Abstract
Background and Aims
Floral development of Cedrela and Toona, the genera comprising the basal tribe Cedreleae of the sub-family Swietenioideae of Meliaceae, is described. The focus was on three endangered, ecologically and economically important species: Cedrela fissilis, Cedrela odorata and Toona ciliata. The aims of the study were to characterize the patterns of floral development in the tribe and to establish apomorphic and plesiomorphic floral characters in relation to other taxa within the family based on the current molecular phylogeny of Meliaceae.
Methods
A detailed floral structural and developmental study was completed using both scanning electron microscopy and visualization of microtome sections with a light microscope.
Key Results
Twelve floral developmental stages were identified. The initial development of the pentamerous flowers of both Toona and Cedrela is strikingly similar. The morphological differences observed between them are due to differential patterns of organ elongation and adnation/connation occurring late in development. Additionally, the formation of functionally male and female flowers was found to occur at specific positions within the inflorescence.
Conclusions
Due to the basal position of the tribe Cedreleae in the phylogeny of Meliaceae, functionally either male or female pentamerous flowers and the presence of (at least partially) free stamens may be considered plesiomorphic traits within the family. In contrast, sympetaly and the absence of nectaries in Cedrela species are synapomorphies.
Key words: Cedrela fissilis, Cedrela odorata, floral development, morpho-anatomy, sex distribution, Toona ciliata
INTRODUCTION
Meliaceae are a large family of tropical woody species, comprised of 50 genera (approx. 575 species; Pennington and Styles, 1975; Mabberley et al., 1995; Chase et al., 1999), which were categorized by Pennington and Styles (1975) into four sub-families: Melioideae (seven tribes, approx. 35 genera); Swietenioideae (three tribes, 13 genera); and the monogeneric Quivisianthoideae and Capuronianthoideae. These authors also considered the secondary xylem to be an important feature separating Swietenioideae and Melioideae.
Meliaceae, compared with other groups of similar size, contain a relatively wide range of floral, fruit and seed morphologies (Pennington and Styles, 1975), and plesiomorphic morphological characters occur side-by-side with derived ones and are frequently connected by intermediate states (Muellner et al., 2003). Molecular data based on the sequences of two plastid genes and one nuclear gene confirmed the sub-familial status of both Melioideae and Swietenioideae (Judd et al., 1999; Muellner et al., 2003). Furthermore, morphological and molecular data both indicate a close relationship between the genera Cedrela and Toona, which are sister taxa forming a monophyletic clade within Swietenioideae (Muellner et al., 2003), justifying their positioning within the same tribe.
Cedrela, the cigar-box cedar tree, is naturally distributed from Mexico throughout the Caribbean islands, the lowland and montane Central and South America to northern Argentina (Verissimo et al., 1998; Cavers et al., 2003). Cedrela trees may reach 40 m in height and 1·2 m in diameter (Newton et al., 1995, 1999; Navarro et al., 2002). The economic value of timber of Cedrela species has resulted in their overexploitation for the past two centuries and has prompted a number of studies concerning the sustainable production and use of Cedrela as a forest crop (Newton et al., 1995, 1999; Navarro et al., 2002; Cavers et al., 2003). An additional threat to the remaining trees and to organized planting programmes is that within its natural area of distribution in the Americas, Cedrela trees are intensely attacked by a species of the mahogany shoot-borer, Hypsipyla grandella Zell, to the extent that use of these trees as a forest crop has been halted until resistant cultivars can be selected for or bred (Keay, 1996; O'Neil et al., 2001). Some species such as Cedrela fissilis Vell are known to be more resistant to H. grandella, and it is hoped that breeding programmes can incorporate this resistance to produce resistant cultivars and/or that hybrids could be developed that allow for resistance in other species.
Similarly, Toona, a genus of large timber trees with a geographical distribution mainly in Asia and Malaysia, is also heavily attacked by the shoot-borer throughout its natural geographical range, but apparently not elsewhere. Therefore, tree breeders in Central and South America have turned their attention to the silviculture and improvement of species belonging to these two closely related genera.
Nevertheless, despite knowledge of the reproductive biology of tropical forest trees being crucial to the success of tree breeding programmes, information is extremely scarce even for the most common and best-known species and, in many instances, it is lacking altogether. In Meliaceae, with the exception of the work by Styles (1972), only the generic monograph on the taxonomy of Meliaceae by Pennington and Styles (1975) describes some of the floral characteristics of the tribe Cedreleae. Thus, as part of a series of papers characterizing the reproductive development in Meliaceae, here the floral development of the two genera comprising the tribe Cedreleae of the sub-family Swietenioideae: Cedrela and Toona, including the ecologically and economically important species C. fissilis Vell., C. odorata L. and Toona ciliata M. J. Roem, is reported. The aims of the study were to characterize the patterns of floral development in the tribe Cedreleae in order to understand better the breeding systems of these two genera and, additionally, to establish apomorphic and plesiomorphic floral characters in relation to other taxa within the family based on the existing molecular phylogeny of the family (Muellner et al., 2003).
MATERIALS AND METHODS
Plant material
Inflorescences of C. fissilis Vell., C. odorata L. and T. ciliata M. J. Roem. (Meliaceae) at various stages of development were collected over a 4-year period from the arboretum of the Escola Superior de Agricultura ‘Luiz de Queiroz’, at the University of São Paulo (Piracicaba, SP, Brazil). Voucher specimens were deposited at the ESA Herbarium and received the numbers ESA78412, ESA78414 and ESA78420, respectively.
Inflorescences containing flowers at all developmental stages were immediately fixed in 4 % paraformaldehyde under vacuum for 24 h, dehydrated to 70 % ethanol and stored at 4 °C until needed for either light microscopy or scanning electron microscopy. A preliminary inspection of inflorescences revealed striking differences in the size and colour of stamens and in the size of gynoecia among opened flowers of the three species. These observations indicated the putative existence of both functionally male and female flowers. Thus the positions were recorded and a putative identity (male or female) was attributed for all flowers from 30 inflorescences for each species studied. Furthermore, 12 developmental stages were established based on the overall meristem/floral bud sizes and arbitrary landmark events for both male and female flowers.
Light microscopy
The samples were further dehydrated to absolute ethanol, embedded in Historesin (Leica, hydroxyetilmethacrilate), followed by polymerization at room temperature for 48 h. After polymerization, serial sections of 5–8 µm thickness were obtained and stained with 0·05 % toluidine blue. Slides were made permanent upon mounting in Entellan (Fluka, Germany). Microtome sections were observed and photographed under a Axioskop-40 Zeiss microscope (Oberkochen, Germany).
Scanning electron microscopy
After dehydration to absolute ethanol, flowers were initially dissected in absolute ethanol under an Olympus dissecting microscope. The resultant material was critical point dried through CO2 (Balzers CPD 020 Critical Point Drier, Schalksmuhle, Germany) and further dissected as required. The samples were mounted on metallic stubs with carbon conductive adhesive tape, sputter coated with colloidal gold and observed at 10–20 kV under a Zeiss DSM 940 A or a LEO 435 VP scanning electron microscope (Oberkochen, Germany).
RESULTS
Inflorescence architecture
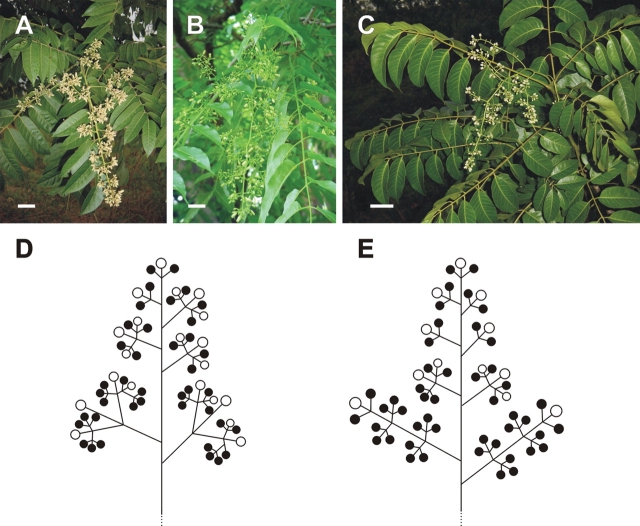
The flowers of the three taxa studied from the tribe Cedreleae are usually borne in a thyrse (a compound cyme or cymose panicle), often with primary, secondary, tertiary and, more rarely, even quaternary branches (Fig. 1A–E). Although the numbers of branches and, consequently, the numbers of flowers, are variable, each ultimate branchlet always ends in a cymule of three (or rarely two) flowers. In Cedrela, the inflorescences may reach >90 cm in length. These are generally more tightly arranged than in Toona (which reach from 25 to 35 cm in length), due to the secondary inflorescence branches of Cedrela frequently containing higher order cymules (generally third order) than those of Toona, where the inflorescence branches generally contain only secondary and, more rarely, third order cymules (Fig. 1).
Fig. 1.
Architecture of Cedrela and Toona inflorescences. (A–C) Thyrses are formed by the conversion of the terminal shoot meristem in C. fissilis (A), C. odorata (B) and T. ciliata (C). (D, E) Schematic diagrams of the thyrses of Cedrela (D) and Toona (E). The circles represent the relative positions of functionally female (white) and male (black) flowers. Scale bars = 5 cm.
Plants are monoecious, and sex distribution within the inflorescences of Cedreleae is not random. Instead, commonly, the centre flower of a cyme or of a three-flowered cymule is functionally female, while the lateral flowers are functionally male. At times, however, cymules may be composed of functionally male flowers only (Fig. 1D, E).
Floral structure in Cedreleae
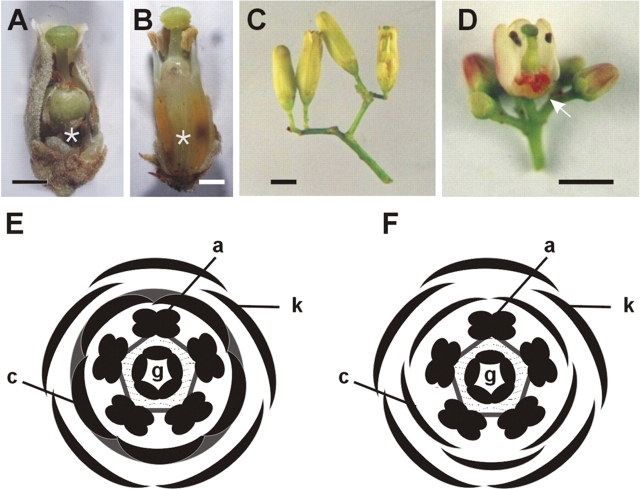
At anthesis, the mature flowers of the two Cedreleae genera, Cedrela (here represented by C. fissilis and C. odorata; Fig. 2A–C) and Toona (T. odorata; Fig. 2D) are morphologically bisexual, but functionally unisexual, with the staminodes or pistillodes being well developed in the functionally female or male flowers, respectively (Fig. 2A, B). Flowers are pentamerous and isomerous (Fig. 2E, F).
Fig. 2.
Floral structure in Cedreleae: C. fissilis, C. odorata and Toona ciliata. (A, B) C. fissilis partially dissected functionally female (A) and male (B) flowers. The asterisks in (A) and (B) indicate the cushion-shaped structure at the base of the gynoecium. (C) Cymule of C. odorata with flowers before anthesis. The partially dissected flower (right) is functionally male. (D) Cymule of Toona ciliata with mature flower buds. The partially dissected flower is functionally female. The arrow points to the nectary. In (A–D), the perianth, as well as the staminal tube were partially removed to reveal the reproductive organs. (E, F) Floral diagrams of Cedrela (E) and Toona (F). k, sepals; c, petals; a, stamens; g, carpels. The grey area connecting the petals (E) indicate synpetally. The grey pentagon connecting the stamens indicate that they are fused at the base of the filaments (E, F). The dotted area between the third and fourth whorls indicates adnation of the base of stamen filaments with the base of the gynoecium (E, F). Scale bars = 2 mm.
The characteristics of sepals and petals are very similar in both male and female flowers of Cedreleae. Sepals are lobed and partially free, connate at their base to half of their length. The abaxial epidermis of sepals shows unicellular filliform trichomes. Petals are much longer than the sepals at anthesis, especially in Cedrela where petals may reach five times the length of sepals. Petals are free in Toona, but connate in Cedrela, the sympetalous region extending for 80–90 % of petal length. In Cedrela, the base of the ventral side of the petals is adnate to the base of the filaments of the stamens, forming a cushion-shaped stalked structure which is also a ‘platform’ to the gynoecium. This structure corresponds to more than one-third of the flower length in Cedrela functionally male flowers (Fig. 2B), while it is shorter in functionally female flowers of both Cedrela species studied (Fig. 2A). In Toona, the petals and stamens are not adnate, but the stamens are joined to the ovary at the ovary base in both male and female flowers. Stamens are connate to each other to various degrees in the three species. In functionally female flowers of both Cedrela and Toona, stamens are present in the form of staminodes. These are about half the length of fertile stamens and their anthers are dark brown in colour (Fig. 2A, D), when compared with the anthers from functional male flowers, which are yellowish (Fig. 2B). In both male and female flowers of Toona, a bright red discoid nectary is present at the base of the gynoecium (Fig. 2D). A morphologically distinct floral nectary is not evident in either male or female flowers of Cedrela (Fig. 2A, B). The gynoecium is entirely syncarpous and placentation is axile. In female flowers of both genera, two series of 6–12 bitegmic anatropus ovules are present in each locule. The stigma is discoid, with the upper surface (topographically speaking, ventral surface morphologically speaking) generally showing the scars of the post-genital fusion of the stigmatic lobes. Nevertheless, transmitting tissue is not visible through these scars. Additionally, stigmatic papillae are restricted to the underside (topographically speaking) of the stigma, the upper surface lacking such cells. The positioning pattern of the papillae on the surface of the stigma may be related to (as yet unknown) pollination mechanisms. Accordingly, no pollen grain was observed germinating on the ‘upper side’ (topographically speaking) of the stigma. Germinating pollen grains were observed only on the surface of the papillae.
In functionally male flowers of both genera, a pistillode is present whose overall appearance closely resembles that of the fertile gynoecium in female flowers; however, it contains aborted ovules. In Toona male flowers the mean width of the ovary is about half of that observed in female flowers, but in Cedrela the differences in ovary width between male and female flowers are not significant.
Floral development in Cedreleae
Based on the main events observed during the development of the Cedreleae flowers, 12 readily identifiable organogenic stages were established (Table 1). The choice of the key features that determine stage transitions was based on the work of Smyth et al. (1990) who described the early reproductive development of the model plant Arabidopsis thaliana. Up to stage 8, when organ elongation and enlargement take place along with the typical organ-specific differentiation of cells, no significant differences in the pattern of floral organ differentiation among the three species studied were observed. Additionally, no significant differences were observed between male and female flowers of both Cedrela and Toona up to stage 8. The only evidence available to define whether a given flower meristem will produce a functionally male or female flower was its relative position in the inflorescence, due to the prior information that the central flower in a given cymule is female and the lateral ones are male.
Table 1.
Stages of flower development in Cedrela and Toona, listing the landmark events that define the beginning of each stage and the respective mean size of the floral meristem or flower bud
| Mean size of the flower meristem or flower bud ± s.d. (mm) |
|||
|---|---|---|---|
| Stage | Landmark event at beginningof stage | Cedrela* | Toona |
| 1 | Flower meristem is detectable on the surface of the inflorescence meristem | 0·05 ± 0·01 | 0·04 ± 0·01 |
| 2 | Flower meristem is individualized | 0·12 ± 0·02 | 0·10 ± 0·03 |
| 3 | Sepal primordia arise | 0·15 ± 0·02 | 0·25 ± 0·04 |
| 4 | Al sepal primordia formed | 0·30 ± 0·04 | 0·35 ± 0·06 |
| 5 | Petal primordia arise | 0·36 ± 0·08 | 0·40 ± 0·08 |
| 6 | Stamen primordia arise | 0·41 ± 0·08 | 0·43 ± 0·09 |
| 7 | Carpel primordia arise | 0·50 ± 0·09 | 0·48 ± 0·08 |
| 8 | Locules are formed in stamen primordia | 1·00 ± 0·12 | 0·50 ± 0·06 |
| 9 | Petal primordia stalked at the base | 1·53 ± 0·18 | 0·55 ± 0·08 |
| 10 | Ovule primordia are initiated | 1·74 ± 0·25 | 0·75 ± 0·09 |
| 11 | Stigmatic papillae appear | 1·85 ± 0·29 | 0·90 ± 0·08 |
| 12 | Anthesis | 2·50 ± 0·31 | 2·00 ± 0·30 |
* The data presented are a raw mean between measurements taken from C. fissilis and C. odorata (ten flowers each).
The floral meristems are produced by the inflorescence meristem, obeying a pattern typical of cymose panicles (Fig. 3A). They are firstly detectable (stage 1) in the inflorescence meristem as a bulge of approx. 45 µm in diameter. At stage 2 (Fig. 3B), the flower meristems start to develop protective bract primordia. The terminal (central, usually female) flower of a given cymule differentiates two opposite bracts almost simultaneously and, later, a third bract is formed. The lateral (male) flowers first develop an abaxially located bract, and then two lateral bracts are formed (Fig. 3B).
Fig. 3.
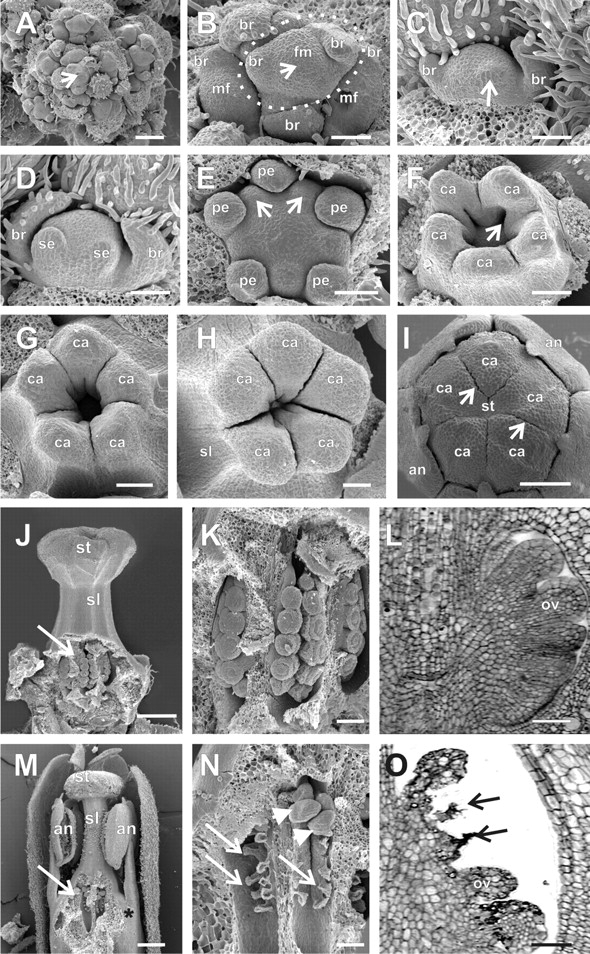
Floral development in Cedrela odorata. (A) The inflorescence meristem (arrow) is surrounded by floral meristems at different developmental stages. (B) A developing cymule (with flowers up to stage 2) is encircled by a dotted line. The arrow points to the floral meristem of the central (female) flower, where bract primordia are differentiating. This central meristem is flanked by two lateral opposing meristems, which will form male flowers. (C) Formation of the first abaxial sepal primordium (arrow) in stage 3 of a lateral (male) flower of a cymule. (D) Formation of the second abaxial sepal primordium in stage 3 of a lateral (male) flower of a cymule. The sepal primordia are formed unidirectionally from the abaxial to the adaxial side. (E) Formation of the stamen primordia (arrows) in stage 6, alternating with the petal primordia in a female flower. (F) Late stage 7 showing the initiation of the placenta as invaginations at the base of each carpel primordium (arrow). (G, H) The carpel primordia of female flowers are elongating and undergoing post-genital fusion of the terminal ends of carpels to form the style and stigma in early (G) or late (H) stage 10 flowers. (I) Late stage 11 female flower. The scar resulting from the post-genital fusion of the carpels remains visible on the surface of the stigma (arrows). (J, K) Partially dissected ovary of female flowers at late stage 10. (L) Longitudinal microtome section of the ovary of a stage 11 female flower, containing developing ovule primordia. (M) Partially dissected functionally male flower at stage 12. The arrow points to aborted ovules. The asterisk indicates the adnation of the base of stamen filament to the gynoecium. (N) Detail of a dissected ovary of a functionally male flower at the same stage as shown in M. A few well-formed ovules (arrowheads) are occasionally observed in ovaries of functionally male flowers, together with the aborted ones (arrows). (O) Longitudinal microtome section of the ovary of a functionally male flower at the same stage as in M and N. The arrows point to the degenerating ovule primordia. (M) Partially dissected functionally female flower at early stage 11. The arrow points to the developing ovules. an, anther; br, bracts; ca, carpel or carpel primordia; fm, female (central) floral meristem of a terminal cymule; mf, central floral meristem of a cymule adjacent to that pointed to by fm; ov, ovule primordia; pe, petal primordia; se, sepal primordia; sl, style; st, stigma. Scale bars: A–I = 250 µm; J, M = 500 µm; K, L, N, O = 10 µm.
The first floral organ to arise (stage 3) is an abaxial sepal primordium (Fig. 3C). Sepals are formed in a unidirectional manner, from the abaxial to the adaxial side (Figs 3D, 4A and 5A). When the second sepal primordium is initiated, trichomes start to differentiate on the abaxial epidermis of the protective bracts (Figs 3D and 4A). All sepal primordia reach a uniform size, despite their staggered initiation (stage 4), before the initiation of petal primordia (Fig. 4B). As the developing sepal primordia elongate and curve inwards towards the centre of the flower, five petal primordia simultaneously develop, alternating with the sepal primordia (stage 5; Figs 4C and D, and 5B and C).
Fig. 4.
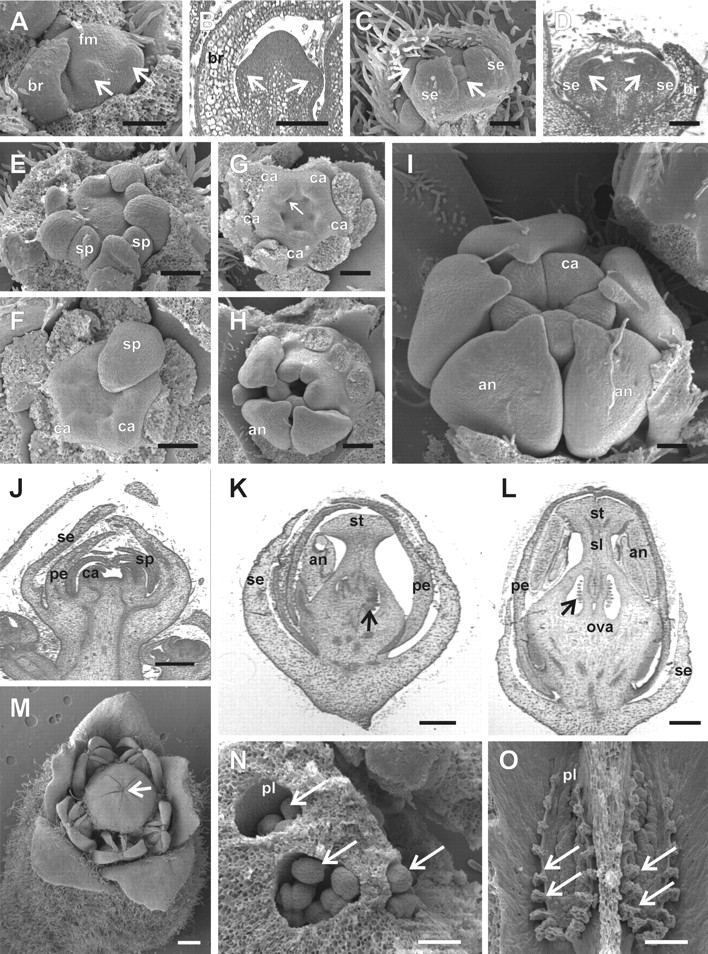
Floral development in Cedrela fissilis. In (E–I) the sepal, petal and/or part of the stamen primordia were removed for a better visualization of the inner structures. (A) Initiation of the abaxial sepal primordia (arrows) in a stage 3 meristem of a lateral male flower of a cymule in lateral view. (B) Longitudinal microtome section of a stage 3 floral meristem, where the abaxial (left arrow) and adaxial (right arrow) sepal primordia are differentiating. (C) Formation of petal primordia (arrows) in the second whorl, intercalary to the first whorl sepal primordia in stage 5. (D) Longitudinal section of a stage 5 flower meristem; the right and left arrows point to abaxial and adaxial petal primordia, respectively. (E) Formation of stamen primordia alternating with the petal primordia at stage 6. (F) Onset of carpel primordia alternating with the stamen primordia at early stage 7. (G) Stage 7, showing the initiation of the placenta as an invagination in the base of each carpel primordium (arrow). (H) Late stage 8, with carpel primordia elongating and undergoing post-genital fusion between carpels to form the ovary. (I) Late stage 10. As the carpels elongate, they displace the developing anthers. At the end of stage 10, the style and stigma begin to differentiate. (J) Longitudinal section of a terminal floral meristem at stage 7. (K) Longitudinal section of a stage 10 meristem of a functionally female flower. The arrow points to the developing ovule primordia. (L) Longitudinal section of a stage 11 male flower, with degenerating ovule primordia (arrow). (M) Stage 12, with a male flower at anthesis. The scars resulting from the post-genital fusion of the carpels remains visible (arrow). (N) Stage 11, female flower. Ovary locules with the top removed to expose developing ovule primordia (arrows). (O) Stage 12, male flower. Longitudinal view of the two exposed ovary locules (dorsal wall removed) showing degenerated ovules (arrows). an, anther; br, bracts; ca, carpel; fm, floral meristem; ova, ovary; pe, petal primordium; pl, placenta; se, sepal primordium; sp, stamen primordium; st, stigma; sl, style. Scale bars: A–D = 250 µm; E–G, M = 500 µm; K = 600 µm; L = 800 µm; N = 200 µm; O = 400 µm.
Fig. 5.
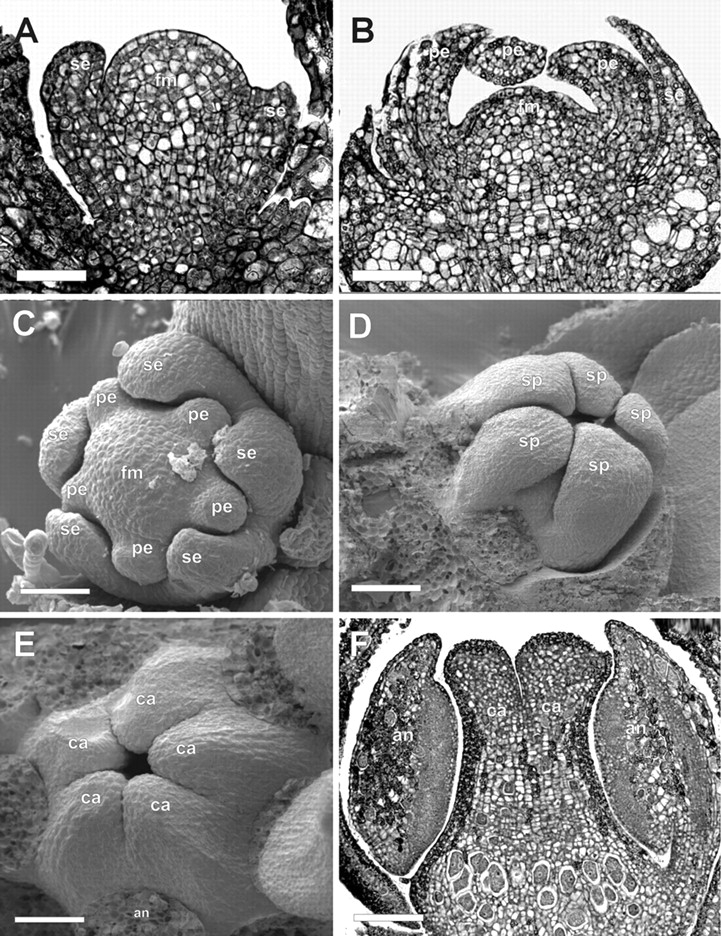
Floral development in Toona ciliata. (A) Longitudinal microtome section of a male flower meristem at stage 3. (B) Longitudinal section of a late stage 5 flower meristem. (C) Flower meristem at stage 5, with differentiating sepal and petal primordia. (D) Flower meristem at stage 9, showing the stamen primordia bending inwards over the developing carpel primordia. (E) Initiation of post-genital fusion between adjacent carpels in an early stage 9 female flower meristem. (F) Longitudinal section of a late stage 9 stage flower meristem. an, anther; br, bract; ca, carpel primordia; fm, flower meristem; pe, petal primordium; se, sepal primordium; sp, stamen primordium. Scale bars: A = 50 µm; B, D, F = 80 µm; C = 90 µm;E = 40 µm.
Five stamen primordia appear, alternating with the petal primordia (stage 6, Figs 3E and 4E). As the stamen primordia develop, they bend towards the centre of the flower, covering the developing carpel primordia (Figs 4F, I, J and 5D) and, later in flower development, the base of heir filaments become fused to the gynoecium (Fig. 3M).
Five carpel primordia are formed alternating with the stamen primordia (stage 7, Fig. 4F, G, J). As they develop, carpel primordia invaginate where the placenta will differentiate (Fig. 3F). The carpel primordia are initially free (Figs 3F and 4G), but undergo post-genital fusion between the carpels as they elongate, to form the syncarpous gynoecium (Figs. 3G–I; 4H, I and 5E). As the carpel primordia elongate upwards (stages 7–10), they push and displace the tips of the anthers that cover them during their development (compare Figs 4H, I and 5F). At the inception of stage 10, 5–8 ovule primordia develop on the placenta in each locule, alternating with each other in two lines (Figs 3J–L; 4K, L; 6A, B, E, F–H). The stigma of Cedrela and Toona is capitate, with the stigmatic papillae differentiation occurring at stage 11 on the undersurface (topographycally speaking) of the stigma (Fig. 6M, N).
Fig. 6.
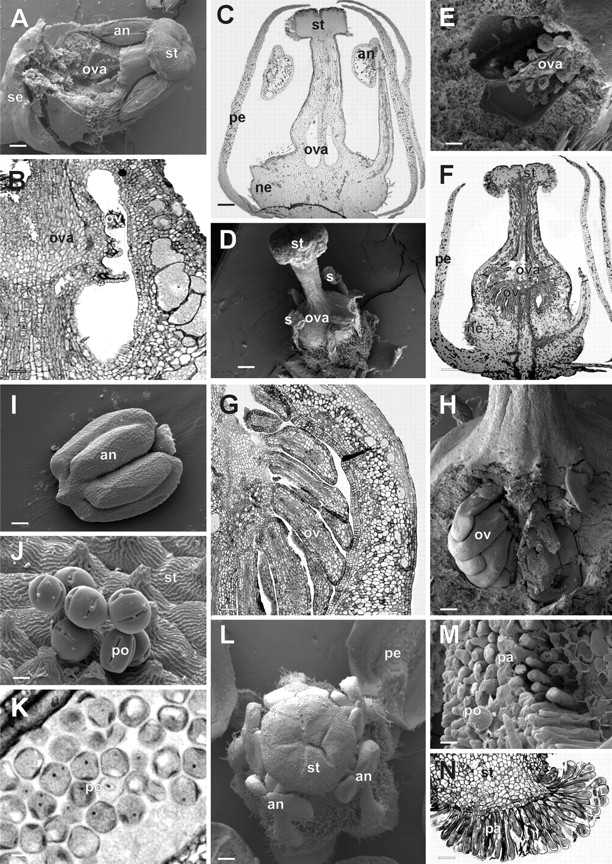
Floral development in Toona ciliata. Partially dissected (A, E) and longitudinal section (B, C) of functionally male flowers at late stage 11 to show the degenerated ovules, observed in detail in (B) and (E). Partially dissected (D, H) and longitudinal microtome section (F, G) of a functionally female flower at late stage 12. A detail of the developing ovules can be observed in (G) and (H). (I) Ventral view of an anther from a late stage 11 male flower. (J) Pollen grains on the outer (rolled out ventral) surface of the stigma of a male flower at anthesis. (K) Detail of a longitudinal section of an anther of a functionally male flower at anthesis, showing mature pollen grains. (L) Upper view of a partially dissected functionally male flower at stage 12 to show the relative positions of the anthers and the stigma. (M, N) Detail of the rolled out ventral surface of the discoid stigma, showing the stigmatic papillae (N is a detail of the longitudinal section shown in F). an, anther; ca, carpel primordia; ne, nectary; ova, ovary; ov, ovule primordium; pa, papillary cells; pe, petal; po, pollen grain; se, sepal; st, stigma. Scale bars: A, C, D, F = 200 µm; B = 50 µm; E = 30 µm; G = 100 µm; H, I = 80 µm; J, K = 5 µm; L = 300 µm; M = 15 µm; N = 3 µm.
Floral organ enlargement and sex differentiation in Cedreleae
With the completion of stage 7, all floral organ primordia have appeared and, from stage 8 onwards, organ elongation and enlargement takes place along with the typical organ-specific differentiation of cells. From stage 11 onwards, the functionally female and male flowers can be distinguished at the macroscopic level.
Most members of sub-family Swietenioideae produce inflorescences with both functionally female and male flowers. This was also observed in Cedrela and Toona during later stages of floral development. At stage 9, the microspore mother cells begin to differentiate within the pollen sacs of both the staminodes of functionally female flowers and the stamens of male flowers which are not distinguishable at this point. However, at the onset of stage 11, the microspores of functionally female flowers collapse and they stain green on microtome sections stained with toluidine blue (in contrast to the microspores of functionally male flowers which stain blue). This differential staining might reflect that secondary compounds, such as tannins, accumulate in the degenerating pollen grains of the female flowers. After stage 11, the anthers of female flowers appear ‘shrunken’ when compared with the anthers of male flowers (compare the anther shown in Fig. 6I with those of Fig. 6D). Eventually, a few pollen grains are formed in the anthers of female flowers, but they are generally deformed and ‘empty’ (data not shown), while those of male flowers are well formed, filled with dense cytoplasm, and cell nuclei may be observed (Fig. 6J, K). The stamen filaments of the male flowers continue to elongate from stage 11 through 12 in such a way that, at anthesis, the terminal portion of the anthers touches the stigmatic papillae (Figs 3I, M, 4M and 6L). However, the elongation of the stamen filaments is halted in functionally female flowers and the anthers do not surpass the base of the style in flowers at stage 12 (Fig. 6D).
Additionally, directly following ovule primordia initiation (stage 10), degeneration of these primordia occurs in male flowers, before the onset of stage 11 (Figs 3M–O, 4L, O and 6A–C, E). Although some ovules in these male flowers show apparent normal morphology (Fig. 3 N), histological sections showed different degrees of degeneration (compare Figs 3L–O and 6B, C with 6F, G).
DISCUSSION
The most comprehensive work on the taxonomy of Meliaceae at present is the generic monograph by Pennington and Styles (1975). Since the completion of this work, a considerable number of new insights (additional studies on morphological characters and molecular phylogenetic work) plus re-evaluations of this circumscription have taken place, which together have led to slight modifications in the classification of the Meliaceae (e.g. Mabberley, 1979; Cheek and Rakotozafy, 1991; Muellner et al., 2003). Among the four Meliaceae sub-families, the two largest, Swietenioideae and Melioideae, have been recognized for over a hundred years (for a review, see Pennington and Styles, 1975). However, their rank and circumscription have been frequently debated, and an additional sub-family, Cedreloideae, has been suggested by Harms (1940) based on morphological differences present in Cedrela and Toona. However, the creation of the sub-family Cedreloideae by Harms (1940) from Swietenioideae was based on a single known characteristic: the filaments of Cedreloideae flowers are free, while in the other genera of Swietenioideae they are united. Here it has been shown that many other important floral structural and developmental features of other tribes of Swietenioideae, such as multiovulate (biseriate) ovary locules and the discoid stigma, are also shared by Cedrela and Toona, not to mention the timing and patterns of floral organ development. It is thus found that the placement of these two genera in a separate sub-family is not justifiable. Moreover, phylogenies based on molecular data (Judd et al., 1999; Muellner et al., 2003) have recently shown that Meliaceae species are arranged in two large sub-families: Melioideae and Swietenioideae, with Quivisianthoideae embedded in the first and Capuronianthoideae embedded in the second. Additionally, based on these same phylogenies, Cedreleae is clearly monophyletic (Muellner et al., 2003). Thus, the resemblances in floral morphology and developmental patterns observed in Cedrela and Toona are not homoplasies.
The recent phylogeny of Meliaceae places Cedreleae as the basal clade within Swietenioideae, and this sub-family also occupies the basal position within the family (Muellner et al., 2003). This indicates that pentamerous flowers that are functionally either male or female may be considered to be basal traits for the family. Thus, reductions in organ number observed elsewhere in the family (e.g. four petals in Guarea and three carpels in Trichilia, both genera from the sub-family Melioideae) might be considered as apomorphies. On the other hand, the sympetalous flowers in Cedrela species is a synapomorphy for the genus, as this characteristic is not shared by Toona and is not observed in other genera within Meliaceae.
According to Pennington and Styles (1975), the androecium provides more characters of diagnostic importance in the classification of Meliaceae than any other organ series. The characteristic feature of the family is the partial or complete fusion of the filaments to form a staminal tube. It is considered that only Cedrela and Toona have consistently free filaments. Thus, the presence of free stamens might be considered a basal character for the family. However, the most basal genera of the Meliaceae are considered to be Chukrasia and Schmardaea (Muellner et al., 2003), and the flowers of both genera show prominent staminal tubes (Pennington and Styles, 1975). Accordingly, it was observed that the basal portions of the filaments actually show a variable degree of connation as well as of adnation to the petals and to the gynoecium both in Cedrela and in Toona (Figs 2A, B and 3M). These observations indicate that the presence of a staminal tube appeared early during the divergence of the family and that the partially free stamens in Cedreleae is a synapomorphy for the members of this tribe. Additionally, as the ontogenesis of the stamen primordia in Cedreleae indicates, the origin of the staminal tube in other species of Meliaceae might rely on the expansion of the connate zone of the filaments upwards to the whole structure. The urceolate staminal tube in Paseudocedrela seems to agree with this hypothesis as the anthers are inserted in the middle of the deltate teeth of the lobes (Pennington and Styles, 1975). Future observations of the morphogenesis of the staminal tube in Swietenieae, the sister tribe of Cedreleae, might help to confirm this supposition.
The presence of a floral nectary is a common feature among the Meliaceae (Pennington and Styles, 1975). Accordingly, a bright red discoid nectary is present at the base of the gynoecium in both male and female flowers of Toona (Figs 2D and 6C, F). Thus, another synapomorphy for the genus Cedrela is the absence of conspicuous nectaries.
The formation of a stalked structure at the base of the gynoecium in Cedrela is the product of the adnation of various floral organs (carpels and the base of the filaments) and is dependent on the late development of the stamens as it corresponds to more than one-third of the flower length in functionally male flowers (Fig. 2B), while it is much shorter in female flowers (Fig. 2A). This structure may be a synapomorphic feature of Cedrela, although similar structures were also observed for some members of the derived tribe Guareeae of sub-family Melioideae (e.g. in Guarea, Heckeldora and Ruagea; Pennington and Styles, 1975). Nevertheless, the ontogeny of this structure has not been described for these other genera.
Cedrela, Toona and Swietenia (C. F. Gouvêa et al., unpubl. res.) and probably all other genera of Swietenioideae share a similar inflorescence arrangement of flowers where the central flower of a cyme or of a three-flowered cymule is female, while the lateral flowers are male (Styles, 1972; C. F. Gouvêa et al., unpubl. res.). Sometimes, however, cymules may have only male flowers or, more rarely, may all be functionally female. Thus, the number of male flowers surpasses the number of functionally female flowers in all Cedrela taxa. This phenomenon has been observed over the 4-year study period and Styles (1972) reported similar findings for other Swietenioideae taxa in Africa and Australia, where it was observed that Swietenia mahogani trees usually have about ten times as many functionally male as female flowers. Additionally, non-synchronized opening of flowers was also observed, in that female flowers reach anthesis prior to male flowers. It has been reported that this prevalence of ‘maleness’ in many plant taxa is related to the ecological and energetic costs of bearing fruits and seeds, especially in extreme environments or in conditions of stress, therefore making it advantageous to disperse genes via pollen dispersal rather than by production of ovules and seeds. Clearly, this observation poses puzzling evolutionary problems as to how the equilibrium of sexes is reached to avoid extinction (Herlihy and Eckert, 2002; Tanurdzic and Banks, 2004). This is also important for tree breeders and for those interested in conservational orchard management. Species with functionally unisexual flowers located at very specific positions within an inflorescence may simplify the technique of emasculation in controlled-pollination experiments. This is especially interesting for species with difficult to reach, small-sized flowers such as those belonging to the Swietenioideae. Moreover, with the possibility of all individuals becoming prevalently ‘male’ in conditions of stress, it is noteworthy that the production of seeds may be seriously compromised during certain periods/years and this situation will influence the size and management of seed orchards, especially with the perspective of a changing global climate (Kerr, 2007).
The present results on the characterization of reproductive development in tribe Cedreleae contribute to the understanding of the reproductive biology of Meliaceae. The observations on the dynamics of the sex distribution within a single inflorescence may have consequences for the breeding as well as for the conservation and/or management of natural populations of these endangered tropical tree species.
ACKNOWLEDGEMENTS
We thank Dr T. C. Fonseca for help with sectioning, Professor G. Bandel for providing an excellent research environment, and Professor E. W. Kitajima, for maintaining the electron microscope facility NAP/MEPA (University of São Paulo, ESALQ, Piracicaba, Brazil). M.C.D. and A.P.M.R. acknowledge CAPES and CNPq (Brazilian government agencies), respectively, for research fellowships. All the authors acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support and a graduate studentship to C.F.G.
LITERATURE CITED
- Cavers S, Navarro C, Lowe AJ. Chloroplast DNA phylogeography reveals colonization history of a Neotropical tree, Cedrela odorata L., in Mesoamerica. Molecular Ecology. 2003;12:1451–1460. doi: 10.1046/j.1365-294x.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- Chase MW, Morton CM, Kallunki JA. Phylogenetic relationships of Rutaceae: a cladistic analysis of the subfamilies using evidence from rbcL and atpB sequence variation. American Journal of Botany. 1999;86:1191–1199. [PubMed] [Google Scholar]
- Cheek MR, Rakotozafy A. The identity of Leroy's fifth subfamily of the Meliaceae, and a new combination in Commiphora (Burseraceae) Taxon. 1991;40:231–237. [Google Scholar]
- Harms H. Meliaceae. In: Engler A, Prandtl K, editors. Die natuerlichen Pflanzenfamilien. Leipzig: Engelmann; 1940. pp. 1–172. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Judd WS, Campbell CS, Kellog EA, Stevens PF. Plant systematics, a phylogenetic approach. Sunderland, MA: Sinauer Associates; 1999. [Google Scholar]
- Keay RWJ. The future of the genus Swietenia in its native forest. Botanical Journal of the Linnean Society. 1996;122:3–7. [Google Scholar]
- Kerr RA. Climate change: global warming is changing the world. Science. 2007;316:188–190. doi: 10.1126/science.316.5822.188. [DOI] [PubMed] [Google Scholar]
- Mabberley DJ. The species of Chisocheton (Meliaceae) Bulletin of the British Museum (Natural History), Botany Series. 1979;6:301–386. [Google Scholar]
- Mabberley DJ, Pannell CM, Sing AM. Meliaceae. Flora Malesiana Series I. 1995;12:1–407. [Google Scholar]
- Muellner AN, Samule R, Johnson SA, Cheek M, Pennington TD, Chase MW. Molecular and phylogenetics of Meliaceae (Sapindales) based on nuclear and plastid DNA sequences. American Journal of Botany. 2003;90:471–480. doi: 10.3732/ajb.90.3.471. [DOI] [PubMed] [Google Scholar]
- Navarro C, Ward S, Hernandez M. The tree Cedrela odorata (Meliaceae): a morphologically subdivided species in Costa Rica. Revista Biologia Tropical. 2002;50:21–29. [PubMed] [Google Scholar]
- Newton AC, Cornelius JP, Mesen JF, Leakey RRB. Genetic variation in apical dominance of Cedrela odorata seedlings in response to decapitation. Silvae Genetica. 1995;44:146–150. [Google Scholar]
- Newton AC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA. Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends in Evolution and Ecology. 1999;14:140–145. doi: 10.1016/s0169-5347(98)01555-9. [DOI] [PubMed] [Google Scholar]
- O'Neill GA, Dawson I, Sotelo-Montes C, Guarino L, Guari-Guata M, Current D, Weber JC. Strategies for genetic conservation of trees in the Peruvian Amazon. Biodiversity and Conservation. 2001;10:837–850. [Google Scholar]
- Pennington TD, Styles BT. A generic monograph of the Meliaceae. Blumea. 1975;22:419–540. [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in. Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles BT. The flower biology of the Meliaceae and its bearing on tree breeding. Silvae Genetica. 1972;21:175–182. [Google Scholar]
- Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. The Plant Cell. 2004;16:S61–S71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verissimo A, Junior CS, Stone S, Uhl C. Zoning of timber extraction in the Brazilian Amazon. Conservation Biology. 1998;12:128–136. [Google Scholar]