Abstract
Background and Aims
When green coffee is stored for a prolonged time the coffee quality decreases distinctively. Apart from well-known ‘off-notes’ that arise from undesired oxidations of lipids, a typical ‘flattening’ of the cup quality is detectable. In order to elucidate the biological causes for this phenomenon, differentially processed coffees (wet, dry, semi-dry processing), were stored under standard conditions for 2 years and analysed comprehensively.
Methods
Wet-processed coffee was stored either as parchment coffee, where the endocarp remained around the beans or as hulled beans. Viability of coffee seeds was estimated using the tetrazolium-test of seed viability. Changes in concentration of free amino acids and soluble carbohydrates were analysed by HPLC.
Key Results
Whereas all other coffees lost viability within the first 6 months of storage, coffee beans stored within the parchment remained viable for >1 year. Glucose and fructose decreased slightly in the course of storage and glutamine content declined significantly. However, the changes observed in sugar and amino acid content were not correlated with the viability of the coffee beans. Consequently, neither typical metabolic reactions occurring within living cells nor characteristic post-mortem reactions could be responsible for the observed changes. As a result of post-mortem reactions in re-imbibed seeds, a characteristic bluish-green colour developed, putatively due to the oxidation of chlorogenic acids and subsequent reactions with primary amino compounds. This coloration might be an appropriate marker to substantiate if coffee seeds had been stored for an expanded time and putative quality losses were not relevant so far.
Conclusions
It is suggested that loss of viability is relevant for the aroma flattening. As neither metabolic nor post-mortem reactions were responsible for the observed changes, it is concluded that Maillard reactions that occur during storage might be the cause of the decrease in potential aroma precursors.
Key words: Coffea arabica; coffee processing; post-harvest treatment; storage, loss of viability; viridinic acid
INTRODUCTION
Coffee represents one of the most important crops of the world. As its volume of sales depends on coffee quality, much attention is paid to quality improvement and maintenance. When green coffee is stored for a prolonged time, its quality decreases distinctively, expressed by a typical flattening and slackening of the cup quality (Wurziger et al., 1982; Scheidig and Schieberle, 2006), apparently related to a reduction in the aroma potential in the green bean. As a consequence, the provenience-specific characteristic features, especially those of top quality coffees, gradually diminish during the course of elongated storage. In contrast to the so-called ‘off-notes’, which also occur during the course of storage, and are mainly caused by undesired changes within the lipid fraction due to oxidation processes (Janícek and Pokorný, 1970; Kurzrock et al., 2005; Speer and Kölling-Speer, 2006), the causes of the progressive weakening of cup quality are still unknown. The appearance of storage-related off-notes can largely be controlled by appropriate storage of the green coffee, whereas the flattening of the cup quality occurs even under optimal storage conditions.
Traditionally, green coffee is produced either by wet or dry processing methods. In wet processing, red, ripe coffee cherries are mechanically depulped and the mucilaginous residues of the pulp are degraded by fermentation. The resulting beans are still covered by the endocarp, termed parchment. The parchment coffee is dried, conditioned and subsequently hulled. In contrast, in dry processing, entire coffee fruits are dried without removal of the pulp and then hulled mechanically.
The chemical composition of the green coffee beans differs significantly depending on the processing method applied (Bytof et al., 2005; Knopp et al., 2006). In both cases, the freshly processed coffee beans remain viable (Huxley, 1964; Cléves, 1998) and exhibit active metabolic processes (Selmar et al., 2005, 2006). During the course of storage, the coffee seeds die (Bacchi, 1958; Huxley, 1964; Coutouron, 1980).
In the past, various authors have argued that the decline in cup quality is linked to the loss of viability (Sivetz and Desrosier, 1979), but scientific proof for this statement is lacking. Although some investigators have analysed the time course for loss of germination capacity, only limited data for changes in composition of stored coffee beans are available (Pokorny et al., 1975). At present, no comprehensive approach to clarify the relationship between the viability of green coffee, the chemical composition and the corresponding cup quality have been published. In this paper, an extensive time course of the viability loss during storage under standard conditions is presented. Furthermore, data for both qualitative and quantitative changes of free amino acids and soluble sugars within the coffee beans during storage are reported. Special emphasis is put to an assumed processing specific behaviour of the stored green coffee beans, in particular with respect to putative influences of the dying procedure. The feasibility of utilizing the information obtained in practice during green coffee production and storage is outlined.
MATERIALS AND METHODS
Green coffee processing
Defined green coffee samples were produced under carefully controlled processings at the facilities of Ipanema Agricola Ltda, Alfenas, Minas Gerais, Brazil. For these sample processings, only sound and fully mature red coffee fruits of C. arabica var. acaiá were used. The coffee fruits were processed in parallel (wet, dry, semi-dry) in three independent repetitions.
In order to use truly ‘identical samples’ for each type of processing, the mature, red and sound fruits, which were used for dry processing, were obtained by careful manual sorting, an operation which in the case of wet processing is performed by flotation based sorting steps and the depulping machine that rejects the green unripe coffee cherries. Subsequently, the dried fruits were mechanically hulled.
Coffee samples were sent by airfreight to the laboratory in Braunschweig within 4 weeks of field processing.
Wet processing
Coffee fruits were mechanically depulped using a Pinhalense drum pulper, and submitted to 22 h of under-water tank fermentation. Subsequently, the washed parchment coffee beans were dried on separate plots of a sun terrace. The desired water content of 11–12 % (wet basis) was achieved after 6 d. About half of the material was mechanically hulled; the other half remained as unhusked parchment coffee.
Semi-dry processing
This method consists of a shortened wet processing sequence, in which the fermentation step to remove the mucilaginous residues of the pulp is omitted. Depulped coffee beans from the same batch used for the wet processing were transferred immediately after depulping to a sun terrace and dried to the desired water content of 11–12 % (wet basis), before the material was mechanically hulled.
Dry processing
Coffee cherries from the same batch as above were dried as whole fruits on a sun terrace until they reached the desired water content of 11–12 % (wet basis).
Storage of green coffees
The hermetic storage of green coffee was performed in two large glass-boxes (200 L) under constant conditions [22 °C and 63 % relative humidity (rh)] for up to 2 years. To ensure that the desired rh was maintained, samples were stored above vessels containing saturated (NH4)2NO3 solution according to Vertucci and Roos (1993). The resulting water vapour pressure corresponds to a rh that is in equilibrium with the 11·5 % moisture of the coffee beans (Stirling, 1981). In order to avoid marked changes in the storage climate when samples are taken, the coffee specimens were previously divided and placed in 500-g samples in small linen bags. In total, 120 bags, from three independent series of processing, each yielding four variations (dry, and semi-dry-processed green coffee, and wet-processed, hulled green coffee and wet-processed, non-hulled or ‘parchment coffee’), were distributed in equal numbers to each box. At selected times (1, 2, 3, 4, 6, 9, 13, 18 and 24 months), one bag of each treatment was withdrawn, corresponding to 12 samples for each date.
Test of viability
Changes in the viability of green coffee seed samples were estimated by the common triphenyl tetrazolium chloride (TTC) test (Dias and da Silva, 1986). Coffee beans allowed to imbibe water overnight were subsequently sliced into thin discs about 2 mm thick. These slices were then incubated for several hours (4–8) in TTC solution (1 % in 50 mm Na-phosphate buffer, pH 7·6). In viable seeds, the cells import TTC, reduce it and form a red dye: whereas this reaction does not occur in dead seeds (Fig. 1).
Fig. 1.
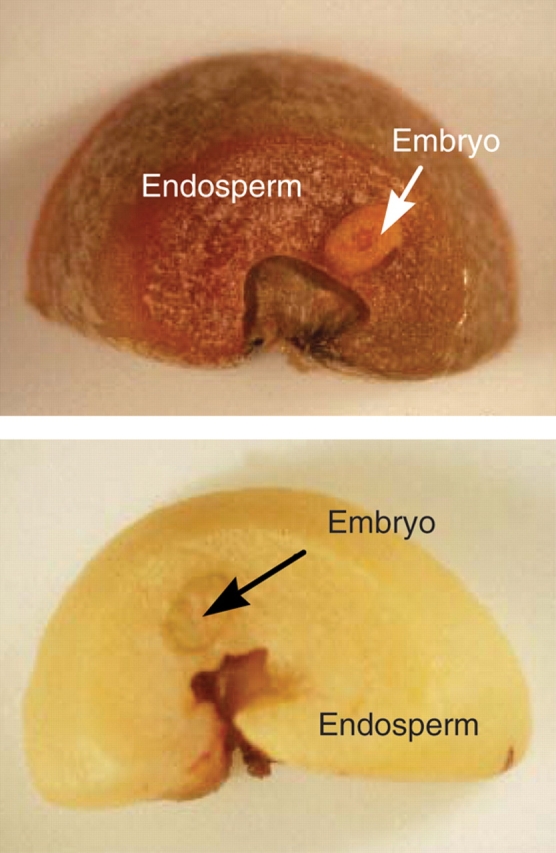
Viable (top) and dead (bottom) coffee seeds. After the coffee beans were sliced in thin discs, they were incubated in TTC solution. In viable seeds the cells import TTC, reduce it and a red dye is formed. In a dead seed this reaction does not occur.
Sensory assessment
Prior to the storage experiments, all samples were evaluated by professional tasters at Ipanema Agricola Ltda, Alfenas, Minas Gerais, Brazil. Sensory assessments of the stored samples were performed by sensory boards of the industrial partners, mainly from Tchibo and Kraft Foods.
Extraction and determination of glucose and fructose
The content of glucose, fructose and other sugars was determined by high performance anion exchange chromatography (HPAEC) according to Knopp et al. (2006). To exclude variability due to individual seed variations, 60 sound and faultless beans were weighed and pooled as one sample, frozen with liquid nitrogen and ground to a fine powder using a Retsch MM 200 ball mill. The powder was then lyophilized and 50-mg aliquots of the freeze-dried powder were transferred to a centrifuge tube. These were mixed with 5 mL of hot ethanol (80 % v/v, 80 °C) and 100 µL internal standard (7 mmol L−1 melecitose in A.dest.). The mixture was extracted in an ultrasonic bath (80 °C, 10 min) and centrifuged (3000 g, 5 min). The pellet was washed twice at room temperature with ethanol (60 % v/v). The supernatants were combined, evaporated to dryness and dissolved in water (10 mL) using ultrasonication (10 min). In order to bind phenolic substances, a small quantity of polyvinylpolypyrrolidone (PVPP) was added prior to ultrasonication. After adjusting the total volume to 50 mL, an aliquot was centrifuged in order to remove the PVPP (3000 g, 5 min). The supernatant was further purified on a Sep-Pak C18-cartridge (Waters). The clear eluate was used for HPAEC (DIONEX® BIO-LC HPAEC-PAD with a DIONEX® PA20 column). Flow was 0·5 mL min−1. The column temperature was 30 °C. Detection was carried out using a DIONEX®-ED50 electrochemical detector with gold electrode. All calculations were made with Chromeleon software (version 6·4) was used. Separation was performed with the following concentration changes of NaOH (step and linear gradients): 17 → 12·5 mmol L−1 in 9·5 min; 12·5 → 28 mmol L−1 as step; 28 mmol L−1 for 8 min; 28 → 108 mmol L−1 as step; 108 mmol L−1 for 11·5 min).
Extraction and determination of free amino acids
The content of free amino acids was determined by high-performance liquid chromatography (HPLC) according to Bytof et al. (2005). As described above, 60 sound and faultless beans were weighed and pooled as one sample in order to exclude variabilities due to individual seed variations. Aliquots (500 mg) of these powders were used for extraction. After adding norvaline (0·8 µmol per sample) as the internal standard, the powder was repeatedly extracted with sulphosalicylic acid (4 % w/v). The extracts were adjusted to 100 mL, centrifuged and filtered.
The amino acids were derivatized with o-phthaldialdehyde prior to HPLC analysis. The o-phthaldialdehyde-derivatization procedure was performed according to Kirchhoff et al., 1989; however, a Spark Holland Midas autosampler was used for derivatization and sample injection. The amino acid derivatives were separated on a C18 column (Nucleosil 100, 5 µm, Macherey & Nagel, 250 × 4·0 mm) using a binary gradient (A: 5 % MeOH, 5 % ACN, 2 % THF, 88 % 50 mm sodium acetate buffer, pH 6·2; B: 40 % MeOH, 40 % ACN, 20 % sodium acetate buffer) at a flow rate of 1·3 mL min−1. The derivatives were detected by means of an RF-551 Shimadzu fluorescence detector (λex = 334 nm; λem = 425 nm) and quantified by external calibration.
RESULTS AND DISCUSSION
Commercial green coffee is stored up to 3 years (Scheidig and Schieberle, 2006). Prolonged storage is accompanied by typical decline of quality, which apparently is caused by changes in the quantitative and qualitative composition of substances present in the coffee beans. It has been argued that the loss of viability is related strongly to the decline in quality (Sivetz and Desrosier, 1979). However, until the present, it is not known, whether the related changes in the chemical composition of the beans are due to either metabolic reactions in the living seeds or to post-mortem processes. In this paper the three different topics, i.e. the decline of viability, and the changes in potential aroma precursors and the cup quality are investigated and discussed comprehensively to establish a basis for new approaches in quality preservation during storage.
Decrease of viability during the course of storage
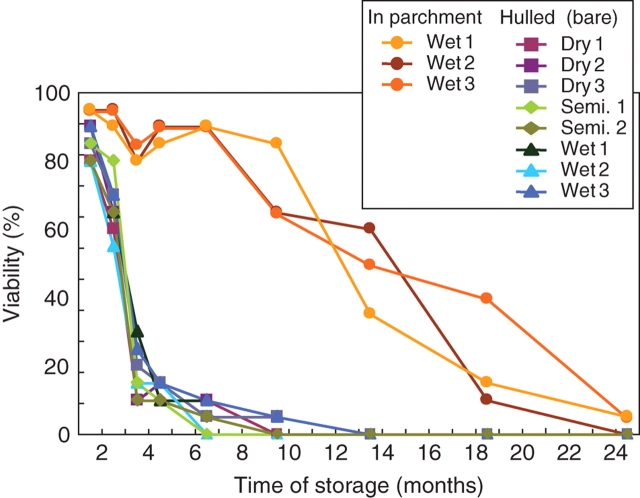
Tetrazolium chloride staining during storage of the green coffee samples revealed a rapid decrease in viability, as noted previously (Huxley, 1964; Coutouron, 1980). In all samples that were hulled prior to storage, >50 % of the seeds lost viability within the first 3 months of storage. After 6 months, the fraction of seeds still viable was <10 %. After 1 year of storage, all seeds were dead (Fig. 2). The mode of processing had no effect on the time course of viability loss. In contrast, the so-called ‘pergamino’ or ‘parchment coffees’ (German: ‘Hornschalenkaffee’) revealed a different pattern. These coffee beans were stored within the parchment (endocarp) and, although these samples were derived from the same processing batch as the corresponding wet-processed samples, the loss of viability was quite different. Even after a longer storage period than 1 year, more than half of the beans stored as parchment coffee were still alive (Fig. 2).
Fig. 2.
Decrease of viability of coffee seeds during storage. Green coffee was stored at 20 °C and 60 % rh for up to 2 years. The viability was determined using the TTC test described in Fig. 1.
Under natural conditions, within dried and slowly rotting fruits, coffee seeds retain their viability for longer than 6 months (Velasco and Guitierrez, 1974; Rojas, 2004) and by applying special storage conditions for each crop, coffee seeds can remain viable even for a longer time period. However, the water content of seeds in the naturally rotting fruits, as well as the water content of the coffee seeds stored as seed material, is far higher than that present in processed green coffees (11 %). Consequently, the much longer period of viability under natural conditions or for breeding purpose is explained by the significantly greater water potential in the seeds (Valio, 1976; Van der Vossen, 1980). This does not, however, explain the differences in viability between parchment coffees and those hulled prior to storage: all samples had the same residual water content of 11·5 %. Thus, the marked increase in the period of viability of coffee seeds must be the consequence of another factor: the parchment. There is no clear explanation for the positive influence of the parchment on the viability. One possible explanation could be based on differences in the microclimate surrounding the seeds, i.e. composition of the air within the parchment might be somewhat different from outside. This would mean that, although all samples were stored in the same box, the composition of the air covering hulled seeds might be different from that surrounding the seeds stored in their parchment. However, as the parchment represents a dead and relatively dry sclerenchymatic tissue, there should be no major diffusion barriers for any of the gases present around the seeds, i.e. CO2, O2 or H2O (Wootton, 1970; Valio, 1980). Alternatively, the reason for the different behaviour of the differentially treated seeds could be mechanical stress during the course of the hulling process. It cannot be excluded that this physical stress resulted in damage or injuries that are subsequently responsible for the relatively fast death of the treated seeds. However, the hulling of the wet-processed parchment coffee and the husking procedure of dry-processed coffee fruits make up quite different mechanical stresses, and yet both wet- and dry-processed seeds are identical with respect to loss of seed viability. Further research is therefore needed to find an explanation for the strong viability-enhancing effect of the parchment, which obviously affects also the cup quality of stored coffees (see below).
Post-mortem reactions in coffee seeds
To estimate the germination rate of coffee seeds that had been stored for various time periods, coffee beans were soaked for 12 h in water and subsequently transferred onto moist filter paper. As expected, most viable seeds germinated in about 2–3 weeks. Surprisingly, these experiments also generated an additional and unusual result. Imbibition by coffee seeds that had died a number of months before resulted in a characteristic appearance. During imbibition by dead seeds, many, mainly brownish substances leaked out, due to the disintegration and decompartmentation of the seeds. In contrast, imbibition of seeds that had been dead for only a few days or weeks resulted in a quite different picture: 2–3 d after imbibition, the dead seeds had acquired a dark blue-green colour (Fig. 3A). The characteristic blue-green colour is known as viridinic acid (German: ‘Viridinsäure’). This results from the oxidation of chlorogenic acids and subsequent reaction with primary amino compounds (Watanabe et al., 1996; Yabuta et al., 2001). Similar reactions are frequently visible as the result of injuries that occur during wet processing, especially in the course of mechanical pulping and demucilation (Fig. 3B). However, their occurrence following imbibition of coffees beans that had died only a few days or weeks before has not been reported so far. Whereas in vitro the corresponding oxidation might be initiated by alkalization (Yabuta et al., 2001), in situ it is catalysed by enzymes, probably polyphenoloxidases and laccases.
Fig. 3.

(A) Formation of a blue-green dye in dead coffee seeds. The seeds shown had been stored for about 4 months and lost their viability between 3 and 4 months of storage. On the left, an unimbibed seed is displayed. The seed on the right was allowed to imbibe for one night and subsequently incubated for 2 d on moist filter paper. (B) Formation of a blue-green dye in injured coffee seeds. After traditional wet processing and mechanical mucilage removal (aquapulping), these seeds had been stored for 1 d under moist conditions in order to improve quality (Selmar et al., 2005). Major and minor injuries result in the typical generation of the chlorogenic acid-based blue-green dye.
Direct contact of enzyme and substrates should be a precondition for such enzymatic oxidation; this may occur in the course of decompartmentation due to cell-death-related disintegration. As mentioned above, the formation of the blue-green compounds only took place in seeds that died within a short time period preceding evaluation. However, in seeds that had lost viability several months before imbibition, these oxidation-induced processes could not observed, although in all cases the tissues of the dead seeds would have disintegrated. Consequently, the enzymes that are responsible for the post-mortem oxidation of chlorogenic acids are still active in seeds that have died within a short period preceding imbibition. In contrast, these oxidative enzymes were already inactive in stale seeds because of denaturation processes that proceeded during the storage of dead seeds.
The conversion of chlorogenic acids was observed only when the dead seeds had imbibed water and did not occur during the course of standard storage. Thus, it can be deduced that the water content of 11 % in the stored seeds is not sufficient to permit the characteristic post-mortem reactions. This might be because either the required comprehensive decompartmentation of cells may not occur before rehydration takes place or the diffusion of enzyme or substrates is strongly limited by water shortage in the stored seeds. In general, it is assumed that in cells with a residual water content of <20 %, water availability is restricted to the degree that the entire metabolism of the cell is shut down. Consequently, in coffee seeds with 11 % residual water, no regulated metabolic reaction should take place. However, even in these coffee seeds some reactions must occur, in particular, those which result in the permanent decrease of viability when seeds with 11 % residual water content are stored. In this context, the so-called after-ripening processes, whose molecular basis is still unknown, could be relevant (Finch-Savage and Leubner-Metzger, 2006). These processes are thought to be the basis for gene expression during seed storage in air-dried, low-hydrated tobacco seeds with an overall moisture content of about 8–13 % (Leubner-Metzger, 2005), and are believed to be responsible for the breaking of seed dormancy in orthodox seeds (Leubner-Metzger, 2005; Finch-Savage and Leubner-Metzger 2006). If this novel developmental mechanism for seed dormancy release could also be relevant in non-orthodox seeds is not known and needs to be clarified.
The observed changes in the composition of substances during green coffee storage may also be due to disintegration processes of biomembranes that take place within the lipid phase. The properties of the biomembranes are not markedly influenced by the residual water content of the cytoplasmic (= water) phase. However, much more research is needed to understand the various processes involved. Nevertheless, since only those coffee beans that have recently died develop this characteristic blue-green colour, the related seeds had been stored only for a very limited period of time as ‘dead seeds with still active enzymes’. In seeds that had been stored markedly longer, the corresponding enzymes are already inactivated. It is assumed that the typical quality losses during prolonged storage might be generated only in dead seeds that had been stored for a prolonged time; therefore, the development of blue-green colour could be a good hint, or even an appropriate marker, to substantiate that coffee seeds had not been stored for an extended time and putative quality losses due to prolonged storage times had not yet occurred.
The influence of prolonged storage on the contents of potential aroma precursors
During roasting of coffee beans, reducing carbohydrates and free amino acids form the majority of the coffee roast aroma compounds in the course of Maillard reactions and subsequent Strecker degradations. These compounds are important aroma precursors (Flament, 2001). In order to estimate if their composition in coffee seeds changes in the course of standard storage, the amounts of amino acids and the low molecular carbohydrates were quantified.
Carbohydrates
As already reported by Knopp et al. (2006), the concentrations of glucose and fructose are strongly influenced by the mode of processing. In dry-processed beans, the concentration of these two sugars is markedly higher than in wet-processed beans. The levels of these sugars in beans originating from a semi-dry process are between those of wet- and dry-processed beans. Consequently, the starting points of the corresponding curves are quite different. The content of soluble sugars in green coffee seeds is subject to high individual variation (Knopp et al., 2006) caused by individual differences in the expression of metabolic processes. Therefore, the corresponding analyses were performed using aliquots of composite samples based on 60 seeds that were mixed during grinding. The resulting data of the three independent experiments still revealed notable amounts of variation, but the characteristic differences due to the certain processing method were clear.
During storage, the relatively high content of glucose present in dry-processed green coffees decreased markedly (Fig. 4A). The same behaviour occurred in semi-dry-processed coffees. A similar tendency might also be recognized for the minor concentration present in wet-processed green coffees, although the corresponding concentration differences are relatively small. Despite the striking differences with respect to viability, there was no notable difference between the wet-processed seeds that had been hulled before storage and those stored within their parchment. Changes in fructose concentration showed a similar pattern to that observed for glucose, although the overall storage-related decrease was not so pronounced in dry and semi-dry-processed seeds and no significant changes were detected for wet-processed beans (Fig. 4B). At first sight, these results contradict the observation of Bucheli et al. (1998), who found an increase in glucose under moist storage conditions, i.e. 85 % rh. However, these authors also detected a significant decrease in sucrose, and they argued that the sucrose is hydrolysed during storage to yield glucose and fructose. In contrast to the storage of green coffee under standard conditions mentioned above (i.e. 63 % rh), where most metabolic reactions are prevented, storage at 85 % rh does not lead to complete inhibition of enzymatic reactions.
Fig. 4.
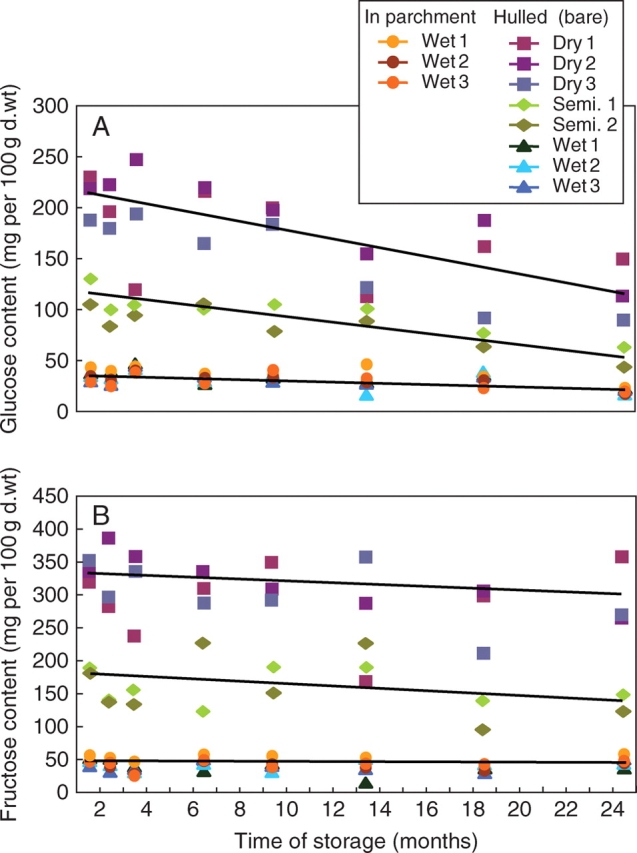
(A) Changes in glucose content during storage of green coffees. The content of glucose was determined by HPAEC. Each value corresponds to a single independent determination of an aliquot of a composite sample of 60 sound and faultless beans for each treatment. Corresponding double determinations revealed that the s.d. of the HPAEC quantification was always <6 %. To demonstrate the extent of natural variations within the single treatments (wet, dry, etc.), not the mean values, but the individual data are presented. The corresponding s.d. varied strongly between <1 % and >20 %. (B) Changes in fructose content during storage of green coffees. The content of fructose was determined by HPAEC. Each value corresponds to a single independent determination of an aliquot of a composite sample of 60 sound and faultless beans for each treatment. Corresponding double determinations revealed that the s.d. of the HPAEC quantification was always <6 %. To demonstrate the extent of natural variations within the single treatments (wet, dry, etc.), not the mean values, but the individual data are presented. The corresponding s.d. varied strongly between <1 % and >20 %.
Sucrose represents the major sugar in green coffee (Bradbury, 2001; Knopp et al., 2006). A content of about 7·5 g 100 g−1 d. wt was estimated as a mean value for the samples used for the storage experiments. This corresponds to a total concentration about 50 times higher than that of glucose. The determination of storage dependent changes in sucrose concentration was affected by high individual variations. Nevertheless, overall, a slight decline in sucrose content from about 7·5 g 100 g−1 d. wt in unstored beans, to about 7·0 g 100 g−1 d. wt in those which had been stored for 2 years, was detected. If this small decline in sucrose concentration might also be due to residual enzymatic hydrolyses as mentioned by Bucheli et al. (1998) is not known. These workers found that, in Robusta coffees stored under moist conditions (85 % rh), up to 40 % of the sucrose is degraded. However, the slight decrease in the sucrose content observed in this work may also have been caused by other reactions (see below).
The three minor sugars, galactose, arabinose and rhamnose, whose concentrations are between 1·0 mg and 4·0 mg 100 g−1 d. wt each, correspond to about 1–2 % of the glucose concentration (Knopp et al., 2006). In contrast to the major sugars, the content of galactose, apart from some marked vacillations, did not show any significant storage-dependent changes (data not presented): The galactose concentration in unstored beans as well as in those that had been stored for 2 years was about 2·5 mg 100 g−1 d. wt. With respect to arabinose, after 24 months, in the dry-processed coffee a storage-dependent increase from about 2·0 mg 100 g−1 d. wt to about 4·5 mg 100 g−1 d. wt was observed, whereas in all other samples the arabinose concentration did not change. A different pattern was observed for rhamnose. From an initial average rhamnose concentration of about 0·25 mg 100 g−1 d. wt the mean value increased 10-fold during storage to a final value of about 2·5 mg 100 g−1 d. wt. With the exception of arabinose, for all carbohydrates so far mentioned, i.e. glucose, fructose, sucrose, galactose and rhamnose, there was no significant difference in the changes observed when wet, dry or semi-dry-processed coffees were compared.
In all cases so far analysed, the pattern of storage-dependent changes of each sugar was not influenced by the presence of the parchment. Moreover, the curves for wet-processed hulled green coffees were identical to those for the corresponding parchment coffees. This finding was surprising if it is considered that in contrast to the hulled beans, the parchment coffees were viable during most of the storage period. From this it must be deduced that the changes in sugar composition during the course of storage cannot be correlated with the fact that the seeds are either dead or alive. Consequently, neither any metabolic reaction nor any post-mortem reaction initiated by cell death-induced decompartmentation could be the only conclusive explanation for the changes observed. An explanation for this phenomenon is still lacking. However, a promising explanation might be based on the knowledge that in foods with little residual water, non-enzymatic browning processes, like Maillard reactions, are known to occur. Pokorny et al. (1975) proposed that these sugar-consuming reactions might occur in green coffee beans. Moreover, these reactions are known to occur also in aged seeds of soybean (Wettlaufer and Leopold, 1991; Sun and Leopold, 1995). Whether Maillard reactions are the causes of the observed changes in the sugar concentration needs to be investigated further.
Free amino acids
As mentioned above, in addition to the soluble carbohydrates, the concentrations of free protein amino acids and their putative changes during prolonged storage were also analysed to register comprehensively the effects of storage on coffee quality. As outlined previously (Arnold et al., 1994; Arnold and Ludwig, 1996; Bytof et al., 2005; Selmar et al., 2005), the individual concentrations of most amino acids, as well as the overall content of all amino acids of green coffee beans, vary markedly, and are strongly affected by various environmental factors (provenances, altitude, etc.), by the maturity of the seeds, and by the processing methods. In this work, these factors can be excluded as identical batches of manually sorted coffee fruits were processed in parallel under defined conditions.
The green coffees used for the storage experiments contained about 4000 mg amino acids kg−1 d. wt, being in the upper range of concentrations determined earlier (Arnold and Ludwig, 1996; Selmar et al., 2005). In contrast to most other batches analysed so far, no major differences between the amino acid content of the differentially processed coffees were observed (data not presented). Typically higher concentrations of amino acids have been detected for wet-processed beans than for dry-processed beans (Selmar et al., 2005). Nevertheless, with respect to the content of γ-amino butyric acid (GABA), reported to be a reliable marker to distinguish between differentially processed green coffees (Bytof et al., 2005), the coffee used for the storage experiments revealed the typical pattern, i.e. high GABA concentrations in the dry-processed beans and minor concentrations in the wet-processed beans. However, the actual GABA concentrations estimated for dry-processed samples (approx. 700 mg kg−1 d. wt) were lower than those reported by Bytof et al. (2005). At present, it is still an open question which additional factors – apart from the intensity and length of drought stress during the drying process – may affect the corresponding GABA production and the corresponding accumulation of this amino acid, respectively.
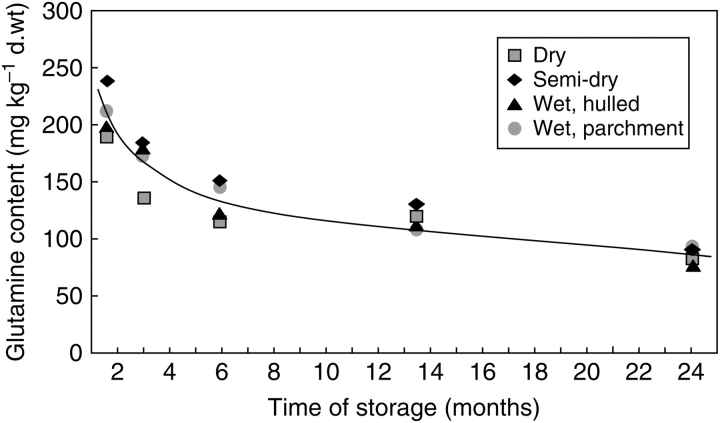
During the course of storage no significant changes, either in the total content of protein amino acids or in the concentration of most individual amino acids, were detected. Moreover, GABA concentrations remained constant; only the concentration of glutamine decreased significantly during storage (Fig. 5). The peculiar behaviour of glutamine in comparison to other amino acids is surprising, although, if it is considered that the decrease in glucose and fructose might be caused by Maillard reactions occurring at room temperature during the course of prolonged storage (see above), at least some amino acids must be consumed. If indeed, under these conditions, glutamine in particular is entering Maillard reactions is not established. More insight into the various reactions that occur during the course of prolonged storage is required.
Fig. 5.
Changes in glutamine content during storage of green coffee. The content of glutamine was determined by HPLC. Each value corresponds to a single independent determination of an aliquot of a composite sample of 60 sound and faultless beans for each treatment. Corresponding double determinations revealed that the s.d. or the HPLC quantification was always <5 %.
Sensory assessment of stored green coffees
The sensory assessment of processing specific differences only makes sense, when identical coffee samples, treated in parallel are used for cupping. This precondition excludes the majority of coffee production sites, since almost all factories are predestined either for wet or for dry processing. At Ipanema Agricola Ltda in Brazil wet, dry and semi-dry processing are all performed routinely. The corresponding coffees used for the storage experiments exhibited the typical processing-specific characteristics, i.e. the dry process coffees were denoted by a full body whereas the wet-processed ones had more acids and revealed a more pronounced aroma. The aroma and taste of semi-dry coffees were in-between these two types.
After 1 year of storage, the coffees exhibited the typical flat aromas and partially some old and woody notes. Although the sensory evaluations were characterized by estimating only slight differences between the various samples, a loss in cup quality was reported for all the green coffees stored for a long time. In many cases, after storage of only 6 months, raspy, woody or stale notes were attributed to the samples. Indeed, in most of cases, the beans that were stored within the parchment were given slightly better quality descriptions than the hulled beans. Due to the large variations and faint differences, only a tendency towards beneficial effects for storage of green coffee within the parchment was perceived. Apart from that, no principal differences in the decrease of quality could be detected between the hulled coffees, regardless of the processing methods used (dry, wet or semi-dry).
In order to establish the advantageous effect of storage of green coffees within the parchment, corresponding experiments with a focus on high-quality coffees, e.g. high-grown washed arabica coffees, should be performed. It is assumed that the sensory differences between coffees stored as either hulled or parchment beans will be far higher because slight differences are likely to cause more drastic effects in the case of high quality coffees.
CONCLUSIONS
The major outcome of this work is the finding that green coffee beans that are stored within the parchment (pergamino) remain viable far longer than hulled beans and that this maintenance of viability apparently has a positive effect on the preservation of green coffee quality during prolonged storage. In addition, gradual decreases in the content of various sugars, notably glucose and fructose, and some amino acids were not correlated with the gradual loss of viability. Thus, the gradual flattening of the aroma during prolonged storage may be correlated with loss of viability and not with changes in the composition of sugars and amino acids. Therefore the gradual loss of viability appears to be the reason for the decline of quality during prolonged storage under standard conditions, although it was not possible to substantiate the changes responsible in the chemical composition.
To preserve the quality of green coffee, storage of wet-processed arabica coffees within parchment is recommended. Even shipping of green coffee as parchment coffee should be considered, although this would mean that the dry mills for hulling and sorting must be available in the destination countries.
Finally, it is suggested that the occurrence of the blue-green colour that develops in post-mortem reactions in re-imbibed seeds might provide a good hint or may be an appropriate marker to confirm that coffee seeds have not been stored for an extended time and putative quality losses due to prolonged storage times have not yet occurred.
ACKNOWLEDGEMENTS
This research project was supported by the FEI (Forschungskreis der Ernährungsindustrie, Bonn, Germany), the Arbeitsgemeinschaft industrieller Forschungsvereinigungen and the Ministry of Economic Affairs (project nos AIF-FV 13234 and 13588). We thank Mrs Yvonne Evers, Technical University Hamburg–Harburg for permission to use the photograph of injured coffee beans (Fig. 4B).
LITERATURE CITED
- Arnold U, Ludwig E. Analysis of free amino acids in green coffee beans. II. Changes of the amino acid content in arabica coffees in connection with post-harvest model treatment. Zeitschrift für Lebensmitteluntersuchung und Forschung. 1996;203:376–384. doi: 10.1007/BF01231078. [DOI] [PubMed] [Google Scholar]
- Arnold U, Ludwig E, Kühn R, Möschwitzer U. Analysis of free amino acids in green coffee beans. I. Determination of amino acids after precolumn derivatization using 9-fluoromethylchloroformate. Zeitschrift für Lebensmitteluntersuchung und Forschung. 1994;199:22–25. doi: 10.1007/BF01192946. [DOI] [PubMed] [Google Scholar]
- Bacchi O. Estudos sobre a conservação de sementes. IV. Café. Bragantia. 1958;17:261–270. [Google Scholar]
- Bradbury AGW. Carbohydrates. In: Clarke RJ, Vitzthum OG, editors. Coffee: recent developments. Oxford: Blackwell Science; 2001. pp. 1–17. [Google Scholar]
- Bucheli P, Meyer I, Pittet A, Vuataz G, Viani R. Industrial storage of green Robusta coffee under tropical conditions and its impact on raw material quality and ochratoxin A content. Journal of Agricultural and Food Chemistry. 1998;46:4507–4511. [Google Scholar]
- Bytof G, Knopp SE, Schieberle P, Teutsch I, Selmar D. Influence of processing on the generation of γ-aminobutyric acid in green coffee beans. European Food Research and Technology. 2005;220:245–250. [Google Scholar]
- Cléves SR. Tecnologia en beneficiado de café. San José, Costa Rica: Tecnicafé Internacional S.A; 1998. [Google Scholar]
- Couturon E. Le maintien de la viabilité des graines de caféiers par le contrôle de la teneur en eau et de la température de stockage. Café, Cacao, Thé. 1980;24:27–32. [Google Scholar]
- Dias MCL, daSilva WR. The tetrazolium test for viability evaluation of coffee bean seed. Pesquisa Agropecuaria Brasileira. 1986;21:1139–1145. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Tansley review: seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Flament I. Coffee flavor chemistry. New York, NY: John Wiley & Sons; 2001. [Google Scholar]
- Huxley PA. Investigations on the maintenance of viability of Robusta coffee seed in storage. Proceedings of the International Seed Test Association (Wageningen); 1964. pp. 423–444. [Google Scholar]
- Janícek G, Pokorný J. Changes of coffee lipids during the storage of coffee beans. Zeitschrift für Lebensmitteluntersuchung und -Forschung A. 1970;144:189–191. [Google Scholar]
- Kirchhoff PM, Biehl B, Crone G. Peculiarity of the accumulation of free amino acids during cocoa fermentation. Food Chemistry. 1989;31:295–311. [Google Scholar]
- Knopp SE, Bytof G, Selmar D. Influence of processing on the content of sugars in green arabica coffee beans. European Food Research and Technology. 2006;223:195–201. [Google Scholar]
- Kurzrock T, Kölling-Speer I, Speer K. Effects of controlled storage on the lipid fraction of green arabica coffee beans. 20ème Colloque Scientifique International sur le Café; 11–15 2004; Bangalore, India. Paris: Association Scientifique Internationale du Café (ASIC); 2005. Oct, pp. 161–168. [Google Scholar]
- Leubner-Metzger G. β-1,3-Glucanase gene expression in low-hydrated seeds as a mechanism for dormancy release during tobacco after-ripening. The Plant Journal. 2005;41:133–145. doi: 10.1111/j.1365-313X.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Con NH, Smidrkalova E, Janícek G. Non-enzymic browning. XII. Maillard reactions in green coffee beans on storage. Zeitschrift für Lebensmitteluntersuchung und Forschung. 1975;158:87–92. doi: 10.1007/BF01460026. [DOI] [PubMed] [Google Scholar]
- Rojas J. Green coffee storage. In: Wintgens JN, editor. Coffee: growing, processing, sustainable production. Weinheim: Wiley-VCH; 2004. pp. 733–750. [Google Scholar]
- Scheidig C, Schieberle P. Einfluss der Lagerung von Rohkaffee auf das Aroma von Rohkaffee, Röstkaffee und Kaffeegetränk. Lebensmittelchemie. 2006;60:55–56. [Google Scholar]
- Selmar D, Bytof G, Knopp SE, Bradbury A, Wilkens J, Becker R. Biochemical insights into coffee processing: quality and nature of green coffees are interconnected with an active seed metabolism. 20ème Colloque Scientifique International sur le Café; 11–15 2004; Bangalore, India. Paris: Association Scientifique Internationale du Café (ASIC); 2005. Oct, [Google Scholar]
- Selmar D, Bytof G, Knopp SE, Breitenstein B. Germination of coffee seeds and its significance for coffee quality. Plant Biology. 2006;8:260–264. doi: 10.1055/s-2006-923845. [DOI] [PubMed] [Google Scholar]
- Sivetz M, Desrosier NW. Hulling, classifying, storing, and grading green coffee beans. In: Sivetz M, Desrosier NW, editors. Coffee technology. Westport, CT: The Avi Publishing Company; 1979. pp. 117–169. [Google Scholar]
- Speer K, Kölling-Speer I. The lipid fraction of the coffee bean. Brazilian Journal of Plant Physiology. 2006;18:201–216. [Google Scholar]
- Stirling H. Storage research on Kenya arabica coffee. 9ème Colloque Scientifique International sur le Café; London. Paris: Association Scientifique Internationale du Café (ASIC); 1981. pp. 189–200. [Google Scholar]
- Sun WQ, Leopold AC. The Maillard reaction and oxidative stress during aging of soybean seeds. Physiologia Plantarum. 1995;94:94–104. [Google Scholar]
- Valio IFM. Germination of coffee seeds (Coffea arabica L. cv. Mundo Novo) Journal of Experimental Botany. 1976;27:983–991. [Google Scholar]
- Valio IFM. Inhibition of germination of coffee seeds (Coffea arabica L. cv. Mundo Novo) by the endocarp. Journal of Seed Technology. 1980;5:32–39. [Google Scholar]
- Van der Vossen HAM. Methods of preserving the viability of coffee seeds in storage. Kenya Coffee. 1980;45:31–35. [Google Scholar]
- Velasco JR, Guitierrez J. Germination and its inhibition in coffee. Philippine Journal of Science. 1974;103:1–11. [Google Scholar]
- Vertucci CW, Roos EE. Theoretical basis of protocols for seed storage. II. The influence of temperature on optimal moisture levels. Seed Science Research. 1993;3:201–213. [Google Scholar]
- Watanabe S, Ushizawa Y, Kusama M. Effect of pH on green pigments produced from chlorogenic acid and glycine. Journal of the Japanese Society of Food Science and Technology. 1996;43:1–6. [Google Scholar]
- Wettlaufer SH, Leopold AC. Relevance of Amadori and Maillard products to seed deterioration. Plant Physiology. 1991;97:165–169. doi: 10.1104/pp.97.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton AE. The storage of parchment coffee. Kenya Coffee. 1970;35:144–147. [Google Scholar]
- Wurziger J, Drews R, Suche B. Über Röstkaffees aus abgelagerten Rohkaffees. Kaffee und Tee Markt. 1982;32:3–5. [Google Scholar]
- Yabuta G, Koizumi Y, Namiki K, Hida M, Nameki M. Structure of green pigment formed by the reaction of caffeic acid esters (or chlorogenic acid) with a primary amino compound. Bioscience, Biotechnology and Biochemistry. 2001;65:2121–2130. doi: 10.1271/bbb.65.2121. [DOI] [PubMed] [Google Scholar]