Abstract
Background and Aims
Most studies on cactus recruitment have focused on the role of woody plants as seedling facilitators. Although the spatial association of cacti with objects had been described, the mechanisms underlying this association remain unknown. The aims of this study were to identify which mechanisms facilitate the establishment of a columnar cactus under the shade and protection of objects and to compare these mechanisms with those involved in plant–plant facilitation.
Methods
Three split-split-plot field experiments were conducted to compare the effects of two microhabitats (inside rocky cavities and beneath plant canopies) on seed removal, germination, seedling survivorship and dry weight. Flat, open spaces were used as the control. For each microhabitat, the effect of seed or seedling protection and substrate limitation were explored; aboveground microclimate and some soil properties were also characterized.
Key Results
The permanence of superficial seeds was greater inside rocky cavities than beneath woody plant canopies or on flat, open areas. Germination was similar in cavities and beneath plant canopies, but significantly higher than on flat, open areas. Seedling survivorship was greater beneath plant canopies than inside cavities or on flat, open spaces.
Conclusions
The mechanisms of plant facilitation are different from those of object facilitation. There are seed–seedling conflicts involved in the recruitment of P. leucocephalus: nurse plants favour mainly seedling survivorship by providing a suitable microenvironment, while nurse objects mainly favour seed permanence, by protecting them from predators.
Key words: Columnar cactus, facilitation, nurse object, nurse plant, Pilosocereus leucocephalus, plant recruitment, seed–seedling conflicts, rocky cavities
INTRODUCTION
Facilitation is one of the most important processes for the survival, growth and fitness of some plants, as well as for the diversity and dynamics of plant communities, especially in harsh ecosystems (reviewed by Callaway, 1995; Flores and Jurado, 2003). Studies of the Cactaceae have significantly contributed to our understanding of how facilitation operates. Most of these studies have shown that woody plants are needed for the successful recruitment of new cactus individuals (reviewed by Godínez-Alvarez et al., 2003). However, there is an increasing number of reports of cacti growing in intercanopy spaces (Parker, 1989; Arriaga et al., 1993; Martínez et al., 1994; Valverde et al., 1999; Rodríguez-Ortega and Ezcurra, 2001; Bashan et al., 2002; López and Valdivia, 2007). Although some of these plants are able to tolerate the stress imposed by direct solar radiation, it has been suggested that others are capable of establishing in open spaces because their recruitment is facilitated by elements of the microrelief such as stones, hollows or crevices (hereafter nurse objects; e.g. Parker, 1989; Valiente-Banuet and Ezcurra, 1991; Reyes-Olivas et al., 2002). While it is well known that microclimatic amelioration and escape from seed and seedling predators are the most common mechanisms underlying plant–plant facilitation in Cactaceae (reviewed by Sosa and Fleming, 2002; Godínez-Alvarez et al., 2003), facilitation by nurse objects is poorly understood. Recent reviews have recommended further research in this topic (Sosa and Fleming, 2002; Flores and Jurado, 2003; Godínez-Alvarez et al., 2003).
Potential mechanisms involved in cactus facilitation by nurse objects are microclimatic amelioration (Parker, 1989; Reyes-Olivas et al., 2002; Kleier and Lambrinos, 2005), protection against mechanical damage (Steenbergh and Lowe, 1969), the accumulation of soil and water (Turner et al., 1966) and/or a reduction in the risk of predation (Niering et al., 1963). However, we know of no study testing these mechanisms experimentally under field conditions. Since the microclimatic amelioration offered by plant canopy shade is one of the most common benefits that nurse plants offer young cacti, perhaps the shade from nurse objects facilitates cactus recruitment in the same way. Although this is likely, nurse plants and nurse objects clearly differ in their physical architecture. Nurse structure clearly influences the area of ground available to a nursed species for establishment and survival (Drezner, 2006). Parker (1989) found that two sympatric species of columnar cactus differ in their association patterns: Carnegiea gigantea is strongly associated with nurse plants and Stenocereus thurberi grows in association with nurse objects. Different association patterns in these plants suggest that nurse plants and nurse objects do not offer the same benefits or that these benefits differ in magnitude. While a plant canopy resembles an umbrella and shades recruits for a long time each day, nurse objects – think of a stone–only offer protection for a shorter time. The solar radiation that reaches seedlings is filtered by green leaves under nurse plant canopies, while it might arrive directly or reflected from stony walls near the object. Other benefits offered by nurse plants, such as nutrient exchange, microbial association or an increase in soil nutrients (see Callaway, 1995 for a review of the mechanisms involved in plant–plant facilitation) could hardly be offered by inanimate nurse objects. On the other hand, an association with nurse objects could be advantageous, because in contrast to plant–nurse plant associations (McAuliffe, 1984; Flores-Martínez et al., 1994, 1998), the protégé does not compete with its nurse (discussed by Resler et al., 2005).
Some authors have indicated that the microenvironments that are suitable for seeds are not necessarily appropriate for seedlings, and vice versa (Schupp, 1995); therefore, studies of plant recruitment have to consider the multistage nature of this demographic process (Jordano and Herrera, 1995; Rey and Alcántara, 2000; Gulias et al., 2004). We are interested in the mechanisms which support plant–nurse object association and the stages of recruitment at which they operate, as well as how different these mechanisms are as compared with those which maintain plant–plant associations. In this study the effects of microclimate and seed or seedling protection beneath plant canopies, in the shelter offered by rocky cavities and on flat, open spaces were experimentally tested during the most critical stages of recruitment: seed permanence, seed germination and early seedling performance (Schupp, 1995).
In addition, the effects of soil limitation (absent or extremely scarce soil) and its interaction with microclimate and protection were explored. The limited availability of soil imposes more xeric conditions on young plants, while soil accumulation promotes deeper penetration of soil water and, therefore, reduces water loss to evaporation (Turner et al., 1966). Soil limitation could affect plant establishment in the study area, as the predominating substrate promotes a degree of edaphic aridity as harsh as that of the desert (Ortega, 1981). The Pilosocereus leucocephalus columnar cactus was used as the study object. To the naked eye, this cactus grows on a wide variety of substrates: from bare rock to developed soils. Pilosocereus leucocephalus is an excellent model for study because it exhibits spatial association with rocky cavities and woody plants (see Study species and its association pattern, below).
It was predicted that seed permanence, seed germination, seedling survival and seedling growth would be higher in the proximity of nurse objects or under nurse plant canopies than on flat, open sites. Minor differences in these variables were also expected between shaded microhabitats (nurse plants and objects), though the task of establishing the direction of these differences was not expected to be a simple one, owing to the scarcity of comparative studies. As such, the approach was exploratory.
MATERIALS AND METHODS
Study area
The cactus population studied here is located at Rancho San Ignacio, in the municipality of Xalapa, state of Veracruz, in south-eastern Mexico [19°26·906′N, 96°58·522′W, 950 m (a.s.l.)]. The climate is the driest of the sub-wet group, with a mean annual temperature of 24 °C. Rain falls from June to September (monthly mean: 209 mm), but sporadic light rains fall in autumn. The dry season extends from November to May (monthly mean precipitation: 22 mm). The vegetation is tropical dry forest growing on a young basaltic substrate formed by a lava flow. Soil, shallow and formed in situ, accumulates primarily in the numerous cavities formed by the release of gases trapped during volcanic activity; however, soil of alluvial origin accumulates in the surroundings of the lava flow. The predominant condition of the substrate propitiates extreme edaphic aridity, so xerophytes are conspicuous (Ortega, 1981). The canopy of this forest is not continuous; in the inter-canopy spaces there are patches of vegetation dominated by annual herbaceous plants, bryophytes or bare rock. Dominant species in the woody stratum are Lysiloma microphylla, Lysiloma acapulcensis and P. leucocephalus (the complete floristic list is given in Castillo-Campos, 2003).
Study species and its association pattern
Pilosocereus leucocephalus is a columnar cactus endemic to Mesoamerica (Guzmán et al., 2003). Adult plants may reach a height of 6 m in this zone. This cactus produces white or pinkish chiropterophilous flowers during spring and summer (April–August). Fructification and seed dispersal occur 3 weeks after each of the three or four peaks of flowering (Munguía-Rosas et al., INECOL, Xalapa, Mexico, unpubl. res.) from late spring to early autumn (May–September). Bats, birds and lizards were observed feeding on fruits, and ants carry the seeds from fruits on the plant as well as from fallen fruits. Seed number per fruit averaged 830 ± 106 (hereafter, mean ± s.e.; n = 35 fruits). Germination success is 65 ± 10·5 % under controlled conditions (this study), and is not enhanced by physical or chemical scarification (Miranda-Jácome, 2005).
To determine the association pattern of P. leucocephalus, all sexually immature individuals <90 cm tall were looked for in ten (10 × 10 m) plots systematically placed 20 m away from each other, along a transect. All the young cacti in each plot were counted, then for each one a table of random numbers was used to obtain the coordinates for a corresponding random point (RP). For each item (cactus and RP), it was recorded whether it was inside a cavity or on a flat surface, as well as if it were or not beneath the canopy of an adult perennial woody plant. A cavity was considered to be any kind of niche or crevice in the rocky substrate delimited laterally by rocky walls <60 cm apart, and a flat surface was any smooth or slightly rough horizontal surface. The following null hypotheses were tested: (a) the incidence of cacti inside cavities is not statistically different from the incidence of RPs in this microhabitat; and (b) the incidence of cactus beneath the canopy of woody plants is not different from the incidence of RPs in the same microhabitat. The incidence of RPs inside cavities and beneath the canopy of woody plants was expected to be a function of the availability of these microhabitats in each plot. A similar method has been used previously for similar purposes (e.g. Suzán et al., 1996).
The mean number of cacti and RPs per plot was 24 ± 3·83. A significant association was detected between the cacti and rocky cavities: the incidence of cacti was higher than the incidence of RPs inside cavities (F1, 463 = 30·46, P < 0·001 for a generalized linear mixed effect model [GLMM]: response variable = incidence of items inside cavities; fixed factor = item identity, cactus or RP; random factor = plot; error, binomial). This cactus is also associated with woody plants: the incidence of cactus beneath woody plant canopies was higher than of RP (F1,463 = 9·24, P = 0·002 for a GLMM: response variable; fixed and random factors; and error as before). There does not appear to be any specificity in the association pattern as most of the plant-shaded items (92 % of cactus and 88 % of RPs) were found under the canopy of the most common woody plants: L. microphylla or L. acapulcensis (indistinguishable to the species level in the field) and there were no statistical differences in the proportion of cacti or RPs shaded by Lysiloma (χ 2 = 1·22, P = 0·26, in a binomial test). So, P. leucocephalus is not as specialist in microhabitat choice as reported for other columnar cacti (Callaway, 1998).
The mean proportion of cavities shaded by a plant canopy and occupied by a cactus (0·77 ± 0·05) was not statistically different from the mean proportion of plant-shaded cavities occupied by an RP (0·61 ± 0·07), according to a GLMM (F1, 9 = 0·011, P = 0·91; response variable = proportion of items inside cavities shaded by plant canopies; fixed and random factors, and error as before). This suggests that cacti that are established in cavities shaded by a nurse plant do not receive additional positive effects.
Seed removal experiment
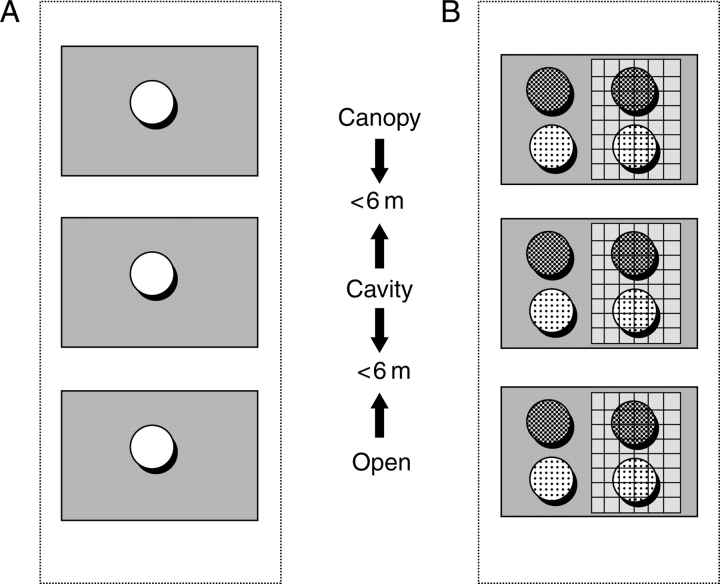
Post-dispersal seed removal was evaluated in three different microhabitats: beneath the canopies of woody plants, in rocky cavities and on flat, open spaces. Seed dishes were exposed to seed removers (potential granivores) in a block design. The block was a spatial grouping factor related to the area within which the three different microhabitats were located. The experiment was set in late summer 2005, as shown in Fig. 1A: three plastic dishes (5 cm radius and 1·5 cm deep) were assigned to each block, one for each type of microhabitat. Each dish was filled with a random sample of 25 depulped seeds. A total of 23 blocks (experimental replicates) were used (25 seeds × 23 blocks = 575 seeds per treatment). Each microhabitat replicate was selected to be as similar as possible for all experiments: nurse plants were adult Lysiloma plants, rocky cavities were limited to niches measuring 20–60 × 20–60 × 10–25 cm deep, and flat, open sites were limited to horizontal surfaces >150 cm away from rock walls, crests or woody plants. Some of the flat, open spaces in some blocks were not entirely devoid of vegetation, and had mosses and/or grasses growing there. These were removed from under and in the proximity of the experimental devices. Microhabitats where the effects were confounded, e.g. where a nurse plant canopy was shading a cavity, were also avoided. A photograph of each microhabitat is provided in the Supplementary Information (available online).
Fig. 1.
Diagrammatic representation of the experimental design used in seed removal (A), germination, seedling survivorship and growth experiments (B). The three microhabitats (grey rectangles) were located in the block (largest dotted-line rectangle): beneath plant canopies (Canopy), inside cavities (Cavity) and on flat, open spaces (Open). The distance between microhabitats was <6 m and the distance beteen blocks was >16 m. Circles represent plastic dishes, open circles in (A) represent plastic dishes with 25 seeds each; circles in (B) represent plastic dishes with seeds (25 each) or seedlings (six each). Different backgrounds of circles in (B) indicate different substrate (ground rock or organic soil). Grids inside grey rectangles in (B) represent protected plastic dishes. Blocks in (A) were replicated 23 times, and blocks in (B) were replicated 10 and 13 times for the germination and seedling performance experiments, respectively.
To facilitate counting the seeds, in this experiment, no substrate was put into the dishes with the seeds. The number of seeds remaining was recorded only once after 72 h of exposure to predators because seed removal was faster than expected. The seed dish technique described here has been used extensively to estimate granivory (Pérez et al., 2006).
Seed germination, seedling survival and growth experiments
In these experiments, the effects of microhabitat, seed and seedling protection, and substrate conditions on seed germination and early seedling performance (survival and biomass measured as dry weight) were assessed. Two full factorial experiments were conducted in a split–split plot design. The former was carried out in early autumn 2005, when natural seed dispersal was nearly over. Seeds from 68 fruits collected from 28 plants were extracted by dissection, washed in tap water, dried at room temperature and mixed to obtain a pool of seeds for the preparation of experimental units. The units were 120 plastic dishes (described in the previous section) filled with approx. 10 cm3 of substrate: 60 with soil and 60 with ground basaltic rock. The substrates were obtained at the study area; soil was taken mainly from cavities and beneath plant canopies because it is quite scarce in flat, open areas. The substrate from these two sources was homogenized before being put into the dishes. Twenty-five seeds were placed on the substrate in each dish, and they were covered with a fine layer of substrate. Dishes were taken to the field and placed in ten blocks (the blocks were a sub-sample of those used in the seed removal experiment). In total, 12 dishes were placed in each block: four per microhabitat, two with soil and two with rock as the substrate. In each microhabitat, pairs of dishes with different substrate types were protected from vertebrate and large invertebrate (Coleopterans, Hemipterans and Orthopterans) predators using 8 mm mesh plastic cages (15 × 12 × 9 cm) and from small invertebrate predators (ants mainly) by spreading Tanglefoot resin on the outer walls of dishes (Fig. 1B). To evaluate a possible effect of cages on microclimate, the temperature inside and outside the cages was recorded using a Li-1000 data logger and a Li-Cor Temperature sensor (LI-COR Bioscience; Lincoln, NE, USA) in 12 blocks during early afternoon (12:30–14:00) on a clear spring day. Within this time interval maximum temperatures are reached at the study site; freezing temperatures, which might also affect columnar cactus survivorship (Nobel, 1984), have not been recorded in historical times. Seed germination, indicated by radicle emergence, was recorded weekly over 5 weeks. At the same time as the germination field experiment, germination trials were completed in a growth chamber by setting up groups of 20 seeds in each of 12 plastic dishes lined with moist filter paper. The dishes were kept in the chamber (24 °C, light–dark period of 12 h) and watered and checked daily for germination. It is common in recruitment ecology studies (e.g. Traveset et al., 2003; Mendez et al., 2004), for such trials to establish the degree of seed viability and provide an idea of the maximum germination potential of the specific seed pool used.
At the same time as the germination experiment, nearly 2000 seeds were also planted in soil inside a greenhouse, in order to obtain a cohort of seedlings and provide continuity for the germination experiment at the seedling stage. Seedlings were replaced instead of maintaining the emerged seedlings from the germination experiment in order to ensure equal intraspecific competition at initial conditions inside the experimental units. After 5 weeks of planting, 936 seedlings (initial size: 4·47 ± 0·01 mm) were taken to the field. The time elapsed from seed planting to the beginning of the seedling survivorship experiment was the duration of the previous germination experiment. Six seedlings in each plastic dish were planted. The dishes were allocated to the microhabitats inside the blocks as in the germination experiment (Fig. 1B). Seedling survivorship (the proportion of surviving seedlings in each container) was recorded weekly for the first 3 months and then monthly or bimonthly for 11 months after that; the experiment lasted 59 weeks. At the end of the experiment, the surviving seedlings were harvested and their aerial dry weight was measured. The same blocks that were selected for the germination experiment were used, plus three additional ones (n = 13). Before starting the experiment, the transplanted seedlings were acclimatized over 2 weeks by gradually decreasing watering and increasing exposure to sunlight. The use of plastic dishes for these experiments allowed both the manipulation of the substrate and testing for any interactions between the substrate and the mechanisms operating aboveground (in this study, microclimate and protection). It was not possible to transplant seedlings to the study area, because the substrate at some microsites was bare rock.
Microclimate and soil properties
At each microhabitat, the microclimate and soil variables which could affect seed germination and/or early seedling performance were measured. The air temperature and relative humidity at ground level were recorded using digital loggers (StowAway XTI temperature and RH relative humidity loggers, respectively; Onset, Computer Corporation. Bourne, MA, USA). The data were collected simultaneously in the three microhabitats of five blocks every 2 min for 48 h in each block. Before data collection was started, each data logger was calibrated and randomly assigned to a microhabitat. Photosynthetically active radiation (PAR) was measured at ground level in all three microhabitats for each of the ten blocks using a Li-1000 data logger and a Li-Cor PAR sensor (LI-COR Bioscience). PAR was measured three times per day: morning (9:00–10:00 h), noon (12:30–13:30 h) and late afternoon (16:00–17:00 h). All measurements were made on a clear autumn day in 2005.
Soil depth was measured in 25 blocks (12 in addition to the 13 previously selected). From these 25 blocks, nine sites per microhabitat type were selected from which approx. 100 g of soil were sampled, after removing the litter. Samples were taken to the laboratory in plastic bags to quantify total nitrogen (N), extractable phosphorus (P) and total carbon (C) content. Nitrogen and C were measured with a Leco TruSpec Analyzer 2000 (Leco Corporation, St Joseph, MI, USA), P using the Bray method, and pH in water (proportion 1:2·5) with a Jenco 1671 pH meter (Jenco Electronics, Taipei Hsien, Taiwan).
Statistical analyses
Because data from random block, split–split plot, and repeated measures designs were collected, the experimental units showed some degree of correlation as a result of being grouped in space or time. To deal with correlated data, mixed effect models (MEMs) were used. These are a mixture of fixed and random effects; the fixed effects are usually the experimental treatments and the random effects are interesting not because of their parameter values per se, but for the amount of variance they explain from the grouping of experimental units (Pinheiro and Bates, 2000). Experiments for which the results are expressed as a proportion (removed seeds, germinating seeds) were analysed with GLMM, using a binomial error distribution and the logit link function. However, the proportion of surviving seedlings was transformed by the arcsine square root function and analysed with linear mixed effect models (LMEs), because matrix calculations did not reach convergence when data were analysed with GLMM. The transformed data met every assumption imposed by LME.
For the seed removal experiment, the response variable was the proportion of seeds inside the plastic dishes after exposure to predators, relative to the original number; microhabitat was modelled as a fixed factor and block as a random one. The model explaining the proportion of germinated seeds was:
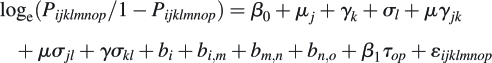 |
where Pijklmnop is the proportion of germinated seeds, β0 refers to the intercept, μ to the microhabitats (j = 3), γ to protection treatments (k = 2), and σ to substrate type (l = 2), all fixed effect factors. All second order interactions between fixed effects μγjk, μσjl, γσkl were included. It was assumed that the effect of third order interactions was negligible, so it was excluded from the model to facilitate matrix convergence. The random part of the model accounts for the hierarchical spatial arrangement of plots as well as the repeated measures: bi (i = 10) refers to the main block, bi,m (m = 3) to the plot where the microhabitat treatment was applied in the main block, bm,n (n = 2) refers to the sub-plot with the protection treatment, bn,o (o = 2) refers to the experimental units (plastic dishes) where the substrate treatment was applied, t is time, and β1 is the slope (repeated measures in MEM is approached as a covariance analysis). Finally, εijklmnop is the within-group error (for an extensive review of model specification in MEM, see Pinheiro and Bates, 2000). The model accounting for the proportion of surviving seedlings included the same terms as the previous model. The only differences were that the response variable was arcsine square root transformed and that the number of replicates was 13 (bi).
Seedling dry weight was fitted to an LME:
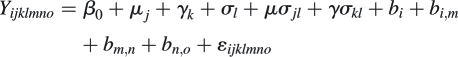 |
where the notation is as in the previous models. It was not possible to estimate the interaction between microhabitat and protection because, by the end of the experiment, all the unprotected plants on flat, open sites and inside cavities had died.
To assess differences in PAR and soil depth among microhabitats, an LME was fitted. In both models, microhabitat was treated as a fixed effect factor and block as a random one. For the PAR model, the time of day was specified as the repeated measure in microhabitat. When significant differences were detected among microhabitat levels, Tukey's honest significant difference (HSD) test was performed to identify the main differences between pairs of levels. GLMMs were fitted by the penalized quasi-likelihood method using the glme function from the correlated data library, and LME by the restricted maximum likelihood method with the lme routine, both available in S-plus software (version 7·0; Insightful Corporation).
Because soil chemical variables did not meet the assumptions for LME or GLMM, comparisons among microhabitats were done with Kruskal–Wallis tests. When the test revealed a significant effect of microhabitat, pair-wise Mann–Whitney tests were applied to assess differences among levels of this factor.
RESULTS
Seed removal experiment
Seed removal was high in the three microhabitats but, after 3 d of exposure to predators, seed permanence differed significantly among microhabitats (Table 1, second column). Rocky cavities protected the greatest number of seeds, while seeds were almost completely removed from flat, open sites and beneath plant canopies. According to the HSD test, removal from cavities differed significantly from that of flat, open sites and from beneath plant canopies. There was no difference in seed permanence beneath plant canopies and on the flat, open areas (Table 2).
Table 1.
Summary of statistical analyses for three experiments with the following response variables: seed permanence, seed germination, seedling survivorship and seedling dry weight of the Pilosocereus leucocephalus columnar cactus
| Response variable |
||||
|---|---|---|---|---|
| Source of variation | Seed permanence (proportion) | Germination (proportion) | Seedling survivorship (proportion, arcsine square root transformed) | Seedling dry weight (g) |
| Microhabitat | F2,42 = 18·88*** | F2,18 = 5·62** | F2,24 = 17·53*** | F2,7 = 0·22 n.s. |
| Protection | – | F1,27 = 23·31*** | F1,36 = 110·87*** | F1,3 = 0·71 n.s. |
| Substrate | – | F1,56 = 0·48 n.s. | F1,72 = 4·75** | F1,141 = 28·25*** |
| Microhabitat × protection | – | F2,27 = 1·98 n.s. | F2,36 = 3·16 n.s. | – |
| Microhabitat × substrate | – | F2,56 = 1·63 n.s. | F2,72 = 0·38 n.s. | F2,141 = 0·92 n.s. |
| Substrate × protection | – | F1,56 = 8·84** | F1,74 = 0·27 n.s. | F1,141 = 0·78 n.s. |
Seedling survivorship and dry weight were evaluated in the same experiment. Data were analysed with mixed effect models. The only factor considered in the seed permanence experiment was microhabitat. The microhabitat × seedling protection interaction was not tested for seedling growth (see text for explanation). Only statistics for the fixed effect factors are shown.
***P < 0·01, **P < 0·05, n.s. = no significant difference.
Table 2.
Final mean ± s.e. of seed permanence, seed germination, seedling survival of the Pilosocereus leucocephalus columnar cactus in three different microhabitats: beneath plant canopies (Canopy), inside cavities (Cavity) and on flat, open areas (Open) when protected or unprotected by cages and resin
| Canopy |
Cavity |
Open |
||||
|---|---|---|---|---|---|---|
| Response variable | Protected | Unprotected | Protected | Unprotected | Protected | Unprotected |
| Seed permanence (%) | – | 0·2 ± 0·2a | – | 10·6 ± 4·7b | – | 1·9 ± 1·2a |
| Germination (%) | 15·8 ± 3·5a | 1·4 ± 0·7a | 18·8 ± 4·2a | 1·8 ± 1·2a | 1·2 ± 0·6b | 0·4 ± 0·4b |
| Seedling survival (%) | 70·51 ± 6·61a | 8·33 ± 4·55a | 24·35 ± 6·61b | 0b | 7·05 ± 3·72b | 0b |
Seed permanence was only recorded for unprotected seeds. Microhabitats sharing a superscript letter are not statistically different, as assessed by Tukey's HSD test in post hoc multiple comparisons.
Seed germination, seedling survival and seedling growth experiments
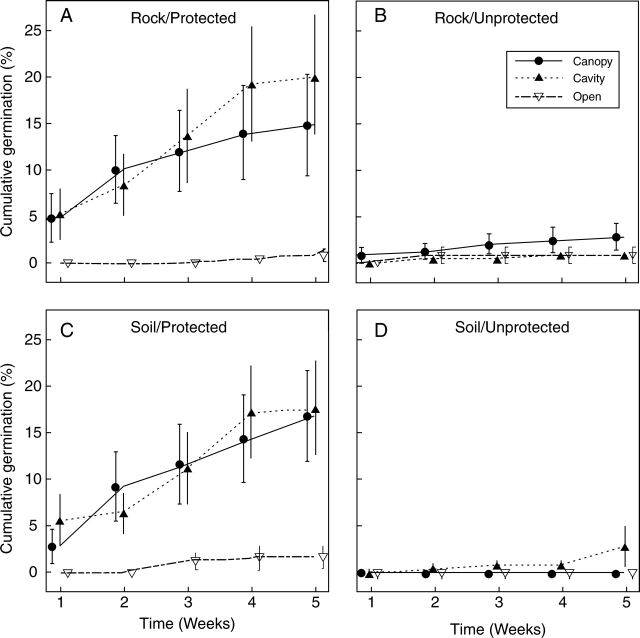
Seeds started to germinate 1 week after the beginning of the experiment; maximum germination occurred in the fourth week in all three microhabitats (Fig. 2). The proportion of seeds that germinated differed significantly among microhabitats and between seed protection treatments. Substrate condition × seed protection was the only significant interaction (Table 1, third column). Germination was far greater beneath the plant canopies and inside cavities than on flat, open areas. Seed germination under controlled conditions (65 ± 10·5 %) was 4 and 3·5 times higher than beneath plant canopies and inside cavities, respectively.
Fig. 2.
Results of the three-factorial experiment on Pilosocereus leucocephalus seed germination. The x-axis represents the elapsed time (in weeks) after the start of the experiment, and the y-axis shows cumulative percentage germination (initial n = 25 seeds). Graphs show seed germination in three microhabitats: beneath plant canopies (Canopy), inside rocky cavities (Cavity) and on flat, open spaces (Open), and combinations of two additional factors: seed or seedling protection (protected or unprotected) and substrate (rock or soil). (A, B) Seed germination on ground rock, (C, D) on organic soil. (A, C) Germination when seeds where protected, (B, D) when unprotected. Symbols and bars show the mean ± s.e.
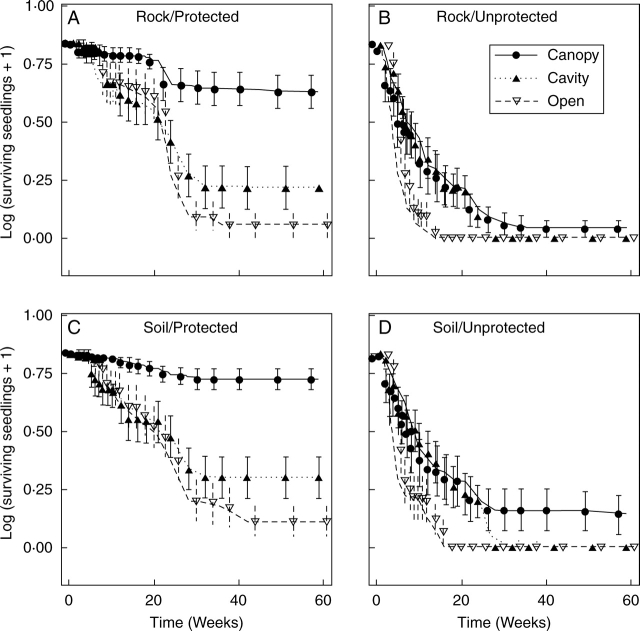
Seedling mortality was greatest during the first 30 weeks in the three microhabitats. Seedling survivorship was greater beneath plant canopies; however, when seedlings were not protected this difference was less evident. Unprotected seedlings died completely on the flat, open spaces and inside the cavities during the experiment. However, all seedlings on flat, open areas died 4 months earlier than those in rocky cavities (Fig. 3). Microhabitat, seedling protection and substrate had a significant effect on seedling survivorship (Table 1, fourth column). HSD tests suggest that there was no difference in seedling survivorship between flat, open spaces and rocky cavities, and that both treatments differ from the plant canopy treatment. Seedling protection promoted higher survivorship in the three microhabitats compared with unprotected plants, especially beneath plant canopies (Table 2). Seedling survivorship at the end of the experiment was 21·36 ± 3·9 and 15·38 ± 3·4 % on soil and ground rock, respectively. Seedling dry weight was only significantly affected by substrate (Table 1, fifth column). Seedlings planted on soil weighed (0·061 ± 0·0059 g) approximately three times more than those on rock (0·019 ± 0·0011 g).
Fig. 3.
Results of three-factorial experiments on Pilosocereus leucocephalus seedling survivorship. The x-axis represents the elapsed time (in weeks) after the start of the experiment, and the y-axis is the number of surviving seedlings plus one on a logarithmic scale (initial n = 6 seedlings). Graphs show seedling survivorship in three microhabitats: beneath plant canopies (Canopy), inside rocky cavities (Cavity) and on flat, open spaces (Open), and two additional factors: seedling protection (protected or unprotected) and substrate (rock or soil). (A, B) Survivorship of seedlings on ground rock, (C, D) on organic soil. (A, C) Seedling survivorship when seedlings were protected, (B, D) when unprotected. Symbols and bars are mean ± s.e.
Microclimate and soil properties
Flat, open spaces had the highest mean maximum air temperature (37·2 ± 1 °C) and the lowest mean relative humidity (11·9 ± 4·1 %) at the hottest time of day. In contrast, cavities had the lowest mean temperature (26 ± 1·8 °C) and the highest humidity (63·2 ± 9 %). Intermediate values were recorded beneath the plant canopy, where the temperature reached 32·8 ± 2·2 °C and relative humidity 39·9 ± 8·2 % (Fig. 4). PAR differed significantly among microhabitats. Mean PAR was highest on flat, open areas and lowest inside cavities. An intermediate value was recorded beneath plant canopies. Variation in PAR was highest on flat, open areas and lowest inside cavities. PAR inside cavities and beneath plant canopies was comparable, but different from PAR on the flat, open areas (Table 3).
Fig. 4.
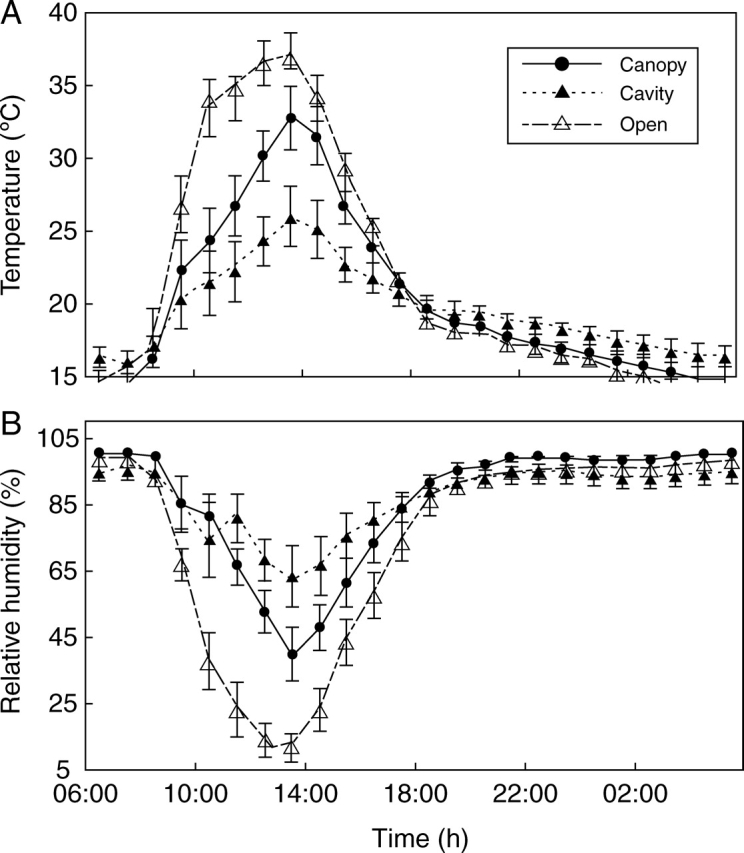
Mean temperature (A) and relative humidity (B) over a 24 h period in the autumn at three microhabitats: beneath nurse plants canopies (Canopy), inside rocky cavities (Cavity) and on flat, open areas (Open) for a deciduous tropical forest patch. Bars show 1 s.e. around the mean.
Table 3.
Mean values ± s.e. of photosynthetically active radiation (PAR) and some soil characteristics for three microhabitats available for Pilosocereus leucocephalus establishment: beneath plant canopies (canopy), inside cavities (cavity) and on flat, open areas (open)
| Microhabitat |
|||||
|---|---|---|---|---|---|
| Analysis | Response variable | Canopy | Cavity | Open | Statistic |
| 1 | PAR (μmol s−1 m−2) | 240·6 ± 63·7a | 222·6 ± 54·5a | 580·6 ± 92·0b | F2,18 = 7·41** |
| 2 | Soil depth (cm) | 8·69 ± 0·8a | 7·06 ± 1·06a | 2·67 ± 0·49b | F2,48 = 24·22*** |
| 3 | Carbon (%) | 35·42 ± 1·02a | 34·56 ± 0·82a | 30·44 ± 2·17b | χ22 = 16·02*** |
| 4 | Nitrogen (%) | 2·57 ± 0·06a | 2·41 ± 0·04b | 2·03 ± 0·17c | χ22 = 14·11*** |
| 5 | pH | 4·32 ± 0·33 | 3·72 ± 0·35 | 4·42 ± 0·52 | χ22 = 1·66 n.s. |
| 6 | Phosphorus (p.p.m.) | 11·4 ± 3·01 | 7·2 ± 1·57 | 10·4 ± 2·37 | χ22 = 1·31 n.s. |
The microhabitat effect was assessed by fitting mixed effect models (1, 2) or by Kruskal–Wallis tests (3, 4, 5 and 6). Different superscript letters indicate differences between treatments according to Tukey's HSD method (1, 2) or a pair-wise Mann–Whitney test (3, 4, 5 and 6).
***P < 0·01, **P < 0·05, n.s. = no significant difference.
Soil depth was significantly different among microhabitats. It was deepest beneath plant canopies, intermediate in cavities and shallow or absent on the flat, open areas. HSD showed that soil depth beneath plant canopies and inside cavities was comparable with but greater than that of flat, open areas (Table 3). There were statistical differences among microhabitats in the values of N and C, but no differences for P or pH. N and C were highest beneath plant canopies and lowest for flat, open areas. Within cavities, N and C were a little lower than they were beneath plant canopies; for N this difference was significant. For the soil below woody plants and in cavities, N was significantly higher than for soil from flat, open areas. Carbon was similar in soil from the cavity and plant canopy microhabitats, and significantly higher than in soil from the open, flat microhabitat (Table 3).
DISCUSSION
Although some authors have described the spatial association of cactus with perennial plants or some elements of the microrelief such as stones, none had addressed the mechanisms underlying cactus–nurse object association. Before the present study, there was a general belief that the cactus–nurse object association arises from mechanisms similar to those of the cactus–nurse plant association. However, the present study reveals that in spite of the microclimatic similarities, the association of the Pilosocereus leucocephalus cactus with woody plants and with rocky cavities is not mediated by the same mechanisms. While both types of shaded microhabitats are suitable for seed germination, only rocky cavities successfully prevented the removal of superficial seeds and only plant canopies offered the best opportunity for seedling survival. Since neither microhabitat was suitable for every recruitment stage, there are ‘seed–seedling conflicts’ (sensu Schupp, 1995) in the recruitment of this species. Soil quantity and quality might be additional factors that tip the balance in favour of an association with nurse plants and nurse objects rather than with flat, open spaces.
Rocky cavities were the safest microhabitats for seed survival. The difference in seed permanence between shaded microhabitats was enormous: the number of seeds inside cavities was 53 times higher than under plant canopies at the end of the seed removal experiment. The avoidance of post-dispersal seed predation is expected to be one of the most important mechanisms underlying the cactus–nurse object association. On the other hand, the effect of protection on germinating seeds was similar in the three microhabitats (protection did not interact with microhabitat). Why is seed germination at odds with seed permanence? In the removal experiment, seeds were not placed within any substrate; quite the contrary, they were only buried in substrate in the germination experiment. The addition of substrate could have reduced the potential seed predators to only those that are able to find seeds below the soil's surface. Data from the germination experiment suggest that granivores that are able to find seeds below ground forage indiscriminately among microhabitats. A similar situation was described in the Israeli desert, where mice and ants are able to find superficial seeds, but only mice are capable of finding buried seeds in both shaded microhabitats and open spaces (Abramsky, 1983). The results of the present seed removal experiment reflect the granivory pattern imposed on superficial seeds, i.e. the pattern expected to occur after seed rain. Most studies conducted to date have pointed out that cactus seed survivorship is either comparable with or lower under plant canopies than on open spaces (reviewed by Sosa and Fleming, 2002). To our knowledge no previous study had demonstrated predator avoidance by natural nurse objects experimentally and under field conditions.
According to the results obtained here, the effects of microhabitat and protection on seed germination are highly significant (Table 1, third column). Seed germination beneath plant canopies and inside cavities is comparable with but greater than germination on flat, open spaces. There is a general agreement that the microenvironmental conditions (shade as a temperature and evaporation buffer) prevailing in shaded microhabitats is the main factor that enhances seed germination. The present findings agree with this notion, since it was found that the temperature inside cavities (range 21–28 °C) and beneath plant canopies (range 21–33 °C) is close to the optimum for germination in Cactaceae, i.e. 25 °C (Rojas-Aréchiga and Vázquez-Yanes, 2000), during the hottest part of the day (12:00–15:00 h). Both shaded microhabitats (nurse canopies and objects) also offer high humidity levels (Fig. 4) and play an important role as radiation (PAR) buffers (Table 3) relative to flat, open areas. A study performed on the Neobuxbaumia tetetzo columnar cactus showed that the shade offered by both perennials and nylon mesh enhances seed germination, and can be considered evidence that inanimate sources of shade are able to increase germination success (Valiente-Banuet and Ezcurra, 1991). In the present study, although germination was similar between shaded microhabitats, the permanence of superficial seeds inside cavities was greater than beneath canopies (Table 2). Differences in seed removal and seed germination beneath plant canopies suggest that recruitment stages are not subject to the same selection pressures in this microhabitat; conditions are favourable for seed germination but not for seed permanence.
It is assumed here, as has been assumed by authors of similar studies (e.g.Valiente-Banuet and Ezcurra, 1991; Méndez et al., 2004; Godínez-Alvarez et al., 2005; Suzán and Sosa, 2006), that the effect of mesh is negligible compared with the experimental factors under study. Temperature inside (38·11 ± 0·41 °C) and outside (38·34 ± 0·38 °C) the cages did not differ significantly (ANOVA, F1, 66 = 0·1637, P = 0·68). Therefore, shade from the cages had a minor effect, if any, on the microenvironment within them, and the exclusion of predators seems to be the main cause of the increase in germination success in the three microhabitats (Table 2). However, some additional benefit of the mesh cage (other than temperature buffering) on seed germination and seedling performance, besides the protection from predators, cannot be ruled out.
In spite of the similarity between microenvironments, seedling survivorship was higher beneath plant canopies than inside cavities. The fact that the microenvironment inside cavities is suitable for seed permanence and germination but not for seedling survivorship also suggests that there is a ‘seed–seedling conflict’ in rocky cavities. This is an unexpected result, as the microclimate variables recorded here (humidity, PAR and temperature) are very similar in these microhabitats. At present, it is not possible to pinpoint which microenvironmental variable is the cause of higher seedling mortality in the cavities. It is speculated that the walls of rocky cavities produce shade (and perhaps albedo) of a different quality from that of plant canopies. It has been demonstrated that even though PAR is constant, a modification in the red: far red ratio can produce a dramatic effect in the development of non-succulent plants (e.g. Thompson and Harper, 2004). Another study with P. leucocephalus and two other species of sympatric columnar cacti (Neobuxbaumia scoparia and Stenocereus griseus) also reported detrimental effects on the seedling survivorship of these species when they were grown under room conditions; the author suspected that light quality was the cause (Miranda-Jácome, 2005). Valiente-Banuet and Ezcurra (1991) found that artificial shade and plant canopies enhance young cactus survivorship similarly. However, their artificial shade source (green nylon net) emulated radiation filtering by plant canopies and not the shade of any microrelief element. Further research should describe light quality and assess its effects on cactus performance in the proximity of nurse objects.
Although seedling survivorship was not enhanced by rocky cavities relative to flat, open sites, it has to be remembered that this difference was evaluated using a small, equal and constant initial number of seedlings. Although unprotected seedlings died during the experiment in both microhabitats (inside cavities and on flat, open spaces), seedlings at the flat, open microhabitat died 4 months earlier. The greater permanence of superficial seeds in cavities might give their seedlings a greater chance to establish than those that germinate on flat, open spaces. The replacement of individuals in each experiment was compulsory as we were interested in detecting ‘seed – seedling’ conflicts, i.e. whether a microhabitat can be differentially suitable for seed or seedling stages. This could not have been assessed if an experiment had been conducted that continued from the seed stage through the seedling stage. Furthermore, such an experiment could be limited by the low germination rate of seeds under natural conditions.
No differences in aerial seedling biomass were found among microhabitats or between protection treatments, as might be expected owing to the slow growth rate of columnar cactus seedlings. Differences might be detected over longer periods of time. However, a red colouring was noticed in most of the surviving seedlings on the flat, open spaces, and this is a symptom of stress from excessive exposure to radiation.
The experimental design allowed the effects of soil limitation on seed germination, seedling survivorship and aerial biomass, as well as their interaction with other factors, to be tested in the field. Substrate treatments significantly affected seedling survival and seedling biomass (Table 1, fifth column). Seedlings growing on soil have a higher probability of surviving and of moving on more quickly from vulnerable life stages than those growing on rock. Although there was no significant interaction between the substrate and microhabitat factors, the soil was the same depth beneath plant canopies and inside cavities, and deeper there than on flat, open areas (Table 3). Therefore, soil accumulation under natural conditions might favour cactus recruitment in shaded microhabitats. Although not measured, one of the benefits of soil accumulation could be water retention, supposing that water content is directly related to soil accumulation (Turner et al., 1966). However, because experimental devices were used, it was not possible to assess the effect of specific water content in each microsite on seedling performance. With respect to nutrient content, the results show that both N and C were higher in shady microhabitats than on flat, open areas. C and N were not very different beneath plant canopies and inside cavities. The faster growth rate of columnar cactus seedlings on nutrient-rich soil in an experimentally shaded microhabitat has been documented (e.g. Pachycereus hollianus; Godínez-Alvarez and Valiente-Banuet, 1998). The present approach allowed the establishment of the effect of soil presence (as a substrate) on seedling performance. However, it was not possible to determine whether the slight differences in N content between shaded microhabitats had a differential effect on seedling performance because the soil used in the experiments was a mixture of soil obtained from under plant canopies and inside cavities.
How seeds arrive in cavities is an open question. Columnar cactus seeds are primarily dispersed by vertebrates; seed rain produced by these organisms frequently falls beneath the canopies of plants used as roosts (see Sosa and Fleming, 2002, and references therein). However, it was proved in this study that seed removal beneath plant canopies was in fact the highest. It is not difficult to think that ants can redistribute seed rain produced by vertebrates. Transportation of P. leucocephalus seed by ants (Solenopsis aurea, and Monomorium sp.) has been observed frequently in the study area, and might be key for seed deposition in rocky cavities. Recent works have shown that interaction between ants and non-mymercochorous seeds has a stronger influence on seed fate than previously thought, even if ants are seed ‘predators’ (see Rico-Gray and Oliveira, 2007, and references therein). Although highly plausible, post-dispersal to nurse objects by ants should be evaluated and compared among all the microhabitats. On the other hand, although seed deposition was scarce inside cavities, given the massive seed permanence in cavities recorded in this study, it would appear that the opportunities for recruitment inside cavities are high.
The mechanisms which could lead to the current spatial association of established P. leucocephalus with nurse objects and nurse plants have been discussed. Some of the previously suggested mechanisms for explaining columnar cactus establishment under perennial plant canopies have been confirmed and some that could be involved in the cactus – nurse object association have been highlighted. In this study, the focus was on the most critical stages for plant establishment: post-dispersal seed permanence, germination and early seedling performance. It can be concluded that of the mechanisms tested, enhancement of germination and seedling survivorship support the association of P. leucocephalus with nurse plants. The causes of association with nurse objects, specifically cavities, are not the same as those described for nurse plants. The greater permanence of superficial seeds and their more successful germination in cavities than on flat, open spaces could explain their association with cavities. Thus, there are different selection pressures for seeds and for seedlings beneath plant canopies and inside cavities. The presence of soil enhances seedling performance relative to bare rock. Although microhabitats can vary in their ‘quality’, the end effect on plant recruitment will be the result of the balance among partial effects during critical stages of plant life. This is the first attempt to identify the mechanisms involved in object- facilitated recruitment; however, it is important to evaluate other mechanisms such as seed deposition to explore their relevance to the trends revealed by this study.
SUPPLEMENTARY INFORMATION
Supplementary information is available online at www.aob.oxfordjournals.org and shows pictures of the three microhabitats compared in the experiments described above: a cactus growing close to the trunk of a common perennial; a young cactus growing inside a rocky cavity; and an intercanopy space.
ACKNOWLEDGEMENTS
We are grateful to O. Briones, F. Molina-Freaner and J. F. Ornelas for valuable comments on an earlier version of this manuscript. S. Galicia, N. Portilla and L. Cruz helped with the soil chemical analyses. Brenda Torales, R. Vega, E. Ruiz, J. Pech-Canche and A. Aguilar-Chama enthusiastically helped with field work. We thank Ignacio Ruiz for allowing us to work on his property. Two anonymous reviewers greatly contributed to improving the quality and focus of the manuscript. B. Delfosse revised the English. This research was partially funded by a CONACyT doctoral scholarship awarded to M.A.M.-R. (Reg. 167292).
LITERATURE CITED
- Abramsky Z. Experiments on seed predation by rodents and ants in the Israeli desert. Oecologia. 1983;57:328–332. doi: 10.1007/BF00377176. [DOI] [PubMed] [Google Scholar]
- Arriaga L, Maya Y, Díaz S, Cancino J. Association between cacti and nurse perennials in a heterogeneous tropical dry forest in northwestern Mexico. Journal of Vegetation Science. 1993;4:349–356. [Google Scholar]
- Bashan Y, Li CY, Lebsky VK, Moreno M, de Bashan LE. Primary colonization of volcanic rocks by plants in arid Baja California, Mexico. Plant Biology. 2002;4:392–402. [Google Scholar]
- Callaway RM. Positive interactions among plants. The Botanical Review. 1995;61:306–349. [Google Scholar]
- Callaway RM. Are positive interaction species-specific? Oikos. 1998;82:202–207. [Google Scholar]
- Castillo-Campos G. Biodiversidad de la selva baja caducifolia en un sustrato rocoso de origen volcánico en el centro del estado de Veracruz. Mexico: Universidad Autónoma Metropolitana; 2003. PhD Thesis. [Google Scholar]
- Drezner TD. Plant facilitation in extreme environments: the non-random distribution of saguaro cacti (Carnegiea gigantea) under their nurse associates and the relationship to nurse architecture. Journal of Arid Environments. 2006;65:46–61. [Google Scholar]
- Flores J, Jurado E. Are nurse–protégé interactions more common among plants from arid environments? Journal of Vegetation Science. 2003;14:911–916. [Google Scholar]
- Flores-Martínez A, Ezcurra E, Sánchez-Colón S. Effects of Neobuxbaumia tetetzo on growth and fecundity of its nurse plant Mimosa luisana. Journal of Ecology. 1994;82:325–330. [Google Scholar]
- Flores-Martínez A, Ezcurra E, Sánchez-Colón S. Water availability and the competitive effect of a columnar cactus on its nurse plant. Acta Oecologica. 1998;19:1–8. [Google Scholar]
- Godínez-Alvarez H, Valiente-Banuet A. Germination and early seedling growth of Tehuacán Valley cacti species: the role of soils and seed ingestion by dispersers on seedling growth. Journal of Arid Environments. 1998;39:21–31. [Google Scholar]
- Godínez-Alvarez H, Valverde T, Ortega-Baes P. Demographic trends in Cactaceae. Botanical Review. 2003;69:173–203. [Google Scholar]
- Godínez-Alvarez H, Rios-Cassanova L, Pérez F. Characteristics of seedling establishment of Stenocerus stellatus (Cactaceae) in the Tehuacán Valley, Mexico. The Southwestern Naturalist. 2005;50:375–407. [Google Scholar]
- Guzmán CU, Arias MS, Dávila P. Catálogo de cactáceas mexicanas. Mexico: Universidad Nacional Autónoma de México; 2003. [Google Scholar]
- Gulias J, Traveset A, Riera N, Mus M. Critical stages in the recruitment process of Rhamnus alaternus L. Annals of Botany. 2004;93:723–731. doi: 10.1093/aob/mch100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordano P, Herrera CM. Shuffling the offspring: uncoupling and spatial discordance of multiple stages in vertebrate seed dispersal. Ecoscience. 1995;2:230–237. [Google Scholar]
- Kleier C, Lambrinos JG. The importance of nurse associations for three tropical alpine life forms. Arctic, Antarctic, and Alpine Research. 2005;37:331–336. [Google Scholar]
- López RP, Valdivia S. The importance of shrub cover for four cactus species differing in growth form in an Andean semi-desert. Journal of Vegetation Science. 2007;18:263–270. [Google Scholar]
- McAuliffe JR. Saguaro nurse tree associations in the Sonoran Desert: competitive effects of saguaro. Oecologia. 1984;64:319–321. doi: 10.1007/BF00379128. [DOI] [PubMed] [Google Scholar]
- Martínez JG, Suzán H, Olivo CS. Aspectos ecológicos y demográficos de Neolloydia pseudopectinata (Backeberg) E. F. Anderson. Cactáceas y Suculentas Mexicanas. 1994;39:27–33. [Google Scholar]
- Méndez M, Durán R, Olmsted I. Population dynamics of Pterocereus gaumeri, a rare and endemic columnar cactus of Mexico. Biotropica. 2004;36:492–504. [Google Scholar]
- Miranda-Jácome A. Mexico: Universidad Veracruzana; 2005. Germinación y supervivencia de Cephalocereus palmeri (Rose), Neobuxbaumia scoparia (Poselger) y Stenocereus griseus en Chichicaxtle, Ver. Bachelor of Science thesis. [Google Scholar]
- Niering WA, Whittaker RH, Lowe CH. The saguaro: a population in relation to environment. Science. 1963;142:15–23. doi: 10.1126/science.142.3588.15. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Extreme temperatures and the thermal tolerances for seedlings of desert succulents. Oecologia. 1984;62:310–317. doi: 10.1007/BF00384262. [DOI] [PubMed] [Google Scholar]
- Ortega R. Vegetación y flora de una corriente de lava (mal-país) al noreste del Cofre de Perote. Biotica. 1981;6:57–84. [Google Scholar]
- Parker KC. Nurse plant relationships of columnar cacti in Arizona. Physical Geography. 1989;10:322–335. [Google Scholar]
- Pérez E, Weisz M, Lau P, Bulla L. Granivory, seed dynamics and suitability of the seed-dish technique for granivory in a neotropical savanna. Journal of Tropical Ecology. 2006;22:255–265. [Google Scholar]
- Pinheiro CJ, Bates D. Mixed-effect models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Resler LM, Butler RD, Malanson PG. Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Physical Geography. 2005;26:112–125. [Google Scholar]
- Rey PJ, Alcántara JM. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology. 2000;88:622–633. [Google Scholar]
- Reyes-Olivas A, García-Moya E, López-Mata A. Cacti–shrub interactions in the coastal desert of northern Sinaloa, Mexico. Journal of Arid Environments. 2002;52:431–445. [Google Scholar]
- Rico-Gray V, Oliveira P. Ecology and evolution of ant–plant interactions. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Rodríguez-Ortega CE, Ezcurra E. Distribución espacial en el hábitat de Mammillaria pectinifera y M. carnea en el Valle de Zapotitlán de las Salinas, Puebla, México. Cactus y Suculentas Mexicanas. 2001;45:4–14. [Google Scholar]
- Rojas-Aréchiga M, Vázquez-Yanes C. Cactus seed germination: a review. Journal of Arid Environments. 2000;44:85–104. [Google Scholar]
- Schupp EW. Seed–seedling conflicts, habitat choice and patterns of plant recruitment. American Journal of Botany. 1995;82:399–409. [Google Scholar]
- Sosa VJ, Fleming TH. Why columnar cacti are associated with nurse plants? In. In: Fleming TH, Valiente-Banuet A, editors. Evolution, ecology and conservation of columnar cacti and their mutualists. Tucson: University of Arizona Press; 2002. pp. 306–323. [Google Scholar]
- Steenbergh WF, Lowe CH. Critical factors during the first years of life of the saguaro (Cereus giganteus) at Saguaro National Monument, Arizona. Ecology. 1969;50:823–834. [Google Scholar]
- Suzán H, Sosa VJ. Comparative performance of the giant cardon cactus (Pachycereus pringlei) seedlings under two leguminous nurse plant species. Journal of Arid Environments. 2006;65:351–352. [Google Scholar]
- Suzán H, Nabhan G, Duncan P. The importance of Olneya tesota as nurse plant in the Sonoran Desert. Journal of Vegetation Science. 1996;7:635–644. [Google Scholar]
- Thompson L, Harper JL. The effect of grasses on the quality of transmitted radiation and its influence on the growth of white clover Trifolium repens. Oecologia. 2004;75:343–347. doi: 10.1007/BF00376935. [DOI] [PubMed] [Google Scholar]
- Traveset A, Gulias J, Riera N, Mus M. Transition probabilities from pollination to establishment in a rare dioecious shrub species (Rhamnus ludovici-salvatoris) in two habitats. Journal of Ecology. 2003;91:427–437. [Google Scholar]
- Turner RM, Alcorn SM, Olin G, Booth JA. The influence of shade, soil, and water on saguaro seedling establishment. Botanical Gazette. 1966;127:95–102. [Google Scholar]
- Valiente-Banuet A, Ezcurra E. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. Journal of Ecology. 1991;79:961–971. [Google Scholar]
- Valverde T, Trejo ML, Castillo S. Patrón de distribución y abundancia de Mammillaria magnimamma en la reserva del Pedregal de San Angel, México D. F. Cactáceas y Suculentas Mexicanas. 1999;44:64–74. [Google Scholar]