Abstract
The natural history of human T-lymphotropic virus type I (HTLV-I) has been shown to differ markedly by geographic area. The differences include contrasting patterns of risk of adult T-cell lymphoma (ATL) and HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP), which may be due in part to differences in host immune response to infection. To characterize variations in host immunity across populations, we compared serologic immune marker patterns in HTLV-I-endemic populations in Japan and Jamaica. We matched 204 participants with archived blood from the Miyazaki Cohort Study (Japan) and the Food Handlers Study (Jamaica)-i.e., 51 HTLV-I-positive (“carriers”) and 51 HTLV-I-negative individuals (“non-carriers”) from each population-by age, sex, and blood collection year. We compared plasma concentrations of markers of T-cell-mediated (antigen-specific) and non-specific immunity using regression models and correlation coefficients. Compared to Jamaican HTLV-I non-carriers, Japanese non-carriers had higher covariate-adjusted mean levels of T-cell activation markers, including antibody to Epstein-Barr virus nuclear antigen-1 (reciprocal titer 27 v. 71, respectively, p=0.005), soluble interleukin-2 receptor-α (477 v. 623 pg/mL, p=0.0008) and soluble CD30 (34 v. 46 U/mL, p=0.0001), and lower levels of C-reactive protein (1.1 v. 0.43 μg/mL, p=0.0004). HTLV-I infection was associated with activated T-cell immunity in Jamaicans but with diminished T-cell immunity in Japanese persons. The observed population differences in background and HTLV-I-related host immunity correspond closely to the divergent natural histories of infection observed among HTLV-I carriers in Japan and Jamaica and corroborate a role for host immune status in the contrasting patterns of ATL and HAM/TSP risk.
Keywords: HTLV-I, epidemiology, natural history, host immunity, viral markers
Introduction
Human T-lymphotropic virus type I (HTLV-I) is associated with adult T-cell lymphoma (ATL) and HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (1). HTLV-I infection is endemic in southern Japan, the Caribbean, and portions of South America, Africa, the Middle East, and Melanesia (1,2). The risk of ATL and HAM/TSP among individuals infected with HTLV-I (“carriers”) varies markedly across Japanese and Caribbean populations. In Japanese carriers, the annual incidence of ATL is comparatively high, peaks around age 60, and is approximately 3 times greater in men than in women (3,4). The incidence of ATL among Caribbean carriers is less than one-third the rate in Japan, peaks in the forties, and does not vary by gender. In contrast, the incidence of HAM/TSP is relatively high among Caribbean carriers, whereas in Japan HAM/TSP occurs at one-tenth the rate in the Caribbean (2,3). HAM/TSP demonstrates a female predominance in both populations.
Genetic variation of the virus does not explain the population differences in HTLV-I-related morbidity (4,5). Instead, factors such as age at infection, route and dose of infection, nutritional status, social environment, co-morbidity, and/or host genetics likely contribute to the geographic differences in natural history (6), in part by influencing the initial host immune response to HTLV-I. For example, ATL appears to develop primarily in persons who acquire HTLV-I perinatally, whereas HAM/TSP often occurs in persons infected later in life (2).
Efficient cellular immunity (7), including an antigen-specific (i.e., virus-specific) cytotoxic T-lymphocyte (CTL) and a type 1 cytokine response, is important for host control of most viral infections. Several serologic markers of HTLV-1 pathogenesis (i.e., “viral markers”) serve as indicators of the host immune response to HTLV-1. One viral marker is provirus load (2,8,9), which represents the proportion of host peripheral blood mononuclear cells with integrated HTLV-I genome (i.e., provirus). Antibodies against HTLV-I structural proteins (anti-HTLV-I), which are found in all HTLV-I carriers, serve as another viral marker and are considered indicative of viral protein expression and of the division of provirus-containing T-lymphocytes (2,3,8). Antibody to Tax (anti-Tax), a product of the HTLV-I tax regulatory gene that can induce viral and host gene transcription (2), is a third viral marker. Anti-Tax detection is associated with an active CTL response to HTLV-I; the absence of anti-Tax is considered to indicate a lack of Tax expression (2). Of interest, anti-Tax is observed in HAM/TSP but not frequently in ATL patients (10-12).
ATL patients are generally considered to have diminished cellular immunity (1,2); patients from Japan had Epstein-Barr virus (EBV)-specific antibody responses suggestive of diminished cellular immunity (13) and increased expression of the cytokine transforming growth factor β1 (14). In contrast to ATL, HAM/TSP is characterized by activated inflammatory and CTL responses to HTLV-I (1,2), including up-regulation of pro-inflammatory and type 1 cytokines (6,14). Ultimately, differential CTL responses to HTLV-I, mediated in part by cytokines, are thought to be an important determinant of the contrasting population risks of ATL and HAM/TSP.
In a recent comparison of HTLV-I viral markers in asymptomatic carriers from Jamaica and Japan (15), we observed similar mean provirus loads in both populations (3.0 v. 3.1 log10 copies/105 cells, respectively, p=0.26), but significantly higher mean anti-HTLV-I titer (3.6 v. 3.2 log10 reciprocal titer, respectively, p=0.03) and anti-Tax seroprevalence (59% v. 39%, respectively, p=0.002) in Jamaican than in Japanese carriers. Those viral marker patterns suggested that Jamaican carriers may have heightened anti-HTLV-I immunity and persistent HTLV-I replication, whereas Japanese carriers have mitotic proliferation of HTLV-I-infected T-lymphocytes in the absence of strong anti-viral immunity (15). Of interest, we (16-18) and others (13,19) have reported immune marker patterns suggestive of subclinical immune deficiency in HTLV-I carriers in Japan. However, the immune statuses of Jamaican and Japanese carriers have not been directly compared. We conducted the present study to perform the first direct comparison of immune status across these populations. Biospecimens appropriate for a direct assessment of anti-viral CTL activity were not available. Thus, we tested a panel of plasma immune markers in the HTLV-I carriers from our virus marker study (15) and in HTLV-I-negative persons (“non-carriers”) from the same study populations (9,20). We also evaluated correlations between immune markers and levels of HTLV-I viral markers among carriers within each population (15).
Methods
Study population
Japanese subjects were participants in the Miyazaki Cohort Study, a prospective study conducted among adult residents of two rural villages in Miyazaki Prefecture between 1984 and 2000 in conjunction with government-sponsored annual health examinations (9). Jamaican individuals were participants in the Food Handlers Study (20), a series of three cross-sectional surveys conducted between 1985 and 1993 among a total of 13,920 applicants for food-handling licenses from all Jamaican parishes. From these two populations, we had previously selected 51 pairs of Japanese and Jamaican HTLV-I carriers, matched by age (± 2 years), sex, and sample collection year (± 2 years), for a comparison of HTLV-I viral markers (15). For the present analysis, we randomly selected one HTLV-I non-carrier from within the same cohort for each of the 51 pairs of carriers, using the same matching criteria as for the carrier pairs. Thus, the analysis included 51 matched sets of 4 participants (N=204) that comprised one carrier and one non-carrier from each study population. Blood samples from these subjects had been stored at -80°C in the respective repositories. The study protocol followed the human-experimentation guidelines of the US Department of Health and Human Services and received approval from the institutional review boards of each collaborating institution. Each participant provided informed consent.
HTLV-I viral markers
Laboratory methods to determine seropositivity for HTLV-I, quantify anti-HTLV-I titers, detect anti-Tax, and quantify HTLV-I provirus load have been described previously (11,15,21).
Immune markers
Matched sets of plasma samples were tested in the same batch for each assay, by technicians blinded to population and HTLV-I status. Plasma EBV antibodies were measured by immunofluorescence assay (IFA) techniques (22-24). Titers were reported as the highest of serial two-fold dilutions to yield a positive IFA reading.
Commercial enzyme-linked immunosorbent assays (ELISA) were performed according to manufacturer’s instructions to quantify peripheral blood levels of the following markers: soluble interleukin (IL)-2 receptor-α (sIL2R; CellFree Human sIL2R, Endogen, Woburn, MA), a molecule cleaved from the surface of activated helper T-lymphocytes (25); soluble CD30 (sCD30; Human sCD30 ELISA, Bender MedSystems, Vienna, Austria), which is a tumor necrosis factor receptor superfamily protein shed from activated B- and T-lymphocytes (26); C-reactive protein (CRP; Virgo CRP 150, Hemagen Diagnostics, Inc., Columbia, MD), a stable marker of chronic inflammation and correlate of circulating IL-6 levels (27); and, neopterin (ELItest Neopterin EIA, BRAHMS Diagnostica GMBH, Berlin, Germany), which is induced by interferon-γ during innate (non-specific) immune responses (28). We also measured concentrations of total human immunoglobulin E (IgE), a marker of B-lymphocyte activation. Total IgE levels were determined by ELISA based on a modified Mouse IgE ELISA protocol (BD Biosciences Pharmingen, San Diego, CA), using anti-human IgE monoclonal antibodies (G7-18, G7-26, 2 μg/mL), and purified human IgE standard (31.2-500 ng/mL, Calbiochem, San Diego, CA); 1 ng/mL of the Calbiochem standard was comparable to 2 IU/mL of human IgE (NIBSC 75/502).
The detection limits of the immune marker assays were 0.25 μg/mL CRP, 6.4 U/mL sCD30, 40 pg/mL sIL2R, 2 nmol/L neopterin, and 31 ng/mL total IgE. Samples with CRP and total IgE levels below the detection limit were assigned a value of half the corresponding assay’s limit (i.e., 0.13 μg/mL CRP or 15.5 ng/mL total IgE).
Statistical analysis
To characterize subjects’ immune marker patterns, we classified levels of antibody to EBV nuclear antigen-2 (anti-EBNA2), EBV viral capsid antigen (anti-VCA), CRP, sIL2R, sCD30, neopterin, and total IgE as greater than (“higher”), or less than or equal to (“lower”), the median levels observed among HTLV-I non-carriers from the same population. Because lower anti-EBNA1 titers are associated with diminished type 1 immunity (22,29), we grouped anti-EBNA1 titers as greater than or equal to (higher), or less than (lower) the respective median titers. We dichotomized titers of antibody to EBV early antigen (anti-EA) as seropositive (≥1:20) or seronegative (<1:20). We computed the ratio of anti-EBNA1 to anti-EBNA2 titers; a ratio of 1.0 or lower (i.e., “low EBNA1:EBNA2 ratio”), which occurs when the anti-EBNA1 titer is less than or equal to the anti-EBNA2 titer, is empirically associated with deficient control of EBV replication (22,29).
All analyses adjusted for the matching factors. Regression models included age and sample collection year as continuous variables. Linear regression models and correlation computations utilized log10-transformations of the continuous immune and/or viral marker levels. To explore underlying population differences in immune status, we computed the geometric mean level of immune markers by population in HTLV-I non-carriers using mixed-effects linear regression analyses that accounted for the matching-related correlations in the data. We assessed population differences in the covariate-adjusted means by the t-test. We used the Mantel-Haenszel test, adjusted for age (<60 v. 60+ years), gender, and sample collection year (1987-1989 v. 1990-1993), to examine population differences in the prevalence of anti-EA seropositivity and a low EBNA1:EBNA2 ratio. Odds ratios (OR) and Wald-type 95% confidence intervals (CI) were computed by logistic regression to examine the association of HTLV-I serostatus with a given immune marker category (i.e., “higher” or “lower” levels), and (in carriers) to assess the immune markers’ association with anti-Tax positivity. We constructed a separate model for each immune marker, stratified by population. We also computed population-specific Spearman partial correlation coefficients (r) to estimate pair-wise correlations between each immune marker and the reciprocal of anti-HTLV-I titer and provirus load among HTLV-I carriers.
We used SAS® statistical software (SAS Institute, Inc., Cary, NC) for the statistical analyses. All statistical tests were 2-tailed. One EBV-negative individual (Japanese) and 12 persons with non-specific anti-EBNA1 reactivity (8 Japanese, 4 Jamaican) were omitted from EBV antibody analyses (22,23).
Results
Age, sex, and blood collection year (i.e., the matching factors) were distributed similarly by study population. The analysis included 45 (22%) persons aged 28-49, 81 (40%) aged 50-59, 53 (26%) aged 60-69, and 25 (12%) aged ≥70 years. Most matched sets (42/51; 82%) were female. We obtained 146 (72%) of the 204 plasma specimens from 1990-1993, and the remainder from 1987-1989.
Immune marker levels in persons uninfected with HTLV-I
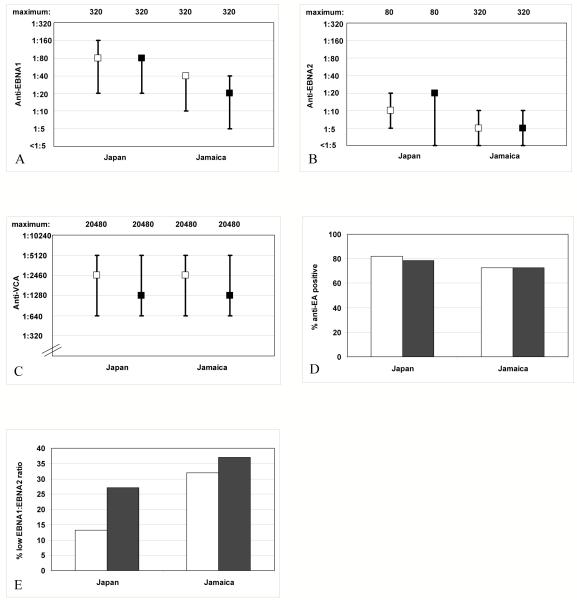
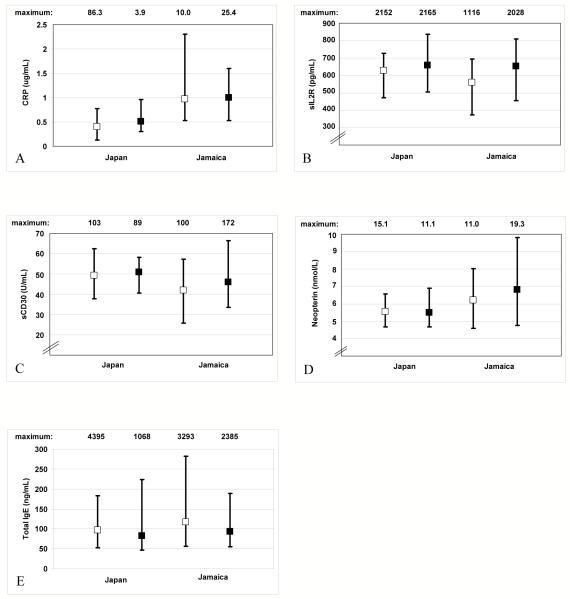
Median EBV antibody titers and the prevalence of anti-EA seropositivity did not differ notably by population in HTLV-I non-carriers (Figure 1A-D). In contrast, a larger proportion of Jamaican than Japanese non-carriers had a low EBNA1:EBNA2 ratio (Figure 1E). After adjustment for the matching factors, population differences in mean anti-EBNA1 titer and prevalence of a low EBNA1:EBNA2 ratio were statistically significant among non-carriers (Table 1). HTLV-I non-carriers from Japan had lower median CRP levels (Figure 2A) and higher median levels of sIL2R (Figure 2B) and sCD30 (Figure 2C) than those from Jamaica. There were no apparent differences in median neopterin (Figure 2D) or total IgE levels (Figure 2E) by population. After adjustment for the matching factors, the population differences in mean CRP, sIL2R, and sCD30 levels were statistically significant (Table 1).
Figure 1.
Median titers, inter-quartile ranges, and maximum observed titers of (A) anti-EBNA1, (B) anti-EBNA2, and (C) anti-VCA and prevalence of (D) anti-EA seropositivity and (E) low EBNA1:EBNA2 ratio, in HTLV-I-uninfected (□) and HTLV-I-infected (■) participants from Japan and Jamaica.
Table 1.
Pair-wise comparison of geometric mean level and prevalence of immune markers among 102 persons uninfected with HTLV-I from Japan and Jamaica
| Population | |||
|---|---|---|---|
| Immune marker | Japan | Jamaica | p-value |
| Anti-EBNA1, GMT† | 71 | 27 | 0.005 |
| Anti-EBNA2, GMT† | 9 | 7 | 0.41 |
| Anti-VCA, GMT† | 2043 | 2120 | 0.87 |
| Anti-EA seropositivity, %‡ | 82.0 | 72.6 | 0.18 |
| Low EBNA1:EBNA2 ratio, %ठ| 13.3 | 31.9 | 0.02 |
| CRP, μg/mL†* | 0.43 | 1.1 | 0.0004 |
| sIL2R, pg/mL† | 623 | 477 | 0.0008 |
| sCD30, U/mL† | 46 | 34 | 0.0001 |
| Neopterin, nmol/L† | 5.6 | 5.6 | 0.94 |
| Total IgE, ng/mL†* | 154 | 146 | 0.82 |
Abbreviations: EBNA1, Epstein-Barr virus nuclear antigen type 1; GMT, geometric mean titer; EBNA2, Epstein-Barr virus nuclear antigen type 2; VCA, viral capsid antigen; EA, early antigen; CRP, C-reactive protein; sIL2R, soluble IL-2 receptor-α; sCD30, soluble CD30; IgE, immunoglobulin E.
Reciprocal of the geometric mean titer, or geometric mean level, from mixed-effects linear regression analyses adjusted for age (continuous, in years), sex, and year of sample collection (continuous); p-values were obtained from the t-test and reflect covariate adjustment.
P-values were obtained from the Mantel-Haenszel test, adjusted for age (<60 v. 60+ years), sex, and year of sample collection (1987-1989 v. 1990-1993).
A low EBNA1:EBNA2 ratio is a ratio of anti-EBNA1 to anti-EBNA2 titers ≤1.0.
Values below assay detection level are assigned a value of one-half the assay detection limit and included in the estimation of the geometric mean.
Figure 2.
Median levels, inter-quartile ranges, and maximum observed plasma levels of (A) C-reactive protein, (B) soluble IL-2 receptor, (C) soluble CD30, (D) neopterin, and (E) total IgE, in HTLV-I-uninfected (□) and HTLV-I-infected (■) participants from Japan and Jamaica.
Immune marker patterns associated with HTLV-I infection
Among Japanese participants, HTLV-I carriers had an increased prevalence of a low EBNA1:EBNA2 ratio compared to non-carriers (Figure 1E). The remaining EBV antibody titers did not vary significantly by HTLV-I status among Japanese subjects (Figures 1A-D). With adjustment for the matching variables, Japanese HTLV-I carriers were three times as likely as Japanese non-carriers to have a low EBNA1:EBNA2 ratio (p=0.06) (Table 2). Japanese carriers also had higher median CRP levels (Figure 2A) and a more than two-fold increased odds of higher CRP levels (p=0.04) (Table 2) compared to Japanese non-carriers. Levels of sIL2R, sCD30, neopterin, and total IgE did not vary markedly by HTLV-I status in Japanese subjects in crude comparisons (Figures 2B-E) or in multivariable regression analyses (Table 2).
Table 2.
Association of HTLV-I seropositivity with immune biomarker levels by study population, with adjustment for age, sex, and year of sample collection
| Japan | Jamaica | |||||||
|---|---|---|---|---|---|---|---|---|
| HTLV-I | HTLV-I | |||||||
| - | + | - | + | |||||
| (N=51) | (N=51) | (N=51) | (N=51) | |||||
| Immune marker category* | % | % | OR§ | (95% CI) | % | % | OR§ | (95% CI) |
| EBNA1:EBNA2 ratio | ||||||||
| >1.0 | 86.7† | 72.9‡ | 1.0 | (reference) | 68.1¶ | 63.0¶ | 1.0 | (reference) |
| ≤1.0 | 13.3† | 27.1‡ | 3.0 | (0.9-9.9) | 31.9¶ | 37.0¶ | 1.3 | (0.5-3.0) |
| CRP | ||||||||
| ≤Median | 51.0 | 31.4 | 1.0 | (reference) | 51.0 | 49.0 | 1.0 | (reference) |
| >Median | 49.0 | 68.6 | 2.4 | (1.1-5.5) | 49.0 | 51.0 | 1.1 | (0.5-2.4) |
| sIL2R | ||||||||
| ≤Median | 49.0 | 39.2 | 1.0 | (reference) | 51.0 | 29.4 | 1.0 | (reference) |
| >Median | 51.0 | 60.8 | 1.6 | (0.7-3.8) | 49.0 | 70.6 | 3.3 | (1.3-8.6) |
| sCD30 | ||||||||
| ≤Median | 51.0 | 45.1 | 1.0 | (reference) | 51.0 | 35.3 | 1.0 | (reference) |
| >Median | 49.0 | 54.9 | 1.3 | (0.5-3.1) | 49.0 | 64.7 | 2.1 | (0.9-5.1) |
| Total IgE | ||||||||
| ≤Median | 51.0 | 52.9 | 1.0 | (reference) | 51.0 | 62.7 | 1.0 | (reference) |
| >Median | 49.0 | 47.1 | 0.8 | (0.4-1.8) | 49.0 | 37.3 | 0.6 | (0.3-1.3) |
| Neopterin | ||||||||
| ≤Median | 51.0 | 52.9 | 1.0 | (reference) | 51.0 | 45.1 | 1.0 | (reference) |
| >Median | 49.0 | 47.1 | 1.0 | (0.4-2.4) | 49.0 | 54.9 | 1.3 | (0.6-3.0) |
Abbreviations:HTLV-I, human T-lymphotropic virus type I; OR, odds ratio; CI, confidence interval; EBNA1, Epstein-Barr virus (EBV) nuclear antigen type 1; EBNA2, EBV nuclear antigen type 2; CRP, C-reactive protein; sIL2R, soluble IL-2 receptor-α (or CD25); sCD30, soluble CD30; IgE, immunoglobulin E.
Above-median categories are defined according to the population-specific median levels observed among non-carriers of HTLV-I.
45 Japanese HTLV-I-seronegative persons were analyzed; we omitted one EBV-seronegative subject and five subjects with non-specific anti-EBNA reactivity.
48 Japanese HTLV-I-seropositive individuals were analyzed; we omitted three subjects with non-specific anti-EBNA reactivity.
From unconditional logistic regression models adjusted for age, sex, and year of sample collection.
49 HTLV-I-seronegative and 49 HTLV-I-seropositive Jamaican persons were analyzed; we omitted two subjects with non-specific anti-EBNA reactivity in each group.
Among the Jamaican participants, neither the prevalence of a low EBNA1:EBNA2 ratio (Figure 1E), nor the concentration of CRP (Figure 2A) varied by HTLV-I serostatus. However, both were higher in Jamaican than in Japanese subjects regardless of HTLV-I status. Compared to Jamaican non-carriers of HTLV-I, Jamaican carriers had higher median sIL2R (Figure 2B) and sCD30 levels (Figure 2C) and three- and two-fold increases in the odds of higher sIL2R (p=0.01) and sCD30 levels (p=0.09), respectively (Table 2). However, median sIL2R levels were similar in HTLV-I carriers from Japan and Jamaica (Figure 2B). Levels of EBV antibodies, neopterin, and total IgE did not differ greatly by HTLV-I status among Jamaican subjects in crude comparisons (Figures 1A-D and 2D-E, respectively) or in multivariable regression analyses (Table 2).
Correlation of HTLV-I viral markers with plasma immune markers
Among Japanese carriers, anti-HTLV-I titer was significantly correlated only with anti-EA titer (Table 3A). In contrast, among Jamaican carriers, anti-HTLV-I titer was significantly correlated with anti-VCA, anti-EA, sIL2R, sCD30 and neopterin levels. Provirus load was not correlated with any immune marker in Japanese carriers but was correlated with anti-EBNA2, anti-EA, sIL2R, sCD30 and neopterin levels in Jamaican carriers (Table 3A). Anti-Tax seropositivity was more prevalent in Japanese carriers with lower anti-EBNA1 titers, higher anti-EBNA2 titers, a low EBNA1:EBNA2 ratio, or detectable anti-EA, although the associations were not statistically significant (Table 3B). Among Jamaican carriers, anti-Tax was more commonly detected in those who had higher sIL2R, sCD30, or neopterin levels. CRP and total IgE levels were not associated with any viral marker in either population (data not shown).
Table 3.
Association of HTLV-I viral markers with immune marker levels among Japanese and Jamaican carriers
| A. Anti-HTLV-I titer and HTLV-I provirus load | ||||
|---|---|---|---|---|
| Geographic population | ||||
| HTLV-I viral marker/Immune marker | Japan | Jamaica | ||
| r† | (p-value) | r† | (p-value) | |
| Anti-HTLV-I titer | ||||
| anti-EBNA2 | 0.07 | (0.66) | 0.28 | (0.06) |
| anti-VCA | 0.26 | (0.08) | 0.32 | (0.03) |
| anti-EA | 0.36 | (0.01) | 0.47 | (0.001) |
| sIL2R | 0.19 | (0.22) | 0.57 | (<0.0001) |
| sCD30 | -0.07 | (0.63) | 0.47 | (0.0009) |
| neopterin | 0.19 | (0.21) | 0.58 | (<0.0001) |
| HTLV-I provirus load | ||||
| anti-EBNA2 | 0.18 | (0.24) | 0.44 | (0.002) |
| anti-VCA | 0.07 | (0.67) | 0.07 | (0.64) |
| anti-EA | 0.22 | (0.15) | 0.31 | (0.03) |
| sIL2R | 0.22 | (0.14) | 0.58 | (<0.0001) |
| sCD30 | 0.08 | (0.61) | 0.49 | (0.0006) |
| neopterin | 0.08 | (0.61) | 0.57 | (<0.0001) |
| B. Anti-Tax seropositivity | ||||
| Immune marker | OR‡ | (95% CI) | OR‡ | (95% CI) |
| Anti-EBNA1 < median | 3.2 | (0.8-12.4) | 1.4 | (0.4-4.6) |
| Anti-EBNA2 > median | 2.0 | (0.6-6.7) | 1.5 | (0.4-5.2) |
| EBNA1:EBNA2 ratio ≤1.0 | 3.6 | (0.8-15.1) | 0.9 | (0.3-3.3) |
| Anti-EA-positive | 2.3 | (0.5-11.0) | 0.7 | (0.2-2.7) |
| sIL2R > median | 1.3 | (0.4-4.5) | 2.7 | (0.7-11.2) |
| sCD30 > median | 1.7 | (0.4-6.4) | 2.8 | (0.7-11.3) |
| Neopterin > median | 1.8 | (0.5-6.9) | 3.4 | (0.9-12.6) |
Abbreviations:HTLV-I, human T-lymphotropic virus type I; sIL2R, soluble IL-2 receptor; sCD30, soluble CD30; OR, odds ratio; CI, confidence interval; EBNA1, Epstein-Barr virus nuclear antigen type 1; EBNA2, Epstein-Barr virus nuclear antigen type 2;VCA, viral capsid antigen; EA, early antigen.
Spearman partial correlations of log10-transformed HTLV-I viral markers with log10-transformed serologic immune marker levels, with mutual adjustment for the immune markers and for age, sex, and year of sample collection.
From logistic regression models adjusted for age, sex, and year of sample collection.
Discussion
We undertook the present analysis to compare patterns of plasma markers of host immune status in Japanese and Jamaican carriers and non-carriers of HTLV-I using standardized laboratory assays. Some cytokines may be unstable, or present at levels below the lower detectable limits of commercial assays, in the plasma specimens available for our investigation (30,31). Thus, we measured soluble protein correlates of cytokine levels that are well accepted as plasma immune markers, including several that we previously measured successfully (32). We included EBV antibody profiles as markers of cellular immunity; studies have shown that the combination of lower anti-EBNA1 and higher anti-EBNA2 titers suggest poor control of EBV infection in the context of a weak CTL response (22, 29). We also measured concentrations of plasma markers of T-cell (sIL2R, sCD30), B-cell (total IgE), and inflammation or non-specific (CRP, neopterin) immune activation (7,25-28,33). Compared to cytokines, the surrogate markers are considered to be more stable, more frequently detectable, and measurable with greater precision in peripheral blood specimens from asymptomatic individuals (30,31).
To characterize underlying population differences in host immunity, we examined immune marker patterns in Japanese and Jamaican non-carriers of HTLV-I. In the Jamaican non-carriers we observed marker patterns that suggest less T-lymphocyte activation (i.e., lower mean anti-EBNA1, sIL2R, and sCD30 levels) (25,26), and diminished CTL control of EBV (i.e., a greater prevalence of a low EBNA1:EBNA2 ratio) (22,29), compared to Japanese non-carriers. This lower level of T-cell activation may confer a lower risk of T-cell transformation and thus contribute to the relatively low risk of ATL in Jamaica. In contrast, higher mean CRP levels in Jamaican non-carriers compared to Japanese non-carriers are consistent with increased levels of inflammation or non-specific immune activation in the Jamaicans (27,28). The Jamaican subjects’ mean CRP levels were lower than those typically associated with inflammation-related disease (34); however, such subclinical increases in inflammation may contribute to the greater risk of HAM/TSP in Jamaica. The small sample sizes in the present analysis, as well as the use of surrogate rather than direct cytokine markers, warrant cautious interpretation. Nonetheless, the contrasting immune marker patterns in Japanese and Jamaican non-carriers of HTLV-I may reflect underlying population differences in host immune status. These differences may be due, in part, to genetics, nutritional status, social environment, and/or co-morbidity such as parasitic burden.
We also observed marked population differences in HTLV-I-associated immune marker patterns. Diminished cellular immunity, as characterized by a low EBNA1:EBNA2 ratio (22,29), was more frequent in Japanese carriers than non-carriers, consistent with previous reports (13,16-19). In contrast, the prevalence of a low EBNA1:EBNA2 ratio did not differ by HTLV-I status in the Jamaican subjects, but was greater in both the carriers and non-carriers from Jamaica than in the Japanese carriers. Thus, the apparent population differences in HTLV-I-related EBV antibody patterns reflect the population differences we observed among the HTLV-I non-carriers.
Similarly, among Japanese subjects, HTLV-I carriers had higher CRP levels than non-carriers, but the median CRP levels were even higher in Jamaican subjects regardless of HTLV-I status. The HTLV-I-related increase in CRP levels in the Japanese subjects may reflect increased IL-6 expression (35), possibly induced by Tax (36), while the higher CRP levels in Jamaican non-carriers may have obscured any Tax-induced effects on CRP or IL-6 in the Jamaican carriers.
HTLV-I infection of T-cells results in up-regulation of activation markers, including IL2R and CD30 (25,37), on infected cells. In addition, elevated plasma sIL2R (38,39) and sCD30 levels (40,41) have been reported in ATL patients and likely reflect tumor burden related to IL2R and CD30 expression by ATL cells (25,26). HAM/TSP patients and asymptomatic HTLV-I carriers were also reported to have significantly higher sIL2R levels than non-carriers in a predominantly Caribbean population (42). We previously reported similar sCD30 levels in asymptomatic Japanese carriers and non-carriers (16), but the levels have not been well studied in Jamaican carriers. In the present analysis, we did not observe differences in sIL2R or sCD30 levels by HTLV-I status in Japanese subjects, whereas levels of both markers were higher in carriers than non-carriers from Jamaica, especially sIL2R. Those apparent HTLV-I-associated differences in sIL2R levels in the Jamaicans were due to lower levels in the Jamaican non-carriers rather than to unusually high levels in the Jamaican carriers. Therefore, the HTLV-I-related increases in these markers among the Jamaicans appear to reflect the lower background population levels of T-cell activation that we observed in the Jamaican non-carriers.
One Jamaican study has previously examined circulating neopterin levels in asymptomatic adults by HTLV-I serostatus (43) and found that the levels did not vary by HTLV-I status, consistent with the present findings. We also did not observe significant differences in total IgE levels by HTLV-I serostatus in either population, in contrast to prior reports of lower total IgE levels in HTLV-I carriers than non-carriers (16,44,45).
In analyses restricted to carriers of HTLV-I, the immune markers generally showed more correlation with viral markers in Jamaican than in Japanese carriers. In the Japanese carriers, EBV antibody patterns consistent with diminished cellular immunity had suggestive associations with anti-Tax seropositivity. We conducted a cross-sectional analysis and therefore cannot determine the temporal relation of the viral and immune marker patterns. However, the observed correlations in Japanese carriers may reflect an increase in viral protein expression and division of HTLV-I-infected T-lymphocytes in persons with diminished type 1 immunity (8). In Jamaican carriers, plasma markers of T-cell (sIL2R, sCD30) (25,26) and non-specific (neopterin) (28) immune activation were correlated with HTLV-I provirus load and anti-HTLV-I titer and had suggestive associations with anti-Tax seropositivity. Those correlations are consistent with the prediction that active host immune responses to HTLV-I in the context of persistent HTLV-I replication characterize the Jamaican carriers in this study population (15).
A synthesis of the immune and viral marker data suggests several hypotheses regarding population differences in host immune status and control of HTLV-I. Among Japanese participants, non-carriers of HTLV-I appeared to have increased T-cell activation, but HTLV-I was not associated with a further increase in T-cell activation marker levels. Concurrently, the HTLV-I viral marker patterns in Japanese carriers did not indicate persistent HTLV-I replication or CTL responses to HTLV-I (15). Thus, the Japanese carriers may not have effective virus-specific immune responses despite the evidence of T-cell activation. This suggestion is consistent with the present and previous observations of diminished type 1 immunity in Japanese carriers (13,16-19). Furthermore, in the absence of evidence for persistent HTLV-I replication, the high provirus loads we observed in Japanese carriers are most likely a result of mitotic division of HTLV-I-infected T-lymphocytes (15). The combination of ongoing but ineffective T-cell activation and mitotic proliferation of HTLV-I-infected T-lymphocytes could contribute to the increased risk of ATL that is characteristic of Japanese carriers. In contrast, in Jamaican carriers and non-carriers of HTLV-I we observed plasma marker levels that imply chronic inflammation. In addition, Jamaican carriers had plasma T-cell activation marker levels similar to those of Japanese subjects. HTLV-I viral marker levels in Jamaican carriers further indicated persistent HTLV-I replication and virus-specific CTL responses. The combination of persistent HTLV-I replication, CTL responses, and inflammation is characteristic of a population at greater risk for HAM/TSP (11,12,15). The lysis of HTLV-I-infected T-cells by a persistent CTL response may also contribute to the comparatively low risk of ATL observed in Jamaica.
The matched study design, direct comparisons of immune marker profiles across populations, and standardized laboratory assays represent unique strengths of the present analysis. Nonetheless, limitations of this study should be noted. The immune marker findings may not be directly applicable to ATL or HAM/TSP patients, as all of the HTLV-I-infected participants were asymptomatic. Also, we could not adjust for factors that may modulate the host immune response to HTLV-I, including age at infection, route and dose of infection, nutritional status, social environment, co-morbidity, and host genetics. The statistical power to detect significant small associations was limited due to the small sample size. However, we included as many participants as could be appropriately matched across populations; we are not aware of ongoing studies that could yield a larger sample size for a similar analysis.
In conclusion, we observed several intriguing new findings related to population differences in host immune status in carriers and non-carriers of HTLV-I. The findings corroborate the view that host immune dysregulation, perhaps even at a modest, subclinical level, contributes to the well established differences in natural history of HTLV-I infection in Japanese and Jamaican carriers. It remains unclear why HTLV-I infection results in contrasting types of immune dysregulation in the two populations. Possible mechanism(s) may be elucidated by longitudinal studies that include ATL and HAM/TSP patients and information on potential co-factors that was not available for the present analysis. The further development of informative, easily measured markers of immune status will also aid future studies. Those studies will provide important information to characterize the asymptomatic carriers of HTLV-I who are at an increased risk of ATL or HAM/TSP.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, and by Public Health Service grants CA115687, CA38450 and training grant CA09001 (BB). We thank Annika Linde for technical and intellectual expertise on the EBV serology; Chung-Cheng Hsieh for statistical consultation; Larry Magpantay for the CRP, sCD30, and total IgE assays; Najib Aziz and the Clinical Immunology Laboratory at UCLA for the sIL2R and NPT assays; Hongchuan Li for quantification of HTLV-I provirus load; Takashi Sawada for detection of anti-Tax; and, Francis Yellin and Christina Raker for database administration. We are also grateful to the members of the Miyazaki Cohort Study and Food Handlers Study populations for their participation in this research.
Abbreviations used
- ATL
adult T-cell leukemia/lymphoma
- CI
confidence interval
- CRP
C-reactive protein
- CTL
cytotoxic T-lymphocyte
- EA
early antigen
- EBNA
Epstein-Barr virus nuclear antigen
- EBV
Epstein-Barr virus
- ELISA
enzyme-linked immunosorbent assay
- HAM/TSP
human T-lymphotropic virus-I-associated myelopathy/tropical spastic paraparesis
- HTLV-I
human T-lymphotropic virus type I
- IFA
immunofluorescence assay
- IL
interleukin
- IL-2R
IL-2 receptor-α
- IgE
immunoglobulin E
- OR
odds ratio
- sCD30
soluble CD30
- sIL2R
soluble IL-2R
- VCA
viral capsid antigen
Footnotes
Novelty: The present study is the first to perform a direct comparison of plasma immune markers measured in centralized laboratories among HTLV-I carriers from Japan and the Caribbean who were carefully matched on several covariates, to explore the contribution of host immune status to the contrasts in risks of adult T-cell lymphoma and HAM/TSP across these populations.
Impact: It is not known which asymptomatic carriers of HTLV-I are risk for ATL and HAM/TSP, and the identification of risk markers is crucial to advance the understanding of the pathogenesis of these conditions, as well as the development of potential strategies for their prevention. In the present study, we observed population differences in immune marker levels among uninfected persons from Japan and Jamaica, as well as in relation to HTLV-I infection in each population, that corroborate a role for differences in host immune status in the contrasting geographic patterns of HTLV-I-related morbidity.
References
- 1.Lairmore MD, Franchini G. Human T-cell leukemia virus types I and II. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2072–105. [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) IARC. Monographs on the evaluation of carcinogenic risks to humans. Vol. 67. IARC; Lyon: 1996. Human T-cell lymphotropic viruses; pp. 261–390. [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller N, Birmann B, Parsonnet J, Schiffman M, Stuver S. Infectious Agents. In: Schottenfeld D, Fraumeni JJ, editors. Cancer Epidemiology and Prevention. 3rd ed. Oxford University Press; New York: 2006. pp. 507–48. [Google Scholar]
- 4.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 5.Li HC, Walters M, Carrington M, Headley C, Amarasinghe A, Goedert J, Cranston B, Hanchard B, Hisada M. Serologic and molecular characteristics of atypical HTLV Western blot banding patterns in Jamaican blood donors [Abstract P56] AIDS Res Hum Retroviruses. 2005;21:479. [Google Scholar]
- 6.Barmak K, Harhaj E, Grant C, Alefantis T, Wigdahl B. Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology. 2003;308:1–12. doi: 10.1016/s0042-6822(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 7.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–62. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akimoto M, Kozako T, Sawada T, Matsushita K, Ozaki A, Hamada H, Kawada H, Yoshimitsu M, Tokunaga M, Haraguchi K, Uozumi K, Arima N, et al. Anti-HTLV-I Tax antibody and Tax-specific cytotoxic T-lymphocyte are associated with a reduction in HTLV-I proviral load in asymptomatic carriers. J Med Virol. 2007;79:977–86. doi: 10.1002/jmv.20807. [DOI] [PubMed] [Google Scholar]
- 9.Mueller N, Okayama A, Stuver S, Tachibana N. Findings from the Miyazaki Cohort Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl 1):S2–7. doi: 10.1097/00042560-199600001-00002. [DOI] [PubMed] [Google Scholar]
- 10.Yokota T, Cho MJ, Tachibana N, McLane MF, Takatsuki K, Lee TH, Mueller N, Essex M. The prevalence of antibody to p42 of HTLV-I among ATLL patients in comparison with healthy carriers in Japan. Int J Cancer. 1989;43:970–4. doi: 10.1002/ijc.2910430603. [DOI] [PubMed] [Google Scholar]
- 11.Kubota R, Nagai M, Kawanishi T, Osame M, Jacobson S. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res Hum Retroviruses. 2000;16:1705–9. doi: 10.1089/08892220050193182. [DOI] [PubMed] [Google Scholar]
- 12.Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I provirus load. J Infect Dis. 2001;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- 13.Imai J, Hinuma Y. Epstein-Barr virus-specific antibodies in patients with adult T-cell leukemia (ATL) and healthy ATL virus-carriers. Int J Cancer. 1983;31:197–200. doi: 10.1002/ijc.2910310210. [DOI] [PubMed] [Google Scholar]
- 14.Tendler CL, Greenberg SJ, Burton JD, Danielpour D, Kim SJ, Blattner WA, Manns A, Waldmann TA. Cytokine induction in HTLV-I associated myelopathy and adult T-cell leukemia: alternate molecular mechanisms underlying retroviral pathogenesis. J Cell Biochem. 1991;46:302–11. doi: 10.1002/jcb.240460405. [DOI] [PubMed] [Google Scholar]
- 15.Hisada M, Stuver SO, Okayama A, Li HC, Sawada T, Hanchard B, Mueller NE. Persistent paradox of natural history of human T lymphotropic virus type I: parallel analyses of Japanese and Jamaican carriers. J Infect Dis. 2004;190:1605–9. doi: 10.1086/424598. [DOI] [PubMed] [Google Scholar]
- 16.Birmann BM, Mueller NE, Okayama A, Hsieh CC, Tsubouchi H, Harn D, Stuver SO. Patterns of serum type 1 and type 2 immune markers in healthy carriers of HTLV-I. J Med Virol. 2006;78:847–52. doi: 10.1002/jmv.20633. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana N, Okayama A, Ishizaki J, Yokota T, Shishime E, Murai K, Shioiri S, Tsuda K, Essex M, Mueller N. Suppression of tuberculin skin reaction in healthy HTLV-I carriers from Japan. Int J Cancer. 1988;42:829–31. doi: 10.1002/ijc.2910420605. [DOI] [PubMed] [Google Scholar]
- 18.Murai K, Tachibana N, Shioiri S, Shishime E, Okayama A, Ishizaki J, Tsuda K, Mueller N. Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J Acquir Immune Defic Syndr. 1990;3:1006–9. [PubMed] [Google Scholar]
- 19.Kwon KW. Epstein-Barr virus-specific immunity in asymptomatic carriers of human T-cell leukemia virus type 1. Hokkaido Igaku Zasshi. 1995;70:315–28. [PubMed] [Google Scholar]
- 20.Murphy EL, Figueroa JP, Gibbs WN, Holding-Cobham M, Cranston B, Malley K, Bodner AK, Alexander SS, Blattner WA. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am J Epidemiol. 1991;133:1114–24. doi: 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 21.Sawada T, Tohmatsu J, Obara T, Koide A, Kamihira S, Ichimaru M, Kashiwaqi S, Kajiyama W, Matsumura N, Kinoshita K, Yano M, Yamaguchi K, et al. High risk of mother-to-child transmission of HTLV-I in p40tax antibody-positive mothers. Jpn J Cancer Res. 1989;80:506–8. doi: 10.1111/j.1349-7006.1989.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennette ET, Rymo L, Yadav M, Masucci G, Merk K, Timar L, Klein G. Disease-related differences in antibody patterns against EBV-encoded nuclear antigens EBNA 1, EBNA 2 and EBNA 6. Eur J Cancer. 1993;29A:1584–9. doi: 10.1016/0959-8049(93)90299-u. [DOI] [PubMed] [Google Scholar]
- 23.Nagy N, Maeda A, Bandobashi K, Kis LL, Nishikawa J, Trivedi P, Faggione A, Klein G, Klein E. SH2D1A expression in Burkitt lymphoma cells is restricted to EBV positive group I lines and is downregulated in parallel with immunoblastic transformation. Int J Cancer. 2002;100:433–40. doi: 10.1002/ijc.10498. [DOI] [PubMed] [Google Scholar]
- 24.Linde A, Andersson J, Lundgren G, Wahren B. Subclass reactivity to Epstein-Barr virus capsid antigen in primary and reactivated EBV infections. J Med Virol. 1987;21:109–21. doi: 10.1002/jmv.1890210203. [DOI] [PubMed] [Google Scholar]
- 25.Rubin LA, Nelson DL. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 1990;113:619–27. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]
- 26.Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer. 2003;98:458–67. doi: 10.1002/cncr.11524. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann G, Wirleitner B, Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res. 2003;52:313–21. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- 29.Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, Marklund G, Rymo L, Wellinder C, Straus SE. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci U S A. 1987;84:570–4. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aziz N, Nishanian P, Fahey JL. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5:755–61. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. Cytokine measurements and interpretation of cytokine assays in human disease. J Clin Immunol. 1994;14:327–39. doi: 10.1007/BF01546317. [DOI] [PubMed] [Google Scholar]
- 32.Birmann BM, Mueller N, Okayama A, Hsieh CC, Tachibana N, Tsubouchi H, Lennette ET, Harn D, Stuver S. Serologic assessment of type 1 and type 2 immunity in healthy Japanese adults. Cancer Epidemiol Biomarkers Prev. 2004;13:1385–91. [PubMed] [Google Scholar]
- 33.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 34.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 35.Yamamura M, Yamada Y, Momita S, Kamihira S, Tomonaga M. Circulating interleukin-6 levels are elevated in adult T-cell leukaemia/lymphoma patients and correlate with adverse clinical features and survival. Br J Haematol. 1998;100:129–34. doi: 10.1046/j.1365-2141.1998.00538.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita I, Katamine S, Moriuchi R, Nakamura Y, Miyamoto T, Eguchi K, Nagataki S. Transactivation of the human interleukin-6 gene by human T-lymphotropic virus type 1 Tax protein. Blood. 1994;84:1573–8. [PubMed] [Google Scholar]
- 37.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 38.Araki K, Harada K, Nakamoto K, Shiroma M, Miyakuni T. Clinical significance of serum soluble IL-2R levels in patients with adult T cell leukaemia (ATL) and HTLV-1 carriers. Clin Exp Immunol. 2000;119:259–63. doi: 10.1046/j.1365-2249.2000.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arisawa K, Katamine S, Kamihira S, Kurokawa K, Sawada T, Soda M, Doi H, Saito H, Shirahama S. A nested case-control study of risk factors for adult T-cell leukemia/lymphoma among human T-cell lymphotropic virus type-I carriers in Japan. Cancer Causes Control. 2002;13:657–63. doi: 10.1023/a:1019511224501. [DOI] [PubMed] [Google Scholar]
- 40.Pfreundschuh M, Pohl C, Berenbeck C, Schroeder J, Jung W, Schmits R, Tschiersch A, Diehl V, Gause A. Detection of a soluble form of the CD30 antigen in sera of patients with lymphoma, adult T-cell leukemia and infectious mononucleosis. Int J Cancer. 1990;45:869–74. doi: 10.1002/ijc.2910450515. [DOI] [PubMed] [Google Scholar]
- 41.Dallenbach F, Josimovic-Alasevic O, Dürkop H, Schwarting R, Diamantstein T, Matsuoka M, Takatsuki K, Stein H. Soluble CD30 antigen in the sera of patients with adult T-cell lymphoma/leukemia (ATL): a marker for disease activity. In: Knapp W, Dorken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, von dem Borne AEG, editors. Leukocyte Typing IV. Oxford University; Oxford, UK: 1989. pp. 426–8. [Google Scholar]
- 42.Tendler CL, Greenberg SJ, Blattner WA, Manns A, Murphy E, Fleisher T, Hanchard B, Morgan O, Burton JD, Nelson DL, Waldmann TA. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci U S A. 1990;87:5218–22. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giusti RM, Maloney EM, Hanchard B, Morgan OS, Steinberg SM, Wachter H, Williams E, Cranston B, Fuchs D, Manns A. Differential patterns of serum biomarkers of immune activation in human T-cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis, and adult T-cell leukemia/lymphoma. Cancer Epidemiol Biomarkers Prev. 1996;5:699–704. [PubMed] [Google Scholar]
- 44.Hayashi J, Kishihara Y, Yoshimura E, Furusyo N, Yamaji K, Kawakami Y, Murakami H, Kashiwaqi S. Correlation between human T cell lymphotropic virus type-1 and Strongyloides stercoralis infections and serum immunoglobulin E responses in residents of Okinawa, Japan. Am J Trop Med Hyg. 1997;56:71–5. doi: 10.4269/ajtmh.1997.56.71. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Miike T, Mizoguchi K, Yamaguchi K, Takatsuki K, Hosoda M, Kawabe T, Yodoi J. Decreased serum levels of IgE and IgE-binding factors in individuals infected with HTLV-I. Clin Exp Immunol. 1990;81:207–11. doi: 10.1111/j.1365-2249.1990.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]