Abstract
Much is known about the immunomodulatory effects of opiate exposure and withdrawal in adult rats. However, little research has delved into understanding the immunological consequences of prenatal opiate exposure and postnatal withdrawal. The purpose of the current study was to measure changes in responding to immune stimulation in adult rats following prenatal opiate exposure. Further, we sought to characterize the role of interleukin (IL)-1β in these changes. Following prenatal exposure to the long-acting opiate l-alpha-acetylmethadol (LAAM), adult male and female rats were assessed for their fever response to lipopolysaccharide (LPS). Blood and tissue samples were collected to measure circulating IL-1β and IL-1β protein in the hypothalamus and spleen. Prenatal LAAM exposure resulted in a blunted fever response to LPS injection without any changes in basal body temperature or in response to saline injection. Circulating IL-1β was not affected by prenatal LAAM exposure, nor was IL-1β protein in the spleen. Interestingly, mature IL-1β protein was elevated in the hypothalamus of prenatally LAAM-treated rats. These results indicate that prenatal opiate exposure blunts the fever response of adult offspring. Direct action of IL-1β is likely not the cause of the dysfunction reported here. However, alterations in signaling mechanisms downstream from IL-1β may play a role in the altered fever response in adult rats treated prenatally with opiates.
Keywords: Lipolysaccharide, LAAM, IL-1β, prenatal, fever
1. Introduction
It is well established that exogenous opiate administration, as well as opiate withdrawal, can alter immune responding. This is often, but not always, manifest as immunosuppression (e.g., McCarthy et al., 2001; Sacerdote et al., 2003; Wei et al., 2003, West et al., 1999). Three major mechanisms have been hypothesized for these effects: direct involvement of opioid receptors, hypothalamic pituitary adrenal (HPA) axis activation, and increased sympathetic nervous system activity. There is clear evidence for a role of opioid receptors, as mu-opiate receptor knockout (MORKO) animals did not display the same degree of immunosuppression as wild-type mice (Gavériaux-Ruff et al., 1998; Roy et al., 1998). Pharmacological studies have likewise implicated mu opiate receptors (e.g., Nelson et al., 2000; reviewed by Carr et al., 1996). Studies using MORKO mice (Wang et al., 2002), as well as adrenalectomy to remove circulating glucocorticoids, demonstrated that HPA axis hormones could modulate immune function following opiate exposure (Fecho et al., 1996). Other studies have implicated mediation by the sympathetic nervous system. For example, chlorisondamine, a nicotinic ganglionic receptor antagonist, altered the effects of morphine exposure on multiple immune parameters (Bencsics et al., 1997; Fecho et al., 1996; Houghtling and Bayer, 2002; Wang et al., 2002). Blockade of beta-adrenergic receptors in the periphery also counteracted morphine’s immunosuppressive effects (Fecho et al., 1993).
Most studies thus far have focused on examining immune competence shortly after opiate exposure and/or withdrawal in adult animals. Like the adult, the developing organism may respond to exogenous opiate exposure by manifesting altered immune competence. The exposure to opiates during pregnancy followed by neonatal withdrawal could have a significant adverse impact on immunity both shortly after birth, as well as throughout life. These effects may be especially profound since the immune system, the HPA axis, and the sympathetic nervous system begin development prior to birth (Kennedy & Ziegler, 2000; Zakharova et al., 2000); therefore, these systems may be perturbed by prenatal insults such as exposure to and/or withdrawal from opiates. A few animal studies in this area have found that the response to immune stimulation in juveniles is dysfunctional following prenatal morphine exposure (Shavit et al., 1998; Martin et al., 1998). In addition, Martin et al. (1996) found that prenatal morphine exposure interacted with the stress response to modulate immune functioning in adult rats. However, to date the mechanisms for these effects have not been explored.
The aim of the present study was two-fold, to replicate the prior studies using an opiate with pharmacological properties that differ from those of morphine and to extend the previous research by beginning to examine potential mechanisms for the immunomodulatory effects of prenatal opiate exposure. First, we determined the immunological consequences of prenatal dependence on the long-acting opiate l-alpha-acetylmethadol (LAAM) followed by postnatal withdrawal. LAAM is a full mu opioid receptor agonist, but differs from other commonly studied opiates in that it is much longer-acting due to the presence of active metabolites (Henderson et al., 1977; York et al., 2002). Morphine has a short half-life in the rat, such that even with twice-daily injections withdrawal signs are present (Gellert and Sparber, 1979). While drugs used in opiate substitution therapy are generally longer acting, methadone has a half-life of less than 3 hr in the rat (Misra et al., 1973). The presence of active metabolites lengthens the duration of LAAM’s action, such that with daily administration, withdrawal signs are not present in rats, unless antagonist-challenged. Another advantage of LAAM is that it has good bioavailability and can be orally administered, eliminating problems associated with other methods of chronic drug administration during pregnancy.
After establishing that prenatal exposure to LAAM produced similar results as that of prenatal morphine exposure on LPS-induced fever, we began to examine potential mechanisms for this effect. We had previously demonstrated in a developing chicken model that the fever response to LPS and the hypersensitivity response to phytohemagglutinin (PHA) were blunted following prenatal exposure to the LAAM metabolite desmethyl-l-α-noracetylmethadol (NLAAM) (Schrott and Sparber, 2004). This suggested alterations in both humoral and cell-mediated immunity, but mechanisms of these changes were not studied. Likewise, mechanisms were not examined in the studies mentioned above that reported attenuated responding to immune stimulation after prenatal morphine exposure. Thus, we extended the previous findings by determining the role of one potential mechanism, namely alterations in IL-1β. We measured circulating IL-1β concentrations, and splenic and hypothalamic IL-1β protein. We chose to examine protein levels in the brain and spleen in lieu of mRNA since IL-1β mRNA undergoes post-translational modification by caspases to convert an inactive precursor protein into the active cytokine (Beuscher et al., 1990; Black et al., 1988; Martinon et al., 2002). Protein analysis has been used successfully by Deak et al. (2005) to examine stress-induced changes in splenic IL-1β. Therefore, by measuring IL-1β protein rather than mRNA, we were able to assess the presence of a functional relationship between IL-1β and fever.
2. Results
2.1 Basal Body Temperature and Response to Saline Injection
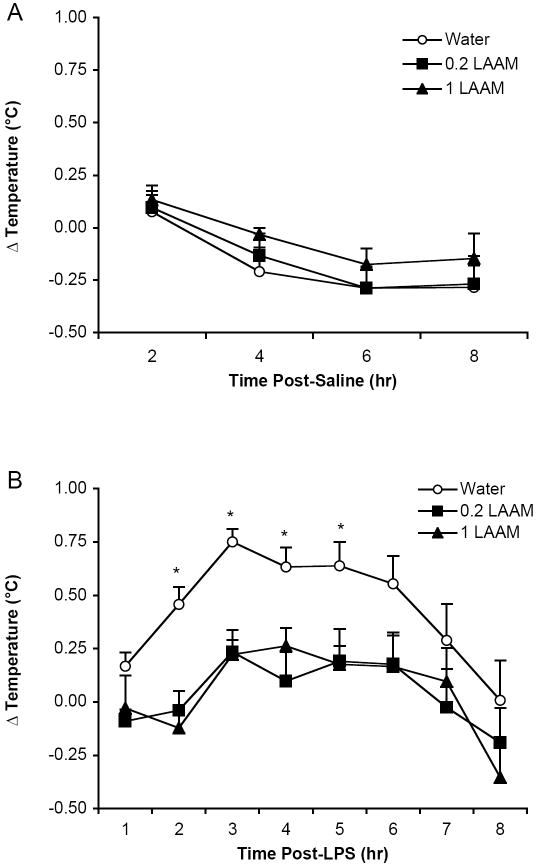
Prior to measuring the fever response, we analyzed the basal temperature and response to saline injection. Females had a basal temperature that was approximately 0.6 °C higher than that of males (F(1,32)=9.54, p<0.005) across all groups. Because there were no interactions between sex and prenatal treatment in subsequent analyses, the data were collapsed across sex. All data are depicted as the mean ± S.E.M. for males and females combined. Basal temperatures were not different across the three prenatal treatments (Figure 1). Although there was a Time effect following saline injection (F(3,105) = 12.25, p<0.0001), with a slight decrease in body temperature over the 8 hr of assessment, there was no difference as a consequence of prenatal treatment (Figure 2A).
Figure 1.
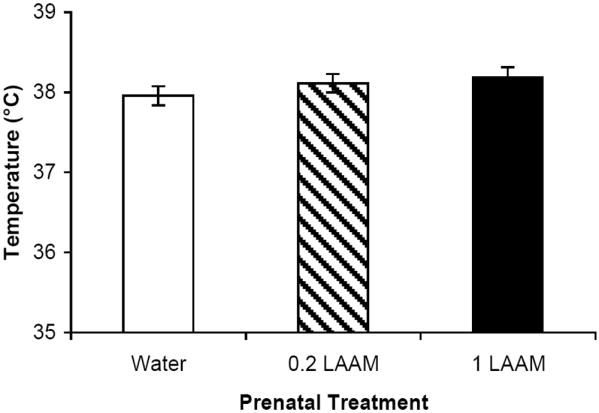
There were no differences in basal body temperature due to prenatal treatment. The mean ± SEM temperature (°C) is depicted for each prenatal treatment group across both sexes (n=12-14 per group).
Figure 2.
Rats’ temperature responses to (A) saline and (B) LPS were assessed over 8 hr. The mean ± SEM change in temperature (Δ°C) is depicted for each prenatal treatment group collapsed across sex (n=12-14 per group). (A) The temperature response to saline injection was not significantly different between the prenatal treatment groups. (B) Prenatally water-treated rats displayed a higher temperature response to LPS than both prenatally LAAM-treated groups; *p<.05 (Fisher’s PLSD).
2.2 Fever Response
Following LPS administration, temperatures were recorded hourly for 8 hr. The peak of the fever response was at 3 hr, and temperatures returned to baseline by 8 hr. The fever response to LPS was blunted by prenatal LAAM exposure (F(2,35)=6.19, p<0.005). Rats that were exposed to LAAM in utero displayed a fever response that was reduced to 33% of prenatal water controls (Figure 2B). Interestingly, this effect was not dose-dependent, as a similar magnitude of attenuation was found for both doses of LAAM prenatally. One-way ANOVAs were conducted to determine when there were significant Treatment effects, and effects were found at 2 hr (F(2,35)=11.70, p<0.0001); 3 hr (F(2,35)= 13.02, p<0.0001); 4 hr (F(2,35)= 6.11, p<0.006); and 5 hr (F(2,35)= 4.42, p<0.02). In all cases, both the 0.2 and 1 mg/kg prenatal LAAM-treated groups differed from the prenatal water-treated controls. In a separate group of rats, we replicated the suppressed fever effect at 3 hr (F(2,33)= 2.48; p<0.05 one-tailed) and obtained blood and tissue samples for analysis of IL-1β. The mean ± SEM fever responses for those groups were as follows: prenatal water = 0.57 °C ± 0.05; prenatal 0.2 mg/kg LAAM = 0.29 °C ± 0.10; and prenatal 1 mg/kg LAAM = 0.20 °C ± 0.10).
2.3 Circulating IL-1β
Rats were sacrificed at the peak of the fever response (3 hr after LPS injection). Prenatal LAAM treatment did not affect circulating IL-1β concentrations as measured by ELISA as this time point (Figure 3).
Figure 3.
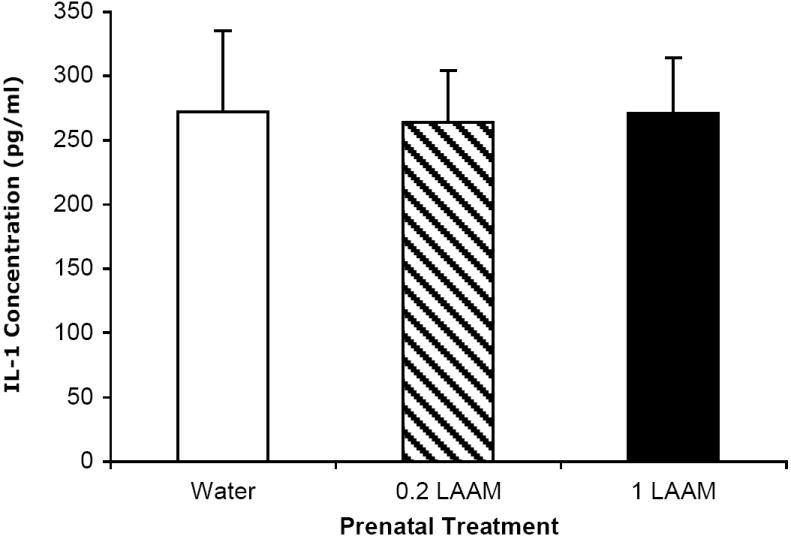
There were no differences in circulating IL-1β across the prenatal treatment groups 3 hr after LPS injection. The mean ± SEM concentration of IL-1β (pg/ml) is depicted for each prenatal treatment group collapsed across sex (n=8-15 per group).
2.4 Splenic and Hypothalamic IL-1β
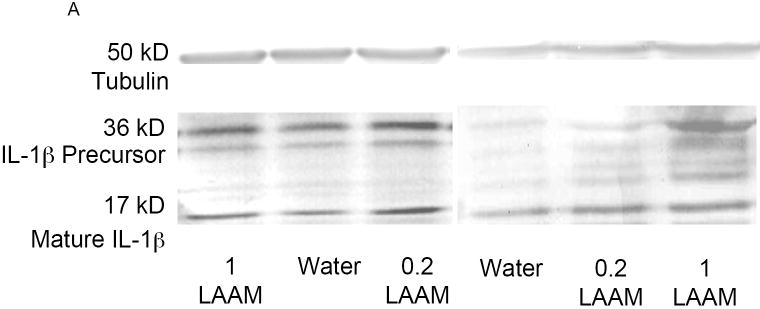
IL-1β protein levels were measured in the spleen and hypothalamus at the 3-hr time point following LPS treatment. Figure 4A depicts a representative Western blot for spleen and hypothalamus samples. Prenatal LAAM exposure did not result in any differences in precursor or mature IL-1β levels in the spleen (Table 1). In contrast, data from the hypothalamus presented a different pattern. While prenatal LAAM treatment did not change the levels of precursor IL-1β (Figure 4B), it resulted in an approximately 1.8-fold increase in mature IL-1β levels in both LAAM-treated groups compared to controls following LPS injection (Figure 4C; F(2,29)=3.82, p<0.05). This was opposite to our expectation since these subjects displayed an attenuated fever response.
Figure 4.
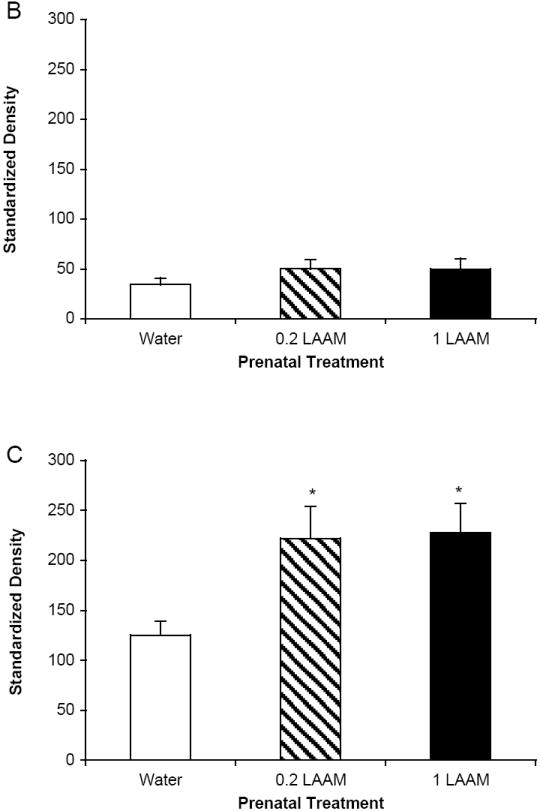
At 3 hr post-LPS injection, brains were harvested to measure precursor and mature IL-1β protein in the hypothalamus. The mean ± SEM standardized density units are depicted for each prenatal treatment group collapsed across sex (n=8-15 per group). (A) A representative Western blot for spleen and hypothalamus from each of the treatment groups. Bands representing both the precursor and mature forms of IL-1β protein are visible. The housekeeping protein tubulin served as a gel loading control. (B) There were no differences in precursor IL-1β protein in the hypothalamus; (C) mature IL-1β protein levels were higher in prenatally LAAM-treated offspring than in prenatally water-treated offspring; *p<.05 (Fisher’s PLSD).
Table 1.
Splenic IL-1β protein measured 3 hr following LPS treatment as quantified from Western blot analysis (n=10-16 per group). Data represent the mean ± SEM of the standardized density.
| Prenatal Treatment | Precursor | Mature |
|---|---|---|
| Water | 27.19 ± 4.46 | 23.86 ± 3.50 |
| 0.2 LAAM | 27.88 ± 3.05 | 33.39 ± 4.40 |
| 1 LAAM | 18.41 ± 3.20 | 28.42 ± 5.76 |
It was then determined if the elevated levels of mature IL-1β protein in the prenatal LAAM groups were specific to the LPS treatment, or if they were a more general characteristic of these animals. Hypothalami from a minimum of 3 prenatal water or 0.2 or 1 mg/kg LAAM-treated subjects that were otherwise untreated were assessed for basal IL-1β levels. There was no prenatal treatment effect for either mature or precursor protein in the hypothalamus, indicating an interaction between prenatal treatment and adult LPS treatment. IL-1β precursor protein means ± SEM for naïve adult rats were: prenatal water = 43.40 ± 5.87; prenatal 0.2 mg/kg LAAM = 34.04 ± 10.02; and prenatal 1 mg/kg LAAM = 40.33 ± 17.47. Means ± SEM for the IL-1β mature protein were: prenatal water = 92.71 ± 13.86; prenatal 0.2 mg/kg LAAM = 129.98 ± 15.10; and prenatal 1 mg/kg LAAM = 90.46 ± 29.63.
3. Discussion
Adult rats that had been exposed prenatally to the long-acting opiate LAAM developed a blunted fever to immune stimulation with LPS, compared to adult rats treated prenatally with water. These effects were not likely due to pre-or postnatal malnutrition because there was no difference in pregnancy or birth parameters (Hamilton et al., 2005). Rather, they are likely the direct result of prenatal opiate exposure and/or postnatal withdrawal. Interestingly, there was no difference between the prenatal groups in their basal temperature or in their response to a saline injection. Similar effects were seen in rats that were first injected with saline and then tested 3-5 days later with LPS and rats that were naïve to injection at the time of assessment with LPS, indicating that prior handling did not influence outcome. This may have been due to the extensive adaptation procedure that was conducted before testing.
The present study replicated and extended previous research in three ways. First, our findings corroborated previous work in both juvenile rodents and chicks (Shavit et al., 1998; Schrott et al., 2004) and indicated that there is a functional change in how the immune system responds to stimulation following prenatal opiate exposure. Importantly, the current study revealed that the dysregulation in fever responding extends into adulthood. This finding is intriguing because in our previous study, we found that the direction of dysregulation of HPA axis following prenatal LAAM exposure was dependent on the age of the rat (Hamilton et al., 2005). Second, the current study revealed that using a long-acting opiate (LAAM) could mimic the pattern of dysfunctional fever responding seen after prenatal morphine exposure (Shavit et al., 1998). Because LAAM has a long duration of action (Henderson et al., 1977), the risk of intermittent prenatal withdrawals is reduced. Thus, it appears that the altered fever response reported here and by Shavit and colleagues (1998) is the result of prenatal opiate exposure, rather than the particular characteristics of morphine or LAAM. Third, the present study further extended the previous work by addressing one potential mechanism of this functional change, alterations in the expression of the pro-inflammatory cytokine IL-1β.
Based upon the lower magnitude of the fever response in the prenatal LAAM-treated rats, we expected a reduction in circulating IL-1β or protein expression in the spleen or hypothalamus in this group of subjects. This hypothesis was based on studies showing that peripheral IL-1β signaling and/or central IL-1β production are involved in generating the pyrogenic effects of LPS (Long et al., 1990; Luheshi et al., 1993). With respect to circulating concentrations, we found that prenatal opiate exposure did not alter the response to LPS at the time of the peak fever response. Because we did not examine earlier time points, it is not possible to rule out a change in the time course of the IL-1β response. However, this would likely not explain the lower fever in prenatal LAAM-treated rats, as there was not a delayed fever onset. We were able to detect both precursor IL-1β protein and the cleaved, mature form of the protein by Western blot analysis. This permitted us to determine if prenatal LAAM affected either protein independently, an important distinction because physiological factors such as glucocorticoid levels and body temperature can affect the cleavage of IL-1β precursor to mature IL-1β (Boneberg et al., 2003; Burns et al., 2003; Knudsen et al., 1987). There was no difference between prenatal treatment groups for mature or precursor IL-1β in the spleen, or for precursor protein in the hypothalamus. In contrast, mature IL-1β protein levels were increased in the hypothalamus following stimulation with LPS in the prenatal LAAM-treated subjects. This was a surprising finding because the fever response was blunted in this group. It is important to note that the IL-1β changes were specific to post-LPS treatment, and there were no differences in IL-1β hypothalamic protein concentrations due to prenatal LAAM in otherwise untreated adult rats.
The results of the present study suggest that an alternative mechanism and/or a mechanism downstream from IL-1β production may be involved in the blunted LPS-induced fever response induced by prenatal opiate exposure. The generation of a fever response following immune system stimulation is complex. Following the production of IL-1, other pro-inflammatory cytokines such as IL-6, and tumor necrosis factor-α (TNF-α) are induced, as well as various thermoregulatory mediators including prostaglandin E2 (PGE2 via the action of cyclooxygenase and phospholipase enzymes) and neuropeptides such as cholecystokinin (CCK) and orexins (Klir et al., 1994; Yoshino et al., 2005; see recent review by Romanovsky et al., 2005). Thus, a reduction in the production of these cytokines and mediators could affect the generation of a fever response. Interestingly, chronic opiate exposure has been shown to inhibit nuclear factor kappa B (NFκB) (e.g., Wang et al., 2005; Welters et al., 2000), a transcription factor involved in the downstream effects of IL-1β (Nadjar et al., 2003) and the production of PGE2 (Pistritto et al., 1999). There is some precedent for effects downstream of IL-1 affecting fever responding following developmental insults. For example, Boissé et al. (2004) and Spencer et al. (2006) reported that neonatal LPS treatment led to an attenuated fever response to LPS in adulthood, possibly by perturbing cyclooxygenase-2 production and the generation of fever-inducing prostaglandins.
In addition to factors that promote fever generation, there are endogenous regulatory factors that dampen fever production, and these may have been enhanced following prenatal opiate exposure. A strong possibility in this regard is alterations in glucocorticoid hormones. We previously reported that rats exposed to LAAM in utero developed an exaggerated corticosterone response to LPS compared with controls. The elevation of corticosterone occurred at the same time point as the blunted fever reported here (Hamilton et al., 2005). Given that glucocorticoids suppress the fever response (Coelho et al, 1992; Morrow et al., 1993), our finding may suggest that the magnified stress response plays a role in the reduced fever response. This effect may be independent of the IL-1β alterations, but result in alterations in prostaglandin production. Evidence for this role for glucocorticoids is derived from Ristimaki and colleagues (1996), who showed that administration of the synthetic glucocorticoid dexamethasone following IL-1 treatment reduced cyclooxygenase-2 mRNA. Another endogenous counterregulatory factor is the receptor antagonist for IL-1 (IL-1ra), which is produced in response to IL-1β (reviewed in Dinarello, 1991). Rahim et al. (2003) found that opiate withdrawal could alter levels of IL-1ra mRNA. Therefore, it is possible that prenatal LAAM exposure and postnatal withdrawal resulted in elevated IL-1ra levels, which could block the action of IL-1β. Endogenous opioids can also modulate fever responding (Benamar, 2000), and it may be possible that prenatal opiate exposure produces long-lasting changes to the endogenous opioid system.
In conclusion, the present study demonstrated that rats prenatally exposed to opiates mounted an inappropriate fever response following immunological stimulation. Importantly, the immune system of the prenatally exposed offspring responded to the stimulation with a comparative over-production of IL-1β protein, but the IL-1β signal did not result in a parallel fever response. However, as discussed above, it is possible that other immunological mechanisms may be disrupted, which would also impact the fever response. Future research aims to determine the relative roles of other cytokine and non-cytokine pathways and downstream mediators of immunological function that may influence responding to immune challenges following prenatal opiate exposure.
4. Experimental Procedures
4.1 Animals
Details of the prenatal treatment and developmental outcome measures have been previously reported (Hamilton et al., 2005). Briefly, group-housed nulliparous female Sprague-Dawley rats (7-8 wks of age; Harlan, Madison, WI) weighing approximately 200 g at the start of the experiment were used as subjects. The animals were habituated to an oral gavage procedure using water before initiation of treatment. Treatments began on day 0 and the rats were gradually made chronically opiate dependent via treatments on days 3, 6, and 8, followed by daily treatments from days 10 to 28. After 28 days of treatment, females were harem-bred, with 1 male placed in each cage of 3 females. Treatment continued through the 10 days of breeding.
Pregnancy was confirmed by a sperm-positive vaginal smear and/or appropriate weight gain. Drug treatment of the females continued during pregnancy until parturition. Females were weighed daily, and throughout gestation they were monitored for signs of opiate withdrawal, such as weight loss, diarrhea, piloerection, and irritability. Dams gained a similar amount of weight throughout gestation (approximately 150-160% of pre-breeding weight), regardless of treatment (Hamilton et al., 2005). Pregnant females were placed in individual nesting cages on approximately gestation day (GD) 14. From GD 20-23 the pregnant dams were checked daily at 0700 and 1400 hr for births. Litters were handled with gloved hands only after pups had been cleaned and fed. At this time, the pups were sexed and weighed. There were no differences in the pups’ body weights across the treatments at birth. Within 12 to 36 hr of parturition, pups from both water-and LAAM-treated dams were fostered to non-treated dams. The foster mothers had been handled and weighed weekly, but were otherwise undisturbed during pregnancy. Pups were culled to 12 per litter at fostering, balancing by sex when possible. Changes in body weight during the first postnatal week were used to index post-parturition opiate withdrawal. Rat pups treated prenatally with either dose of LAAM displayed reduced weight gain during the first 5 days of life, indicative of a transient postnatal withdrawal. By postnatal day (PND) 5, weight gain normalized, and through PND 21, there were no differences in body weight among the prenatal treatment groups (Hamilton et al., 2005).
The pups were weaned at PND 21, with 3-5 same-sex littermates housed per cage. The offspring were weighed weekly until approximately 20 weeks of age, when non-littermate subjects from each treatment were randomly selected for testing. There were no differences in the body weights amongst the prenatal treatment groups (male group averages were 419-437 g and female group averages were 277-280 g) at the time of testing. All animals were maintained on a 12:12 light:dark schedule, with lights on at 0700. Food and water were provided ad libitum throughout the experiment. Animal experimentation was conducted at the University of Minnesota. Experimental procedures conformed to the Guidelines for the Care and Use of Laboratory Animals and were approved by University of Minnesota Animal Care and Use Committee.
4.2 Drugs
4.2.1 LAAM
Two doses of LAAM HCl (kindly provided by the National Institute on Drug Abuse through the Research Triangle Institute, Research Triangle Park, NC) were used: 1.0 mg/kg/day and 0.2 mg/kg/day. The doses were based on prior studies that found that 0.2 mg/kg LAAM led to continuous dependence without significant toxicity (Licthblau and Sparber, 1982). The 1.0 mg/kg dose was chosen because previous studies found that higher doses (e.g., 2.0 mg/kg) increased neonatal morbidity (Licthblau and Sparber, 1982, York et al., 2002). LAAM was dissolved in tap water filtered with a 0.2 μm filter. An 18-gauge, 7.6 cm long gavage tube (Popper and Sons, New Hyde Park, New York) was used for each drug administration. Gavage volumes were 1.0 ml/kg.
4.2.2 Lipopolysaccharide (LPS)
LPS (serotype 0128:B12; Sigma, St. Louis, MO) was dissolved in 0.9% NaCl solution to form a 100 μg/kg dose. LPS was injected i.p. in a volume of 1.0 ml/kg.
4.3 Fever Response
Rats were adapted to the procedure for measuring body temperature prior to initiation of the study. During the first week they were handled, gently restrained by hand, and placed in the position for temperature probe insertion. During the second week, the temperature probe, which was lightly coated in mineral oil, was gently inserted into their anal canal while the rats were lightly restrained by hand. The probe was connected to a digital tele-thermometer that displayed the temperature to the hundredth of a degree (°C). This procedure was repeated twice a day until each rat displayed a consistent temperature across days, typically 3-5 days. All rats adapted well to the procedure, with no struggling or squealing during the testing procedures. The ambient room temperature was measured at each assessment point (mean ± SEM 21.43 °C ± 0.06; range 20.8 to 22.3 °C)
Prior to measuring the fever response, we determined the temperature response following saline administration. A basal temperature was taken at 0800 via insertion of a probe lightly coated with mineral oil into the anus of each rat. Saline was injected, and the rats’ core temperatures were taken every 2 hr for 8 hr. Three to five days later, the same animals were tested for the time course of fever responding following LPS treatment. As before, prior to LPS injection, a basal temperature was measured at 0800. The temperature was then measured every hour for 8 hr. At the 8-hr time point, animals were sacrificed via rapid decapitation without anesthesia. A separate set of rats was adapted to the body temperature assessment procedures and assessed for fever response and sacrificed at 3 hr, the peak of fever response.
4.4 Sera and Tissue Collection
For all subjects, trunk blood was obtained, and the brain and spleen were removed at sacrifice. Blood samples were centrifuged and serum samples were frozen at −80 °C until analysis. At the time of brain removal, the hypothalamus was rapidly dissected from the brain and quick frozen on dry ice. Spleens were also quick frozen on dry ice immediately upon removal. Brain and spleen samples were frozen at −80 °C until analysis.
4.5 ELISA for Circulating IL-1β Concentration
Concentrations of IL-1β were measured using a DuoSet ELISA kit (R&D Systems, Minneapolis, MN). Assays were run in accordance with the manufacturer’s directions, and samples were analyzed in duplicate. Briefly, following plate preparation, serum samples were combined with 50% fetal calf serum in reagent diluent. Plates were washed and 100 μl of detection antibody conjugated to horseradish peroxidase was added to the wells after incubation. Finally, 100 μl of substrate solution was added and absorbance was read at 450 nm using a standard plate reader (Packard SpectraCountT Microplate Photometer). Optical density measures for the standards were used to generate a standard curve, and the concentration of IL-1β in each sample was interpolated from this standard curve.
4.6 Western Blot Analysis for Splenic and Hypothalamic IL-1β Protein
Tissues were homogenized in a Tris-EDTA-EGTA buffer containing protease and phosphatase inhibitors (Sigma, St. Louis, MO). The homogenate was spun at low speed (3000 g) at 4°C for 5 min to remove particulate matter. The supernatant underwent ultracentrifugation (30 min at 100,000 g at 4°C) and the supernatant from that spin reflected the cytosol. A bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) was used to measure total protein in each cytosolic sample. Following quantification, the samples were diluted with 5x Laemmli sample buffer (BioRad, Hercules, CA) and treated with heat and β-mercaptoethanol to denature the proteins. In pilot studies we determined that the optimal protein loading was 10-15 μg protein/cm well. This was defined as clear definition of the IL-1β bands with minimal background and non-specific bands using our electrophoresis, transblotting and detection system.
Protein samples (10 μg/cm well) were loaded onto a 4-20% Tris-glycine gradient gel (BioRad, Hercules, CA). This gradient separates proteins from 4-200 kD, an appropriate range for the proteins of interest. The gel was run at a constant 200 V for 47 min with appropriate buffers using a Criterion system (BioRad, Hercules, CA). After electrophoresis, the proteins were transferred from the gel onto a 0.5-μm nitrocellulose membrane (30 min at 4°C at 100 mA). The membrane was cut into regions using molecular weight markers, such that both the tubulin loading control and the IL-1β protein were assessed on the same membrane for each sample. Following incubation in blocker, the membrane was incubated in primary antibody for IL -1β (Chemicon International, Temecula, CA) or tubulin (Promega, Madison, WI). The IL-1β antibody was a rabbit anti-rat polyclonal that recognized both the precursor form of the protein (approximately 32-36kD), as well as the mature form (17kD). The tubulin antibody was a mouse monoclonal that recognized the c-terminus of rat βIII tubulin. Following washes, the membrane was incubated in secondary antibody that was alkaline-phosphatase conjugated. The secondary antibody for IL-1β was goat anti-rabbit IgG (H+L) (Pierce, Rockford, IL) and for tubulin it was anti-mouse IgG (H+L) (Promega, Madison, WI). The reaction product was visualized using BCIP (5-bromo-4-chloro-3’-indolyphosphate p-toluidine salt) and NBT (nitro-blue tetrazolium chloride) colorometric detection (MP Biomedical, Irvine, CA). The membrane was scanned and band density measured using NIH Image (Bethesda, MD).
4.7 Data Analysis
Data are depicted as mean ± SEM for all measures. For the body temperature data, a two-way analysis of variance (ANOVA) was conducted on the baseline temperatures to determine if there were differences amongst prenatal treatment groups. Sex was included as a fixed factor in that analysis. Repeated measures ANOVAs were conducted to determine if prenatal treatment affected body temperature following saline or LPS injection. Initial analyses included sex, but because there were no sex interactions with treatment, the data were collapsed and all remaining analyses were conducted on combined male and female data. Serum IL-1β optical density readings were converted to pg/ml using the standard curve for each assay and data were analyzed by one-way ANOVA. The IL-1β Western blot data were standardized for gel loading differences using each sample’s tubulin band density. These data are depicted as standardized density units and were analyzed by one-way ANOVA. Planned comparisons were conducted for each set of data comparing each of the prenatal LAAM treated groups to prenatal water-treated controls using Fisher PLSD tests or Dunnett’s contrasts.
Abbreviations
- ANOVA
analysis of variance
- BCA
bicinchoninic acid
- BCIP
5-bromo-4-chloro-3’-indolyphosphate p-toluidine salt
- CCK
cholecystokinin
- GD
gestation day
- HPA
hypothalamic-pituitary-adrenal
- IL
interleukin
- IL-1ra
interleukin-1 receptor antagonist
- LAAM
l-α-acetylmethadol
- LPS
lipopolysaccharide
- MORKO
mu-opiate receptor knockout
- NBT
nitro-blue tetrazolium chloride
- NFκB
nuclear factor kappa B
- PHA
phytohemagglutinin
- PND
postnatal day
- PGE2
prostaglandin E2
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benamar K, Xin L, Geller EB, Adler MW. Effect of central and peripheral administration of a nitric oxide synthase inhibitor on morphine hyperthermia in rats. Brain Res. 2001;894:266–273. doi: 10.1016/s0006-8993(01)02025-x. [DOI] [PubMed] [Google Scholar]
- Bencsics A, Elenkov IJ, Vizi ES. Effect of morphine on lipopolysaccharide-induced tumor necrosis factor-alpha production in vivo: Involvement of the sympathetic nervous system. J Neuroimmunol. 1997;73:1–6. doi: 10.1016/s0165-5728(96)00163-4. [DOI] [PubMed] [Google Scholar]
- Beuscher HU, Gunther C, Rollinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990;144:2179–2183. [PubMed] [Google Scholar]
- Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, Wignall J, Conlon PJ, Cosman D, Hopp TP, et al. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- Boissé L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–4934. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneberg EM, Hartung T. Febrile temperatures attenuate IL-1 beta release by inhibiting proteolytic processing of the proform and influence Th1/Th2 balance by favoring Th2 cytokines. J Immunol. 2003;171:664–668. doi: 10.4049/jimmunol.171.2.664. [DOI] [PubMed] [Google Scholar]
- Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Carr DJJ, Rogers TJ, Weber RJ. The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Exp Biol Med. 1996;213:248–257. doi: 10.3181/00379727-213-44056. [DOI] [PubMed] [Google Scholar]
- Coelho MM, Souza GEP, Pela IR. Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol. 1992;263:R423–R427. doi: 10.1152/ajpregu.1992.263.2.R423. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: A systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Fecho K, Dykstra LA, Lysle DT. Evidence for beta-adrenergic receptor involvement in the immunomodulatory effects of morphine. JPET. 1993;265:1079–1087. [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Evidence for sympathetic and adrenal involvement in the Immunomodulatory effects of acute morphine treatment in rats. JPET. 1996;277:633–645. [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad USA. 1998;95:6326–30. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. Effects of morphine withdrawal on food competition hierarchies and fighting behavior in rats. Psychopharmacology (Berlin) 1979;60:165–172. doi: 10.1007/BF00432288. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Harris AC, Gewirtz JC, Sparber SB, Schrott LM. HPA axis dysregulation following prenatal opiate exposure and postnatal withdrawal. Neurotoxicol Teratol. 2005;27:95–103. doi: 10.1016/j.ntt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Henderson GL, North-Root H, Kuttab SH. Metabolism and disposition of l-alpha-acetylmethadol in the rat. Drug Metab Dispos. 1977;5:321–328. [PubMed] [Google Scholar]
- Houghtling RA, Bayer BM. Rapid elevation of plasma interleukin-6 by morphine is dependent on autonomic stimulation of adrenal gland. JPET. 2002;300:213–9. doi: 10.1124/jpet.300.1.213. This citation needs to stay in. It is cited in the introduction. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Ziegler MG. Ontogeny of epinephrine metabolic pathways in the rat: Role of glucocorticoids. Int J Devl Neuroscience. 2000;18:53–59. doi: 10.1016/s0736-5748(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Klir JJ, McClellan JL, Kluger MJ. Interleukin-1β causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. Am J Physiol. 1994;266:R1845–R1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- Knudsen PJ, Dinarello CA, Strom TB. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987;139:4129–4134. [PubMed] [Google Scholar]
- Lichtblau L, Sparber SB. Congenital behavioral effects in mature rats prenatally exposed to levo-alpha-acetylmethadol (LAAM) Neurobehav Toxicol Teratol. 1982;4:557–565. [PubMed] [Google Scholar]
- Long NC, Otterness I, Kunkel SL, Vander AJ, Kluger MJ. Roles of interleukin 1β and tumor necrosis factor in lipopolysaccharide fever in rats. Am J Physiol. 1990;259:R724–R728. doi: 10.1152/ajpregu.1990.259.4.R724. [DOI] [PubMed] [Google Scholar]
- Luheshi G, Hopkins SJ, Lefeuvre RA, Dascombe MJ, Ghiara P, Rothwell NJ. Importance of brain IL-1 type II receptors in fever and thermogenesis in the rat. Am J Physiol. 1993;265:E585–E591. doi: 10.1152/ajpendo.1993.265.4.E585. [DOI] [PubMed] [Google Scholar]
- Martin JT, Nehlson-Cannarella SL, Gugelchuk GM, Fagoaga O. Prenatal morphine exposure interacts with adult stress to affect type and number of blood leucocytes. Adv Exp Med Biol. 1996;402:89–94. doi: 10.1007/978-1-4613-0407-4_13. [DOI] [PubMed] [Google Scholar]
- Martin JT, Nehlson-Cannarella SL, Gugelchuck GM, Fagoaga O. Morphine during pregnancy in the rat: Studies of cellular immunity in cross-fostered offspring. Adv Exp Med Biol. 1998;437:149–157. [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcoh Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Misra AL, Mule SJ, Bloch R, Vadlamani NL. Physiological disposition and metabolism of levo-methadone-1-3H in nontolerant and tolerant rats. J Pharmacol Exp Therap. 1973;185:287–299. [PubMed] [Google Scholar]
- Morrow LE, McClellan JL, Conn CA, Kluger MJ. Glucocorticoids alter fever and IL-6 responses to psychological stress and to lipopolysaccharide. Am J Physiol. 1993;264:R1010–R1016. doi: 10.1152/ajpregu.1993.264.5.R1010. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Laye S, Tridon V, Dantzer R, Amedee T, Parnet P. Nuclear factor κB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. J Neurochem. 2003;87:1024–1036. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Schneider GM, Lysle DT. Involvement of central mu-but not delta-or kappa-opioid receptors in immunomodulation. Brain Behav Immun. 2000;14:170–84. doi: 10.1006/brbi.1999.0575. [DOI] [PubMed] [Google Scholar]
- Pistritto G, Franzese O, Pozzoli G, Mancuso C, Tringali G, Preziosi P, Navarra P. Bacterial lipopolysaccharide increases prostaglandin production by rat astrocytes via inducible cyclo-oxygenase: Evidence for the involvement of nuclear factor κB. Biochem Biophys Res Commun. 1999;263:570–574. doi: 10.1006/bbrc.1999.1413. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Jr, Zhang L, Adler MW, Rogers TJ, Eisenstein TK. Withdrawal from morphine in mice suppresses splenic macrophage function, cytokine production, and costimulatory molecules. J Neuroimmunol. 2003;144:16–27. doi: 10.1016/s0165-5728(03)00273-x. [DOI] [PubMed] [Google Scholar]
- Ristimaki R, Narko K, Hla T. Down-regulation of cytokine-induced cyclooxygenase-2 transcript isoforms by dexamethasone: Evidence for post-transcriptional regulation. Biochem J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2000;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. Mu-opioid receptor-knockout mice: Role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Limiroli E, Gaspani L. Experimental evidence for immunomodulatory effects of opioids. Adv Exp Med Biol. 2003;521:106–116. [PubMed] [Google Scholar]
- Schrott LM, Sparber SB. Suppressed fever and hypersensitivity responses in chicks prenatally exposed to opiates. Brain Behav Immun. 2004;18:515–525. doi: 10.1016/j.bbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Cohen E, Gagin R, Avitsur R, Pollak Y, Chaikin G, Wolf G, Yirmiya R. Effects of prenatal morphine exposure on NK cytotoxicity and responsiveness to LPS in rats. Pharmacol Biochem Behav. 1998;59:835–841. doi: 10.1016/s0091-3057(97)00532-7. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Boissé L, Mouihate A, Pittman QJ. Long-term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav Immun. 2006;20:325–330. doi: 10.1016/j.bbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor. J Leukoc Biol. 2002;71:782–790. [PubMed] [Google Scholar]
- Wei G, Moss J, Yuan CS. Opioid-induced immunosuppression: is it centrally mediated or peripherally mediated? Biochem Pharmacol. 2003;65:1761–6. doi: 10.1016/s0006-2952(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Welters ID, Menzebach A, Goumon Y, Cadet P, Menges T, Hughes TK, Hempelmann G, Stefano GB. Morphine inhibits NF-kappaB nuclear binding in human neutrophils and monocytes by a nitric oxide-dependent mechanism. Anesthesiology. 2000;92:1677–1684. doi: 10.1097/00000542-200006000-00027. [DOI] [PubMed] [Google Scholar]
- West JP, Dykstra LA, Lysle DT. Immunomodulatory effects of morphine withdrawal in the rat are time dependent and reversible by clonidine. Psychopharmacology (Berlin) 1999;146:320–327. doi: 10.1007/s002130051123. [DOI] [PubMed] [Google Scholar]
- York RG, Denny KH, Moody DE, Alburges ME. Developmental toxicity of levo-alpha-acetylmethadol (LAAM) in tolerant rats. Int J Toxicol. 2002;21:147–159. doi: 10.1080/10915810252866105. [DOI] [PubMed] [Google Scholar]
- Yoshino T, Kimoto A, Kobayashi S, Noguchi M, Fukunaga M, Hayashi A, Miyata K, Sasamata M. Pharmacological profile of celecoxib, a specific cyclooxygenase-2 inhibitor. Arzneimittel-Forschung. 2005;55:394–402. doi: 10.1055/s-0031-1296878. [DOI] [PubMed] [Google Scholar]
- Zakharova LA, Malyukova IV, Proshlyakova EV, Potapova AA, Sapronova AY, Ershov PV, Ugrumov MV. Hypothalamo-pituitary control of the cell-mediated immunity in rat embryos: role of LHRH in regulation of lympocyte proliferation. J Reprod Immunol. 2000;47:17–32. doi: 10.1016/s0165-0378(00)00057-7. [DOI] [PubMed] [Google Scholar]