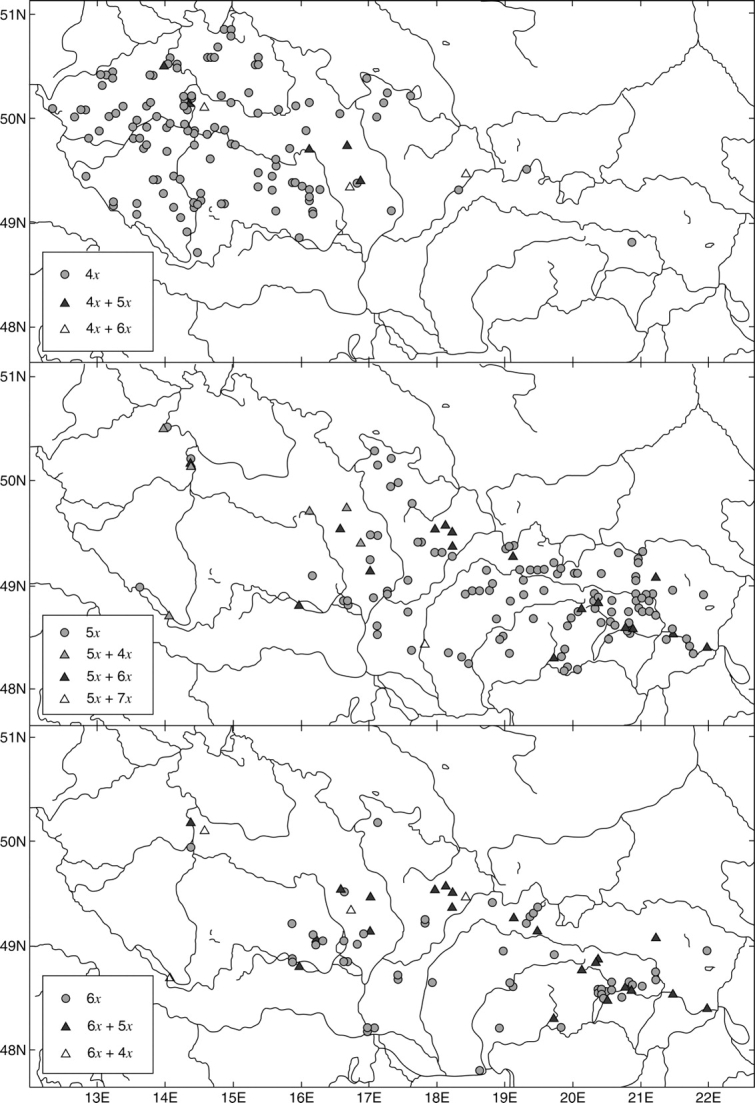
Distribution of ploidy levels of Pilosella officinarum in the Czech Republic, Slovakia and north-east part of Hungary based on present data.
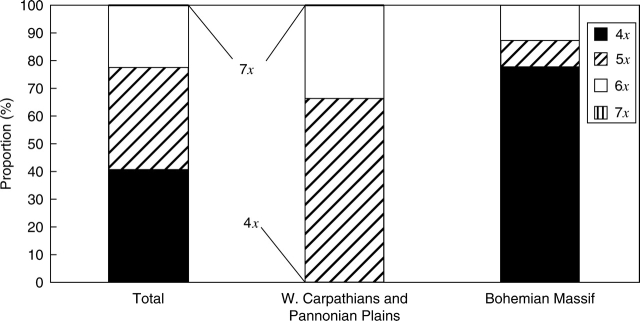
The distribution of ploidy levels in the Czech Republic is not proportional to that in Slovakia. While tetraploids are the most widespread in the Czech Republic, specifically in its western part, penta- and hexaploids predominated in Slovakia and in the eastern part of the Czech Republic (Fig. 2). The boundary between a common occurrence of the tetraploid cytotype and higher ploids is very conspicuous and corresponds well with the natural geological and geomorphological boundary between the Bohemian Massif and the Western Carpathians with the adjacent Pannonian Plain (Král, 1999). If the proportion of the particular ploidy level for each geographic region is taken into account separately, i.e. the Bohemian Massif on one hand and the Western Carpathians with Pannonia on the other, then the differences are very striking (Fig. 3).
Fig. 3.
Proportions of the ploidy level of Pilosella officinarum in the Czech Republic, north-east Hungary and Slovakia based on present data, expressed as a portion of plants of a particular ploidy level compared with the total plants analysed. The proportions over the whole area studied are given (total), together with the proportions found in two different geomorphological regions – the Bohemian Massif and the Western Carpathians with the adjacent part of Pannonia.
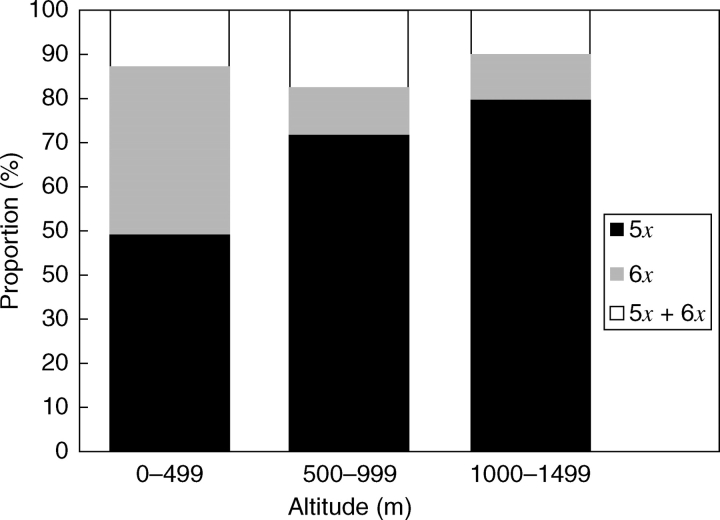
Apart from latitudinal differentiation in ploidy level distribution in the Czech Republic and Slovakia, a statistically significant difference was also found between the proportion of pentaploids and hexaploids across the altitudes in the territory of the Western Carpathians and adjacent Pannonia (Slovakia, north-east Hungary and the eastern part of the Czech Republic) (Table 2). Generally, pure hexaploid populations tend to occur at lower elevations (usually below 500 m), while the pentaploids are very common above 500 m a.s.l. Mixed populations consisting of two different ploidy levels were found relatively evenly along the altitudinal gradient up to 1000 m (Fig. 4).
Table 2.
Means and standard deviations of altitudes of pure pentaploid (5x), pure hexaploid (6x) and mixed populations (5x + 6x) of Pilosella officinarum in the Western Carpathians and adjacent part of Pannonia
| Ploidy level | N | X ± s.d. |
|---|---|---|
| 5x | 86 | 546 ± 292a |
| 6x | 40 | 370 ± 189b |
| 5x + 6x | 21 | 513 ± 253ab |
Only populations with two or more analysed plants were included. The tetraploids and heptaploids were, according to their comparatively low abundance, excluded from this analysis. Altitudinal ranges and means are given in metres above sea level. N, Total number of populations; X, mean; s.d., standard deviation. Means in columns sharing the same superscript letters are not significantly different (Tukey's pairwaise comparisions, P = 0·003, F = 6·19).
Fig. 4.
Proportions of pure pentaploid (5x), pure hexaploid (6x) and mixed populations (5x + 6x) of Pilosella officinarum found at different altitudinal ranges in the Western Carpathians and the adjacent part of Pannonia, expressed as a number of populations of the particular ploidy level compared with the total number of populations analysed in the particular altitudinal range.
General pattern of ploidy level distribution in Europe
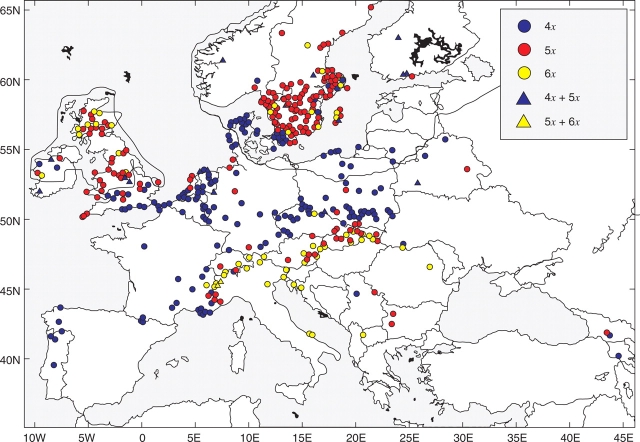
Pilosella officinarum is the European taxon most examined by karyology. Chromosome numbers were counted in plants originating from 655 localities, excluding present data, across the whole continent (refer to Supplementary Information 3, available online, and Table 1). It was possible to localize geographically nearly 600 sites (Fig. 5). The most common cytotype, tetraploid (without present data), was reported in 284 localities (43 %), followed by pentaploid found at 257 collecting sites (40 %). The hexaploid ploidy level is obviously rarer, i.e. detected in 74 cases (11 %). Mixed populations consisting of two or more different cytotypes were found on 40 sites (6 %). However, in most publications the number of plants analysed per population was not given and therefore the proportion of mixed populations would be probably higher if only populations with at least two or more analysed plants per locality were taken in consideration. Tetraploids are distributed mostly in western Europe and the western part of central Europe, being the only cytotype detected in Denmark and Germany. It prevails considerably in the Netherlands (82 %), Poland (71 %) and France (65 %). The pentaploids have two main centres of distribution: at high latitudes in northern Europe (Sweden, 70 %; the British Isles, 64 %) and in major orophytic systems in Europe – the Alps (30 %) and the Carpathian Mountains with the adjacent part of Pannonia (present data for the Western Carpathians indicate 66 % of pentaploids). The predominant ploidy level in the Alps is hexaploid (59 %, in Switzerland even 84 %). The records on diploids and heptaploids are extremely scarce. The former were found only in the south-west Alps (but see Discussion), the latter mostly in northern and central Europe (Sweden, Netherlands and the Czech Republic; heptaploids are not included in Fig. 5). The new data from 18 European localities confirm this general pattern: Bulgaria 4x, 5x and 6x; Georgia 4x and 5x; Ireland 5x and 6x; Italy 6x; Romania 5x, 6x and mixed 5x + 6x; Ukraine 6x (cf. Table 1 and Supplementary Information 4, available online).
Fig. 5.
Cytotype distribution of Pilosella officinarum in Europe (based on data from Supplementary Information 2–4, available online; present data from the Czech Republic, Slovakia and Hungary are not included). The rare heptaploid level and rare mixed cytotype combinations are not given in the map. The bold black line indicates the boundary of Last Glacial Maximum in northern Europe according to Adams (1997).
DISCUSSION
Ploidy level distribution in the Czech Republic and Slovakia
According to the results of the present research, the tetraploids strongly prevail in the western part of the Czech Republic, while the pentaploids and hexaploids represent two main cytotypes in Slovakia and the eastern part of the Czech Republic. This corresponds well with scattered data published in previous studies, with the exception of the tetraploids. Our results contradict the data published by Píšt'anský and Mičieta (2000), who reported a significant predominance of tetraploid populations in the Western Carpathians, but only a rare occurrence of penta- and hexaploid plants (cf. Supplementary Information 2, available online). As preliminary results did not confirm any common occurrence of tetraploids in Slovakia (Šingliarová and Mráz, 2004), in 2004 five localities were visited from which the tetraploids were published by Píšt'anský and Mičieta (2000). However, no tetraploid was detected in any of them. Based on these observations, all data of Píšt'anský and Mičieta (2000) were considered as dubious.
The present study revealed a new heptaploid ploidy level in P. officinarum in the territory of the Western Carpathians. So far, the heptaploids had been detected only in three localities in Sweden (Turesson and Turesson, 1960), in one site in the Netherlands (Gadella, 1984) and one plant in a population of hexaploid plants near Prague in the Czech Republic (Košt'álová, 2004). In the Western Carpathians locality, from four analysed plants three were heptaploid and only one pentaploid. Although pentaploids reproduce the most often via aposporic apomixis, there are some data on facultative apomixis (Turesson and Turesson, 1960; Turesson, 1972) or even full sexuality (Krahulcová et al., 2000; Rotreklová et al., 2002). Moreover, apomictic pentaploids usually produce 2x to 3x pollen grains (Gadella, 1987; Krahulcová and Krahulec, 2000). A possible explanation of an increased ploidy level may be the fusion of reduced and unreduced gametes, as was suggested in the case of a large Dutch heptaploid population situated between two localities – the first occupied by tetra- and the second by pentaploid plants (Gadella, 1988).
A sympatry of two ploidy levels within one population was confirmed in nearly 11 % of populations. The presence of cytotype mixtures is pronounced especially in the Western Carpathians (16·4 %), whereas in the Bohemian Massif a co-occurrence of different ploidy levels is rarer (5·9 %) and confined only to the warmest regions in relict river valleys or in the zone adjacent to the Western Carpathians and the Pannonian Plain. Higher numbers of mixed populations in the Western Carpathians might be explained by a high presence of two different ploidy levels (5x and 6x) in this territory. However, this is not the case for the Bohemian Massif which has only one completely dominating tetraploid cytotype. Whether the presence of mixted cytotypes is mainly due to the more or less stochastic co-existence of different clones with different ploidy levels or to the local formation from one dominant ploidy level is yet unknown. Undoubtedly, the production of fully or partially reduced or unreduced gametes and gene flow between plants in the locality may contribute to the presence of a cytotype mixture in populations (cf. Krahulcová et al., 2000). Mixed cytotype populations were found previously also in other parts of Europe in 37 localities (cf. Supplementary Information 3, available online), as well as in the Western Carpathians (Skalińska, 1967).
Surprisingly, the boundary between a common occurrence of tetraploids and higher ploids of P. officinaum is rather sharp and corresponds well to the natural boundary of two geomorphological units: the Western Carpathians with the adjacent Pannonian Plain and the Bohemian Massif. From the cytological point of view, a similar boundary between two cytotypes of P. bauhini was recorded by Rotreklová (2004), albeit, with the reverse pattern in comparison with P. officinarum. The tetraploids of P. bauhini are more frequent in Slovakia and Hungary and rare in the Czech Republic, Poland or Germany. On the other hand, pentaploid populations prevail in the Czech Republic and Germany. The border between Hercynian (including the Bohemian Massif) and Carpathian regions seems to be an important biogeographic boundary in central Europe as is also seen in the distribution patterns of many plant species. There is a whole set of species reaching this border from the east, from the Carpathian Mountains, e.g. Cardamine glandulosa, Dianthus latifolius, Euphorbia serrulata, Galium rivale, Luzula luzulina, Salvia glutinosa etc. (Hendrych, 1987). On the contrary, some species have migrated eastwards but did not enter, or only rarely, the Carpathian Mountains, like Campanula rotundifolia s.str. (Kovanda, 1977) and Cirsium heterophyllum. Such strong cytological and chorological differentiation between neighbouring geomorphological regions is difficult to explain. Differences in floristic composition suggest that historical processes, such as migration and expansion from different refugial areas using various migration corridors, might have played an important role (see also below). Moreover, the mountainous areas of the Bohemian Massif and the Western Carpathians are separated from each other by the north-west part of the Pannonian Plain (the Intra-Carpathian Basin) and so-called rather narrow Moravia gate, connecting the Pannonian lowland with lowlands of Silesia, Poland. This natural geographic and climatic barrier might have contributed to the different floristic and cytological patterns of these regions.
A significant difference in the proportion of penta- and hexaploid populations across altitude was found within the Western Carpathians and adjacent Pannonia. While pentaploids are more or less evenly present up to 1000 m, the hexaploids usually grow in regions with a warmer climate, usually below 500 m a.s.l. However, there are several regional deviations. Prevailing populations of the pentaploid level are present in the Zemplínske vrchy Mountains (south-east Slovakia) belonging to the warmest region of the Western Carpathians situated in the neighbouring zone with the Pannonian Plain. On the other hand, several hexaploid populations were recorded at a high elevation with a cold and humid climate in the Oravská Magura Mountains. In the Bohemian Massif, rare penta- and hexaploids or mixed populations are confined mostly to the warm, low-elevated, regions, such as river valleys, or to the adjacent zone with the Pannonian Plain (Fig. 2). One hexaploid population was found on the top of the Hrubý Jeseník Mountains (eastern part of the Sudetes range, Czech Republic). It seems that there are at least two hexaploid types in the area studied, differing in distribution and breeding systems (T. Urfus, unpubl. res.). The first one is confined mostly to thermophilous vegetation in the Carpathian Mountains and has an apomictic breeding system; it is probably related to apomictic hexaploids occurring throughout the Carpathian Mountains to the Balkan. The second hexaploid type is confined to relict river valleys in the Bohemian Massif and is sexual; this type is probably related to sexual hexaploids of the Alps (Gadella, 1984).
Amphidiploid origin of Pilosella officinarum?
Diploids of P. officinarum that are considered to have a relict distribution were found by Delcourt (1972) and by Gadella (1972) only in the south-western Alps. However, 16 records of data from the French Alps published by the former author were doubted later due to mis-identification as a closely related but different diploid species from the section Pilosellina – P. peleteriana (Gadella, 1984). Nevertheless, two diploid plants of P. officinarum counted by Gadella might belong to this species, as it is obvious from the photograph of these plants (Gadella, 1972: 362). These diploids originating from a very widely defined locality ‘the valley of Aosta’ (north-west Italy) have long stolons with decreasing leaf size towards the stolon apex. On the other hand, these plants could also represent the hybrids between true P. officinarum and some diploid taxon from P. section Pilosellina. These questionable data on existence of diploids of P. officinarum may suggest that a well-established diploid cytotype within P. officinarum does not really exist in nature. Another fact supports this hypothesis: most of the diploid species of Pilosella that had been counted up to the present, including the closely related taxa from the section Pilosellina, have been found usually in several if not many localities and occupied much wider ranges (e.g. Zahn, 1921–1923; Bräutigam, 1992; Schuhwerk, 1996). Moreover, polyploidy in diploid taxa of section Pilosellina is either unknown or very scattered records of polyploids might be regarded as mis-identifications with P. officinarum or interspecific hybrids. The almost exclusive presence of polyploid populations with the tetraploid ones being the commonest leads to the hypothesis that P. officinarum is likely to be an amphidiploid species originated from one or more crosses between diploid members of section Pilosellina. Both place and time of this hybridization are difficult to estimate. Analysis of ITS sequences showed low differentiation between diploid taxa (Fehrer et al., 2007a), which suggests a relatively low age of the particular members of this group. With respect to chloroplast haplotypes, diploid members of the section Pilosellina share both main types. Pilosella hoppeana, P. macrantha and P. peleteriana share the haplotype typical of steppe and mountain species such as P. onegensis, P. alpicola, P. glacialis, P. echioides, etc., i.e. those species, which occurred together during the Glacial Period in steppic and tundra-like habitats in the area of central Europe. The other diploids confined to southern Europe (P. castellana, P. argyrocoma and P. pseudopilosella) share the haplotype with P. lactucella, P. vahlii and P. breviscapa (Fehrer et al., 2007a). Central European populations of P. officinarum exhibit the same haplotype as P. hoppeana and P. macrantha (Krahulec et al., 2004, Fehrer et al., 2007a, b). The close relationship of diploid P. peleteriana and P. officinarum has been proven by an allozyme pattern observed in the plants originating from Scandinavia (Tyler, 2005). The possible polyphyletic and polytopic origin of polyploid populations of P. officinarum is supported by its enormous morphological variation. Zahn, a monographer of the genus, distinguished about 600 subspecies (Zahn, 1921–1923, 1922–1930). Morphologically, the tetraploid plants of P. officinarum found recently in Bulgaria resemble hybrids between hexaploid P. officinarum and diploid P. macrantha. To understand the origin of P. officinarum it is necessary to know more about the detailed distribution of haplotypes in the whole distribution area of P. officinarum and its diploid relatives. Recently Trewick et al. (2004) included several plants from their natural European range of distribution in their study on the origin of the introduction of P. officinarum into New Zealand. They found a mixed distribution of two common chloroplast haplotypes with no clear geographic pattern. However, three rare haplotypes were distributed mainly in the Alps, Sudeten Mountains, the Carpathian Mountains and Finland.
General pattern of ploidy level distribution in Europe and its relationship with polyploidy and apomixis
Gadella (1984, 1987, 1991) studied the distribution of particular cytotypes of P. officinarum in Europe and tried to explain its pattern. The revision presented in this paper (Fig. 5) showed that this pattern is more complex and fine grained (as was shown in the area of the Slovak and Czech Republics) than that suggested by Gadella. Despite the fact that P. officinarum is the most karyologically studied vascular plant species, it is realized that the published data cover only some parts of its natural distribution range sufficiently (western, central and northern Europe). Large areas in southern, south-eastern and eastern Europe have scarce or almost no data.
In total, four different cytotypes of P. officinarum were found in Europe (see Table 1 and Fig. 5). The records on diploids are highly questionable (see above). The most common ploidy levels are 4x, 5x and 6x. The range of sexual tetraploid cytotypes clearly separates the higher ploidy levels (5x and 6x) into two groups occurring in geographically different regions – into northern Europe and the mountains of central and south-east Europe (the Alps and the Carpathian Mountains, mountains in Bulgaria). Such a pattern of ploidy level distribution suggests an independent origin of penta- and hexaploids. Concerning the results from the Czech Republic and Slovakia, it seems that they match the general pattern in central Europe. While the prevailing tetraploid cytotype in the Bohemian Massif shows linkage to the tetraploid populations in the western part of central Europe, penta- and hexaploid populations in the Western Carpathians and the Pannonian Plain are likely to be related to the high ploids found in the Alps and in the Balkans (cf. Fig. 5).
It was hypothesized that the prevailing occurrence of high ploids (5x and 6x) correlates with either high latitudes or high altitudes and that their common distribution in northern Europe and in the Alps may be the result of the last Pleistocene glaciation (Gadella, 1984, 1987, 1991). The detailed map given in Fig. 5 shows that the tetraploid level is confined to western Europe and the western part of central Europe and that it has a sub-Atlantic distribution character. Northwards, sexual tetraploids are rare or completely missing and they are replaced by apomictically reproducing penta- and hexaploids (cf. Turesson and Turesson, 1960). The boundary of tetraploids and high ploids matches well with the border of the ice-sheet during the Last Glacial Maximum (cf. Adams, 1997) in the British Isles and it is very close to this geographic position in Scandinavia. Interestingly, the same pattern of cytotype distribution was found in Parnassia palustris L. (Parnassiaceae), where the boundary between diploids and tetraploids more or less correlates with the limit of Last Glacial Maximum (Gornall and Wentworth, 1993; Borgen and Hultgård, 2003). It therefore seems that the relationship between the presence of high ploid apomictic plants (5x and 6x) in northern Europe and glaciations might have a real basis and suggests evolutionary advantages of polyploidy associated with apomixis in the colonization of deglaciated areas in Scandinavia (cf. Asker and Jerling 1992). Merxmüller (1975) pointed out that diploid, sexually reproducing taxa of the closely related genera Hieracium and Pilosella are mostly confined to the southern latitudes, while there was a tendency for polyploids, mostly apomictic species, to prevail in northerly situated regions. Such geographically limited parthenogenesis is known also in other sexual–apomictic genera and was summarized by Bierzychudek (1985) (for thorough recent revision on complex causality of geographical parthenogenesis, see Hörandl, 2006). The diploid members of section Pilosellina have a more restrained range of distribution in comparison with polyploid P. officinarum and are confined mostly to southern and central Europe (see Introduction). This recalls the situation of several other groups of polyploid vascular plants associated with apomixis, e.g. Antennaria L. (Bayer and Stebbins 1987), Ranunculus auricomus group (Hörandl, 2006) and Taraxacum (den Nijs et al. 1990), where polyploid apomicts tend to have larger ranges than sexuals. In the present case, the colonizing success of P. officinarum might be attributed to the combinations of different factors, such as its probable allopolyploid origin (see above), increased heterozygosity and the existence of a high number of genetically different clones, the presence of an apomictic mode of reproduction in high ploids (5x and 6x) with the occurrence of residual sexuality, vegetative reproduction via above-ground stolons, the possibility of long-distance dispersal via achenes with a pappus, and the opportunity of recurrent formation of novel genotypes via hybridization. It is possible that all these factors have played an important role in shaping the present cytogeographic patterns of P. officinarum.
SUPPLEMENTARY INFORMATION
Supplementary information is available online at www.aob.oxfordjournals.org and contains the lists of Pilosella officinarum localities accompanied by geographical co-ordinates, ploidy levels and/or chromosome numbers (and references) for data (1) presented in this study for the areas of the Czech Republic, Slovakia and Hungary; (2) for previously reported data from the Czech Republic and Slovakia; (3) for previously published data from the rest of European area; and (4) for new data from Europe outside of Slovakia, Czech Republic and Hungary.
ACKNOWLEDGEMENTS
We thank D. Blanár, K. Čiháková, M. Dudáček and V. Mrázová for their kind help in sampling living plants, and S. and A. Kewish and C. A. Stace for the English revision. This study was financially supported by projects from the Grant Agency of Ministry of the Education of the Slovak Republic and Slovak Academy of Sciences (VEGA 2/6054/26), Slovak Research and Development Agency (APVT-51–006002), Grant Agency of the Academy of Sciences of the Czech Republic (grant no. IAA6005203 to F.K.), Institutional Research Concept no. AV0Z6005908 and Institutional research support MSM 0021620828 from the Ministry of Education, Youth and Sports of the Czech Republic.
LITERATURE CITED
- Adams JM. Global land environments since the last interglacial. TN, USA: Oak Ridge National Laboratory; 1997. [Accessed in February 2006]. http://www.esd.ornl.gov/projects/gen/nerc.html . [Google Scholar]
- Albers F, Pröbsting W. [Report on Hieracium pilosella] In: Wisskirchen R., Haeupler H., editors. Standardliste der Farn-und Blütenpflanzen Deutschlands. Vol. 603. Stuttgart: Verlag Eugen Ulmer; 1998. [Google Scholar]
- Asker SE, Jerling L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Auquier P, Renard R. Dénombrements chromosomiques chez quelques Hieracium d'Europe moyenne et méridionale. Societé pour l'Échange des Plantes Vasculaires de l'Europe Occidentale et du bassin Méditerrannéen, Liége. 1979;17:73–79. [Google Scholar]
- Bayer RJ, Stebbins GL. Chromosome numbers, patterns of distribution, and apomixis in Antennaria (Asteraceae: Inuleae) Systematic Botany. 1987;12:305–319. [Google Scholar]
- Bierzychudek P. Pattern in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- Borgen L, Hultgård U-M. Parnassia palustris: a genetically diverse species in Scandinavia. Botanical Journal of the Linnean Society. 2003;142:347–372. [Google Scholar]
- Bräutigam S. Hieracium L. In: Meusel H, Jäger EJ, editors. Vergleichende Chorologie der zentraleuropäischen Flora 3. Stuttgart/New York: Gustav Fischer/Verlag Jena; 1992. pp. 325–333. 550–560. [Google Scholar]
- Bräutigam S, Bräutigam E. Determination of the ploidy level in the genus Hieracium subgenus Pilosella (Hill) S.F. Gray by flow cytometric DNA analysis. Folia Geobotanica et Phytotaxonomica. 1996;31:315–321. [Google Scholar]
- Cárdenas Vergara CA. Chile: Punta Arenas; 2005. Mecanismos de expansión territorial de pilosela (Hieracium pilosella L.) Tezis, Universidad de Magallanes, Facultad de Ciencias esc. cs. y Tecn. en Rec. Agric. y Acuic. [Google Scholar]
- Chapman HM, Parh D, Oraguzie N. Genetic structure and colonizing success of a clonal, weedy species, Pilosella officinarum (Asteraceae) Heredity. 2000;84:401–409. doi: 10.1046/j.1365-2540.2000.00657.x. [DOI] [PubMed] [Google Scholar]
- Chrtek J. Pilosella (Cichoriaceae) In: Marhold K, Hindák F, editors. Checklist of non-vascular and vascular plants of Slovakia. Bratislava: Veda; 1998. pp. 563–565. [Google Scholar]
- Chrtek J., Jr . 106. Hieracium L. – jestřábník. In: Kubát K, Hrouda L, Chrtek J Jr, Kaplan Z, Kirschner J, Štěpánek J, editors. Klíč ke květeně České republiky. Praha: Academia; 2002. pp. 706–732. [Google Scholar]
- Delcourt E. Contribution á l'étude cytotaxinomique de Hieracium pilosella L. Bulletin de la Société Botanique de France. 1972;119:287–302. [Google Scholar]
- Dmitrieva SA. Kariologicheskaja kharakteristika predstaviteleï sem. Slozhnocvetnykh (Asteraceae Dumort.) Flory Belorusii. Botanika (Minsk) 1987;28:23–33. [Google Scholar]
- Doležel J. Flow cytometric analysis of nuclear DNA content in higher plants. Phytochemical Analysis. 1991;2:143–154. [Google Scholar]
- Doležel J, Göhde W. Sex determination in dioecious plants Melandrium album and M. rubrum using high-resolution flow cytometry. Cytometry. 1995;19:103–106. doi: 10.1002/cyto.990190203. [DOI] [PubMed] [Google Scholar]
- Edmonds JM, Sell PD, Walters SM. [Reports on Pilosella officinarum] Cytology database of Botanical Society of the British Isles. 2005. [Accessed in February 2005]. http://rbg-web2.rbge.org.uk/BSBI .
- Fehrer J, Gemeinholzer B, Chrtek J, Jr, Bräutigam S. Incongruent plastid and nuclear DNA phylogenies reveal ancient intergeneric hybridization in Pilosella hawkweeds (Hieracium, Cichoriaceae, Asteraceae) Molecular Phylogenetics and Evolution. 2007;a 42:347–361. doi: 10.1016/j.ympev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Fehrer J, Krahulcová A, Krahulec F, Chrtek J, Jr, Rosenbaumová R, Bräutigam S. Evolutionary aspects in Hieracium subgenus Pilosella. In: Hörandl E, Grossniklaus U, Sharbel T, van Dijk P, editors. Apomixis: evolution, mechanisms and perspectives. b. Ruggell, Liechtenstein: Gantner Verlag; 2007. [Google Scholar]
- Fernandes A, Queirós M. Contribution á la connaissance cytotaxinomique des Spermatophyta du Portugal. Boletim da Sociedade Broteriana. 1971;45:5–121. [Google Scholar]
- Finch R. [Reports on Pilosella officinarum] Cytology database of Botanical Society of the British Isles. 2005. [Accessed in February 2005]. http://rgb-web2.rbge.org.uk/BSBI .
- Gadella TWJ. Biosystematic studies in Hieracium pilosella L. and some related species of the subgenus Pilosella. Botaniska Notiser. 1972;125:361–369. [Google Scholar]
- Gadella TWJ. Cytology and the mode of reproduction of some taxa of Hieracium subgenus Pilosella. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen: Series C: Biological and Medical Sciences. 1984;87:387–399. [Google Scholar]
- Gadella TWJ. Sexual tetraploid and apomictic pentaploid populations of Hieracium pilosella (Compositae) Plant Systematics and Evolution. 1987;157:219–245. [Google Scholar]
- Gadella TWJ. Some notes on the origin of polyploidy in Hieracium pilosella agg. Acta Botanica Neerlandica. 1988;37:515–522. [Google Scholar]
- Gadella TWJ. Variation, hybridization and reproductive biology of Hieracium pilosella L. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen: Series C: Biological and Medical Sciences. 1991;94:455–488. [Google Scholar]
- Gadella TWJ, Kliphuis E. Chromosome numbers of flowering plants in the Netherlands. Acta Botanica Neerlandica. 1963;12:195–230. [Google Scholar]
- Gornall RJ, Wentworth JE. Variation in the chromosome number of Parnassia palustris L. tin the British Isles. New Phytologist. 1993;123:383–388. [Google Scholar]
- Gottschlich G. Hieracium L. In: Wisskirchen R, Haeupler H, editors. Standartliste der Farn- und Blütenpflanzen Deutschlands. Sttutgart: Eugen Ulmer; 1998. pp. 245–263. [Google Scholar]
- Grime JP, Hodgson JC, Hunt R. [Reports on Pilosella officinarum] Cytology database of Botanical Society of the British Isles. 2005. [Accessed in February 2005]. http://rgb-web2.rbge.org.uk/BSBI .
- Hendrych R. Karpatische Migrationen und Florenbeziehungen in den tschechischen Ländern der Tschechoslowakei. Acta Universitatis Carolinae – Biologica. 1987;1985:105–250. [Google Scholar]
- Holub J. Poznámky k druhu ‘Hieracium hoppeanum’ v Československu. Zprávy Československé botanické společnosti. 1986;21:21–28. [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–535. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hultén E, Fries M. Atlas of North European vascular plants. II North of the tropic of cancer. Koenigstein: Koeltz Scientific Books; 1986. [Google Scholar]
- Jalas J, Pellinen K. Chromosome counts on Erigeron, Hieracium, Pilosella and Sonchus (Compositae), mainly from Finland. Annales Botanici Fennici. 1985;22:45–47. [Google Scholar]
- Košt'álová V. Prague: Charles University; 2004. Vybrané druhy Hieracium subgenus Pilosella na antropogenních stanovištích hlavního města Prahy: variabilita, rozšíření a ekologické podmínky. Diploma thesis, Department of Botany, Faculty of Sciences. [Google Scholar]
- Kovanda M. Polyploidy and variation in the Campanula rotundifolia complex. Part II. (Taxonomic). 2. Revision of the groups Vulgares and Scheuchzerianae in Czechoslovakia and adjacent regions. Folia Geobotanica et Phytotaxonomica. 1977;12:23–89. [Google Scholar]
- Krahulcová A, Krahulec F. Chromosome numbers and reproductive systems in selected representatives of Hieracium subgen. Pilosella in the Krkonoše Mts (the Sudeten Mts) Preslia. 1999;71:217–234. [Google Scholar]
- Krahulcová A, Krahulec F. Offspring diversity in Hieracium subgen. Pilosella (Asteraceae): new cytotypes from hybridization experiments and from open pollination. Fragmenta Floristica et Geobotanica. 2000;45:239–255. [Google Scholar]
- Krahulcová A, Chrtek J, Krahulec F. Autogamy in Hieracium subgenus Pilosella. Folia Geobotanica. 1999;34:373–376. [Google Scholar]
- Krahulcová A, Krahulec F, Chapman HM. Variation in Hieracium subgen. Pilosella (Asteraceae): what do we know about its sources? Folia Geobotanica. 2000;35:319–338. [Google Scholar]
- Krahulcová A, Jr, Krahulec F, Chrtek J., Jr Chromosome numbers and reproductive systems in selected representatives of Hieracium subgen. Pilosella in the Krkonoše Mts (Sudeten Mts) – 2. Preslia. 2001;73:193–211. [Google Scholar]
- Krahulec F, Krahulcová A, Fehrer J, Bräutigam S, Plačková I, Chrtek J., Jr The sudetic group of Hieracium subgen. Pilosella from the Krkonoše Mts: a syntetic view. Preslia. 2004;76:223–243. [Google Scholar]
- Král V. Fyzická geografie Evropy. Praha: Academia; 1999. [Google Scholar]
- Lavrenko AN, Sereditov NP. Chromosome numbers in some plants species from the south-west of the Komi ASSR. Botanicheskiï Zhurnal. 1991;76:769–771. [Google Scholar]
- Lövkvist B, Hultgård UM. Chromosome numbers in South Swedish vascular plants. Opera Botanica. 1999;137:1–42. [Google Scholar]
- Májovský J, et al. Index of chromosome numbers of Slovakian flora (Part 1) Acta Facultatis Rerum Naturalium Universitatis Comenianae, Botanica. 1970;16:1–16. [Google Scholar]
- Merxmüller H. Diploide Hieracien. Anales del Instituto Botanico A. J. Cavanilles. 1975;32:189–196. [Google Scholar]
- Měsíček J, Jarolímová V. List of chromosome numbers of the Czech vascular plants. Praha: Academia; 1992. [Google Scholar]
- Mičieta K. Zytotaxonomischer Beitrag zur Flora des Javorníky – Gebirges II. Acta Facultatis Rerum Naturalium Universitatis Comenianae, Botanica. 1982;29:55–61. [Google Scholar]
- Moore DM. Flora Europaea check-list and chromosome index. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Morton JK. Chromosome numbers of British plants, 3. Watsonia. 1974;10:169. [Google Scholar]
- Morton A. DMAP, Distribution mapping software. 2004. Available from http://www.dmap.co.uk .
- Murín A. Substitution of cellophane for glass covers to facilitate preparation of permanent squashes and smears. Stain Technology. 1960;35:351–353. [PubMed] [Google Scholar]
- Murín A. Karyological study of Slovakian flora. VIII. Acta Facultatis Rerum Naturalium Universitatis Comenianae, Botanica. 1986;33:1–16. [Google Scholar]
- Nazarova E, Ghukasyan A. In: Chromosome numbers of flowering plants of Armenian Flora. Gabrielian E, editor. Yerevan: Institute of Botany National Academy of Sciences of RA; 2004. p. 44. [Google Scholar]
- Nägeli C, Peter A. Die Hieracien Mittel-Europas. Monographishe Bearbeitung der Piloselloiden mit besonderer Berücksichtigung der mitteleuropäischen Sippen. München: R. Oldenburg; 1885. [Google Scholar]
- Natarajan G. Löve Á., editor. [Report on Hieracium pilosella L.] Taxon. 1981;30:699. Chromosome number reports LXXII. [Google Scholar]
- Natarajan G. Etude caryosystématique de quelques dicotyledones de la Garigue languedocienne. Naturalia Monspeliensia. 1988;52:85–123. [Google Scholar]
- den Nijs JCM, Kirschner J, Štěpánek J, Van der Hulst A. Distribution of diploid sexual plants of Taraxacum sect. Ruderalia in East-central Europe, with special reference to Czechoslovakia. Plant Systematics and Evolution. 1990;170:71–84. [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z., editors. Methods in Cell Biology. Vol. 33. New York, NY: Academic Press; 1990. pp. 105–110. [DOI] [PubMed] [Google Scholar]
- Parfenov VI, Dmitrieva SA. Kariologicheskaja kharakteristika predstavitelej flory susodistkykh rastenij Berezinskogo biosfernogo zapovednika. Zapov. Belorusii Issl. 1988;12:8–13. [Google Scholar]
- Pashuk KT. Khromozomnye chisla vidov subal'piyskogo poyasa Chernogory (Ukrainskie Karpaty) Botanicheskiï Zhurnal. 1987;72:1069–1074. [Google Scholar]
- Píšt'anský J, Mičieta K. Príspevok ku karyografickému štúdiu Pilosella officinarum agg. na Slovensku. Acta Facultatis Rerum Naturalium Universitatis Comenianae, Botanica. 2000;40:57–64. [Google Scholar]
- Pogan E, Wcisło H. Cytological investigations on Hieracium pilosella L. from Poland. 1. Karyological studies. Acta Biologica Cracoviensia, Botanica. 1989;31:19–28. [Google Scholar]
- Pogan E, Wcisło H. Embryological analysis of Hieracium pilosella L. from Poland. Acta Biologica Cracoviensia, Botanica. 1995;37:53–61. [Google Scholar]
- Pogan E, Jankun A, Turala K. Further studies in chromosome numbers of Polish Angiosperms. Part XIX. Acta Biologica Cracoviensia, Botanica. 1987;28:65–85. [Google Scholar]
- Rotreklová O. Hieracium bauhini group in Central Europe: chromosome numbers and breeding systems. Preslia. 2004;76:313–330. [Google Scholar]
- Rotreklová O, Krahulcová A, Vaňková D, Peckert T, Mráz P. Chromosome numbers and breeding systems in some species of Hieracium subgen. Pilosella from Central Europe. Preslia. 2002;74:27–44. [Google Scholar]
- Rotreklová O, Krahulcová A, Mráz P, Mrázová V, Mártonfiová L, Peckert T, et al. Chromosome numbers and breeding systems of some European species of Hieracium subgen. Pilosella. Preslia. 2005;77:177–195. [Google Scholar]
- Sell PD, West C. In: Flora Europaea 4. Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, et al., editors. Cambridge: Cambridge University Press; 1976. pp. 358–410. Hieracium. [Google Scholar]
- Schuhwerk F. Published chromosome-counts in Hieracium. 1996. [Accessed in February 2006]. http://www.botanis-chestaatssammlung.de/projects/chrzlit.html .
- Schuhwerk F, Fischer MA. Bestimmungsschlüssel der Untergattung Hieracium subg. Pilosella in Österreich und Südtirol. Neilreichia. 2003;2–3:13–58. [Google Scholar]
- Schuhwerk F, Lippert W. Chromosomenzahlen von Hieracium L. (Compositae, Lactucae) Teil 1. Sendtnera. 1997;4:181–206. [Google Scholar]
- Schuhwerk F, Lippert W. Chromosomenzahlen von Hieracium L. (Compositae, Lactucae) Teil 4. Sendtnera. 2002;8:167–194. [Google Scholar]
- Skalińska M. Cytological analysis of some Hieracium species, subgen. Pilosella from mountains of southern Poland. Acta Biologica Cracoviensia, Botanica. 1967;10:126–140. [Google Scholar]
- Skalińska M. Cytological diversity in the progeny of octoploid facultative apomixis of Hieracium aurantiacum L. Acta Biologica Cracoviensia, Botanica. 1976;19:39–46. [Google Scholar]
- Skalińska M, Pogan E, et al. Further studies in chromosome numbers of Polish Angiosperms. Ninth contribution. Acta Biologica Cracoviensia, Botanica. 1971;14:199–213. [Google Scholar]
- Suda J, Krahulcová A, Trávníček P, Krahulec F. Ploidy level vs. DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Šingliarová B, Mráz P. Cytogeografia druhu Pilosella officinarum (Asteraceae) v Západných Karpatoch a v pril'ahlej časti Panónie (predbežné výsledky) Bulletin Slovenskej Botanickej Spoločnosti, Supplement. 2004;10:176–180. [Google Scholar]
- Trewick SA, Morgan-Richards M, Chapman HM. Chloroplast DNA diversity of Hieracium pilosella (Asteraceae) introduced to New Zealand: reticulation, hybridization, and invasion. American Journal of Botany. 2004;91:73–85. doi: 10.3732/ajb.91.1.73. [DOI] [PubMed] [Google Scholar]
- Turesson B. Experimental studies in Hieracium pilosella L. II. Taxonomy and differentiation. Botaniska Notiser. 1972;125:223–240. [Google Scholar]
- Turesson G, Turesson B. Experimental studies in Hieracium pilosella L. I. Reproduction, chromosome number and distribution. Hereditas. 1960;46:717–736. [Google Scholar]
- Tyler T. Patterns of allozyme variation in Nordic Pilosella. Plant Systematics and Evolution. 2005;250::133–145. [Google Scholar]
- Uhríková A, Feráková V. Löve Á., editor. [Report on Pilosella officinarum] Taxon. 1977;26:257–274. IOPB chromosome number reports LVI. [Google Scholar]
- Vesecký A. Podnebí Československé socialistické republiky. Tabulky. 1961 Hydrometeorologický ústav, Praha. [Google Scholar]
- Watson PM. [Reports on Pilosella officinarum] Cytology Database of Botanical Society of the British Isles. 2005. [Accessed in February 2005]. http://rbg-web2.rbge.org.uk/BSBI .
- Wilson LM, Fehrer J, Brautigäm S, Grosskopf G. A new invasive hawkweed, Hieracium glomeratum (Lactuceae, Asteraceae), in the Pacific Northwest. Canadian Journal of Botany. 2006;84:133–142. [Google Scholar]
- Zahn KH. In: Das Pflanzenreich. 280. Engler A, editor. –1923 4. Leipzig: Wilhelm Engelmann; 1921. Hieracium. [Google Scholar]
- Zahn KH. In: Synopsis der Mitteleuropäischen Flora. 1. Ascherson PF, Graebner P, editors. –1930 12. Leipzig: Bornträger; 1922. pp. 1–492. Hieracium. [Google Scholar]