Abstract
Background and Aims
Considering that few studies on nectary anatomy and ultrastructure are available for chiropterophilous flowers and the importance of Hymenaea stigonocarpa in natural ‘cerrado’ communities, the present study sought to analyse the structure and cellular modifications that take place within its nectaries during the different stages of floral development, with special emphasis on plastid dynamics.
Methods
For the structural and ultrastructural studies the nectary was processed as per usual techniques and studied under light, scanning and transmission electron microscopy. Histochemical tests were employed to identify the main metabolites on nectary tissue and secretion samples.
Key Results
The floral nectary consists of the inner epidermis of the hypanthium and vascularized parenchyma. Some evidence indicates that the nectar release occurs via the stomata. The high populations of mitochondria, and their juxtaposition with amyloplasts, seem to be related to energy needs for starch hydrolysis. Among the alterations observed during the secretory phase, the reduction in the plastid stromatic density and starch grain size are highlighted. When the secretory stage begins, the plastid envelope disappears and a new membrane is formed, enclosing this region and giving rise to new vacuoles. After the secretory stage, cellular structures named ‘extrastomatic bodies’ were observed and seem to be related to the nectar resorption.
Conclusions
Starch hydrolysis contributes to nectar formation, in addition to the photosynthates derived directly from the phloem. In these nectaries, the secretion is an energy-requiring process. During the secretion stage, some plastids show starch grain hydrolysis and membrane rupture, and it was observed that the region previously occupied by this organelle continued to be reasonably well defined, and gave rise to new vacuoles. The extrastomatic bodies appear to be related to the resorption of uncollected nectar.
Key words: Cell ultrastructure, cerrado vegetation, extrastomatic bodies, Fabaceae, floral nectary, Hymenaea stigonocarpa, nectar, plastids, secretion, starch hydrolysis, vacuole
INTRODUCTION
Nectaries have great ecological significance in that they produce substances involved in plant–animal interactions (Davis et al., 1988; Nepi et al., 1996). The position of the nectary within the flower is a determinant in the nature of plant–pollinator interactions (Nepi and Pacini, 1993), and information concerning the structural and ultrastructural aspects of the nectaries are important to understand how cell organelles are involved in the secretory processes, and how the nectar is liberated. Additionally, more detailed information concerning these factors is essential to correlate them with physiological aspects of nectar production and secretion.
The morphology and the anatomy of nectaries have been described for many taxa (see Fahn, 1979) and, in general, nectaries are characterized by the presence of epidermal and parenchyma tissues specialized for the synthesis, accumulation and liberation of nectar.
The ultrastructural organization of floral nectaries varies widely among different species, and the phases of each secretory process show peculiarities and unique ultrastructural changes that defy attempts at defining fixed patterns. As such, the roles of the cell organelles in each of the phases of the secretory process must be analysed in detail in each of the species examined.
The dynamics of cell organelle populations during nectar production have been little studied, and investigations have usually been limited simply to counting them, and at best only superficially recording structural descriptions. Alterations in the form and content of the plastids were described by Peng et al. (2004), however, and opened up perspectives for additional studies that would examine alterations in cell organelles during the secretory processes.
In some species, the starch grains stored in plastids are precursors of the nectar carbohydrates; thus, the breakdown of the stored carbohydrates makes possible high levels of nectar production. In turn, plants can attract and supply pollinators that consume large quantities of nectar (see Pacini et al., 2003; Peng et al., 2004). According to Razem and Davis (1999), studies of nectaries in their secretory phase have generally received greater attention than studies dedicated to ultrastructural aspects of these cells in other stages of development. Nepi et al. (2001) pointed out that examining modifications of the nectary during flower ageing is important to understand the fate of the uncollected nectar; some plants appear to re-absorb uncollected nectar.
Within the Fabaceae, plants of the genus Hymenaea are characterized by the production of resins of commercial value (Langenheim, 2003), edible fruits (Almeida et al., 1998) and abundant quantities of floral (Gibbs et al., 1999) and extrafloral nectar (Paiva and Machado, 2006). Hymenaea stigonocarpa, also known by its common name ‘jatobá-do-cerrado’, is a very typical tree of the Brazilian ‘cerrado’ (savanna). This species produces large flowers and large quantities of nectar at anthesis. The tree is pollinated by bats (Gibbs et al., 1999) and, according to Vogel (1968), has exposed nectaries characteristic of the most primitive chiropterophilous flowers. Considering the importance of H. stigonocarpa in natural ‘cerrado’ communities, and that little is known about floral nectaries in this species, the present study sought to analyse the structure and cellular modifications that take place within its nectaries during the different stages of floral development, with special emphasis on plastid dynamics.
MATERIALS AND METHODS
Plant material
Flowers of H. stigonocarpa Mart. ex Hayne were collected from natural populations occurring in cerrado vegetation in the municipality of Botucatu, São Paulo State, Brazil (22°53′11·4″S, 48°26′07·8″W). Flowers in different stages of development were examined, including floral buds (2 h before anthesis) and open flowers (anthesis, 2 h post-anthesis, 24 h post-anthesis and 5 d post-anthesis). Voucher specimens were deposited in the herbarium of the Botany Department, Instituto de Biociências de Botucatu, UNESP (BOTU) under numbers 23189–23191, 23132 and 23133. Tissue samples were removed from the hypanthium region for anatomical, histochemical and ultrastructural analyses.
Anatomical studies
Samples were fixed in Karnovsky's solution (Karnovsky, 1965) for 24 h, dehydrated in an ethanol series, and embedded in historesin (Leica Embedding Kit). Both transverse and longitudinal (5 µm) sections were made using a rotary microtome with a steel blade, and the sections were subsequently stained in 0·05 % toluidine blue, pH 4·3 (O'Brien et al., 1964). Part of the material was fixed in 70 % ethyl alcohol (Johansen, 1940) and submitted to successive changes of this solvent in order to remove phenolic compounds, followed by embedding in hydroxyl-ethyl-methacrylate resin (Leica). The following histochemical tests were performed in fresh hand-cut sections: aqueous solution of ruthenium red for detection of acidic polysaccharides; ferric chloride for phenolic compounds; Sudan Black B for lipids (Pearse, 1980); bromophenol blue for proteins (Mazia et al., 1953); Schiff's reagent [periodic acid–Schiff's (PAS)] for polysaccharides (McManus, 1948); Fehling's reagent for reducing sugars (Sass, 1951); Lugol reagent for starch (Johansen, 1940); and 0·2 % ninhydrin in acetone (w/v) solution for identifying amino acids in nectar samples (Baker and Baker, 1973).
Ultrastructural studies
For examination under transmission electron microscopy, samples were fixed in Karnovsky's solution (Karnovsky, 1965) for 24 h, post-fixed in osmium tetroxide (1 %, 0·1 m phosphate buffer pH 7·2) and processed in the usual manner (Roland, 1978). Ultra-thin sections were stained with uranyl acetate and lead citrate, and examined under a Philips CM 100 transmission electron microscope at 60 kV. For observation by scanning electron microscopy, samples were fixed in 2·5 % glutaraldehyde (0·1 m phosphate buffer pH 7·2), dehydrated in an ethanol series, dried to the critical point and layered with gold (Robards, 1978). The samples were observed using a Philips 515 model scanning electron microscope.
RESULTS
Nectary structure
The flowers of H. stigonocarpa demonstrate a well-developed, obconical, sub-sessile hypanthium, with extensive nectary tissue on their inner faces (Fig. 1A and B). The gynaecium demonstrates a long stipe crossing the hypanthium region (Fig. 1A). The floral nectar accumulates in the space between the hypanthium and the stipe, and reaches the base of the corolla (Fig. 1A and B). Floral anthesis is nocturnal, occurring just after sunset, with nectar release extending for a number of hours and stopping completely in the first hours of the morning (approx. 12 h after initiation, at anthesis).
Fig. 1.
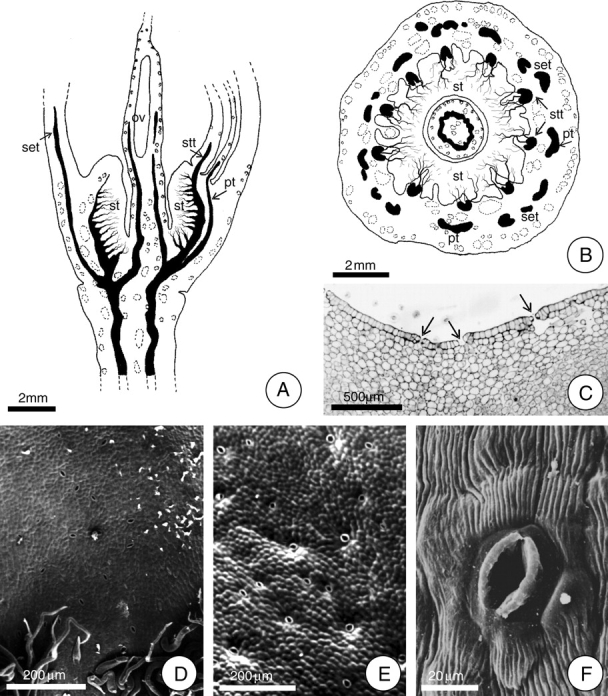
Localization of the secretory face of the floral nectary of H. stigonocarpa. (A and B) Diagrams demonstrating the vascularization of the hypanthium and the position of the floral nectary in longitudinal and transverse section, respectively. In A, the bundle ramifications are exclusively phloem cells. Note in B the position of the ten stamen traces (stt) and the five petal traces (pt) alternating with the sepal traces (set). (C) Transverse section of the median region of the hypanthium. Arrows indicate stomata. (D–F) Detail of the epidermis at the secretory face (SEM). Note the stomatal pore and striated cuticle (F). Scale bars: 2 mm (A, B); 200 µm (D, E); 500 µm (C); 20 µm (F) (ov, ovary; st, secretory tissue).
The entire inner face of the hypanthium constitutes the secretory surface of the nectary. The nectar is apparently liberated through stomata that are distributed over the entire secretory surface (Fig. 1C–F), as suggested by the observation of secretion residues at stomatic pores. These stomata are more concentrated on the basal third of the hypanthium.
The floral nectary consists of an epidermal layer and vascularized parenchyma. The epidermis of the secretory face of the floral nectary is glabrous over much of its length. Trichomes are restricted to the nectary basal portion, near the region of its junction with the stipe (Fig. 1D). The epidermal cells are juxtaposed and covered with a striated cuticle which shows well-developed vacuoles that accumulate phenolic compounds. There were no signs of ruptures or pores in the cuticle that would permit the liberation of nectar (Figs 1F and 5F).
Fig. 5.
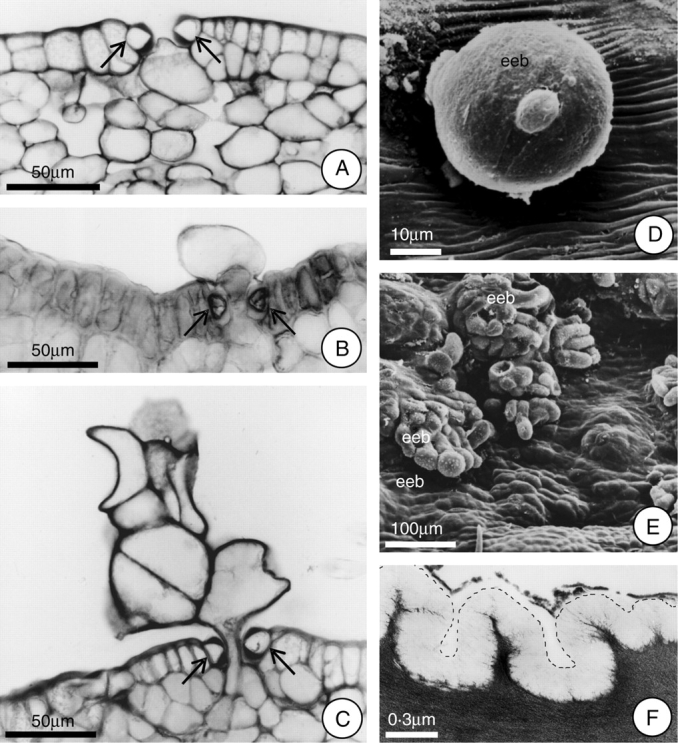
Extrastomatic bodies (eeb) formed after the secretory phase, and detail of the epidermal cell showing the cuticle. (A–C) Proposed sequence of the formation of the extrastomatic bodies (arrows indicate guard cells). Note in A the initial expansion of the cell towards the stomatal pore. (D) Young extrastomatic body, under SEM. (E) Fully differentiated extrastomatic bodies, under SEM. (F) Detail (under TEM) of the epidermal cell showing the cuticle; the dotted line indicates the cuticle outline. Scale bars: 50 µm (A–C); 10 µm (D); 100 µm (E); 0·3 µm (F).
The secretory parenchyma is located between the inner epidermis and the stamen traces, and extends from the base to the apex of the hypanthium (Fig. 1A). The secretory cells are globoid, have only a small volume, and only small spaces exist between them; their cell walls are thin and pectocellulosic, of uniform thickness. The cytoplasm is dense with many starch grains and a low content of phenolic compounds, making this region very distinct from the outer portion of the stamen traces and allowing this secretory tissue to be recognized even by the naked eye.
The stamen traces (ten) are amphicribral, with a predominance of phloem, and they are arranged in a circular pattern that establishes the outer limit of the nectary secretory tissue. Each of the ten stamen traces ramifies continually, from the base of the hypanthium upwards, forming a woven network throughout all of the secretory parenchyma (Fig. 1A and B). The xylem does not accompany the phloem within these ramifications, remaining intact in the centre of the stamen traces. Small druses are frequently observed near the phloem.
Histochemical tests
The secretory parenchyma cells demonstrated strong positive reactions for polysaccharides at anthesis, in large part due to the presence of starch grains. At 5 d after anthesis, the same tissue only demonstrated a weak reaction, and few starch grains could be observed. Samples of the floral nectar mixed with Fehling's reagent demonstrated only weak reactions before acid hydrolysis, but strong reactions after hydrolysis, indicating the presence of non-reducing sugars.
Evidence was observed of a significant accumulation of phenolic compounds in the vacuoles of the parenchyma cells in the outer region of the hypanthium adjacent to the secretory parenchyma, independent of the stage of development of the nectary. Resin secretory cavities were observed in these same regions, especially near the epidermis and the vascularized regions. In the pre-secretory and secretory phases, the secretory parenchyma showed few cells with phenolic compounds; however, 5 d after anthesis a significant increase was noted in the numbers and sizes of the vacuoles and in the amounts of phenolic compounds accumulated.
Lipid-like substances and proteins were absent in the secretory parenchyma of the floral nectary throughout the period analysed. Amino acids were detected only in nectar samples.
Ultrastructure of the secretory cells
In the phase that precedes anthesis (2 h before anthesis), the secretory cells showed large nuclei with irregular shapes, with an evident nucleolus. The cytoplasm was dense, with ribosomes, segments of endoplasmic reticulum, mitochondria and plastids (Fig. 2A). The ribosomes appeared dispersed within the cytosol and associated with segments of the endoplasmic reticulum. The plastids demonstrated poorly developed membrane systems, dense stroma, and starch grains that filled most of the organelle. The starch grains varied in shape from globular to oval and were not very numerous, there rarely being more than four per plastid (Fig. 2A). Vacuoles were small and distributed throughout the protoplasm (Fig. 2A).
Fig. 2.
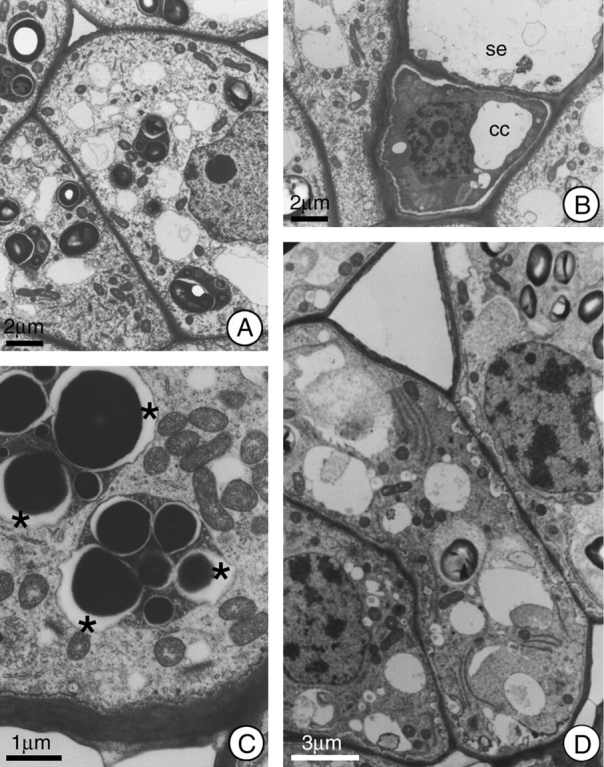
General view of the cells in the secretory region of the nectary of H. stigonocarpa. (A) Secretory cell 2 h before anthesis; note dense cytoplasm, presence of mitochondria, small vacuoles, and plastids with well developed starch grains and dense stroma. (B) Sieve element (se) and companion cell (cc); note absence of symplastic connections between these cells and the secretory cells adjacent to them. (C) Secretory cell at anthesis; note the elevated number of mitochondria and the formation of lacuna (*) next to the starch grains within the plastids. (D) Secretory cell 2 h after anthesis; note the incorporation of the plastids into the cytoplasmic matrix and the increase in periplasmic space. Scale bars: 2 µm (A and B); 1 µm (C); 3 µm (D).
Due to the high density of the plastid stroma within the secretory parenchyma cells, the plastid envelope was not very sharply defined. On the other hand, the maintenance of the equal distances between the double membranes of both the nucleus and the mitochondria over their lengths indicated that the membranes of the organelles were adequately fixed during specimen preparation.
At the phloem, the companion cells demonstrated dense cytoplasm (Fig. 2B), and cytoplasmic connections were restricted to those established with the sieve element.
During anthesis, and up to 2 h afterwards, the ultrastructural characteristics of the plastids did not demonstrate any large changes, except for a reduction in the size of the starch grains and an increase in the numbers of vacuoles. During this phase, the periplasmic space become greater, often with the presence of vesicles that fuse with the plasmatic membrane, which shows a sinuous outline (Fig. 2C and D). The juxtaposition of mitochondria and plastids was commonly observed at anthesis (Fig. 2C).
An abundance of mitochondria was observed during the secretory phase (Fig. 2C). Dictyosomes were infrequent and demonstrated a reduced number of cisternae, these varying in number from two to four. A gradual increase in the sinuous outline of the nucleus was observed, and it became strongly lobate in the post-secretory phase (Fig. 3B). At the end of the secretory phase, the cytoplasm was reduced to a thin layer at the outer edge of the cell, and a significant decrease in the number of cell organelles was observed. Another striking characteristic of the post-secretory phase cells was the presence of multivesicular bodies. In some cells, the reduced cytoplasm appeared dense and dark (Fig. 3C), and phenolic compounds began to accumulate in the vacuoles.
Fig. 3.
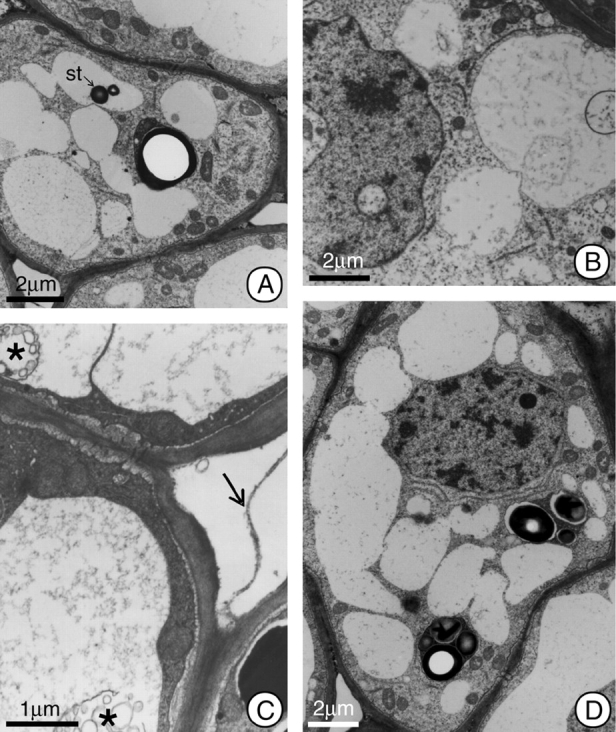
General view of the cells in the secretory region of the nectary at post-anthesis of H. stigonocarpa. (A) Secretory cell 2 h after anthesis; note the increase in size of the vacuoles as a result of incorporating the plastids, and the presence of a remnant starch grain (st) inside the vacuole. (B–D) Secretory cell 12 h after anthesis; in this phase the nucleus appears lobate, and there are myelin figures (*) in the vacuoles. Some cells show electron-dense peripheral cytoplasm, and other show whole plastids (D). Note pectic filaments (arrow) connecting adjacent cells in C. The organelle population appears reduced compared with earlier stages (B–D). Scale bars: 2 µm (A and B); 1 µm (C); 2 µm (D).
Starting 12–24 h after anthesis, a lobate nucleus, increases in the numbers of vacuoles and the apparent reduction in the numbers of organelles constitute the most visible alterations of the secretory cells (Fig. 3B–D).
Communication between the cells of the secretory tissue is maintained throughout the development of the nectary by means of plasmodesmata. Additionally, pectic projections, in the form of filaments, cross the intercellular spaces (Fig. 3C), maintaining the apoplastic communication network between adjacent cells.
In the first 2 h after anthesis the plastids demonstrated a reduction in their stromatic density and in the size of their starch grains. The plastid membranes showed rupture at this time, and the residues of these organelles were incorporated into the cytoplasm matrix. So, the place previously occupied by plastids are recognizable only by the reduced homogeneity of the matrix and by the presence of residual starch grains in their final phases of hydrolysis (Fig. 4A–E). At 12 h after anthesis, the number of plastids was quite reduced, but the plastids that did remain demonstrated structural characteristics similar to those of the pre-anthesis phase (Fig. 3D). These plastids remained in the tissue of the hypanthium after secretory activity had terminated because in this species the nectary region persists throughout the entire period of fruit development, although without demonstrating any continuing secretory activity.
Fig. 4.
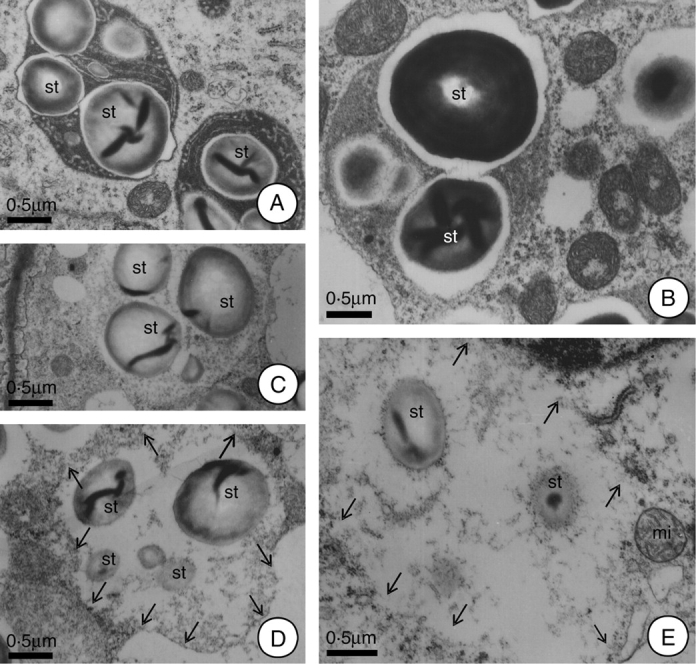
Alterations of the plastids during the secretory and post-secretory phases of H. stigonocarpa. (A) At 2 h before anthesis; plastids with elevated stroma densities, demonstrating whole starch grains and intact plastid membranes. (B) Plastids at anthesis; note the reduction in the density and the organization of the stroma, increase in the lacuna near the starch grains and their association with the mitochondria. (C–E) Plastids 2 h after anthesis; note ruptures in plastid envelope and the incorporation of this organelle into the cytoplasmic matrix, as well as partially hydrolysed starch grains. Note, in E, intact mitochondria next to the plastid remnants. Scale bar: 0·5 µm (A–E). (Arrows indicate the plastids outline.) (st, starch grain.)
Although the residues of the plastids were incorporated into the cytoplasmic matrix, it could be observed that the region previously occupied by this organelle continued to be reasonably well defined, especially in terms of the granular background (Fig. 4E). It was also observed that the plastid envelope completely disappeared (Fig. 4E) and that a new membrane was formed (Fig. 3A and B) that enclosed this region and gave rise to new vacuoles. Some of the new vacuoles formed demonstrated remnants of starch grains and/or the residual granulation of the stroma (Fig. 3A and B).
With the end of the secretory phase, cellular structures of varying shapes and sizes were observed to emerge through some stomata on the inner surface of the hypanthium, especially those at the basal region. These structures are called extrastomatic bodies (Fig. 5C–E), and they project into the space between the hypanthium and the stipe. The cells that comprise these bodies are quite large and irregular in shape, while their walls are thin and the cytoplasm dense.
These extrastomatic bodies begin to form 12 h after the initiation of anthesis, with the expansion of some of the cells that comprise the parenchyma subjacent to the stomata. This expansion occurs in the direction of the stomatal pore (which remains open), allowing this expanding cell to project itself into the space between the hypanthium and the stipe (Fig. 5A and B). Following this, this cell divides, in many different planes, forming the extrastomatic body (Fig. 5C). Soluble carbohydrates were detected in the cytosol of these structures.
DISCUSSION
Nectary structure and histochemistry
According to the classification system proposed by Fahn (1953), the floral nectaries of H. stigonocarpa are placed in the group in which the nectaries are located between the stamens and the ovary, having nectary tissues within the hypanthium. According to this author, this type of nectary is common in the Fabaceae. According to the classification system of floral nectaries proposed by Bonnier (Bonnier, 1879, cited in Fahn, 1952) and modified by Fahn (1952), the floral nectaries of H. stigonocarpa are considered to be tubular nectaries. Relatively large volumes of nectar are produced by H. stigonocarpa, up to 1 mL per nectary in the first hours of secretion (Gibbs et al., 1999). Flowers adapted to pollination by bats, such as H. stigonocarpa, usually furnish significant quantities of nectar, and chiropterophilous flowers in fact produce the largest recorded volumes of nectar in relation to the biomass of their nectaries (Opler, 1983).
The results of the present work indicate that the liberation of nectar in H. stigonocarpa occurs through the stomata, as has been reported for floral nectaries of other species (Davis and Gunning, 1992; Zer and Fahn, 1992; Gaffal et al., 1998; Razem and Davis, 1999; Fahn and Shimony, 2001; Gaffal et al., 2007). The hypothesis that the secretion of nectar occurs exclusively through the stomata is reinforced by the fact that no ruptures were observed in the cuticle, and no sub-cuticular chambers are formed. According to Davis and Gunning (1992), the stomata present in the nectaries are structurally modified and apparently have lost the capacity to close, thus being incapable of regulating the flow of nectar. The presence of secretory tissues and of an epidermis with stomata (as observed in H. stigonocarpa) are common characteristics of floral nectaries (Rachmilevitz and Fahn, 1973; Fahn, 1979; Davis and Gunning, 1992; Zer and Fahn, 1992; Gaffal et al., 2007).
According to Frey-Wyssling (1955), the floral nectaries of the Fabaceae are supplied by ramifications of the adjacent stamen traces, very similar to the situation observed in H. stigonocarpa, and also reported by Horner et al. (2003) in Glycine max (Fabaceae). Frey-Wyssling (1955) stated that there is a positive correlation between the amount of sugars in the nectar and the quantity of phloem elements present in the nectaries. The present results reinforce the hypothesis of Metcalfe and Chalk (1979) that the floral nectaries tend to demonstrate vascularization composed of only phloem elements.
Tests with Fehling's reagent indicated that only small quantities of reducing sugars, but a predominance of non-reducing sugars, were present in the floral nectar. According to Gibbs et al. (1999), the floral nectar of H. stigonocarpa contains from 6 to 19 % sucrose, which supports the observations reported above.
The calcium oxalate crystals observed near the phloem terminations in the floral nectaries of H. stigonocarpa were also reported for other floral nectaries of the Fabaceae, such as Vicia faba (Davis et al., 1988) and G. max (Horner et al., 2003). Calcium oxalate crystals are quite common in floral nectaries vascularized by phloem, and there are even reports of the presence of these crystals in extrafloral nectaries too (Durkee, 1982; Paiva and Machado, 2006).
The presence of phenolic compounds around the secretory tissue, and the increase in the number of cells with these compounds after the secretory phase can be interpreted as a form of protection against herbivory (Taiz and Zeiger, 1998).
Nectary ultrastructure
The abundance of mitochondria, ribosomes and endoplasmic reticulum observed in the secretory cells of the floral nectary in H. stigonocarpa is a common characteristic of nectar-producing tissues (Durkee, 1982; Fahn, 1988).
In the floral nectary of H. stigonocarpa, besides the photosynthates directly derived from the phloem, evidence was found of eccrine secretion of other nectar components from the secretory cells. The large mitochondria population and plastids with starch grains constitute evidence of an energy-requiring process, like eccrine secretion. According to Durkee (1983) and Zer and Fahn (1992), the presence of numerous plastids containing starch grains is further evidence of eccrine secretion, which is reinforced by numerous mitochondria at the secretion stage (see Razem and Davis, 1999). The impressive volume of nectar produced over a relatively short period of time after anthesis, and the predominance of sucrose in the nectar, indicates that phloem contents contribute to the secretion, mixing with and adding to the other nectar components. According to Gaffal et al. (2007), in Digitalis purpurea apoplastic phloem unloading dominates during nectar exudation.
The phloem companion cells that permeate the secretory parenchyma demonstrate ultrastructural characteristics that allow them to be classified as ordinary companion cells. These characteristics suggest apoplastic downloading of phloem components (Taiz and Zeiger, 1998), and they are compatible with the hypothesis of the liberation of a pre-nectar within the intercellular spaces.
The pectic filaments observed in the intercellular spaces and that connect neighbouring cells appear to be functioning as channels for secretion, facilitating the transport of nectar by way of the apoplast (see Carr et al., 1980). The presence of pectic projections connecting adjacent cells is relatively common in many taxa (Carr et al., 1980). These structures appear in many morphological forms, such as filaments (Potgieter and Wyk, 1992).
The presence of multivesicular bodies in the post-secretory phase, as observed in the present study, is an indication of the action of lytic enzymes and of the autophagy of some organelles. Jiang et al. (2002) have demonstrated that the inner vesicles of the multivesicular bodies contain lytic enzymes. A marked characteristic of the post-secretory phase is an increase in the numbers of vacuoles (Durkee, 1982; Fahn, 1988; Razem and Davis, 1999; Fahn and Shimony, 2001).
A decrease in the numbers and the sizes of the starch grains in the floral nectaries of H. stigonocarpa was observed after the opening of the floral bud, at a time when nectar secretion is at its highest and the starch is apparently being hydrolysed. The breakdown of the starch grains in the plastids at the start of the secretory phase suggests that this starch is a principal source of the carbohydrates liberated in the nectar (Rachmilevitz and Fahn, 1973; Durkee et al., 1981; Zer and Fahn, 1992; Razem and Davis, 1999; Pacini et al., 2003).
The juxtaposition of mitochondria and amyloplasts during anthesis was also observed by Nepi et al. (1996) and may be related to energy needs during the hydrolysis of the starch.
The breakdown of the starch and the lysis and re-incorporation of the plastid residues into the cytoplasmic matrix observed in the first hours following anthesis seems to be similar to that observed by Peng et al. (2004) in cucumber flowers, where the plastids appear to evolve into vacuoles. Plastid degradation mediated by vacuoles during programmed cell death was described by Gaffal et al. (2007) in the D. purpurea nectary, and the plastid engulfing by vacuoles seems to occur in others floral nectaries such as those of Eccremocarpus scaber (Belmonte et al., 1994) and Arabidopsis thaliana (Zhu and Hu, 2002). In H. stigonocarpa, it was observed that the region previously occupied by plastids continued to be reasonably well defined, and that a new membrane was formed that enclosed this region and gave rise to new vacuoles.
The persistence of the floral nectary region of H. stigonocarpa during fruit development supports the view that nectar secretion is not predominantly the result of starch hydrolysis, as in these cases the nectary generally degenerates (Pacini et al., 2003).
Extrastomatic bodies
Although the fate of the floral nectar of H. stigonocarpa was not directly investigated, the presence of extrastomatic bodies and the absence of nectar 24 h after anthesis (in protected flowers) both indicate that nectar not collected during anthesis is subsequently re-absorbed. There are numerous examples in the literature of residual floral nectar being re-absorbed (Burquez and Corbet, 1991; Nepi et al., 1996, 2001; Koopowitz and Marchant, 1998). According to Nepi et al. (1996), the floral nectar not collected by pollinators of Cucurbita pepo is re-absorbed. These authors also observed that the epidermis is actively involved in the process of resorption and that this phenomenon allows the plant to recycle part of the energy spent in producing that attractive resource.
Extrastomatic bodies are only observed the day after anthesis, and these structures are restricted to only a few nectaries; additionally, histochemical tests indicate the presence of soluble carbohydrates in the dense cytoplasm of the cells that comprise these extrastomatic bodies – and both observations reinforce the hypothesis that these structures act in the resorption of any nectar that was not collected by pollinators.
In light of the significant volumes of nectar produced, and the absence of a concentration gradient favourable to the re-entry of the nectar into the hypanthium, the resorption of the excess nectar exclusively by way of the stomata appears to be unlikely. The formation of extrastomatic bodies creates a large surface area to re-absorb the residual nectar, although it must be stressed that this is the first report of these structures, and additional studies will be necessary to understand their function fully.
According to Razem and Davis (1999), the occlusion of stomatal pores in the post-secretory phase may be an important mechanism for impeding the entrance of pathogens into these persistent glands. In cases previously reported, however, this occlusion resulted from the accumulation of amorphous material of unknown nature at the stomatal pore, and did not involve the cellular structures observed in H. stigonocarpa. As such, there is no evidence that the extrastomatic bodies act in the occlusion of the stomata pores.
ACKNOWLEDGEMENTS
The authors thank FAPESP (Brazil) for its financial support of this work (Programa Biota 00/12469-3), the technical team of the Centro de Microscopia eletrônica, Instituto de Biociências, UNESP Botucatu, for their help in preparing the samples, and two anonymous reviewers for their helpful comments. S.R.M. is in receipt a research grant from CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil).
LITERATURE CITED
- Almeida SP, Proença CEB, Sano SM, Ribeiro JF. Cerrado – Espécies vegetais úteis. Brasília: Embrapa. 1998. [Google Scholar]
- Baker HG, Baker I. Some anthecological aspects of the evolution of nectar-producing flowers, particularly amino acid production in nectar. In: Heiwood VH, editor. Taxonomy and ecology. London: Academic Press; 1973. pp. 243–264. [Google Scholar]
- Belmonte E, Cardemil L, Arroyo MTK. Floral nectary structure and nectar composition in Eccremocarpus scaber (Bignoniaceae), a hummingbird-pollinated plant of central Chile. American Journal of Botany. 1994;81:493–503. [Google Scholar]
- Burquez A, Corbet SA. Do flowers resorb nectar? Functional Ecology. 1991;5:369–379. [Google Scholar]
- Carr DJ, Oates K, Carr SGM. Studies on intercellular pectic strands of leaf palisade parenchyma. Annals of Botany. 1980;45:403–413. [Google Scholar]
- Davis AR, Gunning BES. The modified stomata of the floral nectary of Vicia faba L. 1. Development, anatomy and ultrastructure. Protoplasma. 1992;164:134–152. [Google Scholar]
- Davis AR, Peterson RL, Shuel RW. Vasculature and ultrastructure of the floral and stipular nectaries of Vicia faba (Leguminosae) Canadian Journal of Botany. 1988;66:1435–1448. [Google Scholar]
- Durkee LT. The floral and extrafloral nectaries of Passiflora. II. The extrafloral nectary. American Journal of Botany. 1982;69:1420–1428. [Google Scholar]
- Durkee LT. The ultrastructure of floral and extrafloral nectaries. In: Bentley B, Elias TS, editors. The biology of nectaries. New York: Columbia University Press; 1983. pp. 1–29. [Google Scholar]
- Durkee LT, Gaal DJ, Reisner WH. The floral and extrafloral nectaries of Passiflora. I – The floral nectary. American Journal of Botany. 1981;68:453–462. [Google Scholar]
- Fahn A. On the structure of floral nectaries. Botanical Gazette. 1952;113:464–470. [Google Scholar]
- Fahn A. The topography of the nectary in the flower and its phylogenetical trend. Phytomorphology. 1953;3:424–426. [Google Scholar]
- Fahn A. Secretory tissues in plants. New York: Academic Press; 1979. [Google Scholar]
- Fahn A. Secretory tissues in vascular plants. New Phytologist. 1988;108:229–257. doi: 10.1111/j.1469-8137.1988.tb04159.x. [DOI] [PubMed] [Google Scholar]
- Fahn A, Shimony C. Nectary structure and ultrastructure of unisexual flowers of Ecballium elaterium (L.) A. Rich. (Cucurbitaceae) and their presumptive pollinators. Annals of Botany. 2001;87:27–33. [Google Scholar]
- Frey-Wyssling A. The phloem supply to the nectaries. Acta Botanica Neerlandica. 1955;4:358–369. [Google Scholar]
- Gaffal KP, Heimler W, El-Gammal S. The floral nectary of Digitalis purpurea L. Structure and nectar secretion. Annals of Botany. 1998;81:251–262. [Google Scholar]
- Gaffal KP, Friedrichs GJ, El-Gammal S. Ultrastructural evidence for a dual function of the phloem and programmed cell death in the floral nectary of Digitalis purpurea. Annals of Botany. 2007;99:593–607. doi: 10.1093/aob/mcm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs PE, Oliveira PE, Bianchi MB. Postzygotic control of selfing in Hymenaea stigonocarpa (Leguminosae-Caesalpinioideae), a bat pollinated tree of the Brazilian cerrados. International Journal of Plant Sciences. 1999;160:72–78. [Google Scholar]
- Horner HT, Healy RA, Cervantes-Martinez T, Palmer RG. Floral nectary fine structure and development in Glycine max L. (Fabaceae) International Journal of Plant Sciences. 2003;164:675–690. [Google Scholar]
- Jiang L, Erickson AH, Rogers JC. Multivesicular bodies: a mechanism to package lytic and storage functions in one organelle? Trends in Cell Biology. 2002;12:362–367. doi: 10.1016/s0962-8924(02)02322-x. [DOI] [PubMed] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw Book Co; 1940. [Google Scholar]
- Karnovsky MJ. A formaldehyde–glutaraldehyde fixative of light osmolality for use in electron microscopy. Journal of Cell Biology. 1965;27:137A–138A. [Google Scholar]
- Koopowitz H, Marchant TA. Postpollination nectar reabsorption in the African epiphyte Aerangis verdickii (Orchidaceae) American Journal of Botany. 1998;85:508–512. [PubMed] [Google Scholar]
- Langenheim J.H. Plant resins. Cambridge: Timber Press; 2003. [Google Scholar]
- Mazia D, Brewer PA, Alfert M. The cytochemistry staining and measurement of protein with mercuric bromophenol blue. Biological Bulletin. 1953;104:57–67. [Google Scholar]
- McManus JFA. Histological and histochemical uses of periodic acid. Stain Technology. 1948;26:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- Metcalfe CR, Chalk L. Anatomy of the dicotyledons. 2nd ed. Vol. 1. New York: Oxford University Press; 1979. [Google Scholar]
- Nepi M, Pacini E. Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Annals of Botany. 1993;72:527–536. [Google Scholar]
- Nepi M, Ciampolini F, Pacini E. Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Annals of Botany. 1996;78:95–104. [Google Scholar]
- Nepi M, Guarnieri M, Pacini E. Nectar secretion, reabsorption, and sugar composition in male and female flowers of Cucurbita pepo. International Journal of Plant Sciences. 2001;162:353–358. [Google Scholar]
- O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue. Protoplasma. 1964;59:368–373. [Google Scholar]
- Opler PA. Nectar production in a tropical ecosystem. In: Bentley B, Elias T.S, editors. The biology of nectaries. New York: Columbia University Press; 1983. pp. 30–79. [Google Scholar]
- Pacini E, Nepi M, Vesprini JL. Nectar biodiversity: a short review. Plant Systematics and Evolution. 2003;238:7–21. [Google Scholar]
- Paiva EAS, Machado SR. Ontogênese, anatomia e ultra-estrutura dos nectários extraflorais de Hymenaea stigonocarpa (Fabaceae-Caesalpinioideae) Acta Botanica Brasilica. 2006;20:471–482. [Google Scholar]
- Pearse AGE. Histochemistry theoretical and applied. 4th edn. II. London: Longman Press; 1980. [Google Scholar]
- Peng Y-B, Li Y-Q, Hao Y-J, Xu Z-H, Bai S-N. Nectar production and transportation in the nectaries of the female Cucumis sativus L. flower during anthesis. Protoplasma. 2004;224:71–78. doi: 10.1007/s00709-004-0051-9. [DOI] [PubMed] [Google Scholar]
- Potgieter MJ, Wyk AE. Intercellular pectic protuberances in plants: their structure and taxonomic significance. Botanical Bulletin of Academia Sinica. 1992;33:295–316. [Google Scholar]
- Rachmilevitz T, Fahn A. Ultrastructure of nectaries of Vinca rosea L., Vinca major L. and Citrus sinensis Osbeck cv. Valencia and its relation to the mechanism of nectar secretion. Annals of Botany. 1973;37:1–9. [Google Scholar]
- Razem FA, Davis AR. Anatomical and ultrastructural changes in the floral nectary of Pisum sativum L. during flower development. Protoplasma. 1999;206:57–72. [Google Scholar]
- Robards AW. An introduction to techniques for scanning electron microscopy of plant cells. In: Hall JL, editor. Electron microscopy and cytochemistry of plant cells. New York: Elsevier; 1978. pp. 343–403. [Google Scholar]
- Roland AM. General preparations and staining of thin sections In. In: Hall JL, editor. Electron microscopy and cytochemistry of plant cells. New York: Elsevier; 1978. pp. 1–62. [Google Scholar]
- Sass JE. Botanical microtechnique. Ames, IA: Iowa State College Press; 1951. [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Vogel S. Chiropterophilie in der neotropischen Flora: neue Mitteilungen. I. Flora B. 1968;157:562–602. [Google Scholar]
- Zer H, Fahn A. Floral nectaries of Rosmarinus officinalis L. Structure, ultrastructure and nectar secretion. Annals of Botany. 1992;70:391–397. [Google Scholar]
- Zhu J, Hu Z-H. Cytological studies on the development of sieve element and floral nectary tissue in Arabidopsis thaliana. Acta Botanica Sinica. 2002;44:9–14. [Google Scholar]


