Abstract
Maintenance of vascular integrity is critical for homeostasis, and temporally and spatially regulated vascular leak is a central feature of inflammation. Sphingosine-1-phosphate (S1P) can regulate endothelial barrier function, but the sources of the S1P that provide this activity in vivo and its importance in modulating different inflammatory responses are unknown. We report here that mutant mice engineered to selectively lack S1P in plasma displayed increased vascular leak and impaired survival after anaphylaxis, administration of platelet-activating factor (PAF) or histamine, and exposure to related inflammatory challenges. Increased leak was associated with increased interendothelial cell gaps in venules and was reversed by transfusion with wild-type erythrocytes (which restored plasma S1P levels) and by acute treatment with an agonist for the S1P receptor 1 (S1pr1). S1pr1 agonist did not protect wild-type mice from PAF-induced leak, consistent with plasma S1P levels being sufficient for S1pr1 activation in wild-type mice. However, an agonist for another endothelial cell Gi-coupled receptor, Par2, did protect wild-type mice from PAF-induced vascular leak, and systemic treatment with pertussis toxin prevented rescue by Par2 agonist and sensitized wild-type mice to leak-inducing stimuli in a manner that resembled the loss of plasma S1P. Our results suggest that the blood communicates with blood vessels via plasma S1P to maintain vascular integrity and regulate vascular leak. This pathway prevents lethal responses to leak-inducing mediators in mouse models.
Introduction
Sphingosine-1-phosphate (S1P), a lipid phosphate produced in the course of sphingosine metabolism in all cell types (1), promotes endothelial cell spreading and barrier function in cell culture (2–5) and in vivo (6, 7). S1P can regulate cell behavior via 5 GPCRs, designated S1P receptor 1 (S1pr1) through S1pr5 (also known as S1P1–S1P5) (1, 4, 8). Models of receptor-dependent roles for S1P in regulating endothelial barrier function have focused on S1P produced by the endothelial cells themselves, casting S1P as a downstream, autocrine/paracrine mediator of the barrier-protective effects of other agents such as activated protein C (9, 10) and angiopoietin (7). However, S1P is present at high concentrations in plasma (11), and the importance of this source of S1P in regulating vascular integrity has not been examined. In addition, GPCR-independent S1P signaling mechanisms and cell-autonomous metabolic effects of disrupting sphingosine conversion to S1P have been reported and may affect vascular integrity (1–5, 7, 12, 13). Central to understanding the physiological roles of S1P in regulating blood vessel function are identification of the sources of S1P that are important for barrier protection in vivo as well as determination of the importance of S1P from blood acting in trans on endothelial cells by receptor-dependent mechanisms versus S1P from endothelial cells acting in cis by autocrine or receptor-independent mechanisms.
Synthesis of S1P from sphingosine requires 2 partially redundant sphingosine kinases (Sphks), Sphk1 and Sphk2 (1). Toward identifying the sources of extracellular S1P important for signaling functions in the adult, and to circumvent the embryonic lethality associated with global loss of S1P synthesis (14), we generated a mouse with one conditional Sphk1 allele and one null Sphk1 allele in an Sphk2-null background and carrying a myxovirus resistance 1–Cre (Mx1-Cre) transgene (15, 16). When Cre expression was induced by poly(I:C) treatment of Mx1-Cre Tg+/0:Sphk1fl/–:Sphk2–/– mice, plasma S1P levels became undetectable (15). Mx1-Cre excises floxed genes efficiently in liver and hematopoietic cells and variably in other cell types (16, 17). Further study of poly(I:C)-induced Mx1-Cre Tg+/0:Sphk1fl/–:Sphk2–/– mice, hereafter referred to here as “pS1Pless” (plasma S1Pless) mice, revealed that hematopoietic cells, particularly erythrocytes, are a major source of S1P in plasma (15). Thus, pS1Pless mice provided an opportunity to assess the importance of S1P supplied to the plasma compartment in maintaining vascular integrity and in regulating permeability responses to inflammatory mediators in adult mice. We provide strong evidence that S1P from plasma plays a central role in these processes.
Results
To determine whether S1P in plasma contributes to the regulation of vascular permeability in vivo, we first examined extravasation of Evans blue/albumin at baseline (Figure 1). Thirty minutes after i.v. injection of Evans blue, lungs from pS1Pless mice had approximately 3.5-fold higher Evans blue content than lungs from their S1P-sufficient littermates, hereafter referred to as “control mice”. Consistent with increased baseline extravasation of Evans blue in lung, hindpaw thickness was increased slightly in pS1Pless mice at baseline (control: 100% ± 3.5%, n = 60; pS1Pless: 102.4% ± 4.9%, n = 42; P = 0.005), perhaps reflecting mild dependent edema.
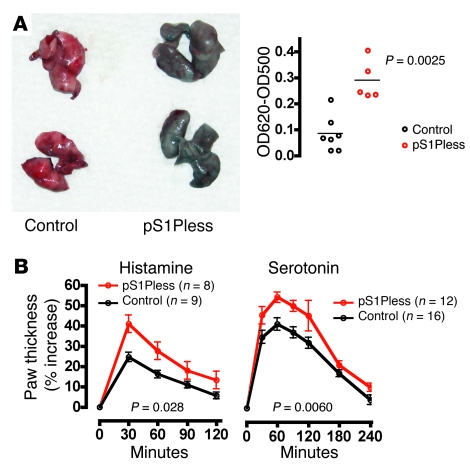
Figure 1. pS1Pless mice exhibit basal vascular leak and increased local response to leak-inducing agents.
(A) Basal leak. Evans blue (1 mg/100 μl saline) was injected i.v., and 30 minutes later, mice were perfused with saline via the right ventricle, lungs were removed and photographed, and Evans blue content was determined. Left: Representative control and pS1Pless lungs. Right: Evans blue quantitation. Each point represents data for a separate mouse. The horizontal bars denote the mean. (B) Induced paw edema. Histamine (60 μg) or serotonin (20 μg) were injected into the hindpaws of pS1Pless mice and their control littermates. The contralateral paw was injected with vehicle. (Agent-injected paw thickness) / (vehicle-injected paw thickness) was determined at the indicated times and expressed as percent increase. Data are mean ± SEM. Note that responses to leak-inducing agents were higher in pS1Pless mice.
pS1Pless mice also showed increased responses to leak-inducing challenges. Hindpaw thickness increased 41% in pS1Pless, compared with 25% in control mice after local injection of histamine (Figure 1). Increase after local serotonin injection was 58% in pS1Pless versus 44% in controls (Figure 1). Basal leak and response to VEGF in a Miles assay were also increased (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI38575DS1).
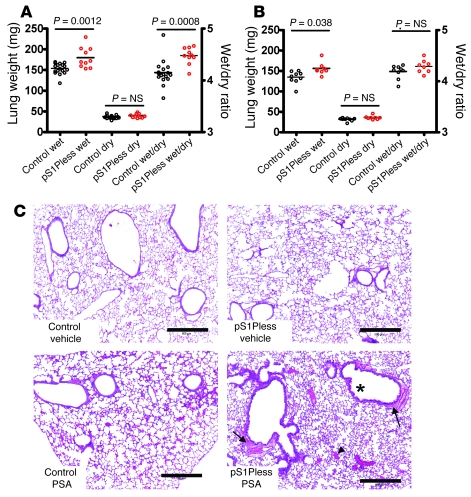
In a model of passive systemic anaphylaxis (PSA), all control mice survived antigen challenge, but 44% of pS1Pless mice died (Figure 2A). Within 90 seconds of antigen administration, hematocrit increased 10% over baseline in control mice and 23% in pS1Pless mice (Figure 2B). Lung wet weight and wet/dry weight ratio also increased more in pS1Pless mice than in controls (Figure 3A), and histological analysis of lungs showed increased peribronchial fluid (Figure 3C). These findings are all consistent with increased extravasation of plasma in pS1Pless mice.
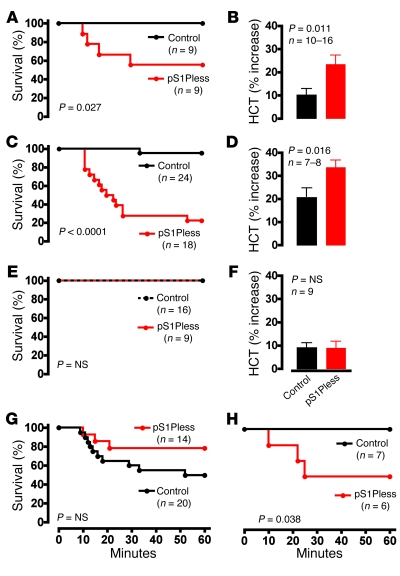
Figure 2. pS1Pless mice exhibit markedly increased sensitivity to systemic challenge with leak-inducing agents and are rescued by erythrocyte transfusion and acute S1pr1 agonism.
(A) Survival after induction of PSA. (B) Hematocrit (HCT) determined 90 seconds after PSA induction. The change from baseline hematocrit, determined more than 7 days previously, is shown (mean ± SEM). (C) Survival after PAF injection (20 μg/kg i.v.). (D) Hematocrit measured 10 minutes after PAF injection. The change from baseline hematocrit, determined more than 7 days previously, is shown (mean ± SEM). (E and F) Mice were transfused with wild-type erythrocytes and studied 2 days later. (E) Survival of transfused mice after PAF injection (20 μg/kg i.v.). (F) Hematocrit determined 90 seconds after induction of PSA. The change from the hematocrit determined 2 hours previously is shown (mean ± SEM). Data were corrected for the drop in hematocrit caused by the blood draw at –2 hours. (G) Mice were injected with the S1pr1 agonist AUY954 (2 mg/kg i.v.) 2 minutes before PAF injection (20 μg/kg i.v.), and survival was followed. (H) Survival of pS1Pless mice and littermate controls after i.v. injection of histamine (200 mg/kg).
Figure 3. pS1Pless mice exhibit increased fluid accumulation in lung during PSA.
(A and B) Lung wet, dry, and wet/dry weights in pS1Pless mice (red circles) and controls (black circles) 90 seconds after DNP-albumin challenge in PSA model without (A) and 2 days after (B) transfusion with wild-type erythrocytes. Note that without transfusion, lung wet/dry weights were increased in pS1Pless mice relative to control mice after PSA, and this difference was absent after transfusion. P values were determined using the Student’s t test. Each point represents data for one mouse. The horizontal bars denote the mean. (C) Histology of lungs fixed 8 minutes after PSA (H&E stain) showing peribronchial fluid accumulation (arrows), likely representing extravasated plasma in lymphatics. This feature was much more prominent in pS1Pless mice. Alveolar wall thickening, suggesting interstitial fluid accumulation, red blood cells in airspace (arrowhead), and epithelial blebbing (asterisk), were also more prominent in pS1Pless mice compared with controls after PAF injection. Scale bars: 500 μm.
The increased sensitivity of pS1Pless mice to PSA could be due to elevated sensitivity to leak-inducing mediators or to their enhanced production. Rises in plasma histamine levels during PSA were similar in control and pS1Pless mice (Supplemental Figure 2), militating against increased production. Conversely, responses to exogenous histamine and to platelet-activating factor (PAF), major mediators of anaphylaxis in mice (18, 19), were greatly exaggerated in pS1Pless mice. After i.v. PAF injection, survival was severely impaired in pS1Pless mice (Figure 2C), and hematocrit again increased significantly more in pS1Pless mice than in wild-type (Figure 2D). Similar results were seen after histamine injection (Figure 2H). The rapid increase in hematocrit after PAF injection was not prevented by splenectomy and hence did not result from mobilization of erythrocytes from spleen; PAF challenge caused Evans blue extravasation in multiple organs including heart, lung, skin, liver, and gut; and repletion of blood volume with i.v. saline prevented death after PAF treatment (Supplemental Figure 3). Thus, death of pS1Pless mice after PAF injection was likely caused by widespread and exaggerated extravasation of plasma with depletion of intravascular volume, and the increased response of pS1Pless mice to PSA was likely due to their enhanced responsiveness to leak-inducing inflammatory mediators.
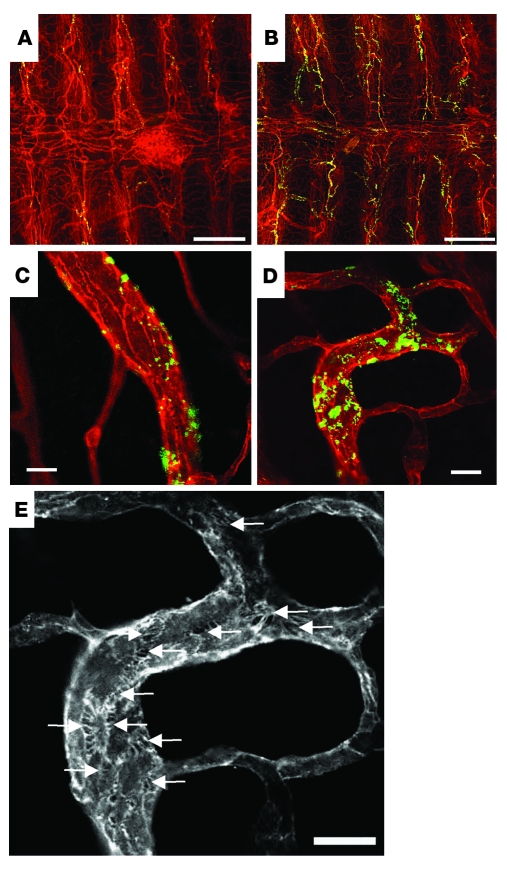
To more directly examine pS1Pless mice for altered control of vascular leak, we assessed extravasation of fluorescent microspheres via interendothelial cell gaps in blood vessels in the trachea (Figure 4 and Supplemental Figure 4), which features stereotyped and readily visualized vascular anatomy (20). No extravasation of microspheres was detected in the absence of PAF challenge. In mice challenged with PAF, microspheres accumulated in the walls of the postcapillary venules that run longitudinally along the tracheal cartilage rings. Collections of microspheres were more numerous and were generally larger in pS1Pless mice than in control mice (Figure 4 and Supplemental Figure 4); the area occupied by microspheres in low-power images of tracheas from PAF-treated pS1Pless mice (determined using NIH ImageJ software) was about 3.4- ± 0.5-fold higher than that in PAF-treated littermate controls (n = 4–5; P = 0.001). The extravasated microspheres were located at the cell-cell junctions, often at large interendothelial cell gaps that were widespread in tracheal preparations from PAF-treated pS1Pless mice but not PAF-treated controls. Analysis of lung tissue by transmission electron microscopy also suggested exaggerated formation of interendothelial cell gaps as well as increased hemoconcentration and vasoconstriction in PAF-treated pS1Pless mice (Supplemental Figure 5).
Figure 4. pS1Pless mice exhibit increased extravasation of fluorescent microspheres via interendothelial cell gaps.
Control (A and C) and pS1Pless (B, D, and E) mice were injected i.v. with 500 nm fluorescent microspheres together with PAF (20 μg/kg i.v.) or vehicle, then perfused with saline 3 minutes later. Tracheas were removed, whole-mount immunostained for the endothelial marker PECAM, opened, laid flat, and imaged using confocal fluorescence microscopy. (A and B) Merged z-stacks at low power with microspheres (green) and PECAM (red). (C and D) Representative single-plane images at high power. (E) Enlarged image of D showing only the PECAM channel. Arrows point to intercellular gaps bridged by filopodia-like extensions. Note widespread accumulation of microspheres in pS1Pless (B) compared with control (A) in venules overlying tracheal rings, which run vertically in the photo. Accumulations were also larger in pS1Pless mice (D versus C) and occurred at sites corresponding to intercellular gaps (D and E). Scale bars: 250 μm (A and B), 10 μm (C–E). See Supplemental Figure 4 for additional representative images from independent preparations.
The results described above demonstrate that Sphk function in Mx1-Cre–sensitive cell types is required to maintain normal vascular integrity and to prevent lethal responses to leak-inducing inflammatory mediators in mice. Mx1-Cre is widely used to trigger excision of floxed alleles in hematopoietic lineages and hepatocytes, and (Mx1-Cre–induced) pS1Pless mice lacked S1P in serum (Supplemental Figure 6) and in platelet-poor plasma (15), consistent with efficient hematopoietic excision and with the notion that the pS1Pless leak phenotype is due to loss of a plasma S1P–dependent function. However, Mx1-Cre–mediated gene excision in endothelial cells has been reported (17), and it was possible that vascular leak in pS1Pless mice might represent an endothelial cell–autonomous phenotype associated with loss of endothelial Sphk activity. We did not detect expression of EYFP or lacZ reporter alleles for Cre-mediated excision in endothelial cells in skin, lung, or trachea from adult mice using our Mx1-Cre induction protocol (Supplemental Figure 7). However, some excision of the floxed Sphk1 allele was detected by PCR and Southern blot analysis of DNA from endothelial cells immunopurified and cultured from the skin of neonatal pS1Pless mice. We therefore used a more direct and functional test of whether vascular leak in pS1Pless mice might be attributable to an endothelial cell–autonomous phenotype as a result of loss of endothelial cell S1P production, by determining whether restoration of S1P to plasma might rescue the pS1Pless phenotype.
Erythrocytes are a major source of plasma S1P, and transfusion of pS1Pless mice with wild-type erythrocytes restores S1P levels in plasma (15). To probe whether supplying S1P to plasma might suffice to prevent the pS1Pless leak phenotype, we asked whether erythrocyte transfusions could reverse the increased sensitivity of pS1Pless mice to PSA and PAF. This was indeed the case (compare Figure 2, E and F, with Figure 2, C and B; compare Figure 3B with Figure 3A). Of note, S1P is highly compartmentalized in tissues — low in interstitial spaces and high in plasma and lymph. Plasma and lymph are themselves distinct S1P compartments supplied by distinct sources; transfusion of pS1Pless mice with wild-type erythrocytes or transplantation with wild-type bone marrow restores S1P specifically to plasma and not to lymph (15). The observation that altered vascular permeability in pS1Pless mice can be rescued by restoring S1P selectively to plasma again suggests a model in which vascular integrity and responses to leak-inducing agents are regulated by plasma S1P acting in trans on the blood vessel wall.
S1pr1 is expressed at relatively high levels in human (21) and mouse (Supplemental Table 1) endothelial cells, and activation of this receptor is barrier protective in endothelial cultures (2). Moreover, it was recently reported that pharmacological S1pr1 antagonism increases basal Evans blue extravasation in lung in a manner similar to that seen in pS1Pless mice (6, 22). The other S1P receptors expressed in mouse endothelial cells, S1pr2 and S1pr3, are found at lower levels than S1pr1 (Supplemental Table 1) and are barrier disruptive in culture studies (2, 23, 24). The concentration of S1P in plasma is approximately 1–3 μM, and it is about 98.5% protein bound (4). This suggests that free plasma S1P concentration is about 15–45 nM, substantially above the approximately 0.5-nM IC50 for S1P inhibition of 32P-S1P binding to S1pr1 (4). In accord with these values, the concentration of S1P in wild-type plasma is at least 10-fold higher than that needed to downregulate S1pr1 on circulating lymphocytes and is sufficiently decreased in pS1Pless plasma to permit upregulation of S1pr1 on these same cells (15). Thus, endothelial cells are probably exposed to S1pr1-saturating concentrations of S1P in wild-type but not in pS1Pless mice, and a lack of endothelial S1pr1 activation might account for the pS1Pless phenotype. Accordingly, we tested the possibility that pharmacological S1pr1 agonism might reverse the increased sensitivity of pS1Pless mice to leak-inducing challenge. Administration of the S1pr1-specific agonist AUY954 i.v. (25, 26) 2 minutes prior to PAF eliminated the increased sensitivity of pS1Pless mice (compare Figure 2G with Figure 2C). The same treatment failed to protect wild-type mice against LD50 PAF (data not shown), perhaps because plasma S1P is already present at S1pr1-saturating concentrations in wild types. The possible reduction in sensitivity to PAF of pS1Pless mice compared with control mice suggested in Figure 2G might be due to rapid partial desensitization of S1pr1 by AUY954 in the controls and/or to relative upregulation of S1pr1 in the pS1Pless mice at baseline due to a lack of plasma S1P (resulting in an increased effect of AUY954 in pS1Pless compared with wild-type mice). Taken together with the results described above, these results suggest that S1P in plasma maintains normal vascular integrity and is necessary to prevent lethal responses to leak-inducing inflammatory mediators such as histamine and PAF, and that S1pr1 signaling over minutes might account for these functions.
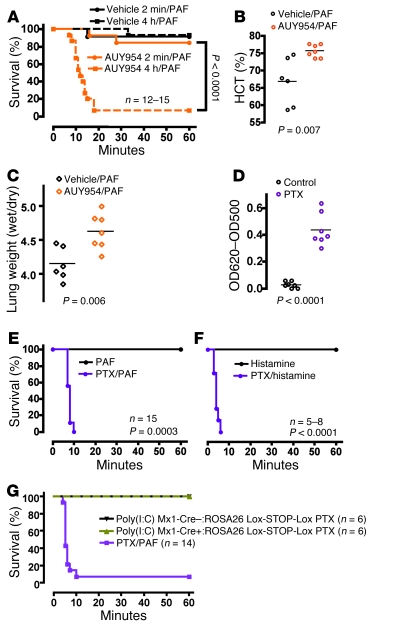
Some pharmacological S1pr1 agonists can be more effective than S1P itself at downregulating S1pr1, likely by increasing the probability that internalized receptors are delivered to lysosomes instead of recycling to the plasma membrane (27). Such agonists can also activate and downregulate S1pr1 in interstitial spaces in which S1P is normally absent (15, 25). Pharmacological activation and downregulation of S1pr1 on lymphocytes in the lymphoid interstitium is associated with failed lymphocyte egress and reduced peripheral lymphocyte counts (25). AUY954 indeed dramatically reduced lymphocyte counts in wild-type mice within 2 hours of administration, with a nadir between 2 and 8 hours (Supplemental Figure 8). Of note, wild-type mice exposed to AUY954 for 4 hours became sensitive to PAF (Figure 5, A–C), similar to untreated pS1Pless mice (Figure 2, C and D), but a 2-minute treatment with AUY954 had no effect (Figure 5A). The ability of prolonged but not brief AUY954 exposure to cause a pS1Pless-like phenotype in wild-type mice is likely due to its ability to downregulate S1pr1 and, together with our other results, is consistent with the model that plasma S1P and S1pr1 comprise a system that conditions or regulates responses to leak-inducing challenge.
Figure 5. Effect of perturbing S1pr1 and Gi function on sensitivity to leak-inducing agents.
(A) Wild-type mice were injected with vehicle or AUY954 (2 mg/kg i.v.) either 2 minutes (solid orange line) or 4 hours (dashed orange line) prior to PAF injection (10 μg/kg i.v.), and survival was followed as a function of time. (B and C) Wild-type mice were injected with AUY954 or vehicle 4 hours before i.v. PAF challenge, and hematocrit (B) and lung weights (C) were determined 10 minutes after PAF. Note that prolonged but not brief exposure to AUY954 sensitized wild-type mice to PAF. Similar results were obtained in 4 separate experiments. Each point represents data for a separate mouse. The horizontal bars denote the mean. (D–F) Mice received vehicle or PTX (400 ng i.v.). Three days later, basal Evans blue extravasation into lung (D) and survival after PAF injection (E; 10 μg/kg i.v.) and histamine (F; 200 mg/kg i.v.) were assessed. In D, each point represents data for a separate mouse, and the horizontal bars denote the mean. (G) Poly(I:C)-induced Mx1-Cre–:ROSA26 Lox-STOP-Lox PTX S1, poly(I:C)-induced Mx1-Cre+:ROSA26 Lox-STOP-Lox PTX S1, or wild-type mice treated with i.v. PTX (400 ng) received PAF (10 μg/kg i.v.), and survival was followed. Note the failure of hematopoietic PTX expression to sensitize mice to PAF.
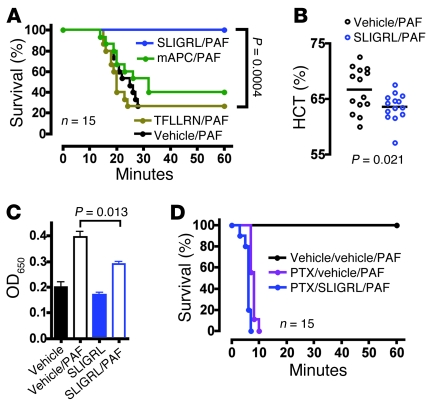
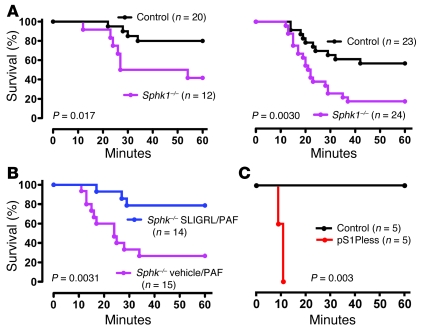
S1pr1 is Gi coupled (21) and activates Rac, actin reorganization, lamellipodial extension, cell spreading, and junction formation in cultured cells (2, 3, 28). If S1P provides barrier-protective signals via S1pr1 and Gi in vivo, pertussis toxin (PTX) should trigger endothelial leak similar to plasma S1P deficiency and S1pr1 downregulation. PTX has long been known to sensitize mice in anaphylaxis models (29), and a single i.v. injection of PTX indeed resulted in constitutive leak in lung as well as increased hemoconcentration and markedly impaired survival to both PAF and histamine (Figure 5, D–F, and Figure 6D) — a phenotype similar to but more severe than that seen in pS1Pless mice (Figure 1A and Figure 2, C and H). Of note, poly(I:C)-induced Mx1-Cre+:ROSA26 Lox-STOP-Lox PTX S1 mice (30), which express the PTX catalytic subunit in all hematopoietic cells, failed to show sensitization to PAF (Figure 5G), suggesting that systemic PTX sensitizes mice to leak by acting on non-hematopoietic cells. These results are consistent with the notion that S1pr1 and other receptors that couple to Gi in endothelial cells limit permeability responses to leak-inducing agents in vivo. They also raised the possibility that, while S1pr1 agonist might not benefit wild-type mice because saturating concentrations of S1P are already present in plasma, agonists for other Gi-coupled receptors might. Par2 (also known as F2rl1) is expressed in microvascular endothelial cells (31) (Supplemental Table 1) and, like S1pr1, activates Gi and Rac in human umbilical venous endothelial (32) and EA.hy926 cells (33). Administration of i.v. SLIGRL, a peptide agonist for Par2, provided striking protection against hemoconcentration and death from high-dose PAF in wild-type mice (Figure 6, A and B). No effect of SLIGRL was seen in Par2–/– mice. SLIGRL decreased the barrier-disruptive effects of PAF in monolayers of mouse endothelial cells grown in culture (Figure 6C), consistent with SLIGRL acting directly on endothelial cells and consistent with previous results (34). In accord with protection by SLIGRL being mediated by Par2 activation of Gi, SLIGRL was no longer protective in mice treated with PTX (Figure 6, compare panels D and A). The protective effect of SLIGRL persisted in Sphk1–/– mice (Figure 7, A and B), suggesting that protective signaling by Par2 and the S1P system converge on Gi signaling rather than being linked in an Sphk1-dependent PAR to S1P receptor cascade, as has been proposed for activated protein C (9). However, in contrast to AUY954, acute treatment with SLIGRL did not rescue the increased sensitivity of pS1Pless mice to PAF (Figure 7C). The difference between AUY954 and SLIGRL in their ability to blunt the pS1Pless phenotype might be due to differences in the onset, duration, magnitude, or location of their actions in vivo or to qualitative or quantitative differences between S1pr1 and Par2 signaling, and S1pr1 signaling might promote or permit the effects of Par2 activation.
Figure 6. Effect of Par2 activation on sensitivity to leak-inducing agents.
(A) Wild-type mice were injected with Par2 agonist peptide (SLIGRL; 12 μmol/kg i.v.), Par1 agonist peptide (TFLLRN; 12 μmol/kg i.v.), or mouse activated protein C (mAPC; 0.4 mg/kg i.v.), followed 2 minutes later with high-dose PAF injection (40 μg/kg i.v.), and survival was followed. Similar results were obtained in 2 additional experiments. (B) Wild-type mice were injected with Par2 agonist peptide (SLIGRL; 12 μmol/kg i.v.), followed 2 minutes later with high-dose PAF injection (40 μg/kg i.v.), and hematocrit levels were determined 10 minutes later. Although hematocrits increased in both cases, the increase was blunted by SLIGRL treatment. Each point represents data for a separate mouse. The horizontal bars denote the mean. (C) Confluent mouse microvascular endothelial cell monolayers in transwells were treated with SLIGRL (100 μM) or vehicle control for 2 minutes prior to PAF (200 nM) or vehicle injection. After 10 minutes, Evans blue/albumin was added to the top chamber. The OD650 of medium in the bottom chamber, determined 10 minutes later, is shown (mean ± SEM; n = 3). Similar results were obtained in 2 additional experiments. (D) Wild-type mice received vehicle or PTX (400 ng i.v.). Three days later, mice received vehicle or SLIGRL (12 μmol/kg i.v.) and PAF (10 μg/kg i.v.) injections as indicated, and survival was followed.
Figure 7. Effect of global Sphk1 deficiency on sensitivity to PAF and of Par2 agonism on sensitivity to PAF in Sphk1–/– and pS1Pless mice.
(A) Survival of Sphk1–/– females (left) and males (right) and littermate controls (Sphk1+/– and Sphk1+/+) after PAF (20 μg/kg i.v.). Note that Sphk1 global knockouts were sensitized to PAF challenge, but much less so than pS1Pless mice. (B) Sphk1–/– females were co-injected with Par2 agonist peptide (SLIGRL;12 μmol/kg i.v.) or vehicle control and PAF (30 μg/kg i.v.), and survival was followed. Note persistence of protection in the absence of Sphk1. (C) pS1Pless mice and littermate controls received SLIGRL (12 μmol/kg i.v.) and PAF (20 μg/kg i.v.), and survival was followed. Note the lack of protection by SLIGRL compared with wild-type (Figure 6A) and Sphk1–/– mice (B).
In contrast to SLIGRL, Par1 agonism by TFLLRN or activated protein C failed to protect wild-type mice from PAF challenge at the doses tested (Figure 6A). Par1 can couple to Gi, but it also couples efficiently to Gq and G12/13 and can promote Rho activation over Rac and barrier disruption, under some conditions (5, 32, 33). Thus, the ability of specific receptors to prevent leak responses may be determined by the balance and/or tempo of activation of different G proteins, the effect of receptor subcellular location and scaffolding proteins on effector pathways, and other factors beyond simply their ability to activate Gi.
Discussion
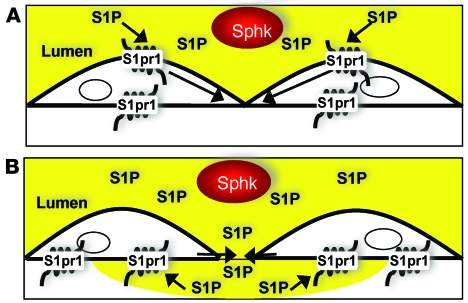
Taken together, our results provide strong genetic and pharmacological evidence that S1P supplied to plasma from erythrocytes and other sources maintains vascular integrity and prevents lethal responses to leak-inducing agents in adult mice, and that it does so by a mechanism that involves moment-to-moment S1pr1 signaling, most likely in endothelial cells. The importance of plasma S1P in this context raises interesting possibilities. S1P in plasma might act upon endothelial S1pr1 continuously to sustain endothelial spreading and cell-cell junctions. Alternatively, differences in S1pr1 signaling at the lumenal versus ablumenal surface of endothelial cells, whether at the level of the receptor or downstream effectors, might permit detection of S1P in plasma entering the subendothelial space and hence dynamic signaling that senses and terminates leaks (Figure 8). These models are not mutually exclusive, and both have theoretical appeal. A constant, barrier-protective tone might set a threshold that must be exceeded for inflammatory stimuli to trigger leak responses. A dynamic leak detector might sense successful extravasation of plasma in response to inflammatory stimuli and provide rapid negative feedback that limits the size of interendothelial cell gaps and/or the duration of gap opening. A need for quick occupancy of ablumenal S1pr1 in response to plasma leak might also account for the high concentration of S1P maintained in plasma (4, 15). We expect our results will stimulate exploration of these ideas.
Figure 8. Working models of how plasma S1P might communicate with endothelial cells.
(A) Tonic signaling model. S1P from plasma (yellow) within the blood vessel lumen continuously interacts with endothelial cell S1pr1, providing a constant signal (arrows) that maintains cell spreading and cell-cell junctions and sets a threshold that decides responses to leak-inducing agents. (B) Dynamic signaling model. Qualitative or quantitative differences in S1pr1 function at the lumenal versus ablumenal plasma membrane allow the endothelial cell to detect S1P in plasma entering the subendothelial space. Such a leak detector mechanism might provide negative feedback to close intercellular gaps opened in response to leak-inducing agents.
Korhonen and colleagues recently demonstrated that Gq/11 signaling in endothelial cells is required for lethal responses to PSA, histamine, and PAF in mouse models (35). Our demonstration that plasma S1P–dependent Gi signaling blunts such responses complements this study and supports the notion that GPCR signaling plays a central role in both triggering and limiting vascular leak.
Reversion of the permeability phenotype in pS1Pless mice by erythrocyte transfusion to restore plasma S1P levels and by acute S1pr1 agonism suggests that endothelial cell–autonomous mechanisms are unlikely to account for this phenotype. However, our results in no way imply that loss of Sphk function in endothelial cells cannot cause important phenotypes. Indeed, global knockout of Sphk1 function, which does not decrease plasma S1P levels enough to permit upregulation of S1pr1 on lymphocytes in plasma but does remove Sphk1 function from endothelial cells, results in a leak phenotype that resembles that seen in pS1Pless mice but that is substantially less dramatic (Figure 7A) (7, 36–38). The ability of Sphk1 deficiency alone to confer some increased susceptibility to PAF is consistent with observations in other models (7, 36). How much of this phenotype is due to toxic/metabolic effects of altered sphingosine metabolism in endothelial cells or other cells, loss of a local source of S1P for autocrine or paracrine signaling to S1pr1 or other receptors, or still other mechanisms is unknown. The fact that plasma S1P levels are only modestly reduced in Sphk1–/– mice (36, 39) (our unpublished observations) suggests that Sphk1 functions independent of supplying S1P to plasma also contribute to vascular integrity.
Angiopoietin 1 from smooth muscle cells and pericytes acting upon endothelial cell Tie2 constitutes another system required for vascular integrity (40). Interestingly, the effect of plasma S1P deficiency on vascular leak was most obvious in postcapillary venules, which do not express Tie2 (41). Thus, the S1P/S1pr1 and Ang1/Tie2 systems may be anatomically separate and complementary, with the former being most important in postcapillary venules where large physiological excursions in permeability occur and are dynamically regulated, and the latter being most important in large vessels and terminal and precapillary arterioles (41) where permeability is constantly maintained at a low level (Figure 4, Supplemental Figure 4, and data not shown).
Drugs that activate and downregulate S1pr1 have the ability to modulate lymphocyte trafficking and immune function and are currently in clinical development (42). The relative importance of S1P and S1pr1 for maintenance of vascular integrity may, of course, differ in mice and humans, the effects of chronic S1pr1 agonism may differ from the relatively short-term effects studied here, and different parts of the concentration response curve may be relevant. Nonetheless, our finding that S1P signaling is important for resisting lethal responses to leak-inducing agents suggests that any indications of altered vascular permeability in clinical trials, particularly in the setting of allergy, should be pursued. Conversely, agonism for Par2 or other Gi-coupled endothelial cell receptors might be explored for acutely limiting endothelial permeability in appropriate settings.
Methods
Mice.
All experiments compared cohorts of mice of the same sex except for that shown in Figure 5G; the groups shown in that study were mixed males and females. In all experiments, mice were more than 8 weeks old at the time of the study and were age matched (within 1 week for studies done with wild-type C57BL/6 mice; littermate controls for the studies with pS1Pless mice and other mutant mice). Wild-type C57BL/6 mice were used for pharmacological experiments in which no mutants were involved. Generation of global (–) and conditional (fl) knockout alleles for these genes and their conditional deletion by poly(I:C) induction in Sphk1fl/–:Sphk2–/– neonates carrying the Mx1-Cre transgene (16), to generate plasma S1P–deficient Sphk1fl/–:Sphk2–/–:Mx1Cre+ (pS1Pless) mice, has been previously described (15). Controls for pS1Pless mice were sex-matched littermates that had at least 1 functional Sphk allele and were also poly(I:C) treated. Most were of the Sphk1fl/+:Sphk2–/–:Mx1Cre+ genotype, but Sphk1fl/+:Sphk2–/–:Mx1Cre– and Sphk1fl/–:Sphk2–/–:Mx1Cre– mice were also examined. No difference in response was observed among these genotypes. Again, all individual experiments compared mice of the same sex, and qualitatively similar results were found whether experiments were done with males or females. Successful Cre-mediated excision in pS1Pless mice was confirmed in several ways. Decreased circulating lymphocyte counts are expected due to the egress defect associated with loss of plasma S1P (15), and loss of plasma S1P was confirmed in each experimental mouse by measuring peripheral lymphocyte counts using a Hemavet (Drew Scientific) and/or by measuring plasma S1P levels directly by mass spectrometry (15). Median peripheral lymphocyte counts from control and pS1Pless mice were approximately 4200 and 800 per microliter, respectively, and median plasma total S1P levels as determined by mass spectrometry were 2454 and 27 nM, respectively (Supplemental Figure 6). Poly(I:C)-induced Sphk1fl/–:Sphk2–/–:Mx1Cre+ mice that had peripheral lymphocyte counts above 2000/μl or total plasma S1P levels above 200 nM were not used in experiments. Excision in hematopoietic lineages was assessed by flow cytometry of platelets from poly(I:C)-induced Mx1-Cre mice carrying the ROSA26-Lox-STOP-Lox-YFP excision reporter (a gift of Nigel Killeen, UCSF; ref. 43) (Supplemental Figure 6).
All experimental procedures were approved by the UCSF Institutional Animal Care and Use Committee.
Paw edema.
Histamine (60 μg; Sigma-Aldrich) or serotonin (20 μg; Sigma-Aldrich) were injected into the right posterior footpad of pS1Pless mice or littermate controls in a volume of 20 μl. Vehicle control was injected into the contralateral footpad. Paw thickness was measured with a caliper (Mitutoyo) before and every 30 minutes after injection for up to 4 hours. Results were expressed as percentage increase in paw thickness in the treated paw minus percentage increase in thickness of the control paw. Control paw thickness typically peaked at 30 minutes, and average maximal increase was 12%.
Evans blue extravasation in lung.
We injected 100 μl of a 1% solution of Evans blue dye (Sigma-Aldrich) in saline into the lateral tail vein of wild-type or pS1Pless mice or wild-type mice pretreated with PTX 3 days beforehand (Calbiochem; 400 ng i.v.), with AUY954 (2 mg/kg for 4 hours), or with vehicle control. Thirty minutes later, mice were anesthetized and perfused with saline via the right ventricle to remove intravascular Evans blue. Lungs were excised and extracted in 1 ml of formamide at 55°C overnight. Evans blue content was determined as OD620 minus OD500 of the formamide extract. AUY954 was a gift from Novartis.
PSA and systemic vascular challenge.
To trigger PSA, mice received 3 μg of anti-dinitrophenol (anti-DNP; Sigma-Aldrich) IgE i.v., then 300 μg of DNP-albumin (Sigma-Aldrich) i.v. the next day. Survival was followed. In separate mice, 90 seconds after antigen injection, blood samples were obtained from the right ventricle and lungs were excised, dabbed dry, and weighed immediately (wet weight) and again after drying at 65°C for 3 days (dry weight). Hematocrits were determined with a Hemavet (DREW Scientific) and plasma histamine levels by ELISA (Beckman Coulter) and were compared with pre-challenge values (obtained approximately 14 days prior, except where otherwise indicated). Where indicated, 350 μl of packed red blood cells were injected i.v. (15) 2 days prior to challenge. For studies of survival, mice were continuously observed for 60 minutes following DNP-albumin injection and the time of death recorded. In modifications of the above protocol, wild-type or pS1Pless mice received i.v. PAF (Calbiochem) or histamine (Sigma-Aldrich), and hematocrits and organ weights or survival times were determined as described above. Doses and time points, as well as whether mice were sensitized with PTX, are indicated in the figure legends. Where indicated, mice were administered i.v. AUY954 or vehicle control 4 hours or 2 minutes prior to challenge or were co-injected with PAF or histamine and SLIGRL, TFLLRN or activated protein C without alterations to the total injection volume.
Vascular leak in mouse trachea.
The anatomic specificity and structural basis for vascular leak was assessed in mouse trachea, essentially as previously described (44). PAF or vehicle control was injected together with 500 nm fluorescent microspheres (Duke Scientific) and allowed to circulate for 3 minutes prior to perfusion with 1% fresh PFA for 8 minutes via the left ventricle. Tracheas were dissected free and postfixed in 1% PFA/PBS for 2 hours, then immunostained with rat (Chemicon) and hamster (Pharmingen) anti-CD31 antibodies, followed by Cy3-conjugated goat anti-rat and anti-hamster antibodies (Jackson ImmunoResearch Laboratories Inc.) to visualize endothelial cells. Trachea were flat mounted and endothelial cells and beads visualized from the luminal surface with an MRC-1024 laser scanning confocal microscope (Biorad Laboratories).
Assessment of endothelial excision.
Mx1-Cre Tg+/0 males were crossed to females homozygous for Lox-STOP-Lox β-galactosidase (ROSA26R; The Jackson Laboratory) or EYFP excision reporters in the ROSA26 locus. Neonates from these crosses were poly(I:C) induced as previously described (15). Tie2-Cre (a gift of Masashi Yanagisawa, University of Texas Southwestern, Dallas, Texas, USA; ref. 45) was crossed to the β-galactosidase reporter as a positive control. To assess excision of the β-galactosidase reporter, adult Cre+ and Cre– control mice were anesthetized and perfused with saline followed by β-galactosidase staining solution (46) via the left ventricle. Trachea, esophagus, brain, small intestine, thymus, and liver were excised, fixed in 2% PFA/0.2% glutaraldehyde, and stained overnight (46). To assess excision of the EGFP reporter, trachea, liver, and thymus were excised, stained for PECAM, and processed for confocal imaging as described above.
Endothelial cell isolation and culture.
Primary mouse microvascular endothelial cells (31) were plated (200,000/well) on fibronectin-coated (25 μg/ml) 0.4-μm pore size polyester inserts in a 6-well, dual-chamber system (Transwells; Costar). Growth medium was supplied to both basal and apical compartments and changed every second day. After 5 days, the endothelial monolayers were washed in serum-free medium, and agonist was added to the apical compartment in serum-free medium at the concentrations indicated in Figure 6C. After 10 minutes of agonist treatment, media in both compartments were replaced with 4 % BSA serum-free medium and, in the upper compartment only, 0.67 mg/ml Evans blue. Ten minutes later, the OD620 of medium sampled from the lower compartment was measured to assess protein leak across the endothelial monolayer. Quantitative PCR profiling of GPCR expression in primary mouse endothelial cells was performed as previously described (30).
Histology.
Mice were anesthetized with ketamine/xylazine and perfused with saline, followed with 4% PFA via the right ventricle. For lung histology, perfusion with fixative was retrograde from the abdominal aorta, so as to leave the chest wall intact and avoid collapse of the lungs prior to fixation. We also instilled 4% PFA into the trachea. Organs were excised, postfixed in 4% PFA overnight, dehydrated, and embedded in paraffin. We stained 5-μm sections with Masson’s trichrome or H&E.
Statistics.
Kaplan-Meier data were analyzed using the log-rank statistic with GraphPad Prism software. Other analyses used t test or ANOVA with t test. Results were considered significant when P < 0.05. A Bonferroni-corrected threshold was applied for multiple comparisons.
Supplementary Material
Acknowledgments
S.R. Coughlin and coworkers are funded by the NIH. The authors thank Linda Prentice, Leonor Patel, and Cherry Concengco for technical support; Donald McDonald and Peter Baluk for advice; and Jason Cyster, Henry Bourne, Orion Weiner, Donald McDonald, and Peter Baluk for critical reading of the manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: DNP, dinitrophenol; Mx1-Cre, myxovirus resistance 1–Cre; PAF, platelet-activating factor; pS1Pless, plasma S1Pless; PSA, passive systemic anaphylaxis; PTX, pertussis toxin; S1P, sphingosine-1-phosphate; S1pr1, S1P receptor 1; Sphk1, sphingosine kinase 1.
Citation for this article: J. Clin. Invest. 119:1871–1879 (2009). doi:10.1172/JCI38575
References
- 1.Spiegel S., Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Garcia J.G., et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M.J., et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/S0092-8674(00)81661-X. [DOI] [PubMed] [Google Scholar]
- 4.Rosen H., Sanna M.G., Cahalan S.M., Gonzalez-Cabrera P.J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Mehta D., Malik A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sanna M.G., et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P(1) antagonist in vivo. Nat. Chem. Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 7.Li X., et al. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–3497. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol. Res. 2003;47:401–407. doi: 10.1016/S1043-6618(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 9.Feistritzer C., Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 10.Finigan J.H., et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 11.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Don A.S., et al. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J. Biol. Chem. 2007;282:15833–15842. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- 13.Mizugishi K., et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizugishi K., et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappu R., et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 17.Leuker C.E., Labow M., Muller W., Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs b cell localization and t cell-dependent humoral immune response. J. Exp. Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii S., et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkelman F.D.2007Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 120506–515; quiz 516–517. . 10.1016/j.jaci.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 20.McDonald D.M. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am. J. Physiol. 1994;266:L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.J., Evans M., Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J. Biol. Chem. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- 22.Foss F.W., Jr. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg. Med. Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez T., et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 24.Gon Y., et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Pan S., et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Niessen F., et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Cabrera P.J., Hla T., Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J. Biol. Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeldt H.M., et al. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 2001;15:2649–2659. doi: 10.1096/fj.01-0523com. [DOI] [PubMed] [Google Scholar]
- 29.Munoz J., Bergman R.K. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol. Rev. 1968;32:103–126. doi: 10.1128/br.32.2.103-126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regard J.B., et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J. Clin. Invest. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camerer E., Kataoka H., Kahn M., Lease K., Coughlin S.R. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J. Biol. Chem. 2002;277:16081–16087. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- 32.Vouret-Craviari V., Grall D., Van Obberghen-Schilling E. Modulation of Rho GTPase activity in endothelial cells by selective proteinase-activated receptor (PAR) agonists. J. Thromb. Haemost. 2003;1:1103–1111. doi: 10.1046/j.1538-7836.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaneider N.C., et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat. Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feistritzer C., Lenta R., Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J. Thromb. Haemost. 2005;3:2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 35.Korhonen H., et al. Anaphylactic shock depends on endothelial Gq/G11. J. Exp. Med. 2009;206:411–420. doi: 10.1084/jem.20082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkataraman K., et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkataraman K., et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tauseef M., et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivera A., et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Thurston G., et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 41.Cho C.H., et al. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ. Res. 2005;97:86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann V., Cyster J.G., Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 43.Srinivas S., et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baffert F., Le T., Thurston G., McDonald D.M. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H107–H118. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kisanuki Y.Y., et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 46.Schlaeger T.M., et al. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.