Summary
Nothing documents better the spectacular adaptive capacity of Staphylococcus aureus than the response of this important human and animal pathogen to the introduction of antimicrobial agents into the clinical environment. The effectiveness of penicillin introduced in the early 1940s was virtually annulled within a decade due to the plasmid epidemics that spread the ß-lactamase gene through the entire species of S. aureus. In 1960 within one to two years of the introduction of penicillinase resistant ß-lactams (methicillin), methicillin resistant S. aureus (MRSA) strains were identified in clinical specimens. By the 1980s, epidemic clones of MRSA acquired multidrug resistant traits and spread worldwide to become one of the most important causative agents of hospital acquired infections. In the early 2000s, MRSA strains carrying the Tn1546 transposon-based enterococcal vancomycin resistant mechanism were identified in clinical specimens, bringing the specter of a totally resistant bacterial pathogen closer to reality. Then, in the late 1990s, just as effective hygienic and antibiotic use policies managed to bring down the frequency of MRSA in hospitals of several countries, MRSA strains began to show up in the community.
Introduction
The purpose of this brief overview is to generate interest among readers in MRSA – a fascinating and dangerous human pathogen continually evolving under our eyes to cope with a constantly changing human environment. In this overview, a special emphasis was paid to the unique staphylococcal chromosome cassettes the structure of which plays a critical role in the epidemiology of both the hospital associated and community acquired MRSA.
The globally spread epidemic clones of MRSA are the product of convergence of several unusual evolutionary processes [1].
The resistance mechanism itself is unusual: it involves acquisition of the mecA gene, the determinant of a unique penicillin binding protein PBP2A which has low affinity for ß-lactam antibiotics and which can function as a surrogate of the native staphylococcal PBPs – cell wall synthetic enzymes – that are inactivated by ß-lactam antibiotics [2].
The resistance gene mecA appears to have evolved from a domestic gene of the fully ß-lactam susceptible Staphylococcus sciuri, a frequent colonizer of the skin of both wild and domestic animals and one of the most abundant staphylococcal species on this planet [3].
Before “arriving” to S. aureus the mecA gene has to be incorporated into a unique molecular vector called the staphylococcal chromosome cassette (SCC) which has its own independent evolutionary history and which is capable of delivering a variety of different resistance or virulence determinants to S. aureus, including mecA.
Successful “grafting” of the SCCmec into an S. aureus cell requires still additional factors: a unique and apparently rare genetic background that allows maintenance and expression of mecA. The relatively few successful SCCmec acquisition events that give rise to an MRSA strain appear to require the absence of a genetic “barrier” that is frequent in the genetic background of many S. aureus lineages [4].
Of the MRSA strains that have managed to maintain and express the SCCmec, only a handful of lineages seem to be able to spread globally. Genetic determinants that define the superior epidemicity of these clones are not known at the present time but may be associated with genes that are responsible for the effective colonization of the host [5].
For the first three decades after their appearance, MRSA strains typically have remained hospital associated pathogens (HA-MRSA). Then, in an unexpected epidemiological “move”, MRSA strains also began to appear in the community among people who had none of the usual risk factors for such infections. Such community acquired MRSA (CA-MRSA) has since been recognized as a major and disturbing reality in many countries, but, the precise scenario that has led to its appearance is still a matter of speculation.
The origin of mecA
Screening of isolates from different staphylococcal species with a DNA probe internal to the mecA of MRSA has led to a staphylococcal species, S. sciuri in which each one of up to 200 epidemiologically unrelated isolates gave strong hybridization signal [3]. The large majority of these isolates were fully susceptible to all ß-lactam antibiotics including penicillin. The gene homologue of mecA was identified as a determinant showing linear structure and conserved motifs typical of a bacterial penicillin binding protein [6], with a transpeptidase (TPase) domain that showed 92% identity with the aminoacid sequence of the TPase domain of mecA from MRSA strains. Recent work identified the mecA homologue as the genetic determinant of an 84 kDa PBP in S. sciuri. Up-regulation of the promoter of this gene was shown to provide wide spectrum ß-lactam resistance to the S. sciuri strain and transfer of the up-regulated S. sciuri mecA homologue on a plasmid to a fully ß-lactam susceptible S. aureus caused substantial increase in the oxacillin MIC value of the S. aureus recipient (Zhou et al. In preparation). It was also possible to show that the plasmid-born S. sciuri gene was able to participate in the synthesis of peptidoglycan of the S. aureus transductant growing in the presence of ß-lactam antibiotics and under these conditions, the S. sciuri mecA homologue produced a peptidoglycan the composition of which was typical of the host strain S. aureus and was completely different from the peptidoglycan of S. sciuri [7].
More recently, the protein product of the S. sciuri mecA homologue was purified to homogeneity and compared to the properties of PBP2A, the product of the S. aureus mecA. The two proteins showed very similar properties which included inhibition by a wide range of ß-lactam antibiotics; the existence of an allosteric site for binding of S. aureus peptidoglycan precursors; both PBP2A and the S. sciuri protein contained a sheltered active site, the access of which to both substrates and ß-lactam inhibitors required a conformational change in the proteins [8].
The first MRSA type mechanism was identified in clinical specimens within a very short – 1-2 years – period after the introduction of the first penicillin resistant – oxazolidine type – ß-lactam into clinical use. Given the complexity of the mecA-based resistance mechanism, the notion that MRSA emerged under the selective pressure of these new ß-lactamase resistant antibiotics is unlikely. It was proposed recently that the evolution of mecA may have occurred over a much longer span of time in a staphylococcal species free of penicillinase under the selective pressure of penicillin [1] which began to be used extensively as a prophylactic agent in veterinary medicine in 1949, i.e., shortly after the introduction of penicillin into human clinical practice [9]. The staphylococcal species involved may have been S. sciuri, which is free of the penicillinase plasmid and is a very frequent inhabitant of the skin of domestic animals. In this scenario, an up-regulated version of the native S. sciuri mecA homologue may have emerged among colonizers of the skin of domestic animals under the selective pressure of prophylactic use of penicillin.
SCCmec core structure and definitions
The mecA gene, is embedded in a large heterologous chromosomal island – staphylococcal cassette chromosome (SCCmec) [10], that integrates into the S. aureus chromosome at a site-specific location (attBscc), located near the origin of replication [11]. The genetic organization of the close mecA vicinity defines the mec gene complex and, in S. aureus, three major classes have been described: class A containing the complete mecA regulon (mecI-mecR1-mecA) and classes B and C containing the mecA regulatory genes disrupted by insertion sequences, ΨIS1272-ΔmecR1-mecA and IS431-ΔmecR1-mecA, respectively [12].
The mobility of SCCmec is in part due to the presence of fully functional recombinases of the invertase/resolvase family and encoded by the ccr gene complex [13]. The ccr gene complex may have two genes, ccrAB, with four known allotypes [14,15], or a single gene not closely related to the ccrAB genes, ccrC [16].
SCCmec types are defined by combining the class of the mec gene complex with the ccr allotype [10,14,16-18] and based on this definition a new nomenclature has been recently proposed [19], so that SCCmec type definitions are as follows (proposed new names in parenthesis): type I – mec class B and ccrAB allotype 1 (1B); type II – class A and ccrAB2 (2A); type III – class A and ccrAB3 (3A); type IV – class B and ccrAB2 (2B); type V – class C and ccrC (5C); and type VI – class B and ccrAB4 (4B).
The remaining parts of SCCmec are called junkyard (J) regions (J1, J2 and J3), which constitute nonessential components of SCCmec; although, in some cases, these regions carry additional antibiotic resistance determinants [20]. Starting clockwise from the origin of replication of S. aureus chromosome, the J3 is the region between the chromosomal left junction and the mec complex; J2 is the region between the mec complex and the ccr complex; and J1 is the region between the ccr complex and the chromosomal right junction. Therefore, SCCmec structural organization may be summarized as: J3-mec-J2-ccr-J1 – see Figure 1. Variations in the J regions (within the same mec-ccr combination) are used for defining SCCmec subtypes or variants.
Figure 1.
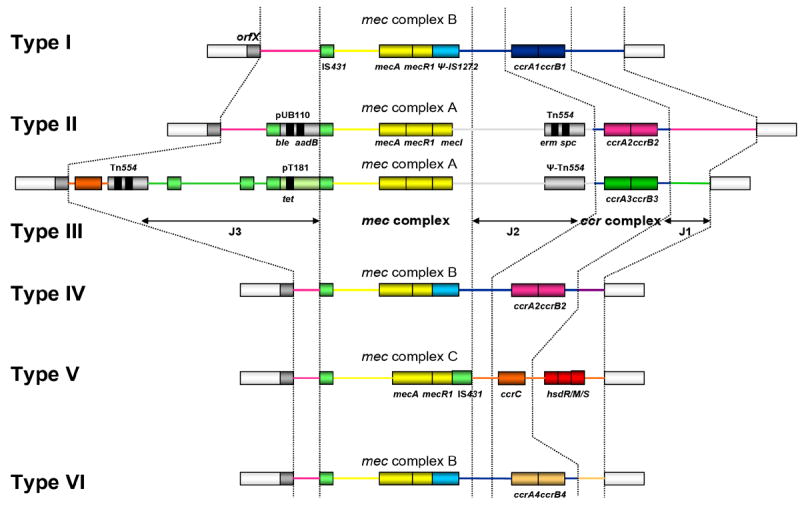
Genetic organization of SCCmec types I – VI (Maria Miragaia, Ph.D. thesis, Universidade Nova de Lisboa, 2006).
CA-MRSA are predominantly characterized by SCCmec type IV or by type V [21](the later described only in 2004 [16]. SCCmec type IV is the smallest structural type of SCCmec and is believed to be the most mobile version [22]. Perhaps as a consequence of its enhanced mobility, SCCmec type IV is also more variable than other SCCmec types and eight subtypes (types IVa through IVh) differing mainly in the J1 region have been described so far [17,20,23-25]. Besides frequently associated to CA-MRSA, SCCmec type IV is also characteristic of some HA-MRSA clones, such as the EMRSA-15, an endemic clone in the UK, which is also spreading in several European countries [26].
For the sake of the extension of this review we will focus on SCCmec typing strategies based on multiplex PCR assays, which are very practical and, as consequence, the most commonly used. Multiplex PCR approaches have a limited number of interrogated loci which may restrict the resolution of the typing strategy but, as they came in different flavors, they can cover different discriminatory needs. Still, no single multiplex PCR reaction can include the 22 out of 34 binary targets required, according to Stephens et al [27], to genotype properly all the 46 SCCmec variants.
In 2006, Kondo et al have proposed a SCCmec typing scheme based on six multiplex PCR reactions [28]. This strategy is very complete and while being able to detect the great majority of SCCmec it also identifies new ones. Still, due the high number of required assays it may not be feasible for routine purposes.
SCCmec typing scheme: a proposal
The choice of the best SCCmec typing strategy depends on the purpose of the study and its setting, such as local versus global surveillance of MRSA clones, clinical or research laboratory, etc. A flexible and feasible SCCmec typing scheme that could provide suitable discriminatory power for most study purposes would have three (sequential) stages and the following design.
ccrB sequencing [29]. This tool, based on the DNA sequencing of an internal fragment of the ccrB gene amplified using a single pair of degenerate primers has the advantage to be easily integrated into the spa and MLST protocols, and, likewise, is amenable for data deposition and analysis in a central web-based database, which is currently being developed (http://www.ccrbtyping.net/).). The sequence data generated may be used for tracing the dissemination of SCCmec elements within the MRSA population or even between S. aureus and coagulase-negative staphylococci (CoNS).
“SCCmec multiplex PCR” [30]. This strategy is able to identify in a single multiplex PCR assay using 20 primers SCCmec types I – VI, which are assigned through the presence of a specific amplification pattern of 2 – 5 bands.
“SCCmec IV multiplex PCR” [24]. This is a multiplex PCR assay for the subtyping of SCCmec type IV strains. SCCmec type IV is highly polymorphic and so far eight variants, differing mainly in the J1 region, have been described (IVa – IVh). Since type IV predominates among CA-MRSA, subtyping this SCCmec element may be critical to trace properly the dissemination of particular MRSA clones. Under this rationale, we have recently developed a single multiplex PCR assay based on the detection of subtype specific J1 sequences able to discriminate most subtypes of SCCmec IV – see Figure 2.
Figure 2.

“SCCmec IV multiplex PCR” (Adapted with permission from reference [24].
Pannel A - Genetic organization of the subtypes of SCCmec type IV. Pannel B – Amplification patterns obtained with the SCCmec multiplex IV PCR for the prototype strains of SCCmec IV subtypes. M, DNA molecular size marker (1 Kb DNA Ladder Plus, Invitrogen Life Technologies, Carlsbad, USA).
Non-mec SCC elements
The SCC element, defined as a mobile chromosomal cassette with dedicated recombinase genes (ccr) and characteristic flanking short sequence repeats, is not restricted to the dissemination of the mecA gene, as several non-mec SCC and ψSCC (without or no functional ccr genes) elements have been described carrying other genetic determinants (for a recent review see [31]. Some of these non-mec SCC elements encode for traits that may contribute to the survival or pathogenic potential of the bacteria, such as resistance to heavy metals (SCC mer [19] and SCC CI [32] or fusidic acid (SCC MSSA476 [33], capsule biosynthesis (SCC cap1 [34] and SCC 15305cap [35], potassium transport (ψSCC h1 [36], cell wall cross-linking (pbb4) and teichoic acid biosynthesis (tagF) (SCCpbp4 [32], DNA protection by restriction-modification systems (SCC CI [32], or arginine deiminase and oligopeptide permease (arginine catabolic mobile element, ψSCC ACME [37]. Many of these non-mec SCC or ψSCC elements have been described in coagulase-negative staphylococci (CoNS), a finding which further implicates these species as responsible for the assembling and dissemination of SCC elements, including SCCmec.
The ACME element is a striking example of SCC dissemination routes and also how these elements may contribute to the phenotypic plasticity of S. aureus clones. In S. epidermidis, a ubiquitous commensal of the human skin, the ACME element is highly prevalent, whereas in S. aureus it is restricted to a single lineage circulating mainly in the USA community but also in Canada and Europe (CA-MRSA clone USA300, ST8-IVa) [37,38] This clone has been implicated in several epidemiologically unassociated outbreaks of skin and soft tissue infections in otherwise healthy individuals and has also been associated with unusually invasive disease [37]. Although, all S. aureus carry a native arginine deiminase metabolic pathway (arc gene cluster), the acquisition of the ACME element was shown to increase the gene dosages, which was suggested to contribute to the unique virulent phenotype of USA300 [37]. The arginine deiminase has been established in Streptococcus pyogenes as a virulence factor involved in the invasion and intracellular survival of host cells [39]. Moreover, since the depletion of L-arginine by arginine deiminase inhibits nitric oxide production, the ACME element is likely to suppress both innate and adaptive host immune responses [37]. The fact that ACME has non-functional ccr genes and is inserted into the chromosome in tandem with SCCmec, suggests that its acquisition was mediated by the SCCmec resident recombinase genes. Since very few MSSA isolates positive for the ACME element have been detected [40], the USA300 MRSA clones may have originated in two sequential steps: first acquiring the SCCmec and then the ACME element.
Number of SCCmec acquisition events and factors that limit their occurrence
While the primary mode of spread of MRSA is clearly clonal expansion, the “birth” of each MRSA strain involves the acquisition of an SCCmec element. Only speculations exist concerning how these genetic elements enter an S. aureus cell. A favored model assumes transduction by one or the other of the enormous number of phages that have been identified in staphylococci. Using the e-BURST method, the types of SCCmec and the genetic backgrounds (sequence types) of MRSA strains, it has been estimated that the number of times SCCmec has been acquired by the species of S. aureus is around 20 [22]. The same method resulted in a substantially higher number of acquisition events (around 54) for the case of methicillin resistant S. epidermidis [41].
One should remember that such acquisition events would only be registered if they lead to the production of a MRSA lineage that can be detected in surveillance studies. Recent experiments by Katayama and Chambers suggest that not all S. aureus genetic backgrounds are hospitable to maintaining the mecA gene in an active form. It was proposed that many S. aureus genetic backgrounds have a “barrier” against maintaining, transcribing and translating a plasmid born mecA gene and bypassing such a barrier appears to be the property of a select group of S. aureus genetic backgrounds [4]. The mechanism and genetic basis of the barrier effect is not known at the present time but its existence could certainly contribute to the relatively low number of MRSA lineages identified in epidemiological studies. A role for the restriction modification system in the spread of various SCCmec types have recently been proposed by Lindsay et al. [42].
Few epidemic MRSA clones cause the largest number of MRSA disease worldwide
One of the surprising findings of the first international molecular epidemiological study on MRSA was the recognition that a handful of MRSA lineages (initially defined by shared PFGE patterns) were responsible for MRSA infections in hospitals located in Europe, USA and the far East in as many sixteen different participating countries [43]. Subsequent and more extensive international surveillance studies confirmed this finding using additional molecular typing techniques, such as MLST and SCCmec typing [44]. These observations indicate the importance of yet another evolutionary process that MRSA clones need in order to successfully compete with drug susceptible lineages of the species. It is interesting that the globally spread and highly epidemic MRSA clones often share a common genetic background with epidemic lineages of MSSA suggesting that critical determinants for epidemicity reside in the genetic background of MRSA clones [45].
Emergence of MRSA in the community
Just as the combination of strict antibiotic use and infection control procedures succeeded in reducing the level of MRSA infections in several hospitals in Nordic countries and the Netherlands, S. aureus “pulled” another surprise: patients from the community without any of the usual risk factors for MRSA infections began to appear in emergency rooms of hospitals most often with skin lesions caused by methicillin resistant S. aureus strains. Initially, and in contrast with the MRSA clones spreading in hospitals (HA-MRSA), these community acquired MRSA strains (CA-MRSA) showed diverse genetic backgrounds; carried – invariably – the relatively small SCCmec type IV or V; were rarely multi-drug resistant and exhibited less defective “fitness” (i.e., grew faster in vitro), than the typical HA-MRSA strains. Soon afterwards, unique clonal lineages of CA-MRSA began to take over, the most prominent ones being clones with the genetic backgrounds of ST30, ST80 and ST8 and often the CA-MRSA lineages also carried the genetic determinant of the PVL toxin which seems to be the critical factor for the overwhelmingly skin and soft tissue infections caused by CA-MRSA strains. (For a recent review see [46].
Conclusion
Contrasting scenarios for the evolution of hospital acquired and community acquired MRSA
In the 1960s the first MRSA was “born” in the hospital environment as an already multidrug resistant pathogen and for over 17 years spread extensively in hospitals as a single clonal type (Archaic clone) [47]. It was only after this period that new clonal types began to appear and spread globally. These epidemic HA-MRSA clones had different genetic backgrounds (defined by MLST) and carried a variety of different SCCmec types (mainly I, II and III).
In contrast, when MRSA was “born again” in the 1990s, it first appeared in the community carrying initially only a single resistance trait (mecA) and exclusively in the form of SCCmec types IV or V and in many diverse genetic backgrounds. Subsequently, a few highly epidemic clones began to emerge, acquire additional resistance traits and spread in the community.
These contrasting features of HA-MRSA and CA-MRSA are intriguing and raise questions about the nature of environmental and microbial factors that contributed to the surprisingly different epidemiological and molecular profiles of the “old” HA-MRSA and the “new” CA-MRSA strains. The scenario of evolution of HA-MRSA has been reviewed recently in some detail [1], and several recent reviews have addressed the various issues involving the CA-MRSA [48,49]. Nevertheless, factors that contributed to the emergence of CA-MRSA remain enigmatic.
On the molecular level the initial appearance of CA-MRSA may have been the consequence of an increased frequency in the encounters between potential SCCmec donors (most likely methicillin resistant coagulase negative staphylococci) and MSSA recipients that most frequently colonize individuals in the community. MRSE strains – frequent colonizers of the human skin – were shown to carry each one of the major SCCmec types identified in various MRSA clones including SCCmec IV [41,50] – which, together with SCCmec type V represents one of the two chromosomal cassettes identified in all CA-MRSA examined so far. The genetic background of MSSA that most frequently colonize humans in the community (such as ST30, ST8) are the same as the genetic backgrounds of the rapidly spreading clones of CA-MRSA (ST30 in the “Oceania” clone; ST8 in the USA300 clone), which eventually emerged from the initially diverse genetic backgrounds of CA-MRSA.
A new and frequent molecular marker of many – but certainly not all – CA-MRSA is the pvl toxin gene which seems to correlate with the high frequency of skin and soft tissue infections caused by these bacteria [46].
A second important factor that may have contributed to the rise of CA-MRSA in our era could be the emergence outside the hospital environment of some new high risk human populations and/or lifestyles that facilitate transmission of infectious agents including MRSA.
The first reports on appearance of CA-MRSA came from Australia from studies on Aboriginal communities [51] followed by reports on CA-MRSA strains spreading in Algerian immigrant populations in Southern France [52] and Indian Reservations in the USA [53]. The crowdedness, lack of hygienic culture and misuse and overuse of antimicrobial agents in such communities has been documented repeatedly.
In the mid 1990s, the carriage rate of MRSA among healthy individuals in the community was exceedingly low: less than 0.1 % in a country where over 50% of S. aureus hospital isolates were resistant to methicillin during the same time period [54]. A more recent survey by the CDC has confirmed that the nasal carriage of MRSA has remained extremely low among healthy individuals in the USA community [55]. On the other hand, surprisingly high carriage rate of MRSA (6-8%) was reported when “under-served” groups were assayed for MRSA carriage such as the homeless, drug addicts [56], inmates in crowded prisons of the USA and attendees of Day Care Centers. A compromised immune system (HIV positive individuals), drug addicts sharing drug paraphernalia, men having sex with men (MSM), but also the proliferation of contact sports combined with inappropriate hygienic conditions may have created an environment in which both the transfer of SCCmec from MRSE to MSSA and also the person to person transmission of infectious agents – including MRSA – is amplified. Thus when MRSA was “born again” it was not in the hospital but in some enclaves of the human community from where such CA-MRSA clones could emerge and spread and were eventually able to make their way back into hospitals both in the USA [57] and in Europe [38]. The entry into the hospitals of such MRSA clones with their relatively small “fitness” burden and frequent association with the PVL toxin may increase the seriousness of drug resistant S. aureus infections and make effective chemotherapy more complex and problematic [49].
Acknowledgments
Partial support for this study was provided by a grant (2 RO1 A1045738-08) from the National Institute of Health, US Public Health Service to Alexander Tomasz and by a contract from Fundação para a Ciência e a Tecnologia, Portugal: POCTI/BIA-MIC/58416/2004 to Hermínia de Lencastre.
Selected References
49. Daum RS: Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007, 357:380-390. (Excellent update on the range of infections caused by CA-MRSA)
1. De Lencastre H, Tomasz A: Multiple stages in the evolution of the methicillin resistant Staphylococcus aureus. In Evolutionary Biology of Bacterial and Fungal Pathogens. Edited by Fernando Baquero CN, and, Gail H. Cassell and, Gutiérrez-Fuentes J-A: ASM Press; 2007:In Press. (A new comprehensive review of the evolution of MRSA clones).
37. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al.: Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367:731-739. (Full genome sequence of the most widely spread CA-MRSA in the USA – highlighting the possible importance of the ACME gene in the epidemicity of the USA-300 clone)
41. Miragaia M, Thomas JC, Couto I, Enright MC, de Lencastre H: Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J Bacteriol 2007, 189:2540-2552. (The first paper addressing the population structure of S. epidermidis – a species suspected to be the source of SCCmec elements).
57. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA: Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006, 355:666-674. (An update on the nature and number of CA-MRSA infected patients that appeared in the emergency rooms in the USA).
46. Tristan A, Bes M, Meugnier H, Lina G, Bozdogan B, Courvalin P, Reverdy ME, Enright MC, Vandenesch F, Etienne J: Global distribution of Panton-Valentine leukocidin--positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis 2007, 13:594-600. (An overview of the clones of CA-MRSA – with special reference to the carriage of pvl toxin genes)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Lencastre H, Tomasz A. Multiple stages in the evolution of the methicillin resistant Staphylococcus aureus. In: Fernando Baquero CN, Cassell Gail H, Gutiérrez-Fuentes J-A, editors. Evolutionary Biology of Bacterial and Fungal Pathogens. ASM Press; 2007. In Press. [Google Scholar]
- 2.Fuda CC, Fisher JF, Mobashery S. Beta-lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cell Mol Life Sci. 2005;62:2617–2633. doi: 10.1007/s00018-005-5148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couto I, de Lencastre H, Severina E, Kloos W, Webster JA, Hubner RJ, Sanches IS, Tomasz A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996;2:377–391. doi: 10.1089/mdr.1996.2.377. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Zhang HZ, Hong D, Chambers HF. Jumping the barrier to beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 2003;185:5465–5472. doi: 10.1128/JB.185.18.5465-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, Husain SE, Butcher PD, Hinds J. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188:669–676. doi: 10.1128/JB.188.2.669-676.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Piscitelli C, de Lencastre H, Tomasz A. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist. 1996;2:435–441. doi: 10.1089/mdr.1996.2.435. [DOI] [PubMed] [Google Scholar]
- 7.Severin A, Wu SW, Tabei K, Tomasz A. High-level (beta)-lactam resistance and cell wall synthesis catalyzed by the mecA homologue of Staphylococcus sciuri introduced into Staphylococcus aureus. J Bacteriol. 2005;187:6651–6658. doi: 10.1128/JB.187.19.6651-6658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuda C, Suvorov M, Shi Q, Hesek D, Lee M, Mobashery S. Shared functional attributes between the mecA gene product of Staphylococcus sciuri and penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. Biochemistry. 2007;46:8050–8057. doi: 10.1021/bi7004587. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen F. Discovery, Isolation, Production and Introduction of Penicillin for Veterinary use in Denmark during World War II. Veterinary History. 2007;13:339–353. [Google Scholar]
- 10.Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 12.Katayama Y, Ito T, Hiramatsu K. Genetic organization of the chromosome region surrounding mecA in clinical staphylococcal strains: role of IS431-mediated mecI deletion in expression of resistance in mecA-carrying, low-level methicillin-resistant Staphylococcus haemolyticus. Antimicrob Agents Chemother. 2001;45:1955–1963. doi: 10.1128/AAC.45.7.1955-1963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira DC, Tomasz A, de Lencastre H. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb Drug Resist. 2001;7:349–361. doi: 10.1089/10766290152773365. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, Daum RS, Hiramatsu K. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira DC, Milheirico C, de Lencastre H. Redefining a structural variant of staphylococcal cassette chromosome mec, SCCmec type VI. Antimicrob Agents Chemother. 2006;50:3457–3459. doi: 10.1128/AAC.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, Jamklang M, Chavalit T, Song JH, Hiramatsu K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006;50:1001–1012. doi: 10.1128/AAC.50.3.1001-1012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updat. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 21.Kluytmans-Vandenbergh MF, Kluytmans JA. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin Microbiol Infect. 2006;12(Suppl 1):9–15. doi: 10.1111/j.1469-0691.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 22.Robinson DA, Enright MC. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:3926–3934. doi: 10.1128/AAC.47.12.3926-3934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon NH, Park KT, Moon JS, Jung WK, Kim SH, Kim JM, Hong SK, Koo HC, Joo YS, Park YH. Staphylococcal cassette chromosome mec (SCCmec) characterization and molecular analysis for methicillin-resistant Staphylococcus aureus and novel SCCmec subtype IVg isolated from bovine milk in Korea. J Antimicrob Chemother. 2005;56:624–632. doi: 10.1093/jac/dki306. [DOI] [PubMed] [Google Scholar]
- 24.Milheirico C, Oliveira DC, de Lencastre H. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex’. J Antimicrob Chemother. 2007;60:42–48. doi: 10.1093/jac/dkm112. [DOI] [PubMed] [Google Scholar]
- 25.Shore A, Rossney AS, Keane CT, Enright MC, Coleman DC. Seven novel variants of the staphylococcal chromosomal cassette mec in methicillin-resistant Staphylococcus aureus isolates from Ireland. Antimicrob Agents Chemother. 2005;49:2070–2083. doi: 10.1128/AAC.49.5.2070-2083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother. 2005;56:455–462. doi: 10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]
- 27.Stephens AJ, Huygens F, Giffard PM. Systematic Derivation of Marker Sets for Staphylococcal Cassette Chromosome mec Typing. Antimicrob Agents Chemother. 2007;51:2954–2964. doi: 10.1128/AAC.01323-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, Hiramatsu K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira DC, Milheirico C, Vinga S, de Lencastre H. Assessment of allelic variation in the ccrAB locus in methicillin-resistant Staphylococcus aureus clones. J Antimicrob Chemother. 2006;58:23–30. doi: 10.1093/jac/dkl208. [DOI] [PubMed] [Google Scholar]
- 30.Milheirico C, Oliveira DC, de Lencastre H. Update to the multiplex PCR strategy for the assignment of mec element types in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3374–3377. doi: 10.1128/AAC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanssen AM, Ericson Sollid JU. SCCmec in staphylococci: genes on the move. FEMS Immunol Med Microbiol. 2006;46:8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 32.Mongkolrattanothai K, Boyle S, Murphy TV, Daum RS. Novel non-mecA-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagF genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:1823–1836. doi: 10.1128/AAC.48.5.1823-1836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luong TT, Ouyang S, Bush K, Lee CY. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J Bacteriol. 2002;184:3623–3629. doi: 10.1128/JB.184.13.3623-3629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda M, Yamashita A, Hirakawa H, Kumano M, Morikawa K, Higashide M, Maruyama A, Inose Y, Matoba K, Toh H, et al. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci U S A. 2005;102:13272–13277. doi: 10.1073/pnas.0502950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, Morimoto Y, Kuroda M, Cui L, Takahashi M, Ankai A, et al. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005;187:7292–7308. doi: 10.1128/JB.187.21.7292-7308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 38.Larsen A, Stegger M, Goering R, Sorum M, Skov R. Emergence and dissemination of the methicillin resistant Staphylococcus aureus USA300 clone in Denmark (2000-2005) Euro Surveill. 2007;12 [Google Scholar]
- 39.Degnan BA, Palmer JM, Robson T, Jones CE, Fischer M, Glanville M, Mellor GD, Diamond AG, Kehoe MA, Goodacre JA. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect Immun. 1998;66:3050–3058. doi: 10.1128/iai.66.7.3050-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goering RV, McDougal LK, Fosheim GE, Bonnstetter KK, Wolter DJ, Tenover FC. Epidemiologic distribution of the arginine catabolic mobile element among selected methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:1981–1984. doi: 10.1128/JCM.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miragaia M, Thomas JC, Couto I, Enright MC, de Lencastre H. Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J Bacteriol. 2007;189:2540–2552. doi: 10.1128/JB.01484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldron DE, Lindsay JA. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol. 2006;188:5578–5585. doi: 10.1128/JB.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira DC, Tomasz A, de Lencastre H. Secrets of success of a human pathogen: molecular evolution of pandemic clones of meticillin-resistant Staphylococcus aureus. Lancet Infect Dis. 2002;2:180–189. doi: 10.1016/s1473-3099(02)00227-x. [DOI] [PubMed] [Google Scholar]
- 44.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aires de Sousa M, Conceicao T, Simas C, de Lencastre H. Comparison of genetic backgrounds of methicillin-resistant and -susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J Clin Microbiol. 2005;43:5150–5157. doi: 10.1128/JCM.43.10.5150-5157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tristan A, Bes M, Meugnier H, Lina G, Bozdogan B, Courvalin P, Reverdy ME, Enright MC, Vandenesch F, Etienne J. Global distribution of Panton-Valentine leukocidin--positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis. 2007;13:594–600. doi: 10.3201/eid1304.061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes AR, Westh H, de Lencastre H. Origins and evolution of methicillin-resistant Staphylococcus aureus clonal lineages. Antimicrob Agents Chemother. 2006;50:3237–3244. doi: 10.1128/AAC.00521-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–390. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 50.Wisplinghoff H, Rosato AE, Enright MC, Noto M, Craig W, Archer GL. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob Agents Chemother. 2003;47:3574–3579. doi: 10.1128/AAC.47.11.3574-3579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coombs GW, Nimmo GR, Bell JM, Huygens F, O’Brien FG, Malkowski MJ, Pearson JC, Stephens AJ, Giffard PM. Genetic diversity among community methicillin-resistant Staphylococcus aureus strains causing outpatient infections in Australia. J Clin Microbiol. 2004;42:4735–4743. doi: 10.1128/JCM.42.10.4735-4743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naimi TS, LeDell KH, Boxrud DJ, Groom AV, Steward CD, Johnson SK, Besser JM, O’Boyle C, Danila RN, Cheek JE, et al. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin Infect Dis. 2001;33:990–996. doi: 10.1086/322693. [DOI] [PubMed] [Google Scholar]
- 54.Sa-Leao R, Sanches IS, Couto I, Alves CR, de Lencastre H. Low prevalence of methicillin-resistant strains among Staphylococcus aureus colonizing young and healthy members of the community in Portugal. Microb Drug Resist. 2001;7:237–245. doi: 10.1089/10766290152652783. [DOI] [PubMed] [Google Scholar]
- 55.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis. 2006;193:172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 56.Pan ES, Diep BA, Charlebois ED, Auerswald C, Carleton HA, Sensabaugh GF, Perdreau-Remington F. Population dynamics of nasal strains of methicillin-resistant Staphylococcus aureus--and their relation to community-associated disease activity. J Infect Dis. 2005;192:811–818. doi: 10.1086/432072. [DOI] [PubMed] [Google Scholar]
- 57.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]


