Abstract
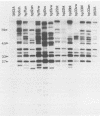
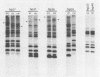
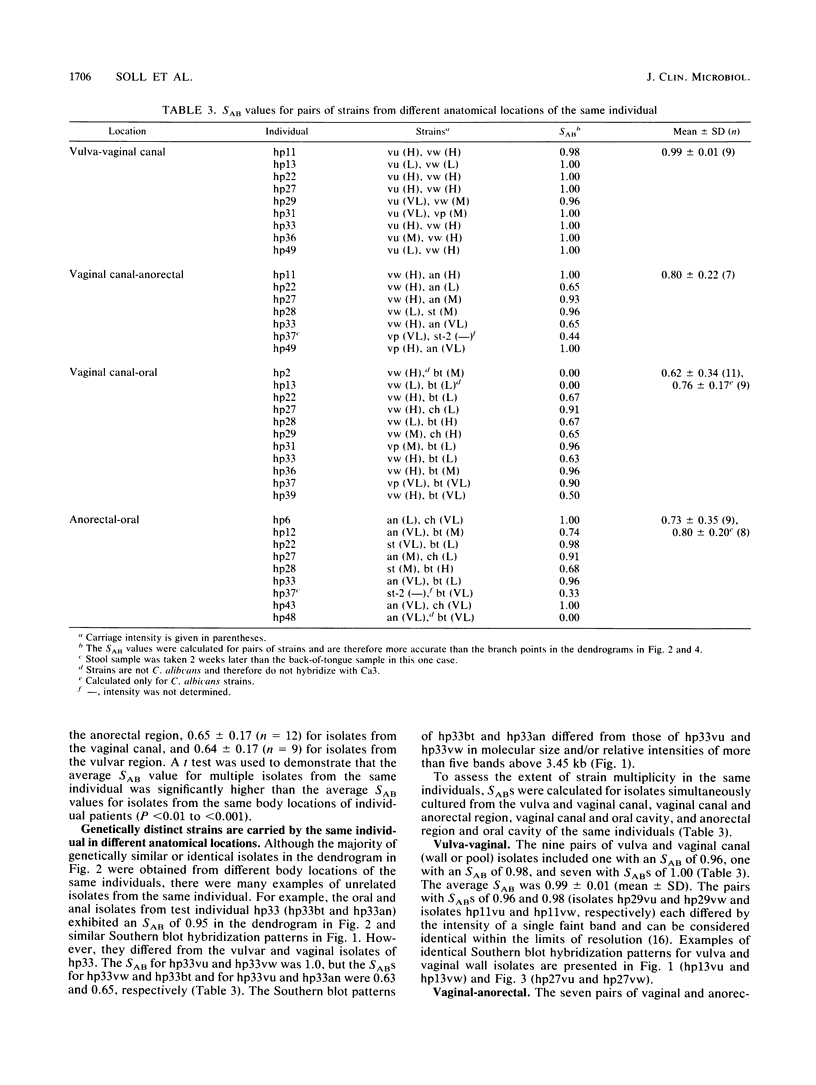
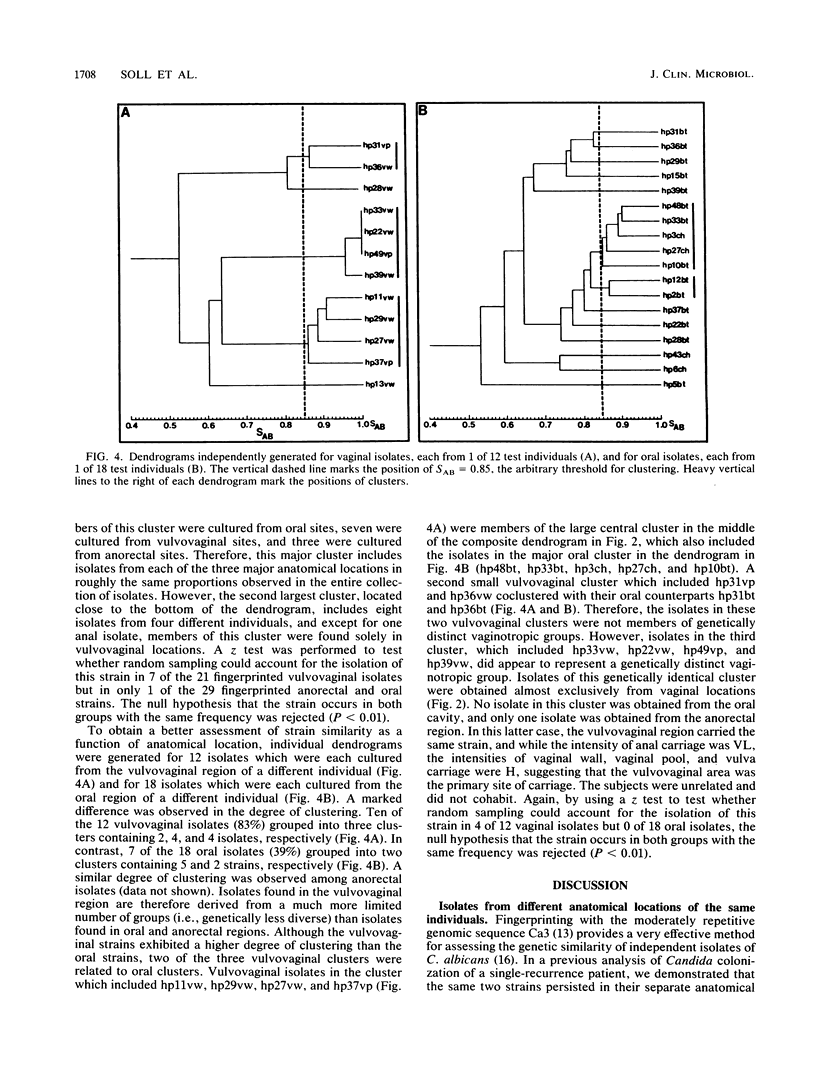
Candida spp. carriage and strain relatedness were assessed in 52 healthy women at 17 anatomical locations by using an isolation procedure which assesses carriage intensity and by using a computer-assisted DNA fingerprinting system which computes genetic similarity between strains on the basis of the patterns of Southern blots probed with the moderately repetitive sequence Ca3. Candida spp. were cultured from 73% of the test individuals, most frequently from the oral (56%), vulvovaginal (40%), and anorectal (24%) regions. Half of the test individuals with Candida spp. carried the organism simultaneously in more than one of the three general areas of carriage. Isolates from different body locations of the same individual were either completely unrelated, identical, or highly similar but nonidentical. In 11 cases in which Candida spp. were simultaneously isolated from the oral cavity and vaginal canal, seven pairs of isolates were genetically unrelated and four pairs were similar but nonidentical. In the latter cases, the isolate pairs each appear to have arisen by genetic divergence from a single progenitor. A comparison of the genetic relatedness of isolates from different individuals further uncovered a single strain which was vaginospecific in the Iowa City, Iowa area and reduced genetic diversity among vulvovaginal strains compared with those isolated from other body locations. These results suggest that strains adapt to different anatomical locations and, conversely, that in a healthy individual there is anatomical selection of vaginotropic, anotropic and orotropic strains of Candida spp.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Cundiff L., Schnars B., Gao M. X., Mackenzie I., Soll D. R. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989 Feb;57(2):458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J., Sobel J. D., Taylor M. L. Zinc status in women with recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1986 Nov;155(5):1082–1085. doi: 10.1016/0002-9378(86)90355-8. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Odds F. C. Genital candidosis. Clin Exp Dermatol. 1982 Jul;7(4):345–354. doi: 10.1111/j.1365-2230.1982.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Webster C. E., Fisk P. G., Riley V. C., Mayuranathan P., Simmons P. D. Candida species and C. albicans biotypes in women attending clinics in genitourinary medicine. J Med Microbiol. 1989 May;29(1):51–54. doi: 10.1099/00222615-29-1-51. [DOI] [PubMed] [Google Scholar]
- Romero-Piffiguer M. D., Vucovich P. R., Riera C. M. Secretory IgA and secretory component in women affected by recidivant vaginal candidiasis. Mycopathologia. 1985 Sep;91(3):165–170. doi: 10.1007/BF00446295. [DOI] [PubMed] [Google Scholar]
- Sadhu C., McEachern M. J., Rustchenko-Bulgac E. P., Schmid J., Soll D. R., Hicks J. B. Telomeric and dispersed repeat sequences in Candida yeasts and their use in strain identification. J Bacteriol. 1991 Jan;173(2):842–850. doi: 10.1128/jb.173.2.842-850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Voss E., Soll D. R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990 Jun;28(6):1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985 Aug 1;152(7 Pt 2):924–935. doi: 10.1016/s0002-9378(85)80003-x. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D. W., Speller C. D., Milne J. D., Hilton A. L., Kershaw P. I. Epidemiological investigation of patients with vulvovaginal candidosis. Application of a resistogram method for strain differentiation of Candida albicans. Br J Vener Dis. 1979 Oct;55(5):357–361. doi: 10.1136/sti.55.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]