Abstract
Background and Aims
Cotyledon number has long been a primary morphological feature distinguishing monocots from other angiosperms. Recent placement of Hydatellaceae near the early-divergent angiosperm order Nymphaeales, rather than in the monocot order Poales, has prompted reassessment of seedling morphology in this poorly known family.
Methods
Seedlings of six species representing all eco-geographical groups of Hydatellaceae are described using light and scanning electron microscopy.
Key Results
Two seedling types were discovered. Material examined of Trithuria submersa, T. bibracteata, T. austinensis and T. filamentosa possess a transparent bilobed sheathing structure that surrounds the main axis below the first foliage leaf. The seed coat is attached to the sheathing structure. Seedlings of Trithuria lanterna and T. konkanensis lack a sheathing structure, and the seed coat is attached to a short, narrow lateral outgrowth on the main axis of the seedling.
Conclusions
The sheathing structure that is present in seedlings of some Hydatellaceae could be homologized with the two united cotyledons of water lilies. It also resembles the single cotyledon of some monocots, and hence demonstrates a possible pathway of the origin of a monocot-like embryo, though no homology is implied. The sheathing structure is reduced in Trithuria lanterna and T. konkanensis, and the short, narrow outgrowth of its seedling could represent a single cotyledon. This synapomorphy suggests that the only Indian species of Hydatellaceae, T. konkanensis, is closer to the northern Australian T. lanterna than to the south-western Australian T. bibracteata.
Key words: Seedling, cotyledon, monocot, dicot, Hydatellaceae, Trithuria
INTRODUCTION
The recent discovery that Hydatellaceae are closely related to the early-divergent angiosperm order Nymphaeales rather than (as previously assigned) to the monocot order Poales (Saarela et al., 2007) has highlighted our inadequate knowledge of many significant morphological characters in this intriguing family. Cotyledon number has long been considered a primary morphological feature distinguishing monocots from other angiosperms, but was not hitherto properly documented in Hydatellaceae (Rudall et al., 2007).
As recently recircumscribed (Sokoloff et al., in press), Hydatellaceae consist of a single genus, Trithuria, with ten species in Australia, one species in New Zealand and one species in India. All species are aquatics with linear leaves in basal rosettes. Two species are perennials, but all other Hydatellaceae are annuals adapted mostly for seasonally wet habitats (Cooke, 1987; Yadav and Janarthanam, 1994; Gaikwad and Yadav, 2003; Sokoloff et al., in press). Mature seeds of Trithuria possess a seed coat with an operculum and contain copious starchy perisperm, a small endosperm and a tiny, few-celled embryo not differentiated into organs (Hamann, 1975; Hamann et al., 1979; P. J. Rudall et al., unpubl. res.).
The only previous investigation of seedlings in Hydatellaceae was that of Cooke (1983a) for Trithuria submersa, who described germination as hypogeal. The green linear primary leaf and the radicle erupt between the operculum and the rest of the seed coat through the upper and lower sides, respectively. The cotyledon (not illustrated by Cooke) forms part of the tissue that remains inside the testa and does not become green. Cooke's report of a single cotyledon agreed with the traditional placement of Hydatellaceae in monocots, but contradicts their novel and robust placement among early-divergent angiosperms. A monocotylar embryo has sometimes been reported for Nymphaeaceae, but closer examination revealed the presence of two cotyledons (for discussion and references to earlier literature, see Tillich, 1990). Thus, since no other early-divergent angiosperm unequivocally possesses embryos with a single cotyledon, more data on seedlings of Trithuria will elucidate the controversy over cotyledon number in Nymphaeales, and improve our understanding of seedling evolution in angiosperms.
MATERIALS AND METHODS
Seedlings of five species of Hydatellaceae from Australia and one species from India were examined. Two were from alcohol-fixed material from the Kew Herbarium (K): (1) T. filamentosa Rodway (K 28269: Talbot de Malahide s.n.), a perennial plant from Tasmania; this species was previously placed in a segregate genus, Hydatella (e.g. Cooke, 1987); (2) T. lanterna D.A. Cooke (K 47115: C.R. Dunlop 4704A), an annual from northern Australia. Material of T. submersa Hook. f. (the type species of Trithuria) was obtained from two sources: (1) seedlings grown at the Royal Botanic Gardens, Kew (RBGK) from seeds collected by one of us (R.E.T.) in south-west Western Australia; (2) FAA-fixed material collected in South Australia (J. G. Conran 961 and P. J. Rudall, voucher deposited at ADU; this material contains late stages only). Trithuria submersa is an annual with disjunct distribution between Tasmania and south-eastern and south-western parts of Australia. Material of two annuals endemic to south-west Western Australia (T. bibracteata Stapf ex D.A. Cooke and T. austinensis D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall) was collected in natural populations by R.E.T. and seedlings were grown and fixed in FAA at Perth. Finally, the annual T. konkanensis Yadav & Janarth. was fixed in 70 % ethanol in a natural population near Kolhapur in India by one of us (S.R.Y.).
The six species investigated represent all eco-geographical groups of Hydatellaceae: (1) T. filamentosa grows in permanent lakes of temperate climates (the New Zealand T. filamentosa also falls in this group); (2) T. submersa, T. bibracteata and T. austinensis occur in mostly ephemeral pools in southern parts of Australia; (3) T. lanterna and T. konkanensis grow in tropical climates in ephemeral pools of northern Australia and India, respectively.
For investigation with the scanning electron microscope (SEM), material was dissected in 70 % ethanol, then dehydrated through absolute ethanol and critical-point dried using a Balzers CPD 020 (BALTEC AG, Liechtenstein) at RBGK. Dried material was further dissected and mounted onto specimen stubs using double-sided tape, coated with platinum using an Emitech K550 sputter coater (Emitech, Ashford, UK), and examined using a Hitachi cold-field emission SEM S-4700-II (Hitachi High Technologies Corp., Tokyo, Japan) at RBGK. For the investigation with the light microscope, material was embedded in paraffin or Tecnovit resin, microtome sections were produced using a Leica rotary microtome and stained with Delafields Haematoxylin and Eosin or Toluidine Blue. Seedlings were drawn by Anton Beer (Figs 5, 8, 10).
RESULTS
Trithuria submersa
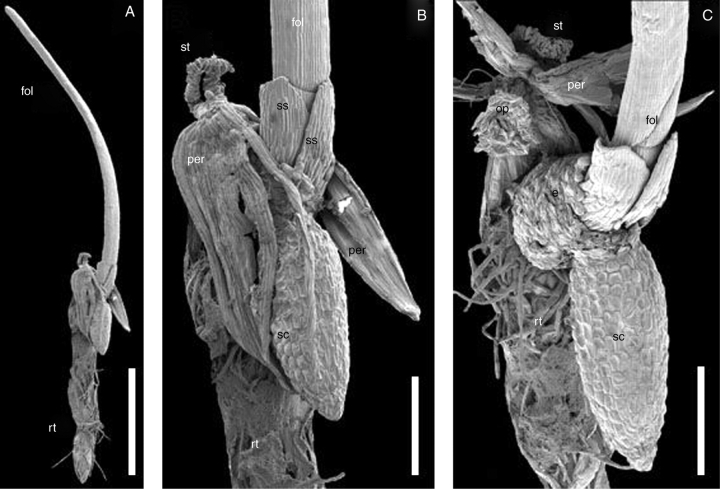
The one-seeded fruit dehisces by separating three longitudinal ribs from the rest of the pericarp. The three ribs of the fruit, as well as the three faces between them, remain connected to each other and to the seed at the top of the fruit. The pericarp and the seed coat remain associated with the seedling (Fig. 1A, B). The seedling emerges between the operculum and the rest of the seed coat (the operculum is attached to the pericarp; Fig. 1C). The radicle appears on one side of the operculum (towards the substrate), while the first green leaf appears on the opposite side.
Fig. 1.
Seedling of Trithuria submersa (SEM). (A) Entire seedling with pericarp and seed coat attached. (B) Detail of (A). (C) The same seedling separated from pericarp; note that the operculum remains attached to the inner surface of the pericarp. Labels: e, endosperm; fol, foliage leaf; op, operculum; per, pericarp; rt, root (note: fragments of agar on which the seedling was cultivated remain attached); sc, seed coat; ss, sheathing structure; st, stigma remaining attached to the fruit apex. Scale bars: A = 1 mm; B, C = 250 µm.
The radicle is always shorter than the green leaf in young seedlings. Numerous root hairs develop on the radicle. The hypocotyl is extremely short and almost exclusively represented by the collar (i.e. the transitional region between the hypocotyl and the primary root: terminology after Tillich, 1995). Numerous collar rhizoids develop on the collar (Fig. 2A); these are longer than root hairs. There is a time interval between the development of collar rhizoids and the first appearance of root hairs on the main root. In older seedlings shoot-borne roots develop on the hypocotyl. These also possess root hairs.
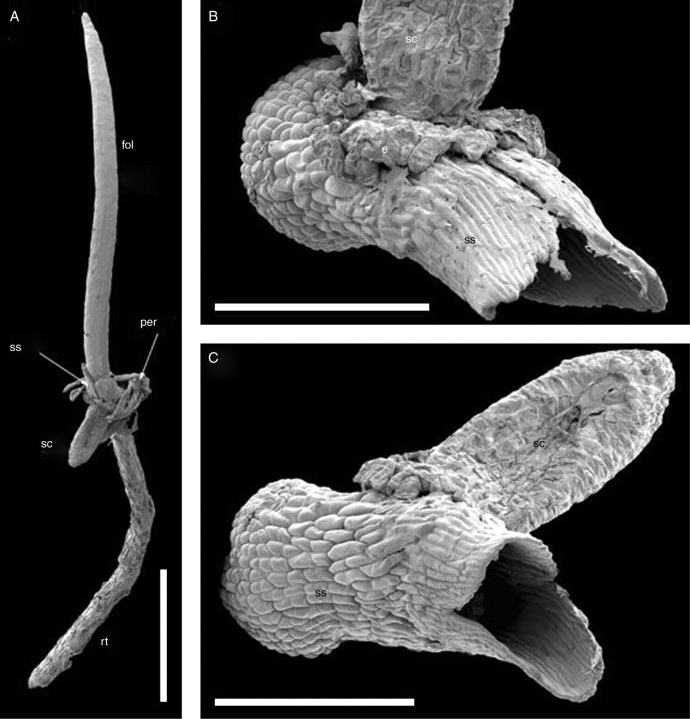
Fig. 2.
Seedling of Trithuria submersa (SEM) with pericarp removed; all figures show the same individual seedling. (A) General view. (B) Portion of (A) to show sheathing structure. (C) View of sheathing structure from the side of the abscised operculum. (D, E) Seed coat and endosperm removed, viewed in (D) from the same angle as in (C), and in (E) from the side of attachment of the (removed) seed coat; the seed coat was attached in the space between the two arrowheads. Labels: cr, collar rhizoids; e, endosperm; fol, foliage leaf; rt, root; sc, seed coat; ss, sheathing structure. Scale bars: A = 1 mm; B = 200 µm; C − E = 300 µm.
The first green leaf is filiform, without a distinct sheathing base or auricles, typically with water stomata at the tip and some normal stomata along the surface. The basal part of the first green leaf is enclosed by a thin, transparent, membranous, non-photosynthetic sheathing structure (Figs 1–3). We believe the sheathing structure has cotyledonary identity (see Discussion), but because other interpretations could be proposed and because the number of cotyledons involved (two or one) is debatable, a morphologically neutral term ‘sheathing structure’ was used here.
Fig. 3.
SEM images of a seedling of Trithuria submersa (A) and details of its sheathing structure viewed from the side of the attached seed coat (B), and from the side of the abscised operculum (C). Labels: e, endosperm; fol, foliage leaf; op, operculum; per, pericarp; rt, root; sс, seed coat; ss, sheathing structure. Scale bars: A = 1 mm; B, C = 300 µm.
The sheathing structure is mostly two cell layers thick (i.e. with inner and outer epidermises only). It is closely appressed to the first green leaf, and is discernible only on careful examination using a dissecting microscope, but is clearly visible in critical-point dried material using SEM. It consists of two transversely oriented parts that are invariably basally connected to form a transparent sheathing tube, which encloses the first green leaf. The seed coat is attached to the base of the sheathing structure on the radius just between the two parts of the sheathing structure (Figs 1–3). The degree of fusion between these two parts on the side opposite seed attachment (i.e. on the side of the operculum) is variable; in some cases they are almost completely united up to the top, and thus appear as a single unit.
The basal part of the sheathing structure is enclosed by a distinct tissue belt (labelled ‘e’ in Fig 2B and C) that is connected on one side to the seed coat. In some cases the belt is incomplete, and the tissue is restricted to a region around the opening of the testa (Fig. 3B). This belt of tissue (here interpreted as endosperm; see Discussion) has no organic connection with the seedling and on careful preparation can be readily separated from it (Fig. 2D, E). Its cells are different in morphology from the testal cells; they are apparently living at this stage, and are of irregular, often amoeboid shape (Fig. 2B, C), in contrast to the dead, rhomboidal testal cells. At this stage the perisperm is completely consumed by the growing seedling.
In young seedlings (Figs 1–3), orientation of the first green leaf with respect to the symmetry plane of the sheathing structure cannot be traced because the leaf base has radial structure and the shoot apex cannot be detected at this stage. Older seedlings (Fig. 4A–C) show that the shoot apex lies between the first green leaf and the side of the sheathing structure to which the seed coat is attached. The divergence angle between the first and the second green leaf is <180°, so that the radius of the second leaf is slightly shifted from the radius of the seed coat attachment (Fig. 4B, C). In the latest available stage, three meristematic bulges are visible after formation of the first two green leaves. If our interpretation of primordia of the third leaf and the fourth leaf (Fig. 4C) is correct, then the radius of seed coat attachment and the radii of the first four leaves are arranged in a manner similar to a Fibonacci spiral.
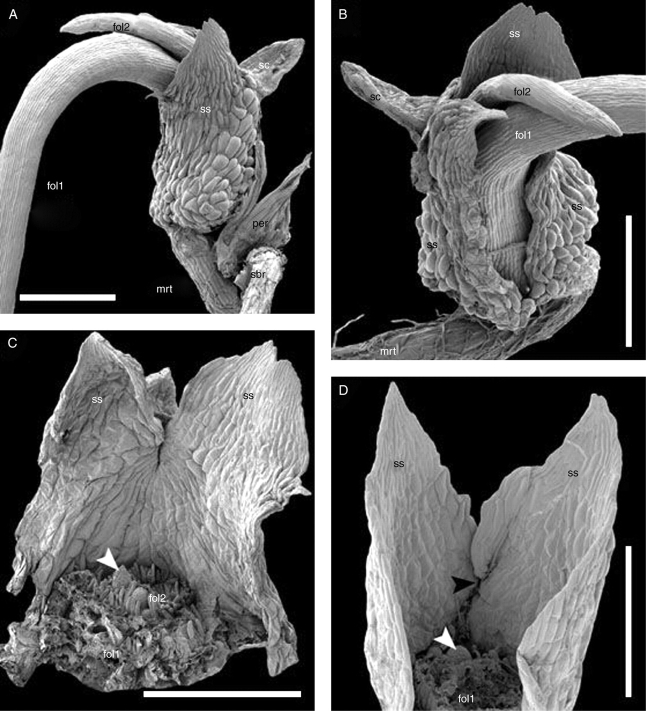
Fig. 4.
SEM images of seedlings showing foliage leaf arrangement on the main axis. (A − C) Trithuria submersa, (D) Trithuria austinensis. (A, B) Different views of the same seedling. (C) The same seedling with the first and the second foliage leaves removed (labelled at their bases); arrowhead indicates the putative primordium of the third foliage leaf. (D) A seedling with the first foliage leaf removed (labelled at base); white arrowhead indicates the primordium of the second foliage leaf, black arrowhead indicates level of seed coat attachment to the reverse side of the sheathing structure. Labels: fol1, fol2, the first and the second foliage leaves; mrt, main root; per, pericarp; sbr, shoot-borne root; sc, seed coat; ss, sheathing structure. Scale bars: A − C = 500 µm; D = 300 µm.
Trithuria bibracteata
This species is similar to T. submersa in its ecology and fruit/seed structure. Germination and seedling structure are also similar to those of T. submersa, though a distinct (endospermous) tissue belt surrounding the base of the sheathing structure was not observed.
Trithuria filamentosa
In the (very limited) material available (Fig. 5), seedling structure was similar to that of T. submersa, but a distinct (endospermous) tissue belt surrounding the base of the sheathing structure was not observed. Also, an elongate part of the hypocotyl was present above the collar. Trithuria filamentosa has indehiscent fruits, but in our material the seed coat attached to the sheathing structure was free from the pericarp. The opened operculum was still weakly attached on one side to the rest of the seed coat.
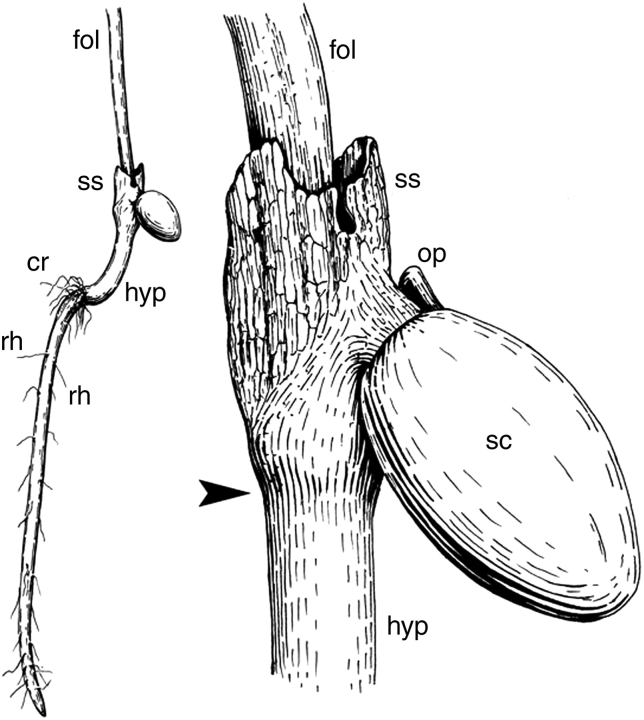
Fig. 5.
Line drawing of a seedling of Trithuria filamentosa, with a detail of seed attachment to the sheathing structure. Labels: cr, collar rhizoids; fol, the first foliage leaf; op, operculum; rh, root hairs on the main root; sc, seed coat; ss, sheathing structure; arrowhead indicates level of attachment of sheathing structure.
Trithuria austinensis
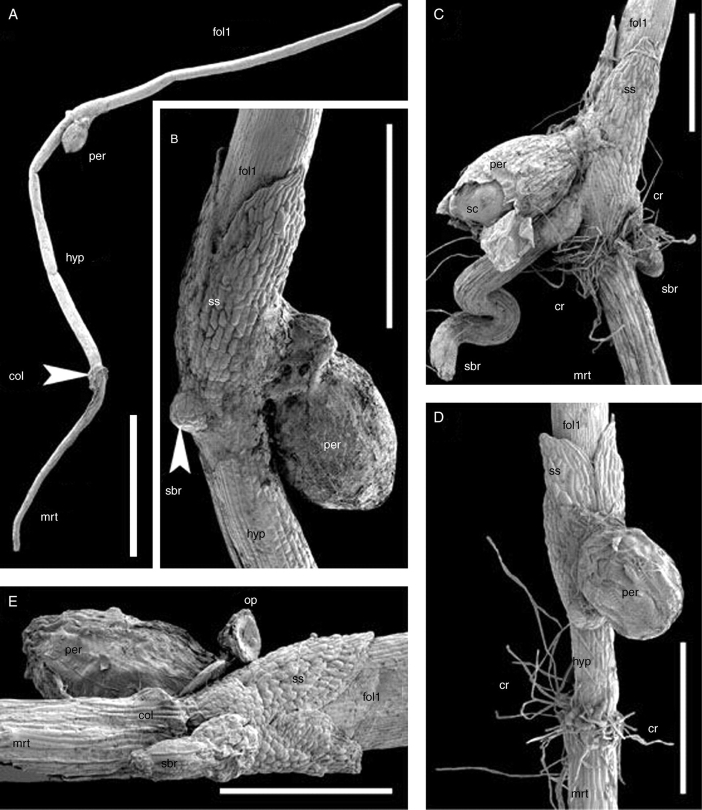
This species (Figs 4D, 6 and 7B, C) has indehiscent fruits. The very thin pericarp remains intact, surrounding the seed coat by the time of seed germination. It breaks together with seed coat when the operculum opens during germination. The operculum is still visible in some seedlings examined; it remains weakly attached by one side to the rest of the seed coat (Fig. 6E). The general bauplan of the seedling is close to that of T. submersa (Figs 4D and 6), though a distinct (endospermous) tissue belt surrounding the base of the sheathing structure was not observed. In addition, both hypocotyl length (Fig. 6) and presence/absence of root hairs (Fig. 7) are variable in the material of T. austinensis examined.
Fig. 6.
SEM images of seedlings of Trithuria austinensis. (A) A seedling with very long hypocotyl; close examination of this seedling revealed the presence of collar rhizoids, but they are not clearly visible at this magnification. (B) The same seedling as in (A), detail from another angle showing sheathing structure. (C) Seedling with short hypocotyl and long collar rhizoids. (D) Seedling with short, but clearly visible hypocotyl and long collar rhizoids. (E) Seedling with short hypocotyl and inconspicuous collar rhizoids; close examination revealed that the collar rhizoids are present but rare and much shorter than in (A − D). Labels: col, collar; cr, collar rhizoids; fol1, first foliage leaf; hyp, hypocotyl; mrt, main root; op, operculum; per, pericarp; sbr, shoot-borne root; sc, seed coat; ss, sheathing structure. Scale bars: A = 2 mm; B − D = 500 µm; E = 400 µm.
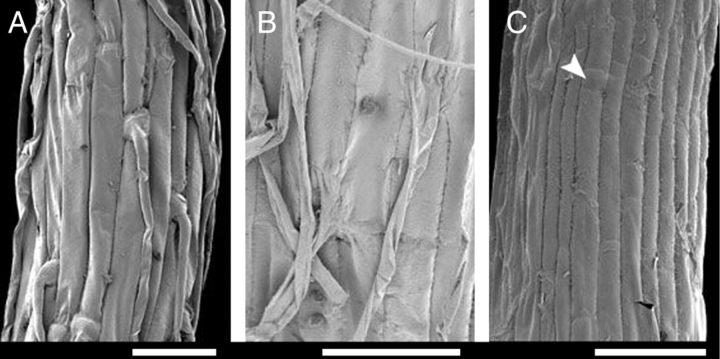
Fig. 7.
Details of main root surface (SEM). (A) Hairy root of Trithuria bibracteata. (B) Hairy root of T. austinensis. (C) Hairless root of T. austinensis; arrowhead indicates short cell. Scale bars = 50 µm.
In the material examined, the hypocotyl length varied between approx. 0·2 mm and approx. 4 mm. Regardless of hypocotyl length, shoot-borne roots develop only near the level of attachment of the sheathing structure. In some seedlings the first shoot-borne root develops on the radius of the seed coat attachment (e.g. Fig. 6B, C) (and the second root is opposite it – Fig. 6C). In other seedlings the first shoot-borne root lies opposite the radius of the seed coat attachment (Fig. 6E). In all seedlings examined, the first shoot-borne root lies in the symmetry plane of the sheathing structure.
Some seedlings have well-developed root hairs on the main root, whereas in others roots hairs are either rare or absent. SEM investigation of entire roots, including their apices, has shown that absence of hairs is not due to hair abscision; also hairless roots were not too young to develop hairs. Most hairless roots of T. austinensis possess characteristic short cells, similar to those that develop hairs in roots of most Hydatellaceae. However, in T. austinensis some hairless roots, in which the surface was composed of long cells only, were also observed. Like the primary root, shoot-borne roots are variable with respect to hair presence. One seedling with a hairy primary root and a hairless shoot-borne root was found; both roots were of the same length.
The collar rhizoids are typically very long and conspicuous in T. austinensis, even in most seedlings with a hairless main root. However, one seedling with very short and inconspicuous (and less numerous) collar rhizoids was found (Fig. 6E). The collar was readily detectable in this seedling because of the characteristic thickening of this part of the main axis.
Trithuria lanterna
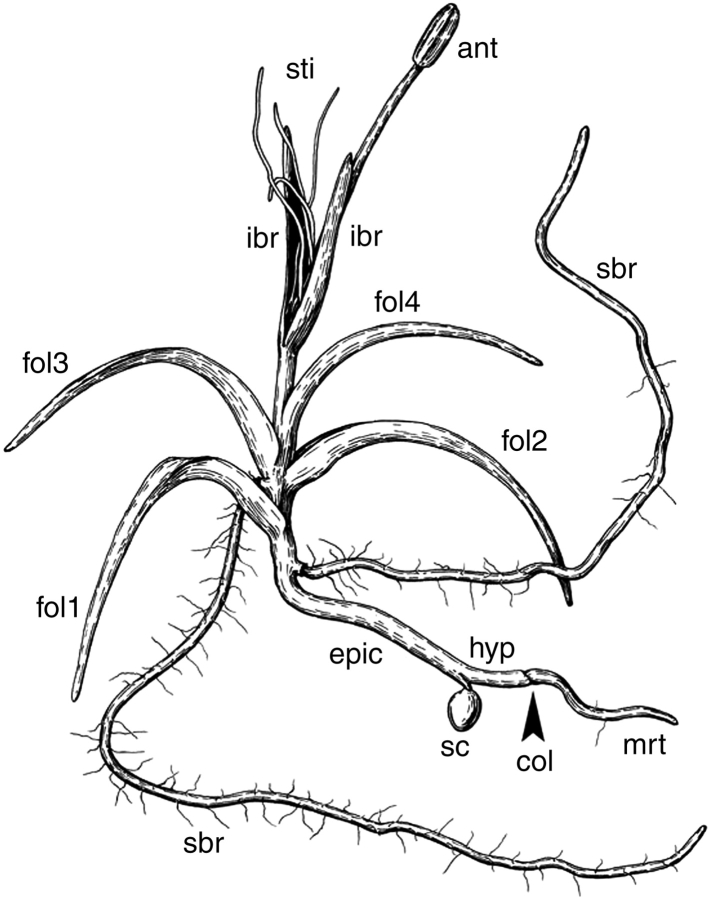
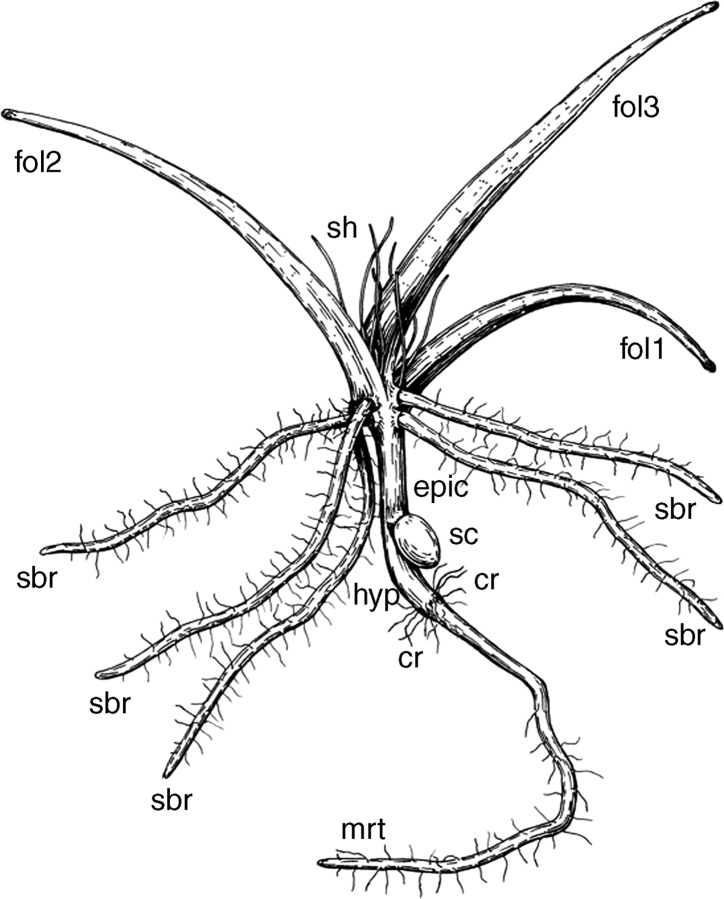
All available seedlings were at later developmental stages than those described above for other species. They possess several roots with root hairs, three or four filiform leaves and a young reproductive unit. However, the seeds were in most cases still attached to the seedlings, and there was no evidence of abscission of any organ, an observation that was confirmed by careful examination of epidermal cells using SEM. The seedlings lack any trace of a sheathing structure near the point of seed coat attachment (Figs 8 and 9B). Above the attachment of the seed coat there is a portion of naked stem of variable length (here interpreted as epicotyl), and then the primary shoot possesses three or four filiform green leaves and finally a reproductive unit. In some seedlings, the internode above the first leaf was elongate, and then a rosette was formed; in other seedlings all leaves were arranged in a rosette. The shoot-borne roots are typically attached just below the leaves. Below the attachment of the seed coat, the seedling axis continues into a hypocotyl and a radicle (Fig. 8). The seed coat is attached to a short, nonvascularized and radially symmetrical outgrowth. Within the seed coat, the outgrowth is often surrounded by the remaining endosperm (Fig. 9A). No traces of perisperm can be found at this stage, and the space occupied by perisperm in the mature seed is empty (Fig. 9A). The hypocotyl has a single central vascular strand, which extends into the main axis of the plant above the nonvascularized outgrowth. The central vascular strand branches to supply a single vascular strand to each leaf and each shoot-borne root. Analysis of leaf traces in serial cross-sections suggests that leaves are arranged according to a Fibonacci spiral. The position of the outgrowth is such that it can be identified as the first member of this spiral.
Fig. 8.
Line drawing of a young plant of Trithuria lanterna. Labels: anth, anther; col, collar; epic, presumed epicotyl; fol1 − fol4, foliage leaves; hyp, hypocotyl; ibr, involucral bract of reproductive unit; sbr, shoot-borne root; sc, seed coat; sti, stigmatic papillae; mrt, main root.
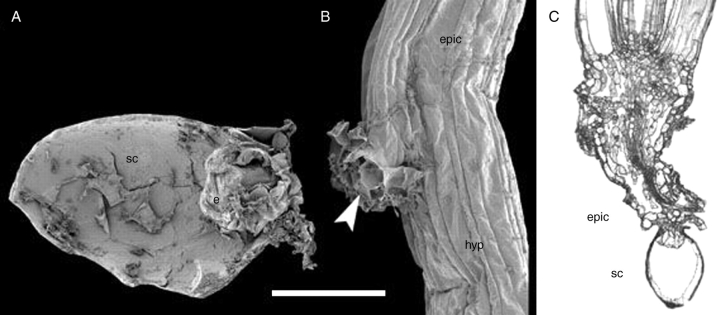
Fig. 9.
Trithuria lanterna. (A) SEM image of longitudinally dissected germinated seed with perisperm consumed. The operculum was attached at the right side of the figure. (B) SEM image of the point of pericarp attachment to a young plant; arrowhead indicates the outgrowth (presumed cotyledon). Scale bar (common to A and B) = 100 µm. (C) Longitudinal section of young plant showing seed coat attachment under a light microscope. Labels: e, endosperm; epic, epicotyl; hyp, hypocotyl; sc, seed coat.
Trithuria lanterna is apparently as variable as T. austinensis with respect to root hair and collar rhizoid presence, though only older stages in T. lanterna were available for examination.
Trithuria konkanensis
The seedlings available were at slightly younger stages than those of T. lanterna. The youngest seedlings examined possessed three green leaves and no mature reproductive unit (Fig. 10). Seedling morphology in this species is generally similar to that of T. lanterna. The sheathing structure is absent, and there is no trace of its having abscised; instead, the seed coat is attached to a very small lateral outgrowth of the main axis. The length of the main axis above the attachment of the seed coat (i.e. the presumed epicotyl length) is as variable as in T. lanterna. The seedling illustrated (Fig. 10) possesses an elongated epicotyl, but in many other seedlings of T. konkanensis the epicotyl was short. The hypocotyl was always elongated. The collar and collar rhizoids are clearly visible in all seedlings available. All roots examined possessed root hairs.
Fig. 10.
Line drawing of a seedling of Trithuria konkanensis. Labels: cr, collar rhizoids; epic, epicotyl; fol1–fol3, foliage leaves; hyp, hypocotyl; mrt, main root; sbr, sc, seed coat; shoot-borne roots; sh, shoot hairs (very long hairs attached to the shortened stem internodes between foliage leaves).
DISCUSSION
Seedling structure is variable in Hydatellaceae
Our data on seedlings of T. submersa differ significantly from Cooke's description (Cooke, 1983a). Cooke did not mention the sheathing structure that we found in all seedlings examined of this species. This discrepancy could be explained either because (a) the sheathing structure is transparent in living material, so it is difficult to discern without examination of critical-point dried material under SEM, or (b) T. submersa is variable in seedling structure (indeed, this is probably the most variable species in the family with respect to other characters; Cooke, 1987; Sokoloff et al., in press). Interestingly, our material of T. lanterna lacks a sheathing structure entirely, and therefore corresponds closely to Cooke's description of T. submersa. We are confident of the precise taxonomic identification of all the material discussed here, because we have examined the voucher specimen of Cooke's study (adult plants), and our material of T. lanterna has reproductive units and is thus readily identifiable (reproductive morphology of plants from the same collection is illustrated in Rudall et al., 2007). Furthermore, our material of T. submersa has the pericarp attached, and the pericarp structure of this species is quite distinctive (Sokoloff et al., in press).
According to our data, seedling morphology varies within Hydatellaceae with respect to presence or absence of the sheathing structure. This structure is present in four of the six species examined here (T. submersa, T. bibracteata, T. austinensis and T. filamentosa) and absent from the other two (T. konkanensis and T. lanterna). Since the highly reduced morphology of Hydatellaceae offers very few useful taxonomic characters, seedling structure merits careful examination in a wider sampling of taxa. However, since we have sampled 50 % of the known species of Hydatellaceae (i.e. 6 out of 12), we propose some preliminary conclusions on variation in this character.
(a) Presence/absence of the sheathing structure varies independently from growth form and seed/fruit morphology. Indeed, T. filamentosa is perennial with indehiscent fruits and smooth seeds, whereas T. submersa is an annual with dehiscent fruits and sculptured seeds, but both species possess the sheathing structure. In terms of fruit/seed morphology, T. lanterna is intermediate between T. filamentosa and T. submersa (Cooke 1981, 1983b, 1987; Sokoloff et al., in press). However, seedlings of T. lanterna lack the sheathing structure and thus differ from both T. filamentosa and T. submersa.
(b) Seedling structure could be important for understanding the relationships of T. konkanensis, the only Indian species of Hydatellaceae (and therefore highly disjunct). This species has been compared with two Australian species, T. lanterna (northern Australia) and T. bibracteata (south-western Australia), based on reproductive morphology: all three species possess bisexual reproductive units with two involucral bracts (Yadav and Janarthanam, 1994). The similarity in seedling structure observed here suggests that T. konkanensis is much closer to T. lanterna than to T. bibracteata, and represents a potential synapomorphy that will be tested in a species-level cladistic analysis.
Morphological interpretation of seedlings in Hydatellaceae
Trithuria lanterna and T. konkanensis
Seedlings of these two species possess a very short cylindrical outgrowth to which the seed coat is attached (arrowhead in Fig. 9B). The outgrowth is attached to the seedling axis below the attachment of the first leaf and above the main root – hypocotyl transition (the collar). Thus, by position in the seedling bauplan, the outgrowth can be interpreted as a cotyledon. The outgrowth is an entire structure and thus we have to postulate the presence of a single cotyledon in T. lanterna and T. konkanensis. The cotyledon is apparently haustorial. The absence of vasculature in this outgrowth (well documented so far only in T. lanterna) is intriguing for an organ of haustorial function (though the outgrowth is very short). Vasculature is generally poorly developed in T. lanterna and other Hydatellaceae; e.g. the hypocotyl bears a single tiny vascular strand.
The presumed cotyledon of T. lanterna and T. konkanensis has a very narrow base, whereas the single cotyledon of monocots has a broad base that completely surrounds the plumule (e.g. Lodkina, 1988; Tillich, 1995). In the few eudicots that possess a single cotyledon (which could be a fusion product of two cotyledons), the cotyledon also has a broad base (Haccius, 1952; Haccius and Hartl-Baude, 1957; Titova, 2000). Among monocots, a superficially similar condition occurs when the basal part of the coleoptile is congenitally fused with the epicotyl, forming a structure termed a mesocotyl (Tillich, 1995). For example, in some Cyperaceae, a cotyledonary haustorium with a narrow base is attached to the seedling axis below the mesocotyl (Tillich, 1995), which resembles the attachment of the presumed cotyledon to the seedling axis in Trithuria lanterna and T. konkanensis. However, T. lanterna and T. konkanensis have no trace of a coleoptile.
An alternative interpretation of the seedlings of T. lanterna and T. konkanensis is that the outgrowth to which the seed coat is attached represents a unique organ sui generis. There is currently no easy way of testing this hypothesis, which can be only accepted if all other possible hypotheses are refuted. It is possible that molecular genetics will help to solve the problem, as suggested by Burger (1998).
Trithuria submersa, T. bibracteata, T. filamentosa and T. austinensis
The most unexpected finding of this study is the bilobed sheathing structure present in seedlings of these species. This structure could be cotyledonary, representing either a fused pair of cotyledons or a single cotyledon. With respect to position, the sheathing structure resembles a pair of cotyledons in seedlings of Nymphaeaceae. Many authors have reported that the cotyledons of water lilies are slightly united at the base, which is more clearly visible at younger stages (reviewed by Haines and Lye, 1975; Titova and Batygina, 1996); hence the embryo of water lilies has often been described as monocotylar with a bifid cotyledon. On the other hand, in favour of its interpretation as a single cotyledon, seedling topography in T. submersa resembles that of some monocots (e.g. Schizocarpa in Taccaceae, Heliconia in Heliconiaceae), which possess a sheathing base continued into two lateral lobes with the haustorial part attached on the radius between the lobes (Tillich, 1995). Taccaceae and Heliconiaceae are not early-divergent monocots, but a bilobed cotyledonary hypophyll is also present in some members of the relatively basal monocot family Araceae (Tillich, 2003). The hypophyll lobes, when present, are typically green and photosynthetic in Araceae, but at least in Dracontioides and Cyrtosperma the cotyledon bears small paired sheath lobes (Tillich, 2003), so that this structure resembles the sheathing structure in Trithuria.
Based on current evidence, a cotyledonary interpretation is our preferred hypothesis. However, possible alternative interpretations are that the sheathing structure represents either (a) the first leaf of the plumule, or (b) two united opposite leaves. The second case is unlikely because fusion of opposite leaves was otherwise never observed in Hydatellaceae (though opposite leaves sometimes occur in reproductive units of many species; Cooke, 1987; Rudall et al., 2007; Sokoloff et al., in press). To interpret the sheathing structure as a single leaf, it should be compared with the sheathing base of foliage leaves. In some species, including T. filamentosa, the sheathing base of some foliage leaves possesses two lateral membranous stipule-like auricles (illustrated for T. australis in Rudall et al., 2007). The two parts of the sheathing structure could be compared with these auricles, and the entire sheathing structure could be viewed as a bladeless leaf. However, this interpretation is also problematic for several reasons. (a) Vegetative leaves of Hydatellaceae never possess a closed sheath; their base is typically narrower than the circumference of the stem. (b) The first green leaf of the seedling lacks a sheathing base, though some subsequent leaves possess one; this would indicate a highly unusual bimodal change of leaf base structure in the leaf series of the shoot. (c) In seedlings of water lilies, the closest relatives to Hydatellaceae, the first leaf after the cotyledons has a filiform blade and scarcely recognizable stipules (summarized by Tillich, 1990). The axillary sheathing stipule is present only from the second leaf onwards. The first leaf of water lilies is closely similar to the first green leaf of Hydatellaceae (and to other leaves of Hydatellaceae; Rudall et al., 2007), but has nothing in common with the sheathing structure of T. submersa and T. filamentosa.
Interpretation of the sheathing structure as the first leaf of the plumule would not in itself contradict a homology between the sheathing structure of Trithuria and the cotyledon of monocots. Indeed, several authors have discussed a hypothesis that the cotyledon of monocots is homologous with the first leaf of the plumule of water lilies (Meyer, 1960; Kudryashov, 1964; Valtzeva and Savich, 1965; Burger, 1998).
Endosperm behaviour in germinating seeds
In most species examined, the endosperm remains within the testa during seed germination. As in water lilies, it apparently helps to transfer nutrients from the copious perisperm to the growing seedling. In our material of T. submersa, the tissue belt surrounding the sheathing structure must be interpreted as endosperm, since these cells have no organic connection with the seedling. Apart from endosperm cells, there are no other living cells surrounding the embryo. Similar endosperm release from the seed coat during germination has been described for some other early-divergent angiospems, such as Myristicaceae, Saururaceae and Piperaceae (Takhtajan, 1988). Like Hydatellaceae, both Saururaceae and Piperaceae possess a well-developed perisperm, and thus similar endosperm behaviour could be of adaptive significance.
CONCLUSIONS
The unusual bilobed sheathing structure – described here for the first time – below the first foliage leaf in four species of Trithuria, is probably cotyledonary. There is evidence to homologize each lobe of the sheathing structure with one of a pair of cotyledons in Nymphaeaceae, though it also resembles the single cotyledon in some monocot seedlings. Since Hydatellaceae are now robustly resolved as the closest relatives of water lilies, the first interpretation is most parsimonious.
In Nymphaeaceae each cotyledon has its own haustorium at the tip (Tillich, 1990), in contrast to Trithuria, in which the tips of the lobes of the sheathing structure are clearly not haustorial. This could mean that the cotyledon tips in water lilies are homologous to the place of testa attachment in Trithuria (which is probably haustorial). If so, the two lobes of the sheathing structure appear to be organs sui generis.
It is possible that the sheathing structure of Trithuria is homologous to the cotyledons of water lilies and represents a single cotyledon. In other words, this could be a case of true syncotyly. The hypothesis that the single cotyledon of monocots is homologous to two united cotyledons has been proposed by many authors, but disputed by others (reviewed by Haines and Lye, 1979). Syncotyly has been reported from several scattered eudicot lineages (reviewed by Titova, 2000), but these cases represent derived transitions that are irrelevant in this context. Reports on syncotyly in water lilies are more relevant here, because of the phylogenetic placement of water lilies far below the major monocot/dicot divergence. However, the degree of syncotyly is weak in water lilies and the general morphology of their cotyledons is very different from that of monocots. If our interpretation proves correct, the sheathing structure of the Trithuria seedling demonstrates how a monocotyledonous condition could arise from the condition that is present in water lilies. It is unlikely that cotyledon structure is synapomorphic between Hydatellaceae and monocots, but shows a possible evolutionary scenario by analogy.
Reduction clearly played an important role in the evolution of seedlings of Hydatellaceae. Their cotyledons are very simple structures in terms of histology. In Trithuria lanterna and T. konkanensis, the sheathing part of the cotyledon is presumably reduced and thus the seedling is very different from that of other species examined. No other family of early-divergent angiosperms shows similar range diversity in seedling morphology. However, seedling diversity in Hydatellaceae does not necessarily represent a primitive polymorphism that elucidates ancestral angiosperm seedling morphology. Rather, it could be caused by a loss of body plan stability, as in some other specialized aquatics, such as Lentibulariaceae (Kondo et al., 1978; Rutishauser and Isler, 2001; Degtjareva et al., 2006). Leaves of many aquatics, especially among early-divergent monocots, show a high degree of developmental plasticity, often dependent on environmental factors (reviewed by Rudall and Buzgo, 2002).
Some authors have suggested that a monocotylar embryo represents the primitive angiosperm condition (reviewed by Tzvelev, 2000). However, we consider it most likely that the dicotylar condition was ancestral for Hydatellaceae, because this condition exists in most other early-divergent angiosperms and in most extant gymnosperms, though little is known about seedlings in extinct stem-group angiosperms. Our observations raise many questions about the evolution of the body plan of the earliest angiosperms. Future work on Hydatellaceae will focus on the development of the embryo from fertilization onwards.
NOTE ADDED IN PROOF
Tillich (2007) has recently described seedlings of Eriocaulaceae and Mayacaceae (Poales), in which cotyledon structure closely resembles that described here for Trithuria lanterna and T. konkanensis.
ACKNOWLEDGEMENTS
We are grateful to Rimma P. Barykina, Galina V. Degtjareva and Vladimir V. Choob for discussion, and to James A. Doyle and an unknown reviewer for constructive reviews of the manuscript. D.D.S. acknowledges funding from President of Russia grant no. MD-1056.2007.4. Two visits by D.D.S. and M.V.R. to Kew were supported by the Bentham-Moxon trust.
LITERATURE CITED
- Burger WC. The question of cotyledon homology in angiosperms. Botanical Review. 1998;64:356–371. [Google Scholar]
- Cooke DA. New species of Schoenus (Cyperaceae) and Trithuria (Hydatellaceae) Muelleria. 1981;4:299–303. [Google Scholar]
- Cooke DA. The seedling of Trithuria (Hydatellaceae) Victorian Naturalist. 1983;100:68–69. [Google Scholar]
- Cooke DA. Two Western Australian Hydatellaceae. Muelleria. 1983;5:123–125. [Google Scholar]
- Cooke DA. Hydatellaceae. In: George AS, editor. Flora of Australia. Vol. 45. Canberra: Australian Government Publishing Service; 1987. pp. 1–5. [Google Scholar]
- Degtjareva GV, Casper SJ, Hellwig FH, Schmidt AR, Steiger J, Sokoloff DD. Morphology and nrITS phylogeny of the genus Pinguicula L. (Lentibulariaceae), with special attention to embryo evolution. Plant Biology. 2006;8:778–790. doi: 10.1055/s-2006-924560. [DOI] [PubMed] [Google Scholar]
- Gaikwad SP, Yadav SR. Further morphotaxonomical contribution to the understanding of family Hydatellaceae. Journal of Swamy Botanical Club. 2003;20:1–10. [Google Scholar]
- Haccius B. Verbreitung und Ausbildung der Einkeimblattrigkeit bei den Umbelliferen. Österreichische Botanische Zeitschrift. 1952;99:483–505. [Google Scholar]
- Haccius B, Hartl-Baude E. Embryologische und histogenetische Studien an “monokotylen Dikotylen”. II. Pinguicula vulgaris L. und Pinguicula alpina L. Österreichische Botanische Zeitschrift. 1957;103:567–587. [Google Scholar]
- Haines RW, Lye KA. Seedlings of Nymphaeaceae. Botanical Journal of the Linnean Society. 1975;70:255–265. [Google Scholar]
- Haines RW, Lye KA. Monocotylar seedlings: a review of evidences supporting an origin by fusion. Botanical Journal of the Linnean Society. 1979;78:123–140. [Google Scholar]
- Hamann U. Neue Untersuchungen zur Embryologie und Systematik der Centrolepidaceae. Botanische Jahrbücher. 1975;96:154–191. [Google Scholar]
- Hamann U, Kaplan K, Rübsamen T. Über die Samenschalenstruktur der Hydatellaceae (Monocotyledoneae) und die systematische Stellung von. Hydatella filamentosa. Botanische Jahrbücher. 1979;100:555–563. [Google Scholar]
- Kondo K, Segawa M, Nehira K. Anatomical studies on seeds and seedlings of some Utricularia (Lentibulariaceae) Brittonia. 1978;30:89–95. [Google Scholar]
- Kudryashov LV. The origin of monocotyly (Helobiae as an example) Botanicheskiy Zhurnal. 1964;49:476–486. [Google Scholar]
- Lodkina MM. Evolutionary relations between mono- and dicotyledons based on embryo and seedling investigation. Botanicheskiy Zhurnal. 1988;73:617–628. [Google Scholar]
- Meyer KI. On the embryology of Nuphar luteum Sm. Bulletin of Moscow Society of Naturalists. Biological Series. 1960;65:48–60. [Google Scholar]
- Rudall PJ, Buzgo M. The evolutionary history of the monocot leaf. In: Cronk QCB, Bateman RM, Hawkins J, editors. Developmental genetics and plant evolution. London: Taylor and Francis; 2002. pp. 432–458. [Google Scholar]
- Rudall PJ, Sokoloff DD, Remizowa MV, Conran JG, Davis JI, Macfarlane TD, et al. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany. 2007;94:1073–1092. doi: 10.3732/ajb.94.7.1073. [DOI] [PubMed] [Google Scholar]
- Rutishauser R, Isler B. Fuzzy Arberian morphology: Utricularia, developmental mosaics, partial shoot hypothesis of the leaf and other FAMous ideas of Agnes Arber (1879–1960) on vascular plant bauplans. Annals of Botany. 2001;88:1173–1202. [Google Scholar]
- Saarela JM, Rai HS, Doyle JA, Endress PK, Mathews S, Marchant AD, et al. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature. 2007;446:312–315. doi: 10.1038/nature05612. [DOI] [PubMed] [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, Rudall PJ. Classification of the early-divergent angiosperm family Hydatellaceae: one genus instead of two, four new species, and sexual dimorphism in dioecious taxa. Taxon. 2008;57 (in press) [Google Scholar]
- Takhtajan AL. Anatomia seminum comparativa. Vol. 2. Leningrad: Nauka; 1988. [Google Scholar]
- Tillich HJ. The seedlings of Nymphaeaceae—monocotylar or dicotylar? Flora. 1990;184:169–176. [Google Scholar]
- Tillich HJ. Seedlings and systematics in monocotyledons. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. London: Royal Botanic Gardens, Kew; 1995. pp. 303–352. [Google Scholar]
- Tillich HJ. Seedling diversity in Araceae and its systematic implications. Feddes Repertorium. 2003;114:454–487. [Google Scholar]
- Titova GE. On the nature of pseudomonocotyly in flowering plants. Botanicheskiy Zhurnal. 2000;85:76–91. [Google Scholar]
- Titova GE, Batygina TB. Is the embryo of Nymphaealean plants (Nymphaeales s.l.) dicotyledonous? Phytomorphology. 1996;46:171–190. [Google Scholar]
- Tzvelev NN. Embryos of Magnoliophyta as primary phytomers. Bulletin of Moscow Society of Naturalists. Biological Series. 2000;105:60–64. [Google Scholar]
- Valtzeva OV, Savich EI. Development of embryo in Nymphaea candida Presl and N. tetragona Georgi. Botanicheskiy Zhurnal. 1965;50:1323–1326. [Google Scholar]
- Yadav SR, Janarthanam MR. Hydatellaceae: a new family to the Indian flora with a new species. Rheedea. 1994;4:17–20. [Google Scholar]
- Tillich H.-J. Seedling diversity and the homologies of seedling organs in the order Poales (Monocotyledons) Annals of Botany. 2007;100:1413–1429. doi: 10.1093/aob/mcm238. [DOI] [PMC free article] [PubMed] [Google Scholar]