Abstract
Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G (APOBEC3G) has recently been identified as a potent antiviral protein. Here, we examined the expression and regulation of APOBEC3G in human brain tissues and the cells of central nervous system (CNS). Similar to the immune cells, human brain tissue and the CNS cells expressed APOBEC3G at both mRNA and protein levels. The expression of APOBEC3G could be up-regulated in human neuronal cells (NT2-N) and astrocytes (U87-MG) by interferons (IFN-α, β and γ), interleukin-1 (IL-1), and tumor necrosis factor. Other cytokines (IL-4, IL-6 and transforming growth factor beta1) and CC-chemokines (CCL3, 4 and 5), however, had little impact on the expression of APOBEC3G. In addition, pseudotyped HIV-1 infection and cytokine/chemokine-enriched supernatants from lipopolysaccharide- stimulated macrophage cultures induced APOBEC3G expression in NT2-N cells. APOBEC3G expressed in the neuronal cells and astrocytes was biologically functional, as the suppression of APOBEC3G expression by the specific siRNA led to increase of pseudotyped HIV-1 replication in these cells. These findings provide direct and compelling evidence that there is intracellular expression and regulation of functional APOBEC3G in the neuronal cells, which may be one of innate defense mechanisms involved in the neuronal protection in the CNS.
Keywords: APOBEC3G, Cytokine, Interferon, Astrocytes, Neuron
1. Introduction
APOBEC3G, a member of the apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC) family of proteins homologous cytidine deaminase domains (Jarmuz et al., 2002), has been identified as a host factor involved in cellular defense mechanism against retroviruses (Harris et al., 2003; Mangeat et al., 2003; Sheehy et al., 2002). APOBEC3G can either edit the newly synthesized viralDNA or inhibit another site(s) of the viral life cycle (Mangeat et al., 2003; Mariani et al., 2003; Turelli and Trono, 2005; Zhang et al., 2003). Recently, inhibition of human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus, equine infectious anemia virus, murine leukemia virus and hepatitis B virus by APOBEC3G has been reported (Mangeat et al., 2003; Navarro and Landau, 2004; Noguchi et al., 2005; Rosler et al., 2005).
Although APOBEC3G resides in HIV-1 permissive cells, such as CD4+ T cells and monocyte-derived macrophages (Chiu et al., 2005), expression of APOBEC3G has also been identified in other human organs, such as lungs, testes, ovaries, liver and kidney (Chen et al., 2006; Jin et al., 2005; Komohara et al., 2007, 2006; Turelli and Trono, 2005). In addition, APOBEC3G could be induced by interferons (IFNs) in human lymphocytes, macrophage, liver and kidney cells (Bonvin et al., 2006; Chen et al., 2006; Komohara et al., 2007, 2006; Peng et al., 2006; Tanaka et al., 2006). Both type I and type II IFNs play a critical role in the host innate immunity against viral infections. IFN-α is a potent inhibitor of HIV-1 infection of CCR5+ CD4+ human macrophages (Baca-Regen et al.,1994; Meylan et al.,1993). IFN-α is also a potent inducer of APOBEC3G, which overrides HIV-1 vif-mediated neutralization of APOBEC3G protein that pose a threat to efficient HIV-1 replication in macrophages (Peng et al., 2006). Although it has been demonstrated that APOBEC3G could be induced by type I IFNs in human lymphocytes, macrophage, liver and kidney (Bonvin et al., 2006; Chen et al., 2006; Komohara et al., 2007, 2006; Peng et al., 2006; Tanaka et al., 2006), there is limited information about the expression and regulation of APOBEC3G in human central nervous system (CNS), an important target for viral infections, including HIV-1. Therefore, we examined whether the CNS cells express functional APOBEC3G, and whether IFNs (alpha, beta and gamma) and other cytokines/chemokines modulate the expression of APOBEC3G in the human neuronal cells.
2. Materials and methods
2.1. Primary cells
Human brain tissues used were obtained from the National Neurological Research Specimen Bank (Los Angeles, CA). Neural precursor cell (NPC)-derived human neuronal cells were kindly obtained from Dr. Hu, Shuxian (The Center for Infectious Diseases and Microbiology Translational Research, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota). NPC cultures were prepared from the telencephalon of 8- to 10-week-old human fetal brain using previously described methods (Hu et al., 2006, 2002; Ni et al., 2004; Uchida et al., 2000). Peripheral blood samples were obtained from healthy adult donors. The Institutional Review Board of the Children's Hospital of Philadelphia approved this investigation. Informed consent was obtained from the subjects. Peripheral blood lymphocytes (PBL) and monocytes were processed as described previously (Hassan et al., 1986). Freshly isolated monocytes were plated in 48-well culture plates at a density of 0.5 × 106 cells per well in DMEM containing 10% fetal bovine serum (FBS) (Invitrogen). Macrophages referred to 7-day-cultured monocytes. Monocyte/macrophage viability was monitored by trypan blue exclusion and maintenance of cell adherence. In all cases, limulus amebocyte lysate assay demonstrated that media and reagents were endotoxin-free.
2.2. Cell lines
Human neuronal cells (NT2-N) were derived from differentiated Ntera-2clD/1 (NT2) cells as described previously (Andrews, 1984). In brief, NT2 cells were plated at a density of 2.3 × 106 per T75 flask and fed twice weekly with DMEM containing high glucose (Gibco, Grand Island, NY, USA) and 10% FBS (Hyclone, Iogan, UT, USA) with 100 U/ml penicillin plus 100 µg/ml streptomycin (Gibco) and 10−5 M retinoic acid (RA) (Sigma-Aldrich, St. Louis, MO, USA) for 5 weeks. The cells were then divided (1:4) and grown for an additional 48 h in identical medium without RA. Neuronal cells growing above a monolayer of non-neuronal cells were dislodged with trypsin and plated at a density of 0.5 × 106 cells per well in a 24-well plate for this study. NT2-N neurons have morphologic features similar to primary human neurons, and have processes that differentiate into axons and dendrites (Andrews, 1984). NT2-N neurons also express cytoskeletal proteins, secretory markers, and surface markers, which are characteristic of neurons. They also express functional neuropeptides (Guillemain et al., 2000) and functional N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptors (Younkin et al., 1993). Undifferentiated human NT2 cells grafted into mouse brain differentiated into neuronal and glial cells (Ferrari et al., 2000).
The neuroblastoma cell lines (CHP212 and SY5Y) were purchased from American Type Tissue Culture (ATCC; Manassas, VA). CHP212 cells were cultured in Eagle's MEM-Ham F12 (1:1) medium containing 10% FCS, 0.1 mM non-essential amino acid and 1.0 mM sodium pyruvate. SY5Y cells were propagated in DMEM with high glucose containing 10% FBS. Human astroglioma cells (U87-MG) and human B lymphoblasts (IM9) were obtained from ATCC (Rockville, MD). U87-MG and IM9 cells were maintained in DMEM with 2mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FBS. The human NK cell line (YTS) was a subclone of the YT lymphoid cell line, derived from a patient with NK cell leukemia and interleukin-2-independent (Cohen et al., 1999). YTS cells were cultured in Iscover's modified Dulbecco's medium (IMDM) supplemented with 10% FCS and 2 mM l-glutamine.
2.3. Reagents
Recombinant human interferon (IFN-α, IFN-β, and IFN-γ), interleukin-1beta (IL-1β), IL-4, IL-6, Transforming Growth Factor Beta1 (TGF-β1), CC chemokine ligand 3 (CCL3), CCL4, CCL5 and tumor necrosis factor-α (TNF-α) were purchased from R&D Systems (Minneapolis, MN, USA). Rabbit polyclonal anti-APOBEC3G antibody was purchased from Immuno Dilagnostics, Inc. (Woburn, MA, USA). Lipopolysaccharide (LPS) was purchased from Sigma (St Louis, MO, USA).
2.4. Treatment of NT2-N or U87-MG cells with cytokines or cytokine-enriched supernatants
NT2-N or U87-MG cells were cultured in the presence or absence of each of the cytokines (IFN-α, IFN-β, IFN-γ, TNF-α, IL-1β, IL-4, IL-6, TGF-β1, CCL3, CCL4 and CCL5). The concentrations of each cytokine used and different time points post-treatment are indicated in the legends for Fig. 3, and Fig.4. We also cultured NT2-N cells in media conditioned with supernatant from macrophage cultures (0.5 × 106 cells/well) with or without LPS (1 ng/ml) stimulation for 12 h.
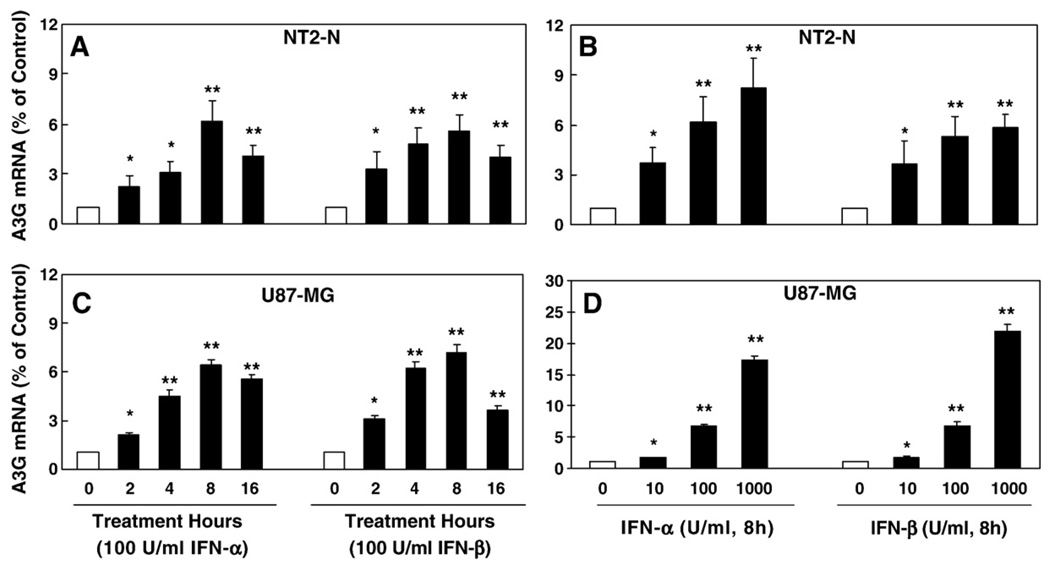
Fig. 3.
Effect of IFN-α and IFN-β on APOBEC3G expression in NT2-N cells and U87-MG cells. (A) Time-course effect of IFN-α and IFN-β on APOBEC3G mRNA expression. The NT2-N cells were treated with IFN-α or IFN-β for the indicated time points. (B) Dose-dependent effect of IFN-α and IFN-β on APOBEC3G expression. The NT2-N cells were treated with IFN-α or IFN-β at indicated concentrations for 8 h. (C) Time-course effect of IFN-α and IFN-β on APOBEC3G mRNA expression. The U87-MG cells were treated with IFN-α or IFN-β for the indicated time points. (D) Dose-dependent effect of IFN-α and IFN-β on APOBEC3G expression. The U87-MG were treated with IFN-α or IFN-β at indicated concentrations for 8 h. For the data shown in A, B, C and D, APOBEC3G mRNA levels were analyzed by the real-time RT-PCR and were expressed as fold increase over untreated NT2-N cells or U87-MG cells (defined as 1). The data shown in A, B, C, and D is expressed as mean±SD of triplicate cultures, representative of three independent experiments (*, p<0.01; **, p<0.001; treated by IFN-α or IFN-β vs. untreated).
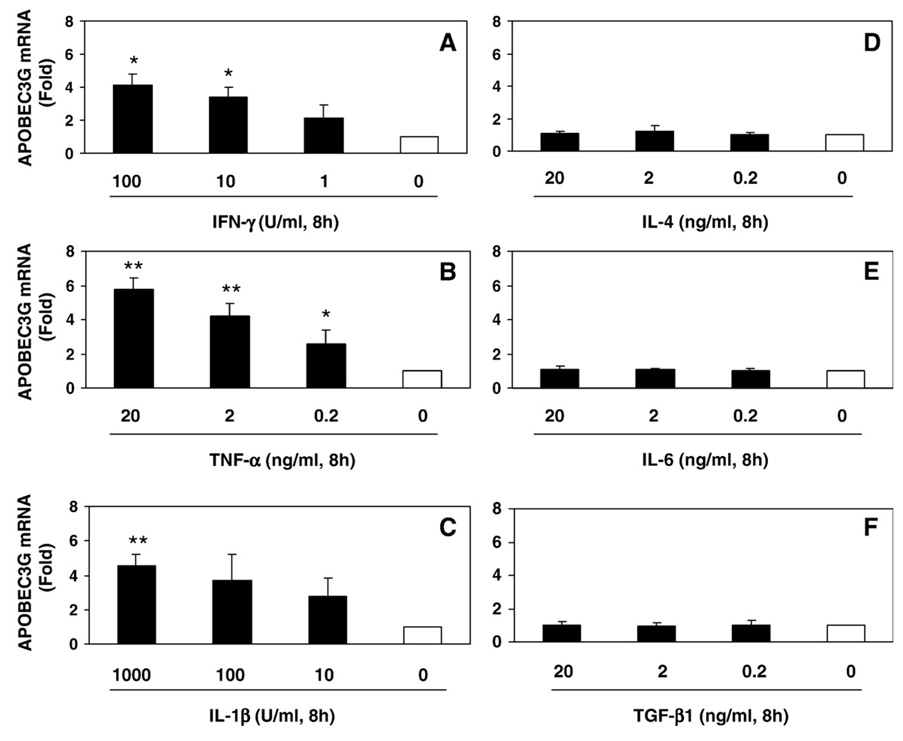
Fig. 4.
Effects of cytokines on APOBEC3G expression in NT2-N cells. NT2-N cells were treated with IFN-γ (A), TNF-α (B), IL-1β (C), IL-4 (D), IL-6 (E) and TGF-β1 (F) at the indicated concentrations for 8 h. Total cellular RNA was subjected to the real-time RT-PCR for APOBEC3G mRNA, which is expressed as fold increase over untreated NT2-N cells (defined as 1). The data is expressed as mean±SD of triplicate cultures, representative of three independent experiments (*, p<0.01; **, p<0.001; treated by indicated cytokines vs. untreated).
2.5. Short interfering RNA transfection and pseudotyped HIV-1 infection of NT2-N neuron and U87-MG astrocytes
APOBEC3G-specific and control siRNAs were chemically synthesized by Dharmacon (Lafayett, Co). NT2-N and U87-MG cells were transfected with 100 nM APOBEC3G-specific siRNA or control siRNA using the transfection reagent (DharmaFect 3, Dharmacon, Lafayett, Co). Twenty-four hours after transfection, half of the cells were collected for APOBEC3G mRNA expression by the realtime RT-PCR. Another half of the cells were infected with MLV-env-pseudotyped HIV-1 (20 ng of p24/ml) for 16 h. The preparation of MLV-env-pseudotyped HIV-1 was described previously (Wang et al., 2002, 2006). The cells were then lysed in 100 µl of 1× Reporter Lysis Buffer (Promega Corp., Madison, WI) 72 h postinfection. Lysate (50 µl) was mixed with 100 µl of luciferase substrate (Promega Corp., Madison, WI) and luciferase activity was determined in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA).
2.6. Real time reverse-transcription-polymerase chain reaction (RT-PCR)
Real time RT-PCR was described previously (Guo et al., 2003). Briefly, total cellular RNA was extracted from the cells (106) and brain tissues using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Total cellular RNA (1 µg) was subjected to reverse transcription using the reverse transcription system from Promega Corporation (Madison, WI). The real-time RT-PCR for mRNA was performed with the Brilliant SYBR Green QPCP Master Mix (Bio-Rad Laboratories, Hercules, CA). The specific oligonucleotide primers used were as follows: APOBEC3G: 5′-TCAGAGGACGGCATGAGACTTAC-3′ (sense) and 5′-AGCAGGACCCAGGTGTCATTG-3′ (anti-sense); GAPDH (the house keeping gene): 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (anti-sense). The oligonucleotide primers were synthesized by Integrated DNA Technologies Inc. (Coralville, IA). After PCR amplification, some samples were separated by 3% ultra pure agarose gel electrophoresis (Gibco-Grand Island, NY).
2.7. Western blot analysis
Total cell lysates from NT2-N cells were prepared using the lysis buffer consisted of 10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM sodium-orthovanadate, 20 µM4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.2% Nonidet P-40, 5 µg/ml leupeptin, phosphatase inhibitor cocktail and protease inhibitor cocktail (Sigma). Protein concentrations were determined by DC protein assay kit (Bio-Rad, Hercules, CA). Proteins were resuspended in NuPAGE LDS Sample Buffer (NOVEX), heated for 5 min at 95 °C and then equivalent amounts of protein (50 µg) for each sample were separated in a 10% Bis-Tris Gel in a NuPAGE Running Buffer with 0.25% Running Buffer Antioxidant for 50 min at 200 V. Proteins were transferred to the nitrocellulose membranes (Bio-Rad) in NuPAGE Transfer Buffer at 100 V for 1 h. The membranes were blocked with 5% fat-free milk in 1 × PBS for 1 h and incubated with appropriate optimal diluted antibodies for actin (1:3000) for 1 h at room temperature or with rabbit polyclonal antibodies to APOBEC3G (1:1000) overnight at 4 °C in the blocking solution. After washing 3 times for 15 min each time with PBST (0.05% Tween 20 in PBS), the membrane was incubated with appropriate HRP-conjugated goat anti-rabbit (1:10,000) IgG antibodies in blocking solution for 1 h at room temperature and washed 3 times as described above. The bound antibodies were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) according to the manufacturer's instruction and exposed to basic Autorad film. Pre-stained molecular markers (Bio-Rad) were used to determine molecular weight of immunoreactive bands.
2.8. Data analysis
Where appropriate, data were expressed as mean±SD. For comparison of the mean of the two groups, statistical significance was measured by Student's t test. Calculations were performed with Stata Statistical Software (StataCorp., College Station, TX). Statistical significance was defined as p < 0.05.
3. Results
3.1. APOBEC3G expression in human CNS cells and immune cells
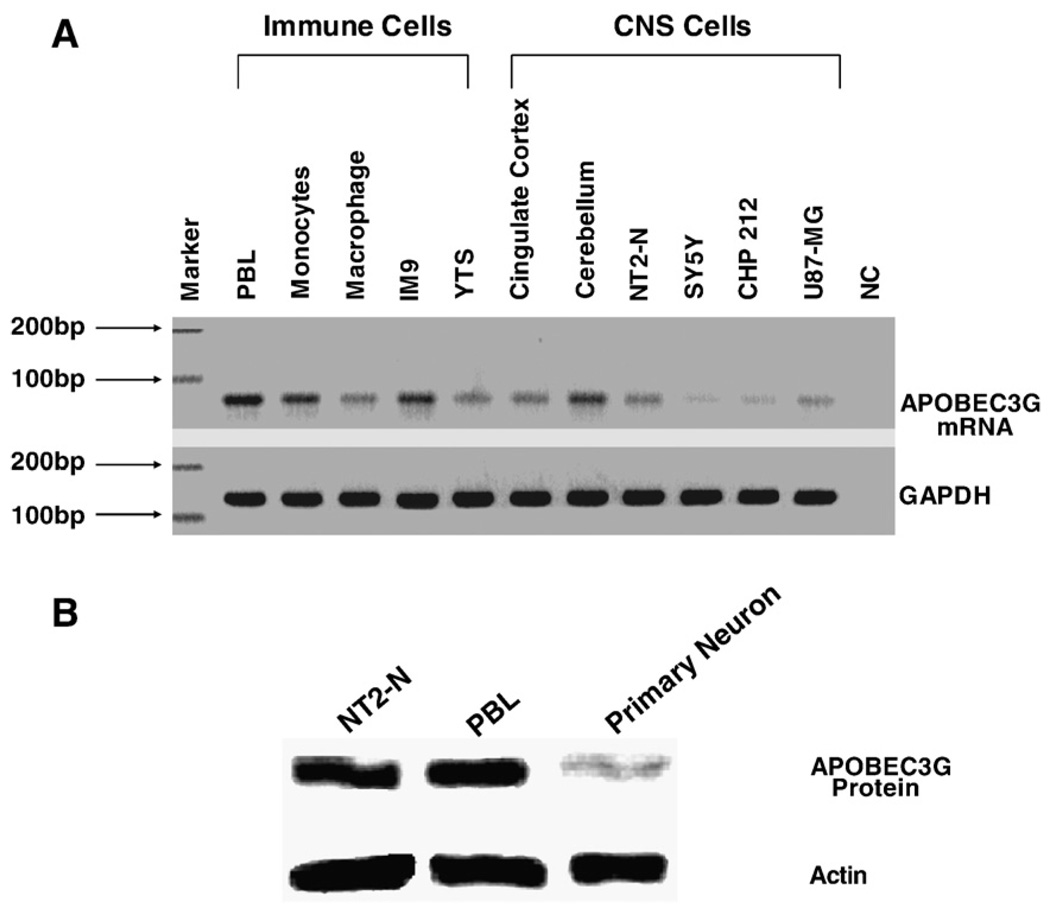
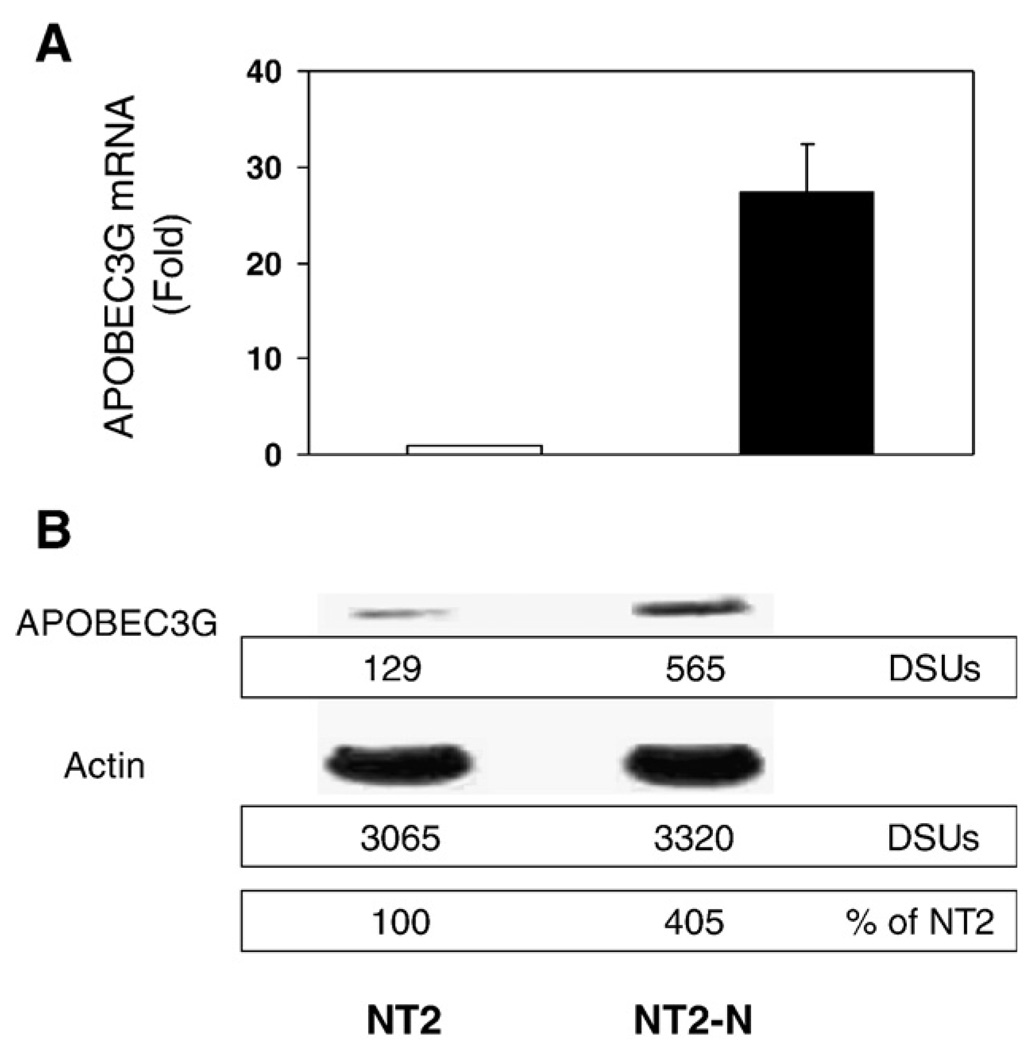
We first examined whether human CNS cells express APOBEC3G mRNA. As shown in Fig. 1, APOBEC3G mRNA was detected in the neuronal cells, including NT2-N, SY5Y, CHP212 and U87-MG cells. Human brain tissues (Cingulate cortex and Cerebellum) also had detectable levels of APOBEC3G mRNA (Fig. 1A). The levels of APOBEC3G mRNA in the CNS cells were comparable with those in the immune cells (Fig.1A). In order to determine that human neuronal cells also express APOBEC3G protein, we performed Western blot using both NT2-N cells and NPC-derived primary human neurons. Similar to peripheral blood lymphocytes (PBL), both NT2-N cells and primary human neurons expressed APOBEC3G protein, although the expression of APOBEC3G in primary human neurons was lower than NT2-N cells (Fig. 1B). Since undifferentiated NT2 cells, grafted into mouse brain, differentiated into neuronal cells and glial cells (Younkin et al., 1993), we also examined whether the cell differentiation affects APOBEC3G expression in NT2-N cells. Compared with undifferentiated NT2 cells, NT2-N cells (5 weeks after RA stimulation) expressed significantly higher levels of APOBEC3G at both mRNA (> 25 fold) and protein (>4 fold) levels (Fig. 2).
Fig. 1.
APOBEC3G expression in the human immune cells, brain tissues and CNS cells. (A) RT-PCR analysis of APOBEC3G mRNA expression. Total cellular RNA extracted from the indicated cells was subjected to the real time RT-PCR using the specific primer pairs for APOBEC3G (70 bp) and GAPDH (127 bp). The amplified (35 cycles) products were then subjected to electrophoresis on 3% ultra pure agarose gel; (B) Western blot analysis of APOBEC3G protein expression. Proteins extracted from NT2-N cells, human peripheral blood lymphocytes (PBL) and primary human neurons (neural precursor cell-derived human neuronal cells) were subjected to Western blot assay using rabbit antibodies against APOPEC3G and actin. One representative of 3 independent experiments with similar results is shown.
Fig. 2.
APOBEC3G expression in undifferentiated NT2 and differentiated NT2-N cells. (A) Real time RT-PCR analysis of expression of APOBEC3G mRNA. Total cellular RNA was extracted from NT2 and NT2-N cells (5-week retinoic acid [RA] -treated NT2 cells), and subjected to the real time RT-PCR using the specific primer pairs for APOBEC3G. The data is expressed as mean±SD of three independent experiments. (B) Western blot analysis of APOBEC3G protein expression. Equal amount of proteins extracted from undifferentiated NT2 cells and NT2-N cells was subjected to Western blot assay using rabbit antibodies against APOPEC3G and actin. The panels show the signal intensities (densitometry scanning units, DSUs) of protein bands of the Western blot. One representative of 3 independent experiments with similar data is shown.
3.2. Regulation of APOBEC3G expression in NT2-N by cytokines/chemokines
In the second set of experiments, we tested the hypothesis that cytokines/chemokines may have the ability to modulate APOBEC3G expression in NT2-N cells. We first examined the impact of type I/II IFNs on APOBEC3G expression in the neuronal cells. Type I IFN-α or IFN-β, when added to NT2-N or U87-MG cell cultures, induced APOBEC3G mRNA expression in a time and dose dependent fashion (Fig. 3). Similar to type I IFNs, IFN-γ also induced the expression of APOBEC3G in NT2-N cells (Fig. 4A). Among other cytokines tested, TNF-α and IL-1β also stimulated APOBEC3G mRNA expression (Fig. 4A, B and C),while IL-4, IL-6 and TGF-β1 had little effects on APOBEC3G mRNA expression (Fig. 4D, E and F). In addition, APOBEC3G expression in NT2-N cells was not affected by CC-chemokines (CCL3, CCL4 and CCL5) (data not shown).
3.3. LPS-activated macrophages produce cytokines/chemokines that induce APOBEC3G
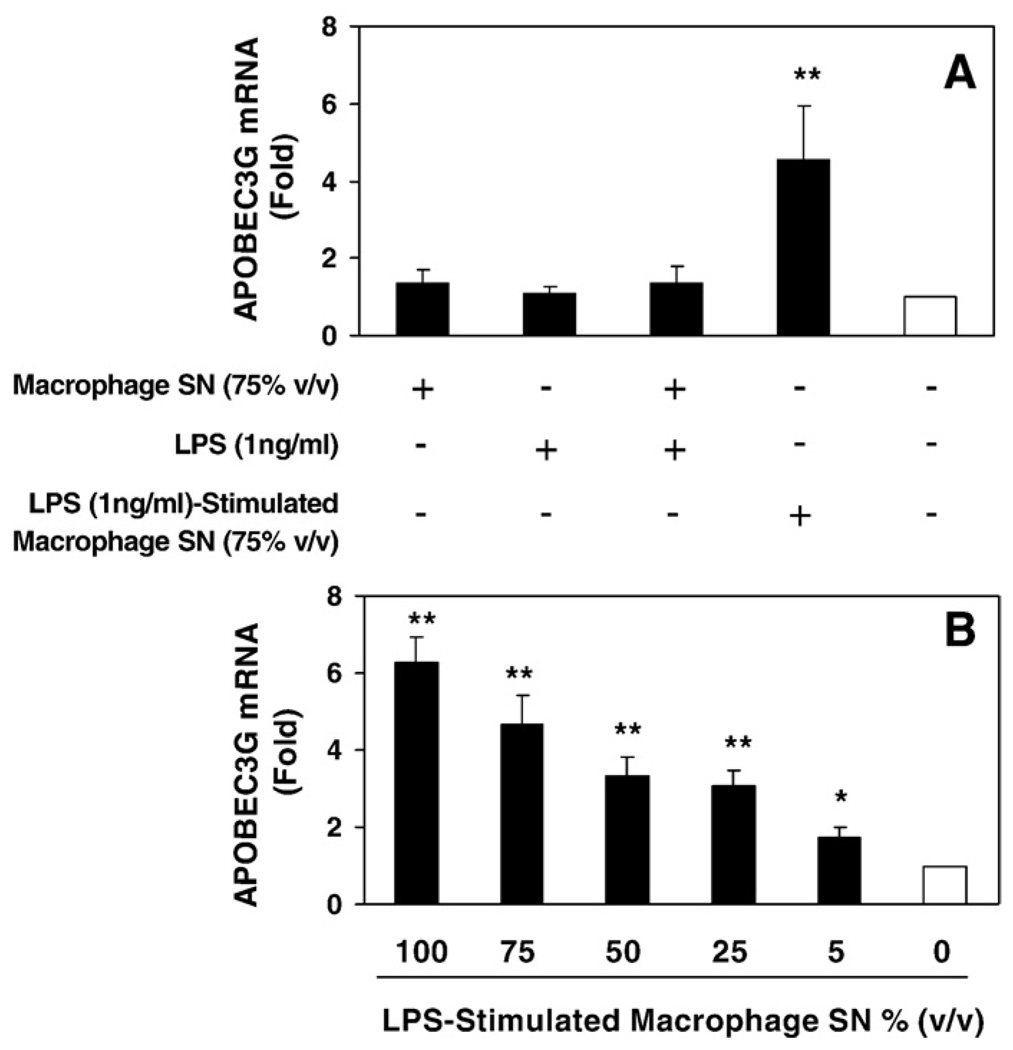
It has been indicated that high levels of circulating LPS (a consequence of microbial translocation from a leaky gut) in AIDS parents increase monocyte activation and trafficking into the CNS, resulting in excessive production of inflammatory cytokines. This process has been suggested as a cause of HIV-associated dementia (HAD). Thus, we examined whether cytokines/chemokines produced by LPS-activated macrophages affect APOBEC3G expression in NT2-N cells. As shown in Fig. 5, NT2-N cells treated with supernatants from LPS-stimulated macrophage cultures produced significantly higher levels of APOBEC3G in NT2-N cells than those of unstimulated macrophage cultures. However, LPS, when directly added to NT2-N cell cultures conditioned with or without unstimulated macrophage cultures, had no effect on the expression of APOBEC3G.
Fig. 5.
Effect of supernatants from LPS-stimulated macrophage cultures on APOBEC3G expression in NT2-N cells. (A) NT2-N cells were incubated in media conditioned with supernatants from unstimulated macrophage cultures and/or LPS or supernatants from LPS-stimulated macrophage cultures as indicated; (B) Dose-dependent effect of supernatants from LPS-stimulated macrophage cultures on APOBEC3G expression. Total cellular RNA was subjected to the real-time RT-PCR for APOBEC3G mRNA, which is expressed as fold increase over untreated NT2-N cells (defined as 1). The data is expressed as mean±SD of triplicate cultures, representative of three independent experiments (*, p<0.01; **, p<0.001; treated by LPS-stimulated macrophage SN vs. untreated).
3.4. Pseudotyped HIV-1 infection induces APOBEC3G expression and the suppression of APOBEC3G enhances HIV-1 replication
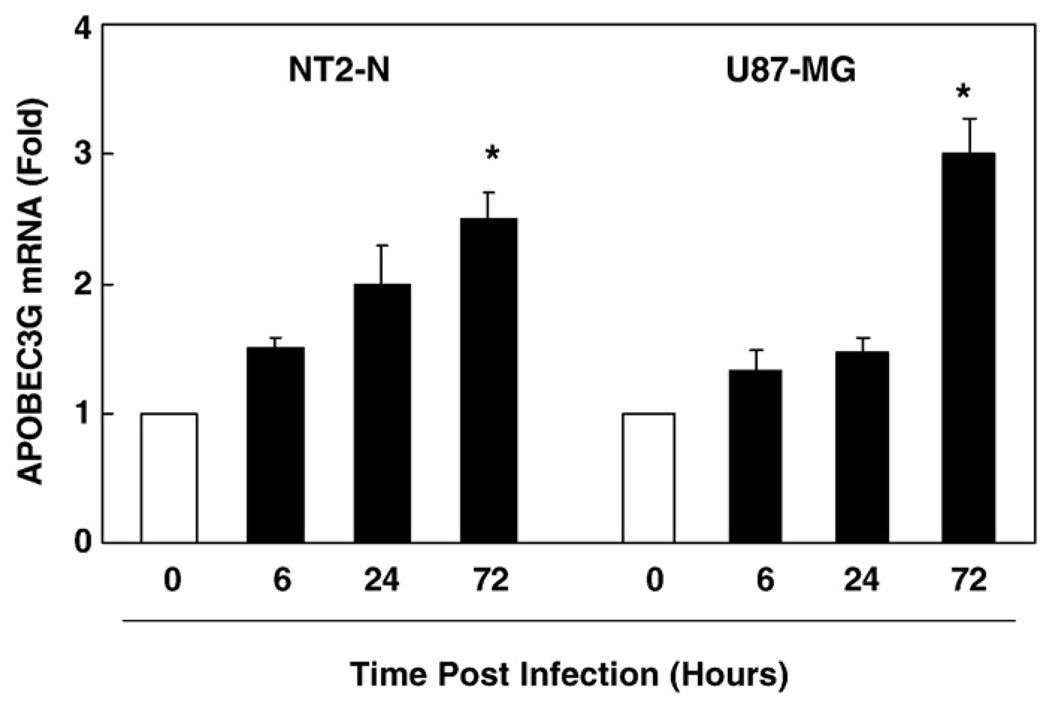
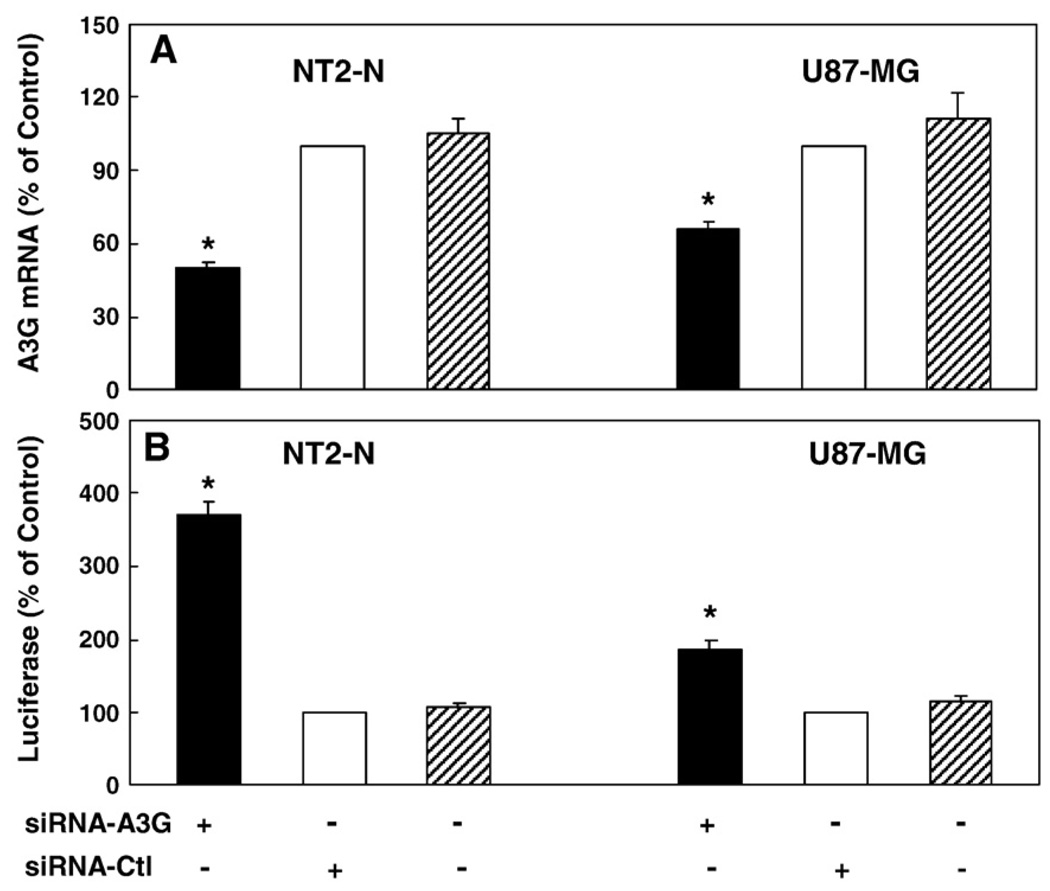
We also examined whether HIV-1 infection could induce APOBEC3G expression in NT2-N and U87-MG cells. When these cells exposed to supernatant from HIV-1 infected macrophage cultures, there was little change of the levels of APOBEC3G (data not shown). We then investigated whether MLV-env-pseudotyped HIV-1 infection could alter the expression of APOBEC3G in NT2-N and U87-MG cells. As shown in Fig. 6, there was graduated increase of APOBEC3G mRNA during the course of infection in both NT2-N cells and U87-MG cells. In order to determine APOBEC3G produced by NT2-N neuron or U87-MG astrocytes is biologically functional, we examined whether APOBEC3G has the antiHIV-1 activity. As shown in Fig. 7A, transfection of specific siRNA against APOBEC3G into NT2-N or U87-MG cells resulted in reduced expression of APOBEC3G mRNA, while negative control siRNA did not alter APOBEC3G expression. This decrease of endogenous APOBEC3G expression was correlated with the increase of MLV-env-pseudotyped HIV-1 replication in NT2-N or U87-MG cells 72 h postinfection (Fig. 7B).
Fig. 6.
Effect of the pseudotyped HIV-1 infection on APOBEC3G expression in NT2-N and U87-MG cells. NT2-N and U87-MG cells were infected with pseudotyped HIV-1. The cells were collected at indicated time points post-infection for analyzing APOBEC3G mRNA by the realtime RT-PCR. The result shown is the mean±SD of mRNA levels activity in triplicate cultures, which is expressed as fold increase over 0 hour cultures (defined as 1, *p<0.01).
Fig. 7.
Effect of APOBEC3G-specific siRNA on APOBEC3G expression and MLV-env-psedoutyped-HIV-1 replication in NT2-N and U87-MG cells. (A) Down-regulation of APOBEC3G mRNA expression in NT2-N and U87-MG cells by the siRNA against APOBEC3G. NT2-N and U87-MG cells were transfected with APOBEC3G-specific siRNA or siRNA-Ctl for 24 h. Total RNA was extracted for analyzing APOBEC3G mRNA expression by the real-time RT-PCR. (B) Effect of APOBEC3G-specific siRNA on MLV-env-psedoutyped-HIV-1 replication in NT2-N and U87-MG cells. The cells were transfected with the siRNAs as described above and then infected with MLV-env-psedoutype-HIV-1. NT2-N or U87-MG cells were collected for luciferase activity 72 h postinfection. The data shown in both A and B is expressed as mean±SD of triplicate cultures, representative of three independent experiments (*, p<0.05; siRNA-A3G treated vs. siRNA-Ctl treated).
4. Discussion
APOBEC3G has recently been identified as an antiviral protein that can suppress HIV-1, hepatitis B virus, murine leukemia virus, simian immunodeficiency virus and equine infectious anemia virus (Mangeat et al., 2003; Navarro and Landau, 2004; Noguchi et al., 2005; Rosler et al., 2005). These new findings prompted us to examine whether human neuronal cells express functional APOBEC3G and whether its expression is regulated by cytokines/chemokines. We demonstrated that both primary brain tissue and human neuronal cells expressed APOBEC3G at both mRNA and protein levels (Fig.1). We also demonstrated that human astroglioma cells (U87-MG) expressed APOBEC3G. These findings support the recent report that human primary neuronal cells and astrocytes expressed APOBEC3G (Argyris et al., 2007). More importantly, we demonstrated that APOBEC3G produced by NT2-N and U87-MG cells had antiviral activity, as evidenced by the observation that the cells transfected with the siRNA against APOBEC3G had higher levels of pesudotyped HIV-1 replication than untransfected or control siRNA-transfected cells (Fig. 7). This finding suggests that human neuronal cells and astrocytes produce functional APOBEC3G. Our further experiments confirmed that APOBEC3G in these cells is involved in the innate immune response to viral infection, as we showed that pseudotyped HIV-1 infection could induce APOBEC3G expression (Fig. 6). We next examined the hypothesis that cytokines/chemokines have the ability to modulate APOBEC3G expression in the neuronal cells. This speculation is based on the observations that human neuronal cells express multiple cytokine receptors (Coughlan et al., 2000; Hopkins and Rothwell,1995; Klein et al., 2000; Mehler and Kessler, 1997; Rothwell et al., 1996). In addition, several studies have demonstrated that both glial and neuronal cells express multiple functional chemokine receptors and chemokines in the CNS (Coughlan et al., 2000; Guo et al., 2003). It is well known now that cytokines and chemokines interacting with specific receptors on the neuronal cells are involved in neuroimmunoregulation.
Our initial studies focused on IFNs, as these cytokines have been demonstrated to have the ability to induce APOBEC3G expression in different cell systems. Our data that IFN-α potently up-regulated the expression of APOBEC3G in NT2-N agrees with the studies by others (Argyris et al., 2007; Bonvin et al., 2006; Chen et al., 2006; Komohara et al., 2007, 2006; Peng et al., 2006; Tanaka et al., 2006), showing that IFN-α induces APOBEC3G expression in other type of cells, including various primary cells (hepatocytes, macrophage, CD4+ T cells, plasmacytoid dendritic cells, kidney cells and brain microvascular endothelial cells) and cell lines (Huh7, HepG2 and 293T cells). In addition to IFN-α, IFN-β also enhanced APOBEC3G expression in the both neuronal cells and astrocytes (Fig. 3). This finding is highly significant since many viruses induce IFN-β prior to IFN-α expression through toll-like receptor (TLR) pathway. Similar to type I IFNs, type II IFN (IFN-γ) also induced the expression of APOBEC3G in the neuronal cells (Fig. 4A). Furthermore, several inflammatory cytokines (TNF-α and IL-1β) also up-regulated APOBEC3G expression in the neuronal cells (Fig. 4B, C). This cytokine-mediated up-regulation of APOBEC3G expression in the neuronal cells is specific, as other cytokines (IL-4, IL-6 and TGF-β1) (Fig. 4D, E and F) and CC-chemokines (data not shown) had little or no effects on APOBEC3G expression in human neuronal cells.
In addition to our attempt to examine the effect of individual cytokine/chemokine on APOBEC3G expression, we also determined the impact of mixture of multi-cytokines/chemokines on the expression of APOBEC3G in the neuronal cells. In order to mimic in vivo microenvironment where macrophages/microglia in the CNS release multiple cytokines/chemokines that affect neuronal functions, we examined whether supernatants from macrophage cultures have impact on APOBEC3G expression in NT2-N cells. We were particularly interested in monocytes/macrophages, as these cells produce cytokines/chemokines that act not only in a paracrine-like fashion in the microenvironment to stimulate immune responses, but also in an endocrine-like fashion on distant organs that participate in inflammatory responses. More importantly, monocytes/macrophages have been shown to play a key role in HIV-mediated neuronal impairment in the CNS. We showed that the expression of APOBEC3G in NT2-N cells was greatly enhanced by supernatants from macrophage cultures stimulated with LPS, a well-known activator of monocytes/macrophages (Ding et al., 2001; Guo et al., 2003; Herbein et al.,1995; Lai et al., 2001; Monari et al., 2005). This activated macrophage-medicated induction of APOBEC3G is not due to the direct effect of LPS, since LPS alone (directly added toNT2-Ncultures) had no effect on APOBEC3G expression. This finding is highly significant and clinically relevant, as LPS has recently been identified as a cause of systemic immune activation in chronic HIV-1 infection (Ancuta et al., 2008; Douek, 2007). In addition, LPS induces monocyte activation, monocyte trafficking across the blood-brain barrier (BBB), leading to the production of inflammatory cytokines in the CNS. It is known that the production of multiple cytokines/chemokines by LPS-activated macrophage/microglia contributes to the disruption of neuronal homeostasis and development of severe neurological abnormalities, including HIV-associated dementia.
Taken together, the present study provides direct and compelling experimental evidence that there is intracellular expression and regulation of functional APOBEC3G in human neuronal cells and astrocytes, which might have a crucial role in glial and neuronal cell-mediated innate defense against viral infections in the CNS. While the mechanisms underlying the regulation of APOBEC3G and biological functions of APOBEC3G in human neuronal cells remain to be determined, the induction of functional APOBEC3G expression by both type I and type II IFNs suggests the possibility that APOBEC3G is involved in neuronal cell-mediated innate protection in the CNS. Further studies are necessary in order to determine the molecular mechanisms by which APOBEC3G is regulated by cytokines and other cellular factors in the CNS. In addition, it is of a great interest to determine whether APOBEC3G has other not yet known biological functions in the CNS.
Acknowledgements
We thank Drs. Shuxian Hu and Phillip K. Peterson (University of Minnesota Medical School, Minneapolis, MN, USA) for providing primary human neurons.
Abbreviation
- APOBEC3G
apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G
- BDNF
brain-derived neurotrophic factor
- BMVECs
brain microvascular endothelial cells
- CCL3
CC chemokine ligand 3
- CCL4
CC chemokine ligand 4
- CCL5
CC chemokine ligand 5
- CNS
central neurons system
- DMEM
Dulbecco's modified Eagle's medium
- DSU
densitometry scanning unit
- FBS
fetal calf serum
- hEGF
human epidermal growth factor
- hFGFb
human fibroblast growth factor-basic
- HIV-1
human immunodeficiency virus type 1
- IFN-α
interferon alpha
- IFN-β
interferon beta
- IFN-γ
interferon gamma
- IL-1β
interleukin-1 beta
- IL-4
interleukin-4
- IL-6
interleukin-6
- IMDM
Iscover's modified Dulbecco's medium
- LPS
lipopolysaccharide
- NMDA
N-methyl-d-aspartate
- NPC
neural precursor cell
- PBL
peripheral blood lymphocytes
- RA
retinoic acid
- TGF-β1
transforming growth factor beta1
- TNF-α
tumor necrosis factor alpha
Footnotes
This work was supported by the National Institutes of Health grants (DA012815 and DA022177 to W.Z.H.).
References
- Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Argyris EG, Acheampong E, Wang F, Huang J, Chen K, Mukhtar M, Zhang H. The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system. Virology. 2007;367:440–451. doi: 10.1016/j.virol.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca-Regen L, Heinzinger N, Stevenson M, Gendelman HE. Alpha interferon-induced antiretroviral activities: restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J. Virol. 1994;68:7559–7565. doi: 10.1128/jvi.68.11.7559-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP, Greeve J. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43:1364–1374. doi: 10.1002/hep.21187. [DOI] [PubMed] [Google Scholar]
- Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J. Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top. HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- Ferrari A, Ehler E, Nitsch RM, Gotz J. Immature human NT2 cells grafted into mouse brain differentiate into neuronal and glial cell types. FEBS Lett. 2000;486:121–125. doi: 10.1016/s0014-5793(00)02251-1. [DOI] [PubMed] [Google Scholar]
- Guillemain I, Alonso G, Patey G, Privat A, Chaudieu I. Human NT2 neurons express a large variety of neurotransmission phenotypes in vitro. J. Comp. Neurol. 2000;422:380–395. doi: 10.1002/1096-9861(20000703)422:3<380::aid-cne5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Douglas SD, Lai JP, Pleasure DE, Li Y, Williams M, Bannerman P, Song L, Ho WZ. Interleukin-1beta stimulates macrophage inflammatory protein-1alpha and -1beta expression in human neuronal cells (NT2-N) J. Neurochem. 2003;84:997–1005. doi: 10.1046/j.1471-4159.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J. Immunol. Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Herbein G, Doyle AG, Montaner LJ, Gordon S. Lipopolysaccharide (LPS) down-regulates CD4 expression in primary human macrophages through induction of endogenous tumour necrosis factor (TNF) and IL-1 beta. Clin. Exp. Immunol. 1995;102:430–437. doi: 10.1111/j.1365-2249.1995.tb03801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Hu S, Cheeran MC, Sheng WS, Ni HT, Lokensgard JR, Peterson PK. Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J. Pharmacol. Exp. Ther. 2006;318:1280–1286. doi: 10.1124/jpet.106.103853. [DOI] [PubMed] [Google Scholar]
- Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–296. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- Jin X, Brooks A, Chen H, Bennett R, Reichman R, Smith H. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J. Virol. 2005;79:11513–11516. doi: 10.1128/JVI.79.17.11513-11516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BD, White HS, Callahan KS. Cytokine and intracellular signaling regulation of tissue factor expression in astrocytes. Neurochem. Int. 2000;36:441–449. doi: 10.1016/s0197-0186(99)00147-3. [DOI] [PubMed] [Google Scholar]
- Komohara Y, Yano H, Shichijo S, Shimotohno K, Itoh K, Yamada A. High expression of APOBEC3G in patients infected with hepatitis C virus. J. Mol. Histol. 2006;37:327–332. doi: 10.1007/s10735-006-9059-0. [DOI] [PubMed] [Google Scholar]
- Komohara Y, Suekane S, Noguchi M, Matsuoka K, Yamada A, Itoh K. Expression of APOBEC3G in kidney cells. Tissue Antigens. 2007;69:95–98. doi: 10.1111/j.1399-0039.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- Meylan PR, Guatelli JC, Munis JR, Richman DD, Kornbluth RS. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology. 1993;193:138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- Monari C, Bistoni F, Casadevall A, Pericolini E, Pietrella D, Kozel TR, Vecchiarelli A. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 2005;191:127–137. doi: 10.1086/426511. [DOI] [PubMed] [Google Scholar]
- Navarro F, Landau NR. Recent insights into HIV-1 Vif. Curr. Opin. Immunol. 2004;16:477–482. doi: 10.1016/j.coi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res. Dev. Brain Res. 2004;152:159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Noguchi C, Ishino H, Tsuge M, Fujimoto Y, Imamura M, Takahashi S, Chayama K. G to A hypermutation of hepatitis B virus. Hepatology. 2005;41:626–633. doi: 10.1002/hep.20580. [DOI] [PubMed] [Google Scholar]
- Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler C, Kock J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizsacker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42:301–309. doi: 10.1002/hep.20801. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol. Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, Ikai I, Chiba T. Anti-viral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem. Biophys. Res. Commun. 2006;341:314–319. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- Turelli P, Trono D. Editing at the crossroad of innate and adaptive immunity. Science. 2005;307:1061–1065. doi: 10.1126/science.1105964. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O'Brien CP, Song L, Davis-Vogal A, Ho WZ. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin. Exp. Res. 2002;26:1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng JS, Metzger DS, O'Brien CP, Zhang T, Ho WZ. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J. Leukoc. Biol. 2006;79:1166–1172. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- Younkin DP, Tang CM, Hardy M, Reddy UR, Shi QY, Pleasure SJ, Lee VM, Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]