Abstract
Background and Aims
Secondary growth via successive cambia has been intriguing researchers for decades. Insight into the mechanism of growth layer formation is, however, limited to the cellular level. The present study aims to clarify secondary growth via successive cambia in the mangrove species Avicennia marina on a macroscopic level, addressing the formation of the growth layer network as a whole. In addition, previously suggested effects of salinity on growth layer formation were reconsidered.
Methods
A 1-year cambial marking experiment was performed on 80 trees from eight sites in two mangrove forests in Kenya. Environmental (soil water salinity and nutrients, soil texture, inundation frequency) and tree characteristics (diameter, height, leaf area index) were recorded for each site. Both groups of variables were analysed in relation to annual number of growth layers, annual radial increment and average growth layer width of stem discs.
Key Results
Between trees of the same site, the number of growth layers formed during the 1-year study period varied from only part of a growth layer up to four growth layers, and was highly correlated to the corresponding radial increment (0–5 mm year–1), even along the different sides of asymmetric stem discs. The radial increment was unrelated to salinity, but the growth layer width decreased with increasing salinity and decreasing tree height.
Conclusions
A patchy growth mechanism was proposed, with an optimal growth at distinct moments in time at different positions around the stem circumference. This strategy creates the opportunity to form several growth layers simultaneously, as observed in 14 % of the studied trees, which may optimize tree growth under favourable conditions. Strong evidence was provided for a mainly endogenous trigger controlling cambium differentiation, with an additional influence of current environmental conditions in a trade-off between hydraulic efficiency and mechanical stability.
Key words: Avicenia marina, cambial marking, mangrove, phloem, salinity, secondary growth, successive cambia, xylem
INTRODUCTION
One of the curious mechanisms of plant growth is secondary wood formation via successive cambia (Wheeler et al., 1989). This means that each growth layer is formed by a new cambium and hence is composed of both xylem and phloem tissue. The cambial variant is especially present in lianas (e.g. Nair, 1993; van Veenendaal and den Outer, 1993; Carlquist, 1996, 1999a; Jacques and De Franceschi, 2007), but is also reported in woody shrub and tree species, with descriptions of the wood anatomy (McDonald, 1992; Carlquist and Gowans, 1995; Carlquist, 1996, 1999b, 2002, 2003a, b) and wood formation (Wheat, 1977; Zamski, 1979, 1981; Fahn and Zimmermann, 1982; Nair and Mohan Ram, 1990; Terrazas, 1991; den Outer and van Veenendaal, 1995; Rajput and Rao, 1999; Carlquist, 2004; Schmitz et al., 2007). However, it remains unclear if the growth layers, originating from the combined action of the several cambia, are formed periodically or continuously, and which factors trigger the formation of a new growth layer.
The mangrove genus Avicennia belongs to this exclusive group of trees showing secondary growth via successive cambia (Schmitz et al., 2007). Early studies speculated on the periodicity of the conspicuous pattern of light- and dark-coloured bands resulting from the alternating xylem and phloem tissue (Baker, 1915; Chapman, 1944, 1947; Gill, 1971). A preliminary study on six Avicennia marina trees in Gazi Bay, Kenya showed the non-annual nature of their growth layers (Schmitz et al., 2007). However, this study suggests that within one site a constant number of growth layers may form per year. While only half a growth layer was formed in a site of high salinity, an average of three growth layers was formed in a site with relatively lower salinity (Schmitz et al., 2007). Therefore, the formation of growth layers seems to be influenced by local environmental factors such as soil water salinity rather than by seasonal climate. In addition, the proportion of phloem tissue increased slightly in parallel with the salinity of the site (Schmitz et al., 2007). In agreement with these findings, Carlquist (2001) and Fahn and Shchori (1967) assume that dispersed phloem tissue over the entire stem offers a functional advantage to trees growing under xeric conditions.
Avicennia tree species tolerate a wide range of soil water salinity levels, related to different inundation frequencies and evaporation of the substrate (Matthijs et al., 1999; Dahdouh-Guebas et al., 2004; Ye et al., 2005; Naidoo, 2006; Sobrado and Ewe, 2006). To study the formation of growth layers, a technique with cellular resolution is offered by mechanically wounding the cambium, leaving a datable scar (Kuroda and Shimaji, 1984). Thanks to the distinct time mark, the cambial mark, the presence of clear tree rings becomes superfluous in studies of wood formation, making the method especially useful for the study of tropical tree species (Détienne, 1989; Nobuchi et al., 1995; Sass et al., 1995; Jalil et al., 1998; Bauch and Dünisch, 2000; Ohashi et al., 2001; Heinrich and Banks, 2002), including mangrove trees (Shiokura, 1989; Verheyden et al., 2004; Schmitz et al., 2007).
In the present study, the cambium of 80 A. marina trees was wounded. The trees were sampled from seven sites in Gazi Bay and one more distant location in Dabaso, both situated in Kenya. The aim was to clarify the secondary growth mechanism of A. marina characterized by successive cambia. This study tested the hypotheses that (a) growth layer formation is periodic, with the number of growth layers formed per year depending on the soil water salinity of the site; and (b) the width of the growth layers decreases with increasing soil water salinity.
MATERIALS AND METHODS
Study sites and sample collection
Wood samples were collected in Gazi and Dabaso, two locations along the Kenyan coast. The mangrove forest of Gazi Bay (39°30'E, 4°25'S) covers about 710 ha (UNEP, 2001) and is situated approx. 50 km south of Mombasa. Sampling was performed in seven sites (Table 1), differing in local environmental conditions (Table 2). For comparison, an additional set of trees was sampled in Dabaso (39°21'E, 03°59'S), in the 1500 ha large mangrove forest of Mida Creek situated about 100 km north of Mombasa (Gang and Agatsiva, 1992). In the rainy season of 2005 and 2006 (May–June) and the dry season of 2006 and 2007 (February–March) soil water was collected at approx. 25 cm depth with a punctured plastic tube connected to a vacuum pump. At each site, 1–3 salinity measurements were carried out with a hand-held refractometer (ATAGO, Tokyo, Japan). One day after spring tide in February 2007, soil water was collected in triplicate at the seven study sites in Gazi Bay. From these water samples, NO3−, NH4+ and P concentrations were measured by standard procedures (APHA-AWWA-WEF, 1995) and expressed relative to soil water content, which was calculated from the fresh and oven-dry weight. Soil texture was determined by standard field characterization methods (GLOBE, 2005). The height above datum was measured with tracing paper, placed at each site before high tide, and the corresponding inundation classes were calculated. According to Tomlinson (1994), inundation classes one, two, three and four correspond to an area being inundated by 100–76, 75–51, 50–26 and 25–5 %, respectively, of the high tides. The leaf area index (LAI), integrated over the zenith angle 0–75 °, was calculated from hemispherical images using the software program Gap Light Analyzer v. 2·0 (Simon Fraser University, British Columbia and the Institute of Ecosystem Studies, New York).
Table 1.
Studied stem discs and outermost wood blocks of Avicennia marina with the corresponding tree characteristics for the eight study sites at two locations in Kenya, Gazi and Dabaso
| Accession no. |
|||||
|---|---|---|---|---|---|
| Location | Discs | Samples | Tree height (m) | R130* (cm) | Rbase† (cm) |
| Gazi | |||||
| Site 1 | Tw58910–13, Tw58927–31 | T72, T76, T85 | 11 (7–14) | 4 (1–13) | 43 (20–100) |
| Site 2 | Tw58905–09, Tw58926, Tw58988 | T40–41 | 3 (2–4) | 2 (1–3) | 9 (5–17) |
| Site 3 | Tw58916–18, Tw58933–35 | T26–28 | 2 (2–4) | 1 (1–3) | 7 (3–11) |
| Site 4 | Tw58943–48, Tw58987 | T14–16 | 6 (4–8) | 3 (2–5) | 17 (5–54) |
| Site 5 | Tw58936–42 | T45, T51, T56 | 6 (6–6) | 3 (1–5) | 7 (3–10) |
| Site 6 | Tw58900–04, Tw58932 | T35,T37 | 4 (2–5) | 3 (1–4) | 6 (6–14) |
| Site 7 | Tw58920–25, Tw58989 | T19-22 | 6 (3–10) | 4 (1–9) | 22 (4–44) |
| Dabaso | |||||
| Site 8 | – | Tw58950–61 | 10 (6–14) | 11 (6–16) | 36 (16–100) |
Values are means with ranges in parentheses.
* Stem radius at 130 cm height.
† Stem radius at the base of the tree.
Table 2.
Stand characteristics of seven study sites in Gazi Bay and one study site in Dabaso, Kenya
| Soil water nutrients (10–3 μmol cm–3 soil) |
Soil water salinity (‰) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Location | NO3− | NH4+ | P | LAI | Soil texture | Minimum | Maximum | Inundation class |
| Gazi | ||||||||
| Site 1 | 0·5 (0·2–0·6) | 3 (0–5) | 1·4 (0·9–1·8) | 1·42 | Silty clay | 20·9 | 46·0 | 1 |
| Site 2 | 0·3 (0·2–0·5) | * | 0 (0–0) | 0·25 | Sandy loam | 40·0 | 70·0 | 2 |
| Site 3 | 0·15 (0·10–0·24) | * | 0·2 (0·1–0·3) | 0·23 | Loamy sand | 38·0 | 86·0 | 3 |
| Site 4 | 0·3 (0·0–0·4) | 1 (0–4) | 2 (0–4) | 1·29 | Loamy sand–sandy loam | 5·0 | 68·2 | 3 |
| Site 5 | 2 (0–6) | 10 (7–14) | 0·1 (0–0·3) | 1·18 | Clay loam | 40·0 | 79·9 | 3 |
| Site 6 | 1 (0–4) | 0·5 (0·1–1·1) | 0·1 (0–0·3) | 0·62 | Sandy loam | 10·0 | 90·0 | 4 |
| Site 7 | 23 (0–68) | * | 2 (0–4) | 1·73 | Loamy sand–sandy loam | 10·0 | 48·0 | 4 |
| Dabaso | ||||||||
| Site 8 | * | * | * | * | * | 33·0 | 72·2 | 3 |
Soil water for nutrient and salinity analyses was taken at about 25 cm depth. Inundation classes are according to Tomlinson (1994). Values are means with range in parentheses.
* No data records.
The cambial marking was performed with a surgical needle of 1·2 mm diameter (Verheyden et al., 2004) in May 2005 (wet season). In view of a potential time mark of the dry season by changes in wood anatomy, the trees were marked a second time in February 2006 (dry season). For two trees (Tw58955, -57) the cambium of both the stem and one branch was wounded. Of these 80 trees, 49 were felled in June 2006 and samples are now part of the Xylarium of the Royal Museum for Central Africa, Tervuren, Belgium (discs, Table 1). From the remaining 31 trees, wood samples were taken with a handsaw of the outermost wood comprising the cambial mark, and 19 of them were subsequently stored in FAA (formalin–acetic acid–alcohol). Before felling or sampling of the trees, the stem circumference at 130 cm height and the stem diameter at the base were measured. The height was calculated trigonometrically.
Climate description
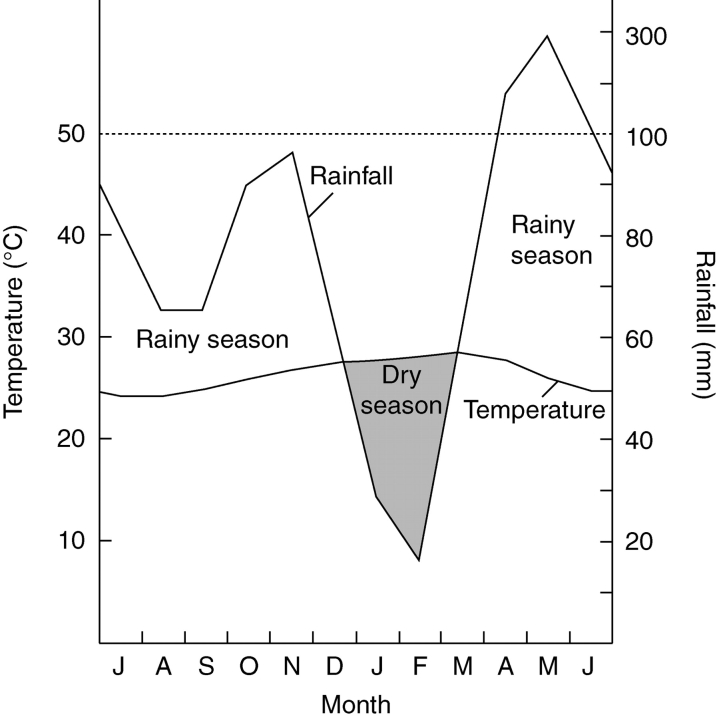
The climate along the Kenyan coast is characterized by a bimodal distribution of precipitation. A distinct dry season (January–February) is followed by a long (April–July) and a short rainy season (October–November; Fig. 1). During the wet season, the rivers Mkurumuji and Kidogoweni provide an important freshwater source for the mangroves of Gazi Bay (Kitheka, 1997). The average temperature at the Kenyan coast ranges from 22 to 30 °C, with a mean relative humidity of 65–81 % (annual averages of minima and maxima for Mombasa for the period 1972–2001, data from the Kenyan Meteorological Department, Mombasa, Kenya).
Fig. 1.
Climate diagram of Mombasa (39°36'E, 4°0'S) adapted from Lieth et al. (1999), showing the long (April–July) and short (October–November) rainy season and one distinct dry season (January–February). The precipitation axis is reduced to one-tenth scale above the dotted horizontal line.
Sample preparation and microscopic analysis
In this study, the following terms and definitions are used: phloem band designates a zone of phloem strands united in a band of parenchyma tissue and bordered on the outside by a layer of sclereids; xylem band designates the zone in between two phloem bands; and growth layer designates one ontogenetic unit of phloem and xylem (Figs 2 and 3).
Fig. 2.
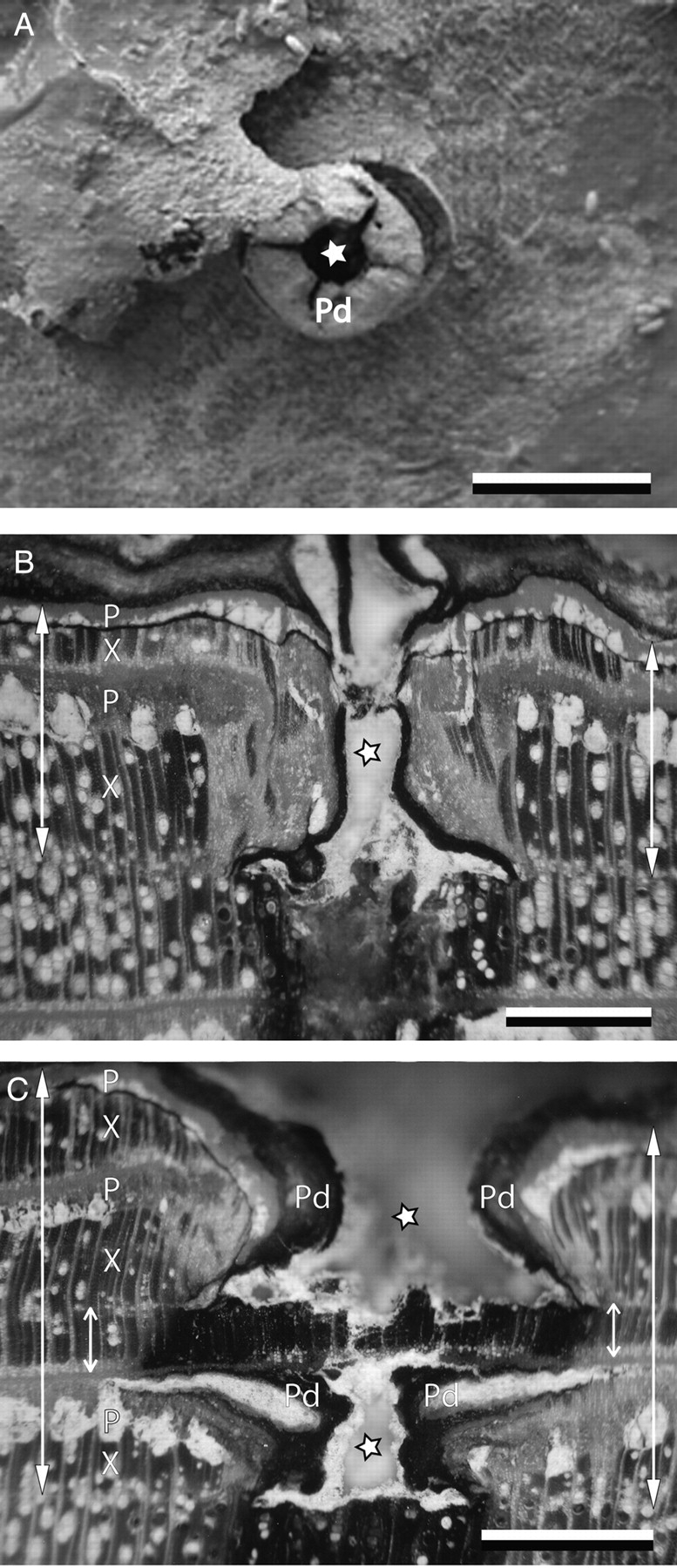
Macro- and microscopic characteristics of Avicennia marina stem wood after wounding the cambium with a needle of 1·2 mm diameter. (A) Pinhole encircled by wound periderm, scale bar = 1 cm. (B, C) Detail of sanded stem discs showing (B) a decreased vessel density and vessel diameter in the zone around the pinhole, marking the time of cambial wounding, and (C) a double cambial wound illustrating the simultaneous formation of the two growth layers. Scale bars = 1 mm. P, phloem band; Pd, periderm; X, xylem band. Arrows, radial increment from May 2005 to June 2006. Small arrows, part of the growth layer already formed at the time of cambial wounding. Asterisks, pinhole.
Fig. 3.
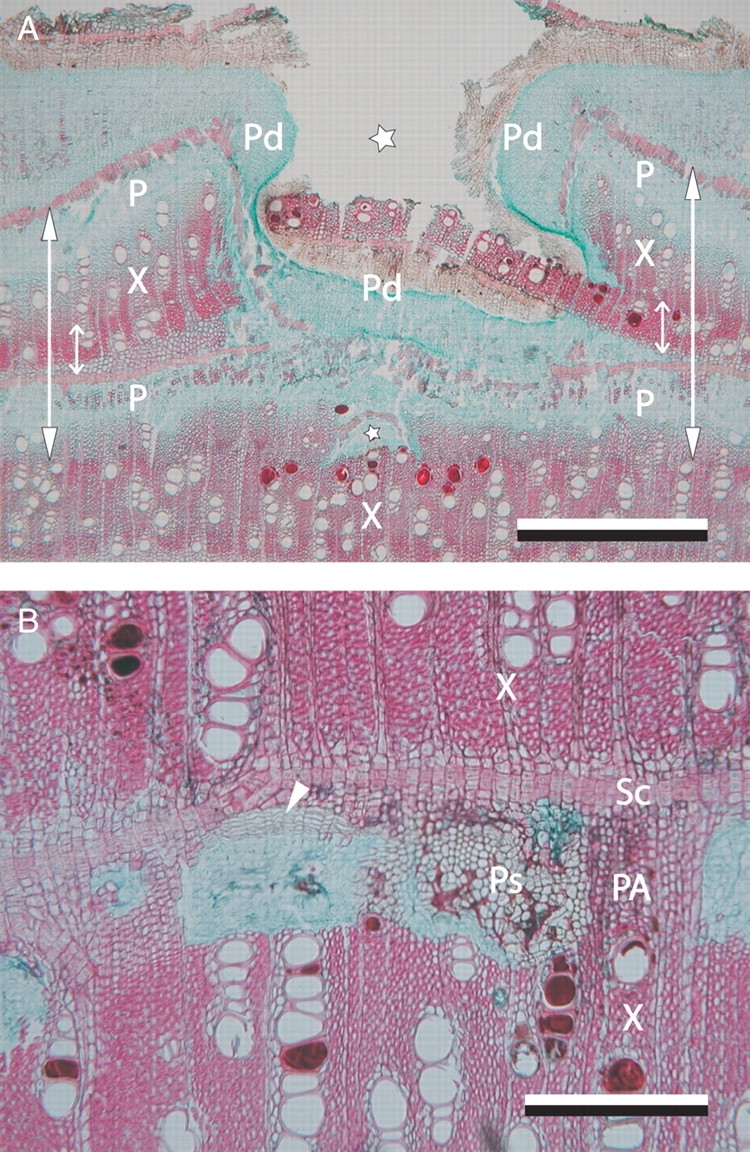
Transverse microsections of the stem wood of Avicennia marina after wounding the cambium with a needle. (A) Microscopic wood structure after wounding of the two outermost cambia. Scale bar = 1 mm. (B) Detail of two phloem strands located inward from the pinhole showing a coating of the cells and an induced meristem (arrowhead) in the right and left phloem strand, respectively. Scale bar = 500 µm. P, phloem band; PA, parenchyma; Pd, periderm; Ps, phloem strand; Sc, sclereids; X, xylem band. Arrows, radial increment from February 2006 to June 2006. Small arrows, part of the growth layer already formed at the time of cambial marking. Asterisks, pinhole.
To investigate the periodicity of growth layer formation, the number of growth layers formed in the period from17–26 May 2005 to 9 June–9 July 2006 was counted from 79 cambially marked trees. Wood discs and wood samples were prepared as described by Schmitz et al. (2007). In brief, wood discs were sanded with a series of sandpaper from 100 to 1200 grit, while wood samples were dehydrated in an ethanol series and embedded in PEG1500. Transverse sections were made with a sliding microtome and double stained with safranin–fast green. The number of growth layers from the cambial mark onwards was counted using an Olympus BX 60 microscope. This was done at both sides of the wound and the growth layer count was averaged (Figs 2B, C and 3A). The growth layer network of one stem, Tw57798 (see also Schmitz et al., 2007), was visualized with a CT scan (Fig. 4; CT-scan Brilliance 64 slice, Philips, The Netherlands).
Fig. 4.
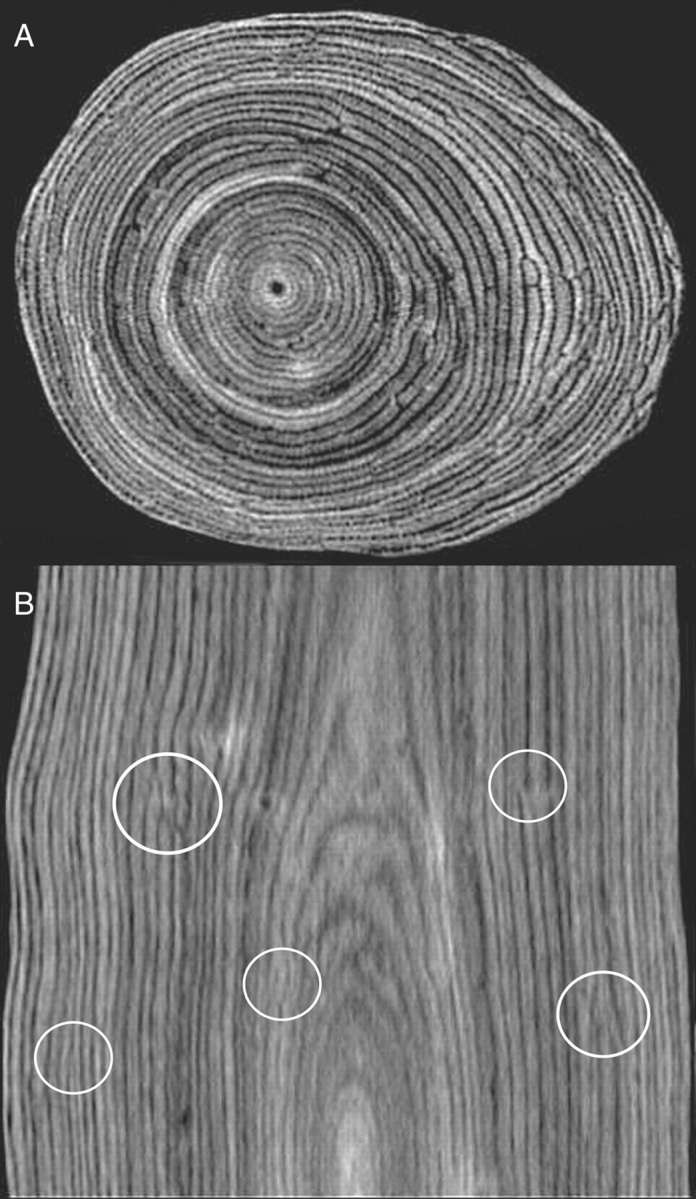
Networking pattern of the growth layers in the (A) transverse and (B) longitudinal plane as visualized by a CT scan of a tree stem portion of 7 cm diameter (Tw57798, Tervuren xylarium). Circles indicate the positions of branching phloem bands.
In addition, the increment during the period of the cambial marking experiment was measured with the image analysis software AnalySIS Pro 3·2 (Soft Imaging System GmbH, Münster, Germany) via a camera connected to a microscope (Olympus BX60). The increment was measured at about 2 mm distance from the abnormal growth at the wound, which is the furthest position showing a cambial mark. For trees showing a double wound, resulting from two simultaneously dividing cambia, the increment was corrected for the part of the xylem tissue of the outermost growth layer already formed at the time of wounding (Figs 2C and 3A).
On each of 48 stem discs, the width of the xylem and phloem bands of each growth layer was measured along three transects from pith to bark at the maximum, minimum and medium sized disc radius. Because of the networking pattern of the growth layers, a pencil line was drawn and all growth layers crossing the line were measured.
Statistics
To quantify relationships between different tree and growth layer characteristics, Pearson correlation coefficients were calculated. Simple linear regressions were used to study relationships in growth increment with environmental and tree characteristics. One-way analyses of variance (ANOVAs) were used to test (a) the difference in radial increment between the different study sites and (b) the relationship between the average ring width on different sides of asymmetric stem discs and corresponding radii. A t-test for independent variables was performed to compare the growth increment at opposite radii of asymmetric trees and between trees with and without signs of die-back (with separate variance estimates). To comply with the assumptions of a normal distribution of the data and homogenous variances, data were transformed with either logarithmic or square root functions. If transformations failed, Spearman R correlation coefficients were calculated (STATISTICA, StatSoft Inc.).
RESULTS
Wood anatomical description of the cambial mark
On a macroscopic level, the pinhole was regularly encircled by an extra periderm (Fig. 2A). Microscopically, the position of the cambium at the time of wounding could be determined from a variable combination of wood anatomical changes (Figs 2B and C, and 3). In agreement with earlier reports, the time mark was formed by an initial parenchyma band in 53 % of the trees (n = 80) (Shiokura, 1989; Nobuchi et al., 1995; Ohashi et al., 2001; Stobbe et al., 2002) and relatively smaller vessels (Nobuchi et al., 1995; Schmitt et al., 2000; Ohashi et al., 2001) at a lower density (Carlquist, 2001) in an area of about 1 mm2 on both sides of the pinhole. The canal created by wounding was closed by periderm (Carlquist, 2001; Stobbe et al., 2002), with an extra internal wound periderm in 14 % of the studied trees (n = 79) showing a double wound (Figs 2C and 3A). Regularly, a coating that stained pink with safranin was observed in the phloem strands inward from the canal caused by wounding (Fig. 3B). Suberin has been mentioned to coat parenchyma tissue of the reaction zone (Schmitt and Liese, 1993). It is known as a barrier-forming agent protecting against invading pathogens (Franke and Schreiber, 2007) and necrosis of the deeper lying tissues (Schmitt and Liese, 1993). In addition, in 53 % (n = 19) of the microsections, the phloem strands showed a meristematic zone (Fig. 3B), corresponding to the finding in poplar of secondary phloem cells dedifferentiating into meristematic cambium cells (Frankenstein et al., 2005; Frankenstein, 2006). Finally, a dark zone of protection wood (Carlquist, 2001) was observed inward from the wound canal (Fig. 2B, C) with partly occluded vessels and fibres (Schmitt et al., 2000).
Annual increment
From only a tenth of a growth layer up to four completed growth layers was formed in the period of the cambial marking experiment from May 2005 to June 2006 (Fig. 5, Table 3). The number of growth layers formed during the 1-year study period was highly correlated with the corresponding radial increment (r2 = 0·73, n = 73, P < 0·0001). Also at the level of the entire stem disc, the number of growth layers showed a strong correlation with its radius (r2 = 0·89, n = 141, P < 0·0001). In agreement with this high correlation, the difference between the maximum and minimum disc radius of asymmetrical stem discs was correlated with the corresponding difference in number of growth layers (r2 = 0·37, n = 42, P < 0·0001). Growth layer width increased from a minimum, through a medium to a maximum disc radius (F = 50·79, n = 2663, P < 0·0001; linear trend analysis: F = 101·57, n = 2663, P < 0·0001).
Fig. 5.
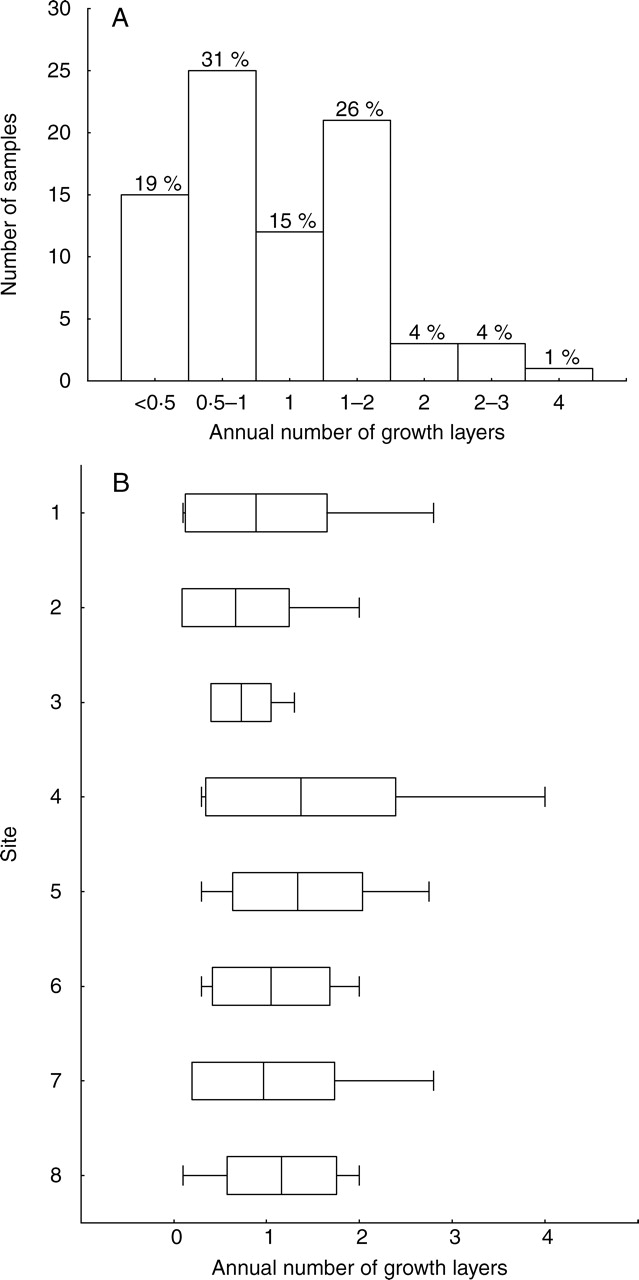
Number of growth layers of 79 Avicennia marina trees formed in the period from May 2005 to June 2006 (A), distribution of the annual growth layer number over the eight study sites (B) (see also Table 2). Line, mean; box, s.d.; whiskers, min.-max.
Table 3.
Characteristics of the secondary growth of Avicennia marina averaged over the 1-year study period between May 2005 and June 2006 (n = 80) or averaged over the entire stem disc (n = 49)
| Growth characteristic | Mean ± s.d. | Minimum | Maximum |
|---|---|---|---|
| 1-year study period | |||
| Growth rate (mm year−1) | 1 ± 1 | 0 | 5 |
| Included growth* (μm year−1) | 249 ± 105 | 64 | 376 |
| Growth layers (nr year−1) | 1·0 ± 0·7 | 0·1 | 4 |
| Disc average | |||
| D(M – m) (nr) | 4 ± 3 | 0 | 12 |
| Growth layer width (μm) | 898 ± 137 | 402 | 1564 |
| Xylem width (µm) | 657 ± 107 | 257 | 1198 |
| Phloem width (µm) | 241 ± 35 | 117 | 403 |
| % Phloem growth layer−1 | 28 ± 2 | 20 | 44 |
D(M – m), difference in number of growth layers between the minimum and maximum disc radius; nr, number of growth layers.
* Growth of the last but one growth layer (n = 10, see Figs 2C and 3A).
No significant difference was observed in the number of growth layers formed in the 1-year study period between the eight study sites (Fig. 5B; F = 1·48, n = 79, P = ns). By analysing different environmental factors separately, characterizing the different study sites, no or only very weak relationships were found with the radial increment of the study period (Table 4). Also stem radius at the sampling height of the trees and tree height showed a weak but significant correlation with the radial increment measured from the cambial markings onwards (Table 4). The growth rate during the 1-year study period was significantly larger in trees showing a double cambial mark, resulting from the additional radial increment of the last but one growth layer (Figs 2C and 3A; t = –3·2, n1 = 62 and n2 = 11, P < 0·01). The cambium of this inner growth layer produced on average 249 ± 105 µm (n = 10) of xylem tissue during the 1-year study period (Table 3). Within trees Tw58955 and Tw58957, radial growth was 370 and 1065 % higher, respectively, in branches compared with stems. Trees without dead leaves or branches had a significantly larger growth increment compared with trees with dead leaves and/or branches dispersed in the crown (t = –3·68, n1 = 15 and n2 = 57, P < 0·01). The growth from the cambial mark in February 2006, the middle of the dry season, until the felling in June 2006, the end of the long rainy season, was mostly small and restricted to the zone around the wound, hindering relevant measurements and study of potential wood anatomical changes in the dry season.
Table 4.
Results of simple linear regressions for the May 2005–June 2006 growth increment of Avicennia marina trees of different size and growing under different environmental conditions (see Table 1)
| Factor | F-value | P-value | n | r2‡ |
|---|---|---|---|---|
| Environment | ||||
| Minimum salinity | 3·00 | ns | 73 | 0·03 |
| Maximum salinity | 0·04 | ns | 73 | −0·01 |
| Inundation class | 1·47 | ns | 73 | 0·01 |
| [NO3−] | 1·11 | ns | 62 | 0·00 |
| [NH4+] | 0·02 | ns | 37 | –0·03 |
| [P] | 1·09 | ns | 54 | 0·00 |
| LAI | 6·05 | <0·05 | 62 | 0·08 |
| Tree | ||||
| R130* | 16·87 | <0·001 | 73 | 0·18 |
| Rb† | 0·34 | ns | 73 | −0·01 |
| Height | 9·13 | <0·01 | 73 | 0·10 |
Data were transformed with a logarithmic function.
* Stem radius at 130 cm height.
† Stem radius at the base.
‡ Adjusted correlation coefficient.
Growth layer characteristics
The 2005–2006 growth increment was significantly correlated with the average growth layer width of a stem disc (r2 = 0·31, t = 4·37, n = 44, P < 0·0001). The average growth layer width was strongly correlated with tree height (r2 = 0·43, F = 36·35, n = 47, P < 0·0001) and weakly but significantly with soil water salinity, showing a higher correlation with maximum salinity (r2 = 0·16, F = 9·60, n = 47, P < 0·01) than with minimum salinity (r2 = 0·10, F = 5·97, n = 47, P < 0·05). No correlation was observed between growth layer width and age, represented by growth layer number, with the first growth layer at the pith having number 1 and the last growth layer at the bark having the highest number (partial correlation, controlling for tree: r2 = 0, t = 0·0067, n = 2663, P = ns). The width of a xylem band showed a strong positive correlation with the phloem band width of the same growth layer (Table 3, r2 = 0·53, t = 54·78, n = 2663, P < 0·0001). However, the increase in phloem width was only 22 % of the corresponding xylem width, resulting in a negative relationship between the percentage of phloem and the growth layer width (Spearman r2 = 0·14, n = 2663, t = –21·26, P < 0·0001) with a lower limit at 20 % phloem per growth layer (Table 3).
DISCUSSION
The patchy growth hypothesis
The first hypothesis of a periodic growth with the number of growth layers produced during the year depending on site conditions was rejected. Instead, a patchy growth mechanism is proposed based on circumstantial evidence: (a) a network pattern of growth layers (Fig. 4); (b) two simultaneously forming growth layers without the occurrence of wood cracks or crushed phloem tissue indicating the absence of internal strains (Figs 2C and 3A); (c) a remarkable variation in growth layer formation within the different study sites (Fig. 5B); and (d) an extensive variation of the radial increment, seemingly independent of any of the environmental factors considered (Tables 3 and 4). The patchy growth hypothesis states a basic growth rate all around the tree,with a more vigorous growth at one or several positions around the stem circumference. The side of active growth changes with time to end up with a tree of regular circumference. A patchy growth would clarify the three-dimensional phloem network, first reconstructed from serial branch microsections by Zamski (1979) and here for the first time visualized in situ from a mature tree trunk. Not only around (Fig. 4A) but also along the stem (Fig. 4B), growth patches instead of rings were observed. Nair and Mohan Ram (1990) propose a similar explanation for the growth layer network of Dalbergia paniculata, resembling that of A. marina. They suggest it could be due to the outermost parenchyma cells that do not invariably become meristematic. More recently, discontinuous new cambia have been observed in some species of Amaranthaceae (Rajput and Rao, 2000) and a liana species of the Menispermaceae (Jacques and De Franceschi, 2007).
The earlier suggestion of a simultaneous development of two growth layers (Schmitz et al., 2007) was illustrated by a double cambial mark (Figs 2C and 3A). Cracks in the lignified part of the outermost growth layer can be overcome by collapse of phloem cells (Carlquist, 2007). Supporting the patchy growth hypothesis, this was not observed in the present study nor in A. resinifera and A. germinans in which at least the four outer phloem bands were uncrushed (Zamski, 1979). Moreover, the finding of 14 % (n = 79) of the trees with more than one active cambium could be an underestimation. The depth of wounding was not standardized because the depth needed to wound both cambia varies with the width of the outer growth layer. Recently, more than one active cambium was observed in the herbaceous and shrubby plants of Aizoaceae (Carlquist, 2007), questioning the existence of two mechanisms for successive cambia formation. Unlike Avicennia (Zamski, 1979) and some other species (Esau, 1969), Carlquist (1999a, b, 2003a, 2004) found in members of the Caryophyllales successive cambia originating from one lateral meristem in the cortex that also produces the rays and the tissue in between the phloem strands. This would be hindered by the partially formed outer growth layer in between the lateral meristem and the inner forming growth layer. Moreover, the patchy differentiation of new cambia makes the existence of one circular meristem, giving rise to the vascular cambia, unlikely in A. marina.
Secondary growth in discrete units would explain the variation in growth between A. marina trees (Fig. 5). On the side of wounding, the cambial activity and the duration of active growth during the study period would determine the number of growth layers produced and the annual radial increment. Therefore, radial increment was not a reflection of past environmental conditions (Table 4). However, the total increase in stem width is expected to be influenced by external factors such as soil water nutrients (Feller et al., 2003; Lovelock et al., 2004), salinity (Lin and Sternberg, 1992; Tuffers et al., 2001; Ball, 2002) and inundation periods (Worbes, 1989; Callado et al., 2001; Menezes et al., 2003; Verheyden, 2004). In favour of this idea, the average growth layer width of stem discs did show a decrease with increasing salinity. Despite the underestimation of total secondary growth, radial increment mirrored differences in leaf area related to crown die-back or to site-specific LAI (Table 4). Although only slightly, compared with tree species from Cameroon (Worbes et al., 2003), tree size (Table 4) was also reflected in A. marina's growth rate for 2005–2006 as supported by the overall positive effect of tree height on mangrove biomass production (Saenger and Snedaker, 1993). The absence of a relationship between radial increment during the study period and basal diameter (Table 4) agrees with earlier findings of tree growth being determined by tree structural characters rather than by age (e.g. Pélissier and Pascal, 2000; Sterck et al., 2001; Worbes et al., 2003; King et al., 2005).
Additional support for the patchy growth is given by the slower radial growth, on average 0·57 mm year–1 (see also Fig. 5B), at site 5 during the previous study (Schmitz et al., 2007). The longer study period of 2·5 years in combination with a cambial mark at a single position around the stem would have increased the underestimation of the total increase in stem width if growth takes place at discrete and changing positions around the stem. Therefore, when comparing the growth rate of A. marina with that of other tree species, one has to be cautious. The measured growth rate concerns the radial increment on the side where wounding occurred. Depending on the number of positions around the stem circumference active during the study period, this value might underestimate the total increase in stem width. On the other side, growth layers growing at the time of wounding have already produced (part of) their phloem band, which might have resulted in an overestimation of the growth rate. Among mangrove species, the present findings on radial growth corresponded quantitatively with the radial growth of Rhizophora mucronata growing in the same forest in Gazi Bay, <0·5–4·81 mm year–1 (Verheyden, 2004), and of Heritiera fomes and Sonneratia apetala from Bangladesh, 1·4 ± 0·5 and 2 ± 1 mm year–1, respectively (Md Q Chowdhury, Wageningen University, The Netherlands, unpubl. res.). Shiokura (1989) and Thampanya (2006) mention a higher growth rate of 3·8–5·7 mm year–1 for Avicennia species from Japan and Thailand. A soil water salinity level rarely exceeding 40 ‰ (see also Table 2) in these countries' mangrove forests (Wakushima et al., 1994a, b) might be responsible for the inconsistency. In India, three A. officinalis trees even had a diameter growth of 25·3 mm year–1 (Baker, 1915). The growth layers there are suggested to be monthly (Baker, 1915) and thus are approx. 1 mm wide, as are those of A. marina (Table 3) and of A. germinans from Japan (Sun and Suzuki, 2000).
What triggers cambium differentiation?
The second hypothesis of the growth layer width being determined by soil water salinity was only partially accepted. The similar width of the growth layers of different Avicennia species together with the influence of radial growth on the formation of a new growth layer is in favour of a mainly endogenous control of cambium differentiation. An additional external influence of tree height and soil water salinity was recorded. Also, in other species, stem radius and number of growth layers are highly related (Ogden, 1981; Ruthrof et al., 2003), but in A. marina the relationship applies even along the different radii of a single asymmetric stem disc. Moreover, the radial increment of 2005–2006 was only slightly related to the width of the corresponding growth layers as compared with trees with one vascular cambium. A strictly controlled growth layer formation results in a regular dispersion of the phloem tissue over the entire stem disc. Within one growth layer, the ratio of xylem to phloem tissue is lower compared with trees with conventional secondary growth (ratio: 2–9; Table 3) (Artschwager, 1950; Waisel et al., 1966; Rajput and Rao, 2000). Besides, they accumulate only a limited number of phloem increments, making the total phloem fraction of an Avicennia stem considerably higher (Esau, 1969). The functional advantage of such a three-dimensional phloem network lies in its proposed role in embolism repair and water storage (Mauseth and Plemons-Rodriguez, 1997; Salleo et al., 2004, 2006; Scholz et al., 2007), supported by the decreasing growth layer width with increasing soil water salinity. The smaller the growth layers are, the higher is the percentage of phloem tissue and the lower the fraction of thick-walled xylem fibres, since the increase in phloem tissue was only 22 % of the xylem production. Therefore, the disappearance of growth layers at the smaller radius of asymmetric stems, instead of becoming smaller, might ensure the mechanical stability of the tree. The finding of wider growth layers in larger trees confirms this idea though no age trend was observed. Nevertheless, the considerable variation in growth layer width within a tree, with an average range of 402 ± 92 to 1564 ± 354 µm (n = 47), underscored the existence of an external control on the development of a new cambium superimposed on a genetically defined basic growth layer width.
In conclusion, a patchy growth via successive cambia may offer a functional advantage under xeric and fluctuating environmental conditions as occur in the mangrove environment. It is proposed that growth layer width is partly predetermined by mature tree height and partly controlled by the micro-environmental conditions via a trade-off between hydraulic safety and mechanical stability. Optimal growth conditions could prematurely stimulate the differentiation of a new cambium, resulting in two simultaneously forming growth layers. In this way, beneficial growth conditions could be exploited, which is supported by the significantly larger increment of the trees showing a double wound. To test this hypothesis and the proposed patchy growth, future studies should focus on controlled eco-physiological experiments and address the cambial activity on an intra-annual level, considering intra-tree variations and the relationship with tree phenology. Further insight into the functioning of successive cambia in different tree species and the link with environmental conditions is needed to reveal this growth strategy as a functional adaptation or a mere structural oddity.
ACKNOWLEDGEMENTS
We thank Dr Samuel Teissier for assistance with the nutrient analyses, Dr J. De Mey of the UZ-Brussels, Jette for the CT scan photographs, Hassan Said Attuar and Hamisi Ali Kirauni for their invaluable help during the fieldwork, and all the people of Mwamba (A Rocha Kenya) and Gazi for their assistance and hospitality. This research was financially supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen), the Belgian Federal Science Policy (project MO/37/015), travel grants from the National Fund for Scientific Research (FWO, Belgium), the Schure-Beijerinck-Popping Fonds (Koninklijke Nederlandse Akademie van Wetenschappen, The Netherlands) and the Flemish Interuniversity Council (VLIR).
LITERATURE CITED
- APHA-AWWA-WEF. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; 1995. [Google Scholar]
- Artschwager E. The time factor in the differentiation of secondary xylem and phloem in pecan. American Journal of Botany. 1950;37:16–24. [Google Scholar]
- Baker RT. The Australian ‘grey mangrove’ (Avicennia officinalis, Linn.) Journal and Proceedings of the Royal Society of New South Wales. 1915;49:257–281. [Google Scholar]
- Ball MC. Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees. 2002;16:126–139. [Google Scholar]
- Bauch J, Dünisch O. Comparison of growth dynamics and wood characteristics of plantation-grown and primary forest Carapa guianensis in Central Amazonia. IAWA Journal. 2000;21:321–333. [Google Scholar]
- Callado CH, da Silva Neto SJ, Scarano FR, Costa CG. Periodicity of growth rings in some flood-prone trees of the Atlantic rain forest in Rio de Janeiro, Brazil. Trees. 2001;15:492–497. [Google Scholar]
- Carlquist S. Wood, bark, and stem anatomy of gnetales: a summary. International Journal of Plant Sciences. 1996;157:S58–S76. [Google Scholar]
- Carlquist S. Wood anatomy, stem anatomy and cambial activity of Barbeuia (Caryophyllales) IAWA Journal. 1999;a 20:431–440. [Google Scholar]
- Carlquist S. Wood and stem anatomy of Stegnosperma (Caryophyllales); phylogenetic relationships; nature of lateral meristems and successive cambial activity. IAWA Journal. 1999;b 20:149–163. [Google Scholar]
- Carlquist S. Comparative wood anatomy. 2nd edn. Berlin: Springer-Verlag; 2001. [Google Scholar]
- Carlquist S. Wood and bark anatomy of Salvadoraceae: ecology, relationships, histology of interxylary phloem. Journal of the Torrey Botanical Society. 2002;129:10–20. [Google Scholar]
- Carlquist S. Wood and stem anatomy of woody Amaranthaceae s.s.: ecology, systematics and the problems of defining rays in dicotyledons. Botanical Journal of the Linnean Society. 2003;a 143:1–19. [Google Scholar]
- Carlquist S. Wood anatomy of Polygonaceae: analysis of a family with exceptional wood diversity. Botanical Journal of the Linnean Society. 2003;b 141 [Google Scholar]
- Carlquist S. Lateral meristems, successive cambia and their products: a reinterpretation based on roots and stems of Nyctaginaceae. Botanical Journal of the Linnean Society. 2004;146:129–143. [Google Scholar]
- Carlquist S. Successive cambia in Aizoaceae: products and process. Botanical Journal of the Linnean Society. 2007;153:141–155. [Google Scholar]
- Carlquist S, Gowans DA. Secondary growth and wood histology of. Welwitschia. Botanical Journal of the Linnean Society. 1995;118:107–121. [Google Scholar]
- Chapman VJ. The morphology of Avicennia nitida Jacq. and the function of its pneumatophores. Journal of the Linnean Society of London: Botany. 1944;52:487–533. [Google Scholar]
- Chapman VJ. Secondary thickenings and lenticels in. Avicennia nitida. Proceedings of the Linnean Society. 1947;158:2–6. [Google Scholar]
- Dahdouh-Guebas F, De Bondt R, Abeysinghe PD, Kairo JG, Cannicci S, Triest L, et al. Comparative study of the disjunct zonation pattern of the grey mangrove Avicennia marina (Forsk.) Vierh. in Gazi Bay (Kenya) Bulletin of Marine Science. 2004;74:237–252. [Google Scholar]
- Détienne P. Appearance and periodicity of growth rings in some tropical woods. IAWA Bulletin n.s. 1989;10:123–132. [Google Scholar]
- Esau K. The phloem. 2nd edn. Berlin: Gebrüder Borntraeger; 1969. [Google Scholar]
- Fahn A, Shchori Y. The organization of the secondary conducting tissues in some species of the Chenopodiaceae. Phytomorphology. 1967;17:147–154. [Google Scholar]
- Fahn A, Zimmermann MH. Development of the successive cambia in Atriplex halimus (Chenopodiaceae) Botanical Gazette. 1982;143:353–357. [Google Scholar]
- Feller IC, Whigham DF, McKee KL, Lovelock CE. Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River Lagoon, Florida. Oecologia. 2003;134:405–414. doi: 10.1007/s00442-002-1117-z. [DOI] [PubMed] [Google Scholar]
- Franke R, Schreiber L. Suberin – a biopolyester forming apoplastic plant interfaces. Current Opinion in Plant Biology. 2007;10:252–259. doi: 10.1016/j.pbi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Frankenstein C. 2006. Strukturelle und topochemische Untersuchungen zu Wundreaktion und Kallusbildung von Pappel (Populus tremula L. × Populus tremuloides Michx., Salicaceae) [Google Scholar]
- Frankenstein C, Schmitt U, Waitkus C, Eckstein D. Wound callus formation – a microscopic study on poplar (Populus tremula L. × Populus tremuloides Michx.) Journal of Applied Botany and Food Quality-Angewandte Botanik. 2005;79:44–51. [Google Scholar]
- Gang PO, Agatsiva JL. The current status of mangroves along the Kenyan coast: a case study of Mida Creek mangroves based on remote sensing. Hydrobiologia. 1992;247:29–36. [Google Scholar]
- Gill AM. Endogenous control of growth-ring development in. Avicennia. Forest Science. 1971;17:462–465. [Google Scholar]
- GLOBE. Soil characterization protocol. Field guide. 2005. GLOBE website at http://www.globe.gov/
- Heinrich I, Banks J. Using the pinning method to track intra-seasonal growth in Toona ciliata (Australian Red Cedar) IAWA Journal. 2002;23:458. [Google Scholar]
- Jacques FMB, De Franceschi D. Menispermaceae wood anatomy and cambial variants. IAWA Journal. 2007;28:139–172. [Google Scholar]
- Jalil NRA, Itoh T, Sahri MH, Jusoh MZ. Periodicity of xylem growth of rubberwood (Hevea brasiliensis) grown in Malaysia. Holzforschung. 1998;52:567–572. [Google Scholar]
- King DA, Davies SJ, Nur Supardi MN, Tan S. Tree growth is related to light interception and wood density in two mixed dipterocarp forests of Malaysia. Functional Ecology. 2005;19:445–453. [Google Scholar]
- Kitheka JU. Coastal tidally-driven circulation and the role of water exchange in the linkage between tropical coastal ecosystems. Estuarine, Coastal and Shelf Science. 1997;45:177–187. [Google Scholar]
- Kuroda K, Shimaji K. The pinning method for marking xylem growth in heartwood species. Forest Science. 1984;30:548–554. [Google Scholar]
- Lin G, Sternberg LdaSL. Effect of growth form, salinity, nutrient and sulfide on photosynthesis, carbon isotope discrimination and growth of Red Mangrove (Rhizophora mangle L.) Australian Journal of Plant Physiology. 1992;19:509–517. [Google Scholar]
- Lovelock CE, Feller IC, McKee KL, Engelbrecht BMJ, Ball MC. The effect of nutrient enrichment on growth, photosynthesis and hydraulic conductance of dwarf mangroves in Panamá. Functional Ecology. 2004;18:25–33. [Google Scholar]
- Matthijs S, Tack J, van Speybroeck D, Koedam N. Mangrove species zonation and soil redox state, sulphide concentration and salinity in Gazi Bay (Kenya), a preliminary study. Mangroves and Salt Marshes. 1999;3:243–249. [Google Scholar]
- Mauseth JD, Plemons-Rodriguez BJ. Presence of paratracheal water storage tissue does not alter vessel characters in cactus wood. American Journal of Botany. 1997;84:815–822. [PubMed] [Google Scholar]
- McDonald JA. Evolutionary implications of typical and anomalous secondary growth in arborescent Ipomoea (Convolvulaceae) Bulletin of the Torrey Botanical Club. 1992;119:262–267. [Google Scholar]
- Menezes M, Berger U, Worbes M. Annual growth rings and long-term growth patterns of mangrove trees from the Bragança peninsula, North Brazil. Wetlands Ecology and Management. 2003;11:233–242. [Google Scholar]
- Naidoo G. Factors contributing to dwarfing in the mangrove. Avicennia marina. Annals of Botany. 2006;97:1095–1101. doi: 10.1093/aob/mcl064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MNB. Structure of stem and cambial variant in Spatholobus roxburghii (leguminosae) IAWA Journal. 1993;14:191–204. [Google Scholar]
- Nair MNB, Mohan Ram HY. Structure of wood and cambial variant in the stem of Dalbergia paniculata Roxb. IAWA Bulletin n.s. 1990;11:379–391. [Google Scholar]
- Nobuchi T, Ogata Y, Siripatanadilok S. Seasonal characteristics of wood formation in Hopea odorata and. Shorea henryana. IAWA Journal. 1995;16:361–369. [Google Scholar]
- Ogden J. Dendrochronological studies and the determination of tree ages in the Australian tropics. Journal of Biogeography. 1981;8:405–420. [Google Scholar]
- Ohashi Y, Sahri MH, Yoshizawa N, Itoh T. Annual rhythm of xylem growth in rubberwood (Hevea brasiliensis) trees grown in Malaysia. Holzforschung. 2001;55:151–154. [Google Scholar]
- den Outer RW, van Veenendaal WLH. Development of included phloem in the stem of Combretum nigricans (combretaceae) IAWA Journal. 1995;16:151–158. [Google Scholar]
- Pélissier R, Pascal J-P. Two-year tree growth patterns investigated from monthly girth records using dendrometer bands in a wet evergreen forest in India. Journal of Tropical Ecology. 2000;16:429–446. [Google Scholar]
- Rajput KS, Rao KS. Structural and developmental studies on cambial variant in Pupalia lappacea (Amaranthaceae) Annales Botanici Fennici. 1999;36:137–141. [Google Scholar]
- Rajput KS, Rao KS. Secondary growth in the stem of some species of Alternanthera and Achyranthes aspera (Amaranthaceae) IAWA Journal. 2000;21:417–424. [Google Scholar]
- Ruthrof KX, Loneragan WA, Yates CJ. Comparative population dynamics of Eucalyptus cladocalyx in its native habitat and as an invasive species in an urban bushland in south-western Australia. Diversity and Distributions. 2003;9:469–484. [Google Scholar]
- Saenger P, Snedaker SC. Pantropical trends in mangrove above-ground biomass and annual litterfall. Oecologia. 1993;96:293–299. doi: 10.1007/BF00317496. [DOI] [PubMed] [Google Scholar]
- Salleo S. Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel) Functional Plant Biology. 2006;33:1063–1074. doi: 10.1071/FP06149. [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Trifilò P, Nardini A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell and Environment. 2004;27:1065–1076. [Google Scholar]
- Sass U, Killmann W, Eckstein D. Wood formation in two species of Dipterocarpaceae in peninsular Malaysia. IAWA Journal. 1995;16:371–384. [Google Scholar]
- Schmitt U, Liese W. Response of xylem parenchyma by suberization in some hardwoods after mechanical injury. Trees. 1993;8:23–30. [Google Scholar]
- Schmitt U, Möller R, Eckstein D. Seasonal wood formation dynamics of Beech (Fagus sylvatica L.) and Black Locust (Robinia pseudoacacia L.) as determined by the ‘pinning’ technique. Journal of Applied Botany. 2000;74:10–16. [Google Scholar]
- Schmitz N, Verheyden A, Kairo JG, Beeckman H, Koedam N. Successive cambia development in Avicennia marina (Forssk.) Vierh. is not climatically driven in the seasonal climate at Gazi Bay, Kenya. Dendrochronologia. 2007;25:87–96. [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer F, Franco AC, Miralles-Wilhelm F. Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant, Cell and Environment. 2007;30:236–248. doi: 10.1111/j.1365-3040.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- Shiokura T. A method to measure radial increment in tropical trees. IAWA Bulletin n.s. 1989;10:147–154. [Google Scholar]
- Sobrado MA, Ewe SML. Ecophysiological characteristics of Avicennia germinans and Laguncularia racemosa coexisting in a scrub mangrove forest at the Indian River Lagoon, Florida. Trees. 2006;20:679–687. [Google Scholar]
- Sterck FJ, Bongers F, Newbery DM. Tree architecture in a Bornean lowland rain forest: intraspecific and interspecific patterns. Plant Ecology. 2001;153:279–292. [Google Scholar]
- Stobbe H, Schmitt U, Eckstein D, Dujesiefken D. Developmental stages and fine structure of surface callus formed after debarking of living lime tree (Tilia sp.) Annals of Botany. 2002;89:773–782. doi: 10.1093/aob/mcf137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Suzuki M. Wood anatomy of mangrove plants in Iriomote Island of Japan: a comparison with mangrove plants from lower latitudes. Acta Phytotaxonomica et Geobotanica. 2000;51:37–55. [Google Scholar]
- Terrazas T. Origin and activity of successive cambia in cycas (cycadales) American Journal of Botany. 1991;78:1335–1344. [Google Scholar]
- Thampanya U. Mangroves and sediment dynamics along the coasts of southern Thailand. The Netherlands: Wageningen University; 2006. PhD thesis. [Google Scholar]
- Tomlinson PB. The botany of mangroves. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Tuffers A, Naidoo G, von Willert DJ. Low salinities adversely affect photosynthetic performance of the mangrove. Avicennia marina. Wetlands Ecology and Management. 2001;9:225–232. [Google Scholar]
- UNEP. Eastern African Database and Atlas Project (EAF/14) 2001. The Eastern African Coastal Resources Atlas: Kenya. United Nations Environmental Program.
- van Veenendaal WLH, den Outer RW. Development of included phloem and organisation of the phloem network in the stem of Strychnos millepunctata (Loganiaceae) IAWA Journal. 1993;14:253–265. [Google Scholar]
- Verheyden A. Belgium: Vrije Universiteit Brussel; 2004. Rhizophora mucronata wood as a proxy for changes in environmental conditions. A study of the wood anatomy, stable isotope chemistry and inorganic composition of a Kenyan mangrove species. PhD thesis. [Google Scholar]
- Verheyden A, Kairo JG, Beeckman H, Koedam N. Growth rings, growth ring formation and age determination in the mangrove. Rhizophora mucronata. Annals of Botany. 2004;94:59–66. doi: 10.1093/aob/mch115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisel Y, Noah I, Fahn A. Cambial activity in Eucalyptus camaldulensis Dehn. The production of phloem and xylem elements. The New Phytologist. 1966;65:319–324. [Google Scholar]
- Wakushima S, Kuraishi S, Sakurai N. Soil salinity and pH in Japanese mangrove forests and growth of cultivated mangrove plants in different soil conditions. Journal of Plant Research. 1994;a 107:39–46. [Google Scholar]
- Wakushima S, Kuraishi S, Sakurai N, Supappibul K, Siripatanadilok S. Stable soil pH of Thai mangroves in dry and rainy seasons and its relation to zonal distribution of mangroves. Journal of Plant Research. 1994;b 107:47–52. [Google Scholar]
- Wheat D. Successive cambia in the stem of. Phytolacca dioica. American Journal of Botany. 1977;64:1209–1217. [Google Scholar]
- Wheeler EA, Baas P, Gasson PE. IAWA list of microscopic features for hardwood identification. IAWA Bulletin n.s. 1989;10:219–332. [Google Scholar]
- Worbes M. Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the neotropics. IAWA Bulletin n.s. 1989;10:109–122. [Google Scholar]
- Worbes M, Staschel R, Roloff A, Junk WJ. Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. Forest Ecology and Management. 2003;173:105–123. [Google Scholar]
- Ye Y, Tam NFY, Lu CY, Wong YS. Effects of salinity on germination, seedling growth and physiology of three salt-secreting mangrove species. Aquatic Botany. 2005;83:193–205. [Google Scholar]
- Zamski E. The mode of secondary growth and the three-dimensional structure of the phloem in. Avicennia. Botanical Gazette. 1979;140:67–76. [Google Scholar]
- Zamski E. Does successive cambia differentiation in Avicennia depend on leaf and branch initiation? Israel Journal of Botany. 1981;30:57–64. [Google Scholar]