Abstract
It took more than 100 years before it was established that the proteins that form intermediate filaments (IFs) comprise a unified protein family, the members of which are ubiquitous in virtually all differentiated cells and present both in the cytoplasm and in the nucleus. However, during the past 2 decades, knowledge regarding the functions of these structures has been expanding rapidly. Many disease-related roles of IFs have been revealed. In some cases, the molecular mechanisms underlying these diseases reflect disturbances in the functions traditionally assigned to IFs, i.e., maintenance of structural and mechanical integrity of cells and tissues. However, many disease conditions seem to link to the nonmechanical functions of IFs, many of which have been defined only in the past few years.
Introduction
Intermediate filaments (IFs) represent one of the main cytoskeletal systems found in virtually all vertebrate cells. Depending upon the cell type, IFs are composed of different members of the cytoskeletal IF protein family (Figure 1). IF proteins are also present in the nucleus, where they are the main components of the nucleoskeleton.
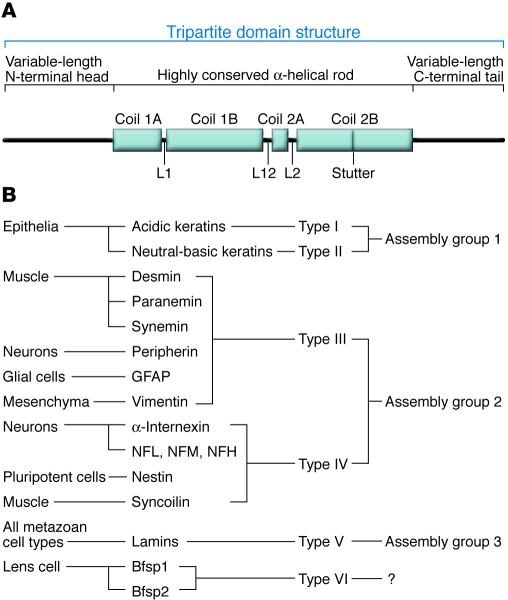
Figure 1. Overview of the IF protein family.
(A) All IF proteins share a tripartite domain structure consisting of a highly conserved α-helical central rod domain flanked by variable N-terminal head and C-terminal tail domains. (B) Based on central rod domain amino acid sequences, net acidic charge, and secondary structure predictions, cytoskeletal IF proteins were grouped into 4 sequence homology classes (types I–IV). Nuclear IF proteins, the lamins, constitute a fifth class. The sixth class of IF proteins (also referred to as orphans), beaded filament structural protein 1 (Bfsp1; also known as filensin) and Bfsp2 (also known as phakinin and CP49), form highly specialized IFs found only in the lens of the eye. Based on their abilities to copolymerize, the IF proteins of the 6 types are further subdivided into assembly groups 1–3. NFL, NFM, NFH, low–, middle–, and high–molecular weight neurofilament subunits, respectively.
In this Review we present a historical overview of the discovery and characterization of cytoplasmic and nuclear IFs. Furthermore, we explore many of the features of cytoplasmic IFs that underlie their relevance to disease (Table 1), including their dynamic properties and participation in cytoskeletal crosstalk as well as their roles in signaling, mechanical stabilization, and motility. Although IFs have been studied in various species, such as squid, Xenopus laevis, and Caenorhabditis elegans, we focus here mainly on data obtained from studies in mammalian systems.
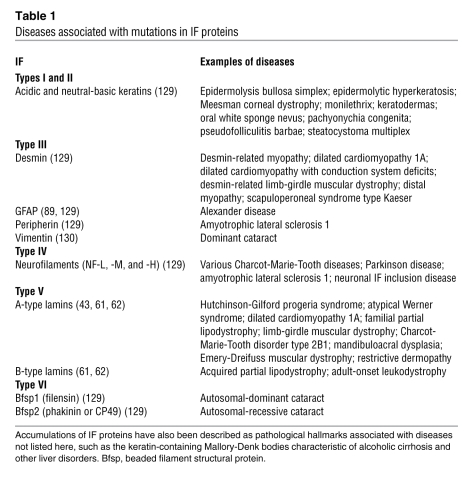
Table 1 .
Diseases associated with mutations in IF proteins
Historical overview of IFs
Early difficulties in the recognition of IFs as a distinct cytoskeletal system.
Retrospectively, the first observations of IFs were made by cytologists and histologists during the period when silver staining was used to generate contrast for observations with bright-field microscopy. For example, the structures called tonofibrils that were described in various silver-stained epithelial tissues (1) were likely the same tonofibrils described much later with antibodies directed against keratin (2) and that we now know are composed of densely packed bundles of IFs (see below).
Interest in the composition of natural fibers also stimulated early studies of IFs, which began with X-ray diffraction analyses of human hair, sheep wool, and porcupine quills (3). These studies led to the first descriptions of the α-type diffraction patterns generated by keratin IFs. Subsequently, these patterns were shown to represent coiled/coil interactions of 2 or more α-helical proteins (4). EM studies provided the first ultrastructural details of bundles of IFs (tonofibrils) in thin sections of wool, hair, and various epithelial cells, including epidermal keratinocytes (5). Over the next 20 years or so, EM revealed fibers with an average diameter of approximately 10 nm in many cell types — including those comprising skeletal, smooth, and cardiac muscle; those forming epithelial tissues, such as the epidermis; and those found in nerve tissue — as well as in cultured cells such as fibroblasts (6–11). During this period, 10-nm filaments were given many different names, including square filaments, round filaments, neurofilaments, and β filaments (12).
In addition to the variety of names, alternate hypotheses regarding the origins of the 10-nm filaments delayed the recognition of IFs as a unique protein family. For example, it was suggested that IFs were an alternately assembled form of microtubules (MTs). This idea arose from EM observations that MT inhibitors, such as colchicine and colcemid, apparently increased the number of 10-nm filaments coincident with MT depolymerization (13, 14). However, we now know that after MT depolymerization, some types of IFs retract from the cell surface and reorganize into large bundles around the nucleus (8, 9, 15, 16). At the time, it was suggested that the IFs within the bundles were another polymerized form of tubulin (13, 14). Further confusion regarding the differences between IFs and MTs stemmed from the fact that the major IF protein in fibroblasts, vimentin, has a molecular weight similar to that of tubulin, which made them difficult to separate by gel electrophoresis (17, 18). In addition, it was even considered for a brief period that IFs contained a microfilament core (19, 20). However, Ishikawa et al. suggested that the 10-nm filaments they observed in cells from developing muscle might represent a distinct class of cytoskeletal structures, which they called intermediate-sized filaments (7). This suggestion was supported by other studies in nonmuscle cells (8).
By the mid- to late 1970s, several different types of IFs had been isolated, solubilized, and purified by cycles of assembly and disassembly in vitro (21–23). These studies took advantage of the unique properties of IFs, namely, that they are insoluble under physiological conditions and are able to repolymerize after disassembly into their subunits under conditions that would irreversibly denature many proteins. Furthermore, in contrast to actin and tubulin, IFs do not require nucleoside triphosphates such as ATP or GTP for assembly, but rather self assemble under very simple conditions.
Classifying IFs.
IFs all share a common tripartite structure, consisting of a highly conserved central α-helical rod domain and variable N-terminal head and C-terminal tail domains (Figure 1A). As IF research progressed, it became evident that different cell types possessed different types of cytoskeletal IF proteins (24). For instance, epithelial cells contain mainly keratins; muscle cells, desmin; mesenchymal cells, vimentin; and neurons, neurofilaments (Figure 1B). It also became apparent that some cell types express more than one IF protein (Figure 2), and that the expression of IF proteins is frequently developmentally regulated (e.g., vimentin is detected in early developing muscle, but not in terminally differentiated muscle; refs. 25, 26). Because of this, classifying IFs based on cell type–specific expression patterns was difficult. Eventually, similarities in the α-helical rod domain sequences and assembly properties of the large number of different keratin proteins prompted the classification of cytoskeletal IFs into 4 major types according to central rod domain amino acid sequences, net acidic charge, and secondary structure predictions (27). According to this classification, type I cytoskeletal IF proteins are the acidic keratin proteins, and type II cytoskeletal IF proteins are the neutral-basic keratin proteins. At least one type I keratin and one type II keratin are needed to form heteropolymeric keratin IFs. Type III IF proteins, which include vimentin and desmin, can form homopolymeric IFs as well as heteropolymeric IFs in combination with other type III or type IV IFs. The type IV IF proteins include nestin, synemin, and the neurofilament triplet proteins.
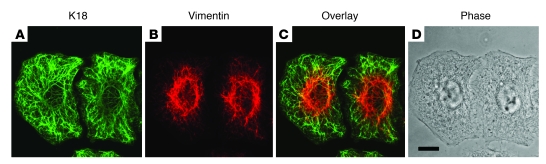
Figure 2. Coexistence of distinct keratin and vimentin IFs in an individual cell.
Human alveolar carcinoma cells were processed for immunofluorescence using antibodies against K18 (A) and vimentin (B). (C) Merged image of A and B. (D) Phase contrast image. Scale bar: 10 μM.
Type V IFs: the nuclear lamins.
The nuclear lamins are the major components of a filamentous layer, the nuclear lamina, that is closely associated with the inner nuclear membrane. This layer was originally described in ultrastructural studies of the protozoans Amoeba proteus (28, 29) and Gregarina melanoplus (30), as well as in neurons of the leech Hirudo medicinalis (31, 32), and often appeared to have a honeycomb structure. The first descriptions of a fibrous lamina in vertebrate cells came from EM observations of smooth muscle cells obtained from the guinea pig epididymis, intestinal epithelial cells from the Congo eel, and interstitial cells from the cat (33). These studies revealed a 15- to 20-nm fibrous lamina between the inner nuclear membrane and the underlying peripheral heterochromatin. Several other laboratories described a similar structure, which became known as the nuclear lamina, in various cell types and species (34, 35). Isolation of whole nuclei and of nuclear fractions resistant to detergent and high salt (termed lamina/pore complex fractions) from rat liver revealed the close association of the nuclear lamina with heterochromatin and nuclear pore complexes (36–38). These cell-free preparations also revealed that the nuclear lamina is persistent throughout the entire nuclear periphery as a 15-nm layer (37, 38), as had been observed in situ (33, 35).
Biochemical analyses revealed that the lamina/pore complex fractions isolated from rat liver contained 3 major polypeptides of approximately 60–70 kDa (37–39). Further biochemical characterization of these proteins, eventually named lamins A, B, and C according to their descending weights (40), revealed that they were closely related structurally, with lamins A and C sharing extensive sequence homology (41, 42). This latter finding suggested that lamin C might be a cleavage product of lamin A (41). However, later studies showed that lamins A and C are encoded by different mRNAs, although they are derived from a single gene (LMNA) by alternative splicing (reviewed in ref. 43). Furthermore, it was discovered that lamin A is first expressed as a precursor protein (pre-lamin A), which is posttranslationally processed to become mature lamin A (reviewed in ref. 44). With respect to lamin B, it was shown that in vertebrate somatic cells, there are 2 isoforms, lamin B1 and lamin B2, encoded by different genes (43). Similar to lamin A, lamins B1 and B2 are synthesized as precursors and subsequently modified into their mature forms (44). Besides lamins A, C, B1, and B2, other, less abundant, lamin isoforms (lamins AΔ10, C2, and B3) have been described (43). Although at least one lamin isoform appears to be present in all metazoan cells, lamins, at least as we have defined them, seem to be absent from unicellular organisms and from plants (reviewed in refs. 45, 46). It is also of interest to note that comparison of the gene structures of lamin genes and genes encoding cytoplasmic IFs suggests that all IF proteins are derived from a lamin-like progenitor (47, 48).
Early cross-linking experiments showed that the nuclear lamins can form oligomeric structures (42, 49). Even before the nuclear lamins were recognized as members of the IF protein family, sucrose-gradient density centrifugation and gel-filtration analyses of solubilized fractions of nuclear lamina preparations led to the hypothesis that the basic building blocks of lamin structures were rod-shaped dimers (50). This was supported 3 years later by the finding that lamins belong to the IF protein family, most of which are assembled from rod-shaped, parallel, and in-register dimers (51–55). Because of structural differences between the rod domains of lamins and those of cytoplasmic IF, lamins were designated as type V IF proteins (27, 55).
The generation of distinct antibodies specific for each of the lamins led to the observation that lamins A, B, and C localize mainly to the periphery of interphase nuclei (Figure 3) and that the nuclear lamina is reversibly disassembled during mitosis (40, 56, 57). Further studies revealed that the disassembly of the nuclear lamina during mitosis requires the phosphorylation of lamins at specific sites by several kinases, including p34cdc2 (reviewed in ref. 58).
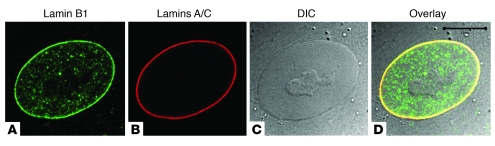
Figure 3. Lamin localization at the nuclear periphery and within the nucleoplasm.
Human dermal fibroblasts were processed for indirect immunofluorescence using antibodies against lamins B1 (A) and A/C (B). (C) Differential interference contrast (DIC) image of the cell, shown to visualize the nucleus. (D) Merged image. Scale bar: 10 μM.
Little is known about the actual structure of the nuclear lamina and of lamins in an intact nucleus. Early EM studies of the nuclear lamina of germinal vesicles from Xenopus oocytes revealed an orthogonal network of regularly spaced 10- to 15-nm fibers (51), whereas in mammalian cells, only irregular filamentous meshworks have been observed (59, 60). Besides their presence at the nuclear periphery, lamins have also been described in the nucleoplasm (Figure 3) (reviewed in ref. 61). Beginning in 1994, a series of studies demonstrated roles for lamins in DNA replication, DNA transcription, DNA repair, cell signaling, cell proliferation, and cell differentiation as well as in the structural, functional, and epigenetic organization of chromatin (61). Furthermore, it has recently been revealed that mutations in LMNA are associated with many different diseases (Table 1) (reviewed in ref. 62). The diversity of lamin-associated processes suggests that the lamins are major components of a nuclear structural framework necessary for the assembly of multiprotein complexes involved in various nuclear functions.
A turning point in IF research: IFs are dynamic cytoarchitectural elements
With the generation of specific antibodies and the widespread use of immunofluorescence, it soon became obvious that cytoskeletal IF networks formed extensive, complex arrays that pervaded most of the cytoplasm of cultured cells (17, 24). These IF networks were presumed by numerous investigators to be static, an assumption based on the finding that after extraction of cells in solutions containing relatively high concentrations of salts and detergents, IFs are retained, while most other cellular proteins (including tubulin and actin) are extracted (17). In addition, there are relatively small pools of cytosolic soluble IF proteins (63), which suggested to some that there is very little subunit exchange within IFs.
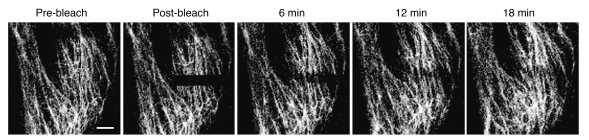
However, beginning in the late 1980s, studies revealed that ectopically expressed keratin and vimentin readily became incorporated into endogenous IFs through a process termed dynamic subunit exchange (64, 65). Similar results were obtained when IF proteins were microinjected into cultured cells (66, 67). Soon after, advances in live cell imaging technology permitted assays of IF subunit exchange in vivo using fluorescence recovery after photobleaching (FRAP; refs. 68–70). The results of these FRAP studies confirmed that subunit exchange took place all along the length of vimentin and keratin IFs (Figure 4), providing evidence that they are apolar in vivo. Subsequent studies have demonstrated specific phosphorylation sites that are involved in regulating the dynamic exchange of subunits (reviewed in refs. 71, 72). The phosphate turnover maintaining this dynamic exchange is driven by a high protein phosphatase activity on IFs, something that is also necessary for maintaining the structural integrity of IF polymers (73, 74). Further evidence supporting the dynamic properties of IFs have been derived from observations of mesenchymal cells treated with MT-depolymerizing drugs such as colchicine. As MTs disappear, IFs move toward the center of the cell, where they form a perinuclear cap (8, 9, 15, 16). There is also evidence showing that this colchicine-induced IF reorganization requires ATP and microfilaments, which suggests that it is driven by molecular motors (75–77). The redistribution of IFs from a perinuclear cap into the cytoplasmic extensions that form in spreading fibroblasts also suggested early on that IFs are involved in spreading and shape formation (9).
Figure 4. Vimentin subunit exchange as determined by FRAP.
After photobleaching (post-bleach, shown by the dark area), the exchange of GFP-tagged vimentin protein subunits within IFs occurs over the length of the bleached zone. Scale bar: 10 μM.
With the use of GFP fusion proteins as reporters for time-lapse observations, it has been shown that individual IFs change shape and propagate wave forms along their lengths (69, 70, 78). Measuring the rate of subunit turnover on adjacent fibrils in FRAP experiments revealed that filaments move at different speeds relative to each other (69, 70). Subunit exchange as well as changes in IF shape require energy, intact MTs, and intact microfilaments (69, 70, 78–80). Furthermore, the translocation of vimentin IFs is dependent on the MT-based molecular motors kinesin and dynein (80–82). These results confirmed that IFs are highly dynamic structures and brought into focus a new level of functional and regulatory capacity, discussed below (see The switch in the IF paradigm: from structural proteins to multifunctional organizers of cellular processes).
Beginning in the late 1980s and early 1990s, cytoskeletal IF proteins were shown to exist in nonfilamentous forms and in IFs of different lengths. This was especially evident in mitotic cells, in which different types of IFs disassembled into nonfilamentous aggregates that were dispersed throughout the cytoplasm (83–85). Similar nonfilamentous keratin and vimentin particles were seen immediately following their microinjection into cells. At longer times after microinjection, fewer IF particles were seen, and short IFs emerged as particles were incorporated into the endogenous IF network, further demonstrating the dynamic nature of IFs with respect to subunit incorporation (67, 68). Direct observations of these different forms of IF were also seen in cells transfected with GFP-tagged IF proteins (69, 70, 80, 86, 87). In the early stages of spreading, fibroblasts contain numerous nonfilamentous vimentin particles; these appear to form short IFs, also called squiggles, which then anneal to form long IFs (80). In epithelial cells, keratin also forms particles and squiggles (70, 78, 87). Many of the vimentin particles and squiggles move rapidly in the cytoplasm, at speeds up to 3 μm/s, whereas keratin particles and squiggles move at much slower speeds (approximately 0.3 μm/s; refs. 69, 87). The movements of the various forms of vimentin and keratin depend on MTs and microfilaments and their associated motors (70, 80, 82, 87), which suggests that IFs may be important mediators of crosstalk among all 3 cytoskeletal systems. Crosstalk among IFs, MTs, and/or microfilaments is also mediated by linker proteins, such as plectin, which can bind to multiple cytoskeletal systems (reviewed in ref. 88). In addition to molecular motors and linker proteins, numerous other proteins have been found to interact with IFs, many of which have well-described functions in distinct cellular processes.
The switch in the IF paradigm: from structural proteins to multifunctional organizers of cellular processes
Although it was clear from the above-mentioned studies that, similar to the other cytoskeletal elements, IFs are highly dynamic structures, the prevailing view of IFs during the 1990s was still that their role was primarily to maintain the structural and mechanical integrity of cells. However, this view began to change, especially with the realization that many of the diseases associated with mutations in the genes encoding IF proteins and aberrations in IF proteins could not easily be related to structural functions, but rather pointed toward involvement in a broader spectrum of functions, such as organizing cytoplasmic architecture, signaling, and/or regulating transcription (Table 1). This more recent perception is strongly supported by results obtained from studies of laminopathies, a group of diseases resulting from mutations in the genes encoding nuclear lamins. Individuals with these mutations exhibit a broad range of symptoms, from metabolic disturbances to rapidly accelerated aging in children (62). A dramatic example is Alexander disease, which is a rare, neurodegenerative disorder harming the growth of the myelin sheath (89). It is caused by mutations in glial fibrillary acidic protein (GFAP) and characterized by the formation of cytoplasmic GFAP inclusions, termed Rosenthal fibers, in astrocytes. Alterations of the myelin sheath have been suggested to result from the astrocyte dysfunction and disturbed interactions between astrocytes and other specialized brain cells (89). Furthermore, the role of IFs in maintaining cellular homeostasis has been well exemplified by numerous studies revealing that mutations in keratin 8 (K8) or K18 cause or predispose to various liver diseases (90). Cytoprotective, nonmechanical functions of keratins include protection from apoptosis.
Vimentin is the major IF protein found in mesenchymal cells. It is familiar to many researchers, as it is frequently used as a developmental marker of cells and tissues. The advances in cell biology research on vimentin are an example of how the knowledge of novel IF functions has evolved. Vimentin shows a very high degree of sequence homology among species (91, 92), implying important and evolutionary conserved physiological roles. However, until recently, the functions of this IF protein remained elusive, as the phenotype of vimentin-deficient mice seemed to be relatively mild (93). More recently, detailed analyses of these mice have revealed significant defects pointing to involvement of vimentin in several cellular functions, including regulation of the morphology of glial cells (94). In addition, vimentin-knockout fibroblasts have deficiencies in motility and directional migration that affect wound healing and fibroblast migration (95, 96). Vimentin also has a role in the mechanotransduction of shear stress, as reflected by effects on the resistance of arteries (97). Furthermore, the homing, transmigration, and extravasation of lymphocytes and the integrity of the vascular endothelium are severely affected by a lack of vimentin (98). Subsequent studies shed light on the molecular mechanisms underlying these vimentin-related defects, as vimentin has been shown to participate in a number of critical cellular processes related to the organization and regulation of proteins involved in adhesion, migration, and cell signaling. These functions explain well the observed defects in vimentin-deficient mice. These and other novel functions of IFs are further highlighted here: the examples below are not intended to be comprehensive, but rather present illustrative models of how IFs may function in regulating cellular functions and signal processing (for details on the signaling-related functions of IFs, see refs. 71, 99–102).
IFs as signaling organizers.
There is now ample evidence that IFs can act as a scaffold for and as functional determinants of signaling molecules, such as kinases (71, 99–102). In many cases, posttranslational modifications by phosphorylation or glycosylation act as regulators of the IF-related signaling functions, as they regulate IF interactions with individual signaling proteins and affect the dynamic properties of the IFs, which in turn has an effect on these interactions (71, 100, 102).
An example of an IF-kinase interaction is the interaction of vimentin with the RAF-1/RhoA signaling pathway (103, 104), which, through Rho-binding kinases (ROKs), is involved in mediating actin dynamics and focal adhesion formation (105). Activated ROKα phosphorylates vimentin, which leads to filament collapse and consequent release of ROKα from the vimentin IFs and its translocation to the periphery of the cell (106). Thus, the presence and organization of vimentin affects the outcome of RhoA-mediated signaling.
The cytoprotective effects of IFs are related to their mechanical properties. However, cytoprotection may also stem from the capacity of IFs to interact with signaling pathways involved in determining cell survival. For example, the IF protein nestin forms a scaffold with cyclin-dependent kinase 5 (Cdk5; ref. 107). In this way, nestin regulates the apoptosis-inducing activity of Cdk5 during oxidant-induced cell death (108). This function could be related to a cytoprotective role for nestin in asymmetric cell division of neuronal stem cells, during which one daughter cell survives and the other undergoes apoptosis. The surviving daughter cell has high nestin content, in contrast to the apoptotic cell, which is low in nestin content but has large amounts of the proapoptotic protein Par-4 (109). Similarly, K8 sequesters the proapoptotic protein JNK after death receptor stimulation (110). This interaction seems to prevent JNK from phosphorylating proapoptotic transcription factor targets, as c-Jun phosphorylation was clearly shown to be reduced under these conditions (110).
IFs may also regulate survival signaling by transport of stress-induced signaling molecules. This functional modality does not require polymer structures, but relies upon nonfilamentous IF precursors. It has been shown that vimentin IF precursors are able to travel along MTs with the help of kinesin and dynein motor proteins, transporting cellular cargo by “walking” on MT tracks (reviewed in ref. 111); moreover, these precursors carry along signaling molecules critical for survival processes. This transport mechanism is activated upon nerve injury, when soluble vimentin subunits transport phosphorylated ERK1 and ERK2 from the site of the axonal lesion to the nerve cell body (112). Phosphorylated ERK1/2 is linked by means of vimentin to the nuclear import factor importin, which complexes with dynein and is thereby subjected to dynein-mediated retrograde transport toward the nucleus. This is thought to be a prime reason for the elevated vimentin levels observed specifically in injured neurons (112), as vimentin is then used as a long-distance messenger for the stress-mediated signaling.
In addition to the regulation of the cytoplasmic signaling components associated with cell death and/or survival, IFs can influence cell fate by directly controlling death receptor complexes. In particular, K8/K18 filaments have been described to protect liver hepatocytes during apoptosis-promoting stress conditions, either by regulating the targeting of death receptors at the cell surface (120) or by controlling the formation of the death-inducing signaling complex (DISC) through protein sequestration and thereby controlling downstream signaling (113–115). A similar regulatory role based on the sequestration of DISC components has been suggested for hair follicle K17 in the modulation of TNF-α signaling (116). The importance of IFs has been also observed downstream in death receptor pathways, since keratins have been suggested to form platforms for caspase activation, to limit the intracellular distribution of active caspases (117), and to regulate the ability of death receptor–associated proteins to activate survival-related signaling pathways (118).
Members of the 14-3-3 protein family typically modulate the functions of signaling proteins through direct phosphorylation-dependent interactions. IFs have been suggested to have a number of roles in regulating 14-3-3 functions. For example, selective phosphorylation of K18 Ser33 during mitosis promotes the interaction between 14-3-3ζ and K18, thereby changing K18 organization and distribution (119, 120). Absence of K8, and thereby loss of K8/K18 IFs, shifts 14-3-3ζ distribution toward accumulation in the nucleus, arresting cells in S/G2 phase. The K18/14-3-3 interaction seems to regulate 14-3-3 binding to phosphorylated Cdc25, a crucial mitotic checkpoint regulator. Cdc25 — the main function of which is to remove the inhibitory phosphate residues from target cyclin-dependent kinases and thereby regulate progression of the cell cycle — can be suppressed by interaction with 14-3-3, which prohibits Cdc25 from interacting with its specific cyclin-dependent kinase. Whether 14-3-3 is bound to K18 affects the ability of 14-3-3 to interact with Cdc25. This implies that the keratin network has the capacity to prevent uncontrolled and potentially harmful 14-3-3 interactions (121).
Another intriguing example of IF/14-3-3 interactions is the interaction between K17 and 14-3-3σ. Cell growth requires strict orchestration during development and in response to various injuries. K17, an IF protein rapidly induced in wounded stratified epithelia, has been shown to regulate cell growth through binding to 14-3-3σ. The interaction with K17 regulates the Akt/mammalian target of rapamycin (Akt/mTOR) signaling pathway, which is key in the control of protein synthesis and thereby the growth of cells, tissues, and organisms (122). These findings revealed an interesting feedback loop between a wound-induced IF and a signaling pathway that regulates cell growth and size by controlling protein synthesis during the healing process.
IFs as buffers of cellular stress.
Beyond their roles in maintaining cell integrity and serving as regulators for stress-activated kinases, IFs have been assigned roles as stress proteins. For example, K8 and K18 are phosphorylated during different forms of cell stress (reviewed in ref. 100). It has been shown that the K18 Ser52→Ala52 (S52A) mutation, which makes this residue phosphorylation deficient, predisposes transgenic mice to hepatotoxic injury, leading to the postulation that this keratin phosphorylation has a hepatoprotective effect (123). The idea of protective keratin phosphorylation was recently shown to be applicable in the context of the human liver disease–associated mutation K8 Gly61→Cys61 (G61C; ref. 124). Transgenic mice overexpressing K8 with this mutation were more sensitive to liver injury and apoptosis than were wild-type mice (124). Interestingly, the G61C mutation inhibited K8 phosphorylation at Ser73, which is targeted by stress-activated protein kinases, such as p38 kinase and JNK (124). Although the susceptibility to injury of mice overexpressing a Ser73→Ala73 (S73A) mutant was the same as that of mice overexpressing the G61C mutant, it was concluded that keratins protect tissues from injuries by serving as a phosphate “sponge” that absorbs stress-induced kinase activity (124). The amino acid sequence surrounding Ser73 in K8 resembles phosphorylation motifs found in other type II keratins that become phosphorylated during stress (125), which indicates that this type of phosphate buffering could be a general phenomenon of type II keratins (124).
IFs and migration.
Focal contacts participate in the movement of cells on a substratum. While focal contacts are considered to anchor actin filaments, vimentin has also been reported to accumulate at adhesion sites at focal adhesions (126). These structures are termed vimentin-associated matrix adhesions (VMAs). Because VMAs assemble in actively migrating cells, it has been suggested that they support motility (126). The architecture of focal contacts is disturbed in vimentin-deficient fibroblasts (96), which supports a role for vimentin in regulating focal adhesions. Further support of this role came from the demonstration that the vimentin cytoskeleton regulates focal contact size and helps to stabilize cell-ECM adhesions in endothelial cells (127). Interestingly, the transcellular migration of lymphocytes through vimentin-deficient endothelial cell barriers is severely impaired when endothelial cells or lymphocytes lack vimentin. It was shown that this effect was due to the disturbed distribution of adhesion molecules (integrin β1 in lymphocytes and both ICAM1 and VCAM1 in endothelial cells) in the vimentin-deficient cells (98). Interestingly, vimentin is thought to be an important organizer of integrins, as it participates in the PKCε-mediated trafficking of integrins to the plasma membrane. In this way, vimentin modulates cell motility that is dependent on the cycling of adhesion site proteins (128).
Conclusions
The literature reveals very early published descriptions of IFs, but it took a long time for the realization that this is a very large family of proteins with highly conserved common structural and biochemical features that determine their assembly into polymers. After the paradigm of IFs as a unified protein family was established, it still took many years before it was realized that these proteins form highly dynamic structures in the same way that other cytoskeletal elements do. Recent developments have shed light on numerous disease-related functions of IFs, many of which are likely to be associated with their nonmechanical functions (Table 1). These broad-ranging, previously unknown functions of cytoplasmic IF proteins all relate to the capacity of IFs to actively sequester, position, or act as scaffolds for signaling molecules, including developmentally active and cell fate–determining kinases. The interaction with stress-activated kinases also makes them highly suitable to act as phosphate sponges in stressed cells. IFs are in rich supply in most cells, making them efficient both as stress buffers and as signaling scaffolds, even for high-abundance signaling molecules such as 14-3-3. While the knowledge of the functions of these proteins has rapidly accumulated during the past 2 decades, it seems more than likely that many important functions of IFs are yet to be discovered. In light of the numerous recently discovered signaling-related functions of IFs, it seems probable that many of these functions will be related to a broad range of signaling pathways that are important for normal homeostasis as well as for developmental and stress-related processes. Given the importance of signaling in disease onset and progress, deciphering these functions will either reveal direct IF-related links to disease processes or pave the way toward a better understanding of different disease processes.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: FRAP, fluorescence recovery after photobleaching; GFAP, glial fibrillary acidic protein; IF, intermediate filament; K, keratin; MT, microtubule.
Citation for this article: J. Clin. Invest. 119:1763–1771 (2009). doi:10.1172/JCI38339
References
- 1. Wilson, E.B. 1928.The cell in development and heredity . 3rd edition. Macmillan Company. New York, New York, USA. 1232 pp. [Google Scholar]
- 2.Jones J.C., Goldman R.D. Intermediate filaments and the initiation of desmosome assembly. J. Cell Biol. 1985;101:506–517. doi: 10.1083/jcb.101.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astbury W.T., Street A. X-ray studies of the structure of hair, wool, and related fibres. I. General. Philos. Trans. R. Soc. Lond. A. 1930;230:75–101. doi: 10.1098/rsta.1932.0003. [DOI] [Google Scholar]
- 4.Crick F.H. Is alpha-keratin a coiled coil? Nature. 1952;170:882–883. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- 5.Fraser R.D., Macrae T.P. The molecular configuration of alpha-keratin. J. Mol. Biol. 1961;3:640–647. doi: 10.1016/s0022-2836(61)80027-2. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt F.O. Fibrous proteins — neuronal organelles. Proc. Natl. Acad. Sci. U. S. A. 1968;60:1092–1101. doi: 10.1073/pnas.60.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J. Cell Biol. 1968;38:538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman R.D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 1971;51:752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman R.D., Follett E.A. Birefringent filamentous organelle in BHK-21 cells and its possible role in cell spreading and motility. Science. 1970;169:286–288. doi: 10.1126/science.169.3942.286. [DOI] [PubMed] [Google Scholar]
- 10.Rice R.V., Moses J.A., McManus G.M., Brady A.C., Blasik L.M. The organization of contractile filaments in a mammalian smooth muscle. J. Cell Biol. 1970;47:183–196. doi: 10.1083/jcb.47.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J. Cell Biol. 1976;68:539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson A., Thornell L.E. Intermediate (skeletin) filaments in heart Purkinje fibers. A correlative morphological and biochemical identification with evidence of a cytoskeletal function. J. Cell Biol. 1979;80:231–247. doi: 10.1083/jcb.80.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisniewski H., Shelanski M.L., Terry R.D. Effects of mitotic spindle inhibitors on neurotubules and neurofilaments in anterior horn cells. J. Cell Biol. 1968;38:224–229. doi: 10.1083/jcb.38.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes K.V., Choppin P.W. On the role of microtubules in movement and alignment of nuclei in virus-induced syncytia. J. Cell Biol. 1968;39:526–543. doi: 10.1083/jcb.39.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blose S.H., Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J. Cell Biol. 1976;70:459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blose S.H., Meltzer D.I., Feramisco J.R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J. Cell Biol. 1984;98:847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starger J.M., Brown W.E., Goldman A.E., Goldman R.D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J. Cell Biol. 1978;78:93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starger J.M., Goldman R.D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc. Natl. Acad. Sci. U. S. A. 1977;74:2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley I.K., Raju T.R., Stewart M. Heavy meromyosin labeling of intermediate filaments in cultured connective tissue cells. J. Cell Biol. 1978;78:644–652. doi: 10.1083/jcb.78.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley I.K., Raju T.R., Stewart M. Claims that intermediate filaments contain F-actin are unwarranted. J. Cell Biol. 1981;90:309–311. doi: 10.1083/jcb.90.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zackroff R.V., Goldman R.D. In vitro reassembly of squid brain intermediate filaments (neurofilaments): purification by assembly-disassembly. Science. 1980;208:1152–1155. doi: 10.1126/science.7189605. [DOI] [PubMed] [Google Scholar]
- 22.Zackroff R.V., Goldman R.D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc. Natl. Acad. Sci. U. S. A. 1979;76:6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinert P.M., Gullino M.I. Bovine epidermal keratin filament assembly in vitro. Biochem. Biophys. Res. Commun. 1976;70:221–227. doi: 10.1016/0006-291X(76)91131-1. [DOI] [PubMed] [Google Scholar]
- 24.Franke W.W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. U. S. A. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gard D.L., Bell P.B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc. Natl. Acad. Sci. U. S. A. 1979;76:3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger B.L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979;18:1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- 27.Steinert P.M., Roop D.R. Molecular and cellular biology of intermediate filaments. Annu. Rev. Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- 28.Mercer E.H. An electron microscopic study of Amoeba proteus. Proc. R. Soc. Lond. B Biol. Sci. 1959;150:216–232. doi: 10.1098/rspb.1959.0016. [DOI] [PubMed] [Google Scholar]
- 29.Pappas G.D. The fine structure of the nuclear envelope of Amoeba proteus. J. Biophys. Biochem. Cytol. 1956;2:431–434. doi: 10.1083/jcb.2.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beams H.W., Tahmisian T.N., Devine R., Anderson E. Ultrastructure of the nuclear membrane of a gregarine parasitic in grasshoppers. Exp. Cell Res. 1957;13:200–204. doi: 10.1016/0014-4827(57)90071-X. [DOI] [PubMed] [Google Scholar]
- 31.Coggeshall R.E., Fawcett D.W. The fine structure of the central nervous system of the leech, Hirudo medicinalis. J. Neurophysiol. 1964;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- 32.Gray E.G., Guillery R.W. An electron microscopical study of the ventral nerve cord of the leech. Z. Zellforsch. Mikrosk. Anat. 1963;60:826–849. doi: 10.1007/BF00339095. [DOI] [PubMed] [Google Scholar]
- 33.Fawcett D.W. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J. Anat. 1966;119:129–145. doi: 10.1002/aja.1001190108. [DOI] [PubMed] [Google Scholar]
- 34.Patrizi G., Poger M. The ultrastructure of the nuclear periphery. The zonula nucleum limitans. J. Ultrastruct. Res. 1967;17:127–136. doi: 10.1016/S0022-5320(67)80025-X. [DOI] [PubMed] [Google Scholar]
- 35.Oryschak A.F., Ghadially F.N., Bhatnagar R. Nuclear fibrous lamina in the chondrocytes of articular cartilage. J. Anat. 1974;118:511–515. [PMC free article] [PubMed] [Google Scholar]
- 36.Barton A.D., Kisieleski W.E., Wassermann F., Mackevicius F. Experimental modification of structures at the periphery of the liver cell nucleus. Z. Zellforsch. Mikrosk. Anat. 1971;115:299–306. doi: 10.1007/BF00324932. [DOI] [PubMed] [Google Scholar]
- 37.Aaronson R.P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc. Natl. Acad. Sci. U. S. A. 1975;72:1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J. Cell Biol. 1976;70:581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berezney R., Coffey D.S. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 1974;60:1410–1417. doi: 10.1016/0006-291X(74)90355-6. [DOI] [PubMed] [Google Scholar]
- 40.Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- 41.Cochran D.L., Yeoman L.C., Egle P.M., Shelton K.R. Comparison of the major polypeptides of the erythrocyte nuclear envelope. J. Supramol. Struct. 1979;10:405–418. doi: 10.1002/jss.400100404. [DOI] [PubMed] [Google Scholar]
- 42.Kaufmann S.H., Gibson W., Shaper J.H. Characterization of the major polypeptides of the rat liver nuclear envelope. J. Biol. Chem. 1983;258:2710–2719. [PubMed] [Google Scholar]
- 43.Broers J.L., Ramaekers F.C., Bonne G., Yaou R.B., Hutchison C.J. Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 44.Rusinol A.E., Sinensky M.S. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 2006;119:3265–3272. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- 45.Meier I. Composition of the plant nuclear envelope: theme and variations. J. Exp. Bot. 2007;58:27–34. doi: 10.1093/jxb/erl009. [DOI] [PubMed] [Google Scholar]
- 46.Melcer S., Gruenbaum Y., Krohne G. Invertebrate lamins. Exp. Cell Res. 2007;313:2157–2166. doi: 10.1016/j.yexcr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Dodemont H., Riemer D., Weber K. Structure of an invertebrate gene encoding cytoplasmic intermediate filament (IF) proteins: implications for the origin and the diversification of IF proteins. EMBO J. 1990;9:4083–4094. doi: 10.1002/j.1460-2075.1990.tb07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doring V., Stick R. Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J. 1990;9:4073–4081. doi: 10.1002/j.1460-2075.1990.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelton K.R., Guthrie V.H., Cochran D.L. Oligomeric structure of the major nuclear envelope protein lamin B. J. Biol. Chem. 1982;257:4328–4332. [PubMed] [Google Scholar]
- 50.Havre P.A., Evans D.R. Disassembly and characterization of the nuclear pore complex-lamina fraction from bovine liver nuclei. Biochemistry. 1983;22:2852–2860. doi: 10.1021/bi00281a013. [DOI] [PubMed] [Google Scholar]
- 51.Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 52.Fisher D.Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. U. S. A. 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldman A.E., Maul G., Steinert P.M., Yang H.Y., Goldman R.D. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc. Natl. Acad. Sci. U. S. A. 1986;83:3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKeon F.D., Kirschner M.W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 55.Parry D.A., Conway J.F., Steinert P.M. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem. J. 1986;238:305–308. doi: 10.1042/bj2380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J. Cell Biol. 1978;79:546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krohne G., Franke W.W., Ely S., D’Arcy A., Jost E. Localization of a nuclear envelope-associated protein by indirect immunofluorescence microscopy using antibodies against a major polypeptide from rat liver fractions enriched in nuclear envelope-associated material. Cytobiologie. 1978;18:22–38. [PubMed] [Google Scholar]
- 58.Fields A.P., Thompson L.J. The regulation of mitotic nuclear envelope breakdown: a role for multiple lamin kinases. Prog. Cell Cycle Res. 1995;1:271–286. doi: 10.1007/978-1-4615-1809-9_22. [DOI] [PubMed] [Google Scholar]
- 59.Shimi T., et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schermelleh L., et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320:1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dechat T., et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Worman H.J., Fong L.G., Muchir A., Young S.G. Laminopathies and the long strange trip from basic cell biology to therapy. J. Clin. Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soellner P., Quinlan R.A., Franke W.W. Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc. Natl. Acad. Sci. U. S. A. 1985;82:7929–7933. doi: 10.1073/pnas.82.23.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albers K., Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J. Cell Biol. 1989;108:1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ngai J., Coleman T.R., Lazarides E. Localization of newly synthesized vimentin subunits reveals a novel mechanism of intermediate filament assembly. Cell. 1990;60:415–427. doi: 10.1016/0092-8674(90)90593-4. [DOI] [PubMed] [Google Scholar]
- 66.Vikstrom K.L., Borisy G.G., Goldman R.D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller R.K., Vikstrom K., Goldman R.D. Keratin incorporation into intermediate filament networks is a rapid process. J. Cell Biol. 1991;113:843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vikstrom K.L., Lim S.S., Goldman R.D., Borisy G.G. Steady state dynamics of intermediate filament networks. J. Cell Biol. 1992;118:121–129. doi: 10.1083/jcb.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon K.H., et al. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J. Cell Biol. 2001;153:503–516. doi: 10.1083/jcb.153.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon M., Moir R.D., Prahlad V., Goldman R.D. Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 1998;143:147–157. doi: 10.1083/jcb.143.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyder C.L., Pallari H.M., Kochin V., Eriksson J.E. Providing cellular signposts — post-translational modifications of intermediate filaments. FEBS Lett. 2008;582:2140–2148. doi: 10.1016/j.febslet.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 72.Sihag R.K., Inagaki M., Yamaguchi T., Shea T.B., Pant H.C. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007;313:2098–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eriksson J.E., Opal P., Goldman R.D. Intermediate filament dynamics. Curr. Opin. Cell Biol. 1992;4:99–104. doi: 10.1016/0955-0674(92)90065-K. [DOI] [PubMed] [Google Scholar]
- 74.Toivola D.M., Goldman R.D., Garrod D.R., Eriksson J.E. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J. Cell Sci. 1997;110:23–33. doi: 10.1242/jcs.110.1.23. [DOI] [PubMed] [Google Scholar]
- 75.Rodionov V.I., et al. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J. Cell Biol. 1993;123:1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hollenbeck P.J., Bershadsky A.D., Pletjushkina O.Y., Tint I.S., Vasiliev J.M. Intermediate filament collapse is an ATP-dependent and actin-dependent process. J. Cell Sci. 1989;92:621–631. doi: 10.1242/jcs.92.4.621. [DOI] [PubMed] [Google Scholar]
- 77.Tint I.S., Hollenbeck P.J., Verkhovsky A.B., Surgucheva I.G., Bershadsky A.D. Evidence that intermediate filament reorganization is induced by ATP-dependent contraction of the actomyosin cortex in permeabilized fibroblasts. . J. Cell Sci. 1991;98:375–384. doi: 10.1242/jcs.98.3.375. [DOI] [PubMed] [Google Scholar]
- 78.Windoffer R., Leube R.E. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J. Cell Sci. 1999;112:4521–4534. doi: 10.1242/jcs.112.24.4521. [DOI] [PubMed] [Google Scholar]
- 79.Ho C.L., Martys J.L., Mikhailov A., Gundersen G.G., Liem R.K. Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 1998;111:1767–1778. doi: 10.1242/jcs.111.13.1767. [DOI] [PubMed] [Google Scholar]
- 80.Prahlad V., Yoon M., Moir R.D., Vale R.D., Goldman R.D. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gyoeva F.K., Gelfand V.I. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 1991;353:445–448. doi: 10.1038/353445a0. [DOI] [PubMed] [Google Scholar]
- 82.Helfand B.T., Mikami A., Vallee R.B., Goldman R.D. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 2002;157:795–806. doi: 10.1083/jcb.200202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou Y.H., Rosevear E., Goldman R.D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones J.C., Goldman A.E., Yang H.Y., Goldman R.D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J. Cell Biol. 1985;100:93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lane E.B., Goodman S.L., Trejdosiewicz L.K. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1:1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Windoffer R., Woll S., Strnad P., Leube R.E. Identification of novel principles of keratin filament network turnover in living cells. Mol. Biol. Cell. 2004;15:2436–2448. doi: 10.1091/mbc.E03-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woll S., Windoffer R., Leube R.E. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur. J. Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Sonnenberg A., Rojas A.M., de Pereda J.M. The structure of a tandem pair of spectrin repeats of plectin reveals a modular organization of the plakin domain. J. Mol. Biol. 2007;368:1379–1391. doi: 10.1016/j.jmb.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 89.Quinlan R.A., Brenner M., Goldman J.E., Messing A. GFAP and its role in Alexander disease. Exp. Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ku N.O., Strnad P., Zhong B.H., Tao G.Z., Omary M.B. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–1649. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- 91.Herrmann H., Fouquet B., Franke W.W. Expression of intermediate filament proteins during development of Xenopus laevis. I. cDNA clones encoding different forms of vimentin. Development. 1989;105:279–298. doi: 10.1242/dev.105.2.279. [DOI] [PubMed] [Google Scholar]
- 92.Schaffeld M., Herrmann H., Schultess J., Markl J. Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 2001;80:692–702. doi: 10.1078/0171-9335-00206. [DOI] [PubMed] [Google Scholar]
- 93.Colucci-Guyon E., et al. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 94.Colucci-Guyon E., Gimenez Y.R.M., Maurice T., Babinet C., Privat A. Cerebellar defect and impaired motor coordination in mice lacking vimentin. Glia. 1999;25:33–43. doi: 10.1002/(SICI)1098-1136(19990101)25:1<33::AID-GLIA4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 95.Eckes B., et al. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000;113:2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 96.Eckes B., et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- 97.Henrion D., et al. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J. Clin. Invest. 1997;100:2909–2914. doi: 10.1172/JCI119840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nieminen M., et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 99.Ivaska J., Pallari H.M., Nevo J., Eriksson J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 100.Omary M.B., Ku N.O., Tao G.Z., Toivola D.M., Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem. Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Pallari H.M., Eriksson J.E. Intermediate filaments as signaling platforms. Sci. STKE. 2006;2006:pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 102.Toivola D.M., Tao G.Z., Habtezion A., Liao J., Omary M.B. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15:608–617. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Ehrenreiter K., et al. Raf-1 regulates Rho signaling and cell migration. J. Cell Biol. 2005;168:955–964. doi: 10.1083/jcb.200409162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janosch P., et al. The Raf-1 kinase associates with vimentin kinases and regulates the structure of vimentin filaments. FASEB J. 2000;14:2008–2021. doi: 10.1096/fj.99-0883com. [DOI] [PubMed] [Google Scholar]
- 105.Amano M., Fukata Y., Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 106.Sin W.-C., Chen X.-Q., Leung T., Lim L. RhoA-Binding Kinase alpha Translocation Is Facilitated by the Collapse of the Vimentin Intermediate Filament Network. Mol. Cell. Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sahlgren C.M., et al. Cdk5 regulates the organization of Nestin and its association with p35. Mol. Cell. Biol. 2003;23:5090–5106. doi: 10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sahlgren C.M., et al. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bieberich E., MacKinnon S., Silva J., Noggle S., Condie B.G. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J. Cell Biol. 2003;162:469–479. doi: 10.1083/jcb.200212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He T., Stepulak A., Holmstrom T.H., Omary M.B., Eriksson J.E. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J. Biol. Chem. 2002;277:10767–10774. doi: 10.1074/jbc.M111436200. [DOI] [PubMed] [Google Scholar]
- 111.Helfand B.T., Chou Y.H., Shumaker D.K., Goldman R.D. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005;15:568–570. doi: 10.1016/j.tcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 112.Perlson E., et al. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 113.Caulin C., Ware C.F., Magin T.M., Oshima R.G. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J. Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gilbert S., Loranger A., Daigle N., Marceau N. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J. Cell Biol. 2001;154:763–773. doi: 10.1083/jcb.200102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Inada H., et al. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J. Cell Biol. 2001;155:415–426. doi: 10.1083/jcb.200103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tong X., Coulombe P.A. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev. 2006;20:1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dinsdale D., Lee J.C., Dewson G., Cohen G.M., Peter M.E. Intermediate filaments control the intracellular distribution of caspases during apoptosis. Am. J. Pathol. 2004;164:395–407. doi: 10.1016/S0002-9440(10)63130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilbert S., Loranger A., Marceau N. Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol. Cell. Biol. 2004;24:7072–7081. doi: 10.1128/MCB.24.16.7072-7081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ku N.O., Liao J., Omary M.B. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao J., Omary M.B. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J. Cell Biol. 1996;133:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Toivola D.M., et al. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology. 2001;34:1174–1183. doi: 10.1053/jhep.2001.29374. [DOI] [PubMed] [Google Scholar]
- 122.Kim S., Wong P., Coulombe P.A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 123.Ku N.O., et al. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J. Cell Biol. 1998;143:2023–2032. doi: 10.1083/jcb.143.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ku N.O., Omary M.B. A disease- and phosphorylation-related nonmechanical function for keratin 8. J. Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Toivola D.M., Zhou Q., English L.S., Omary M.B. Type II keratins are phosphorylated on a unique motif during stress and mitosis in tissues and cultured cells. Mol. Biol. Cell. 2002;13:1857–1870. doi: 10.1091/mbc.01-12-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gonzales M., et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsuruta D., Jones J.C. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. . J. Cell Sci. 2003;116:4977–4984. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- 128.Ivaska J., et al. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Szeverenyi I., et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 130.Müller M., et al. Dominant cataract formation in association with a vimentin assembly-disrupting mutation. Hum. Mol. Genet. 2009;18:1052–1057. doi: 10.1093/hmg/ddn440. [DOI] [PubMed] [Google Scholar]